An exquisitely preserved tiny bark-gnawing beetle (Coleoptera: Trogossitidae) from mid-Cretaceous Burmese amber and the phylogeny of Trogossitidae
Contributing authors: Yan-Da Li ([email protected],), Erik Tihelka ([email protected]), Richard A. B. Leschen ([email protected]), Yali Yu ([email protected]), Adam Ślipiński ([email protected]), Hong Pang ([email protected]), Diying Huang ([email protected]), Jiří Kolibáč ([email protected])
ZooBank link: LSID: http://zoobank.org/urn:lsid:zoobank.org:pub:3694913C-FA40-4520-88F8-3C42E441EDC6
Online ISSN: 1439-0469
Abstract
The first fossil representative of the cleroid family Trogossitidae is described from mid-Cretaceous Burmese amber. Microtrogossita qizhihaoi Li & Cai gen. et sp. nov. is unique among Trogossitidae in the relatively widely separated procoxal and mesocoxal cavities, weakly asymmetrical antennal clubs, coarsely facetted eyes, coarse sculpture of dorsal and ventral surfaces of thorax in comparison with tiny body size, and the absence of spines along side margin of tibiae. Morphological characters of the fossil were analyzed together with representatives of 44 extant genera of Cleroidea (Peltidae, Lophocateridae, and Trogossitidae) in a matrix of 93 characters. Microtrogossita qizhihaoi was resolved as a member of Trogossitini within Trogossitidae. The tribal composition of Trogossitidae is discussed in light of our re-analysis of a previously published four-gene dataset under a site-heterogeneous model. The recently described lophocaterid Mesolophocateres pengweii Yu, Leschen & Ślipiński syn. nov. from Burmese amber is suggested to be a junior synonym of Burmacateres longicoxa Kolibáč & Peris.
1 INTRODUCTION
The former beetle family Trogossitidae (e.g., Barron, 1971; Kolibáč, 2006, 2008; Ślipiński, 1992) (Cleroidea) had been revealed to be paraphyletic in several molecular phylogenies (e.g., Bocak et al., 2014; Bocakova et al., 2016; Kolibáč et al., 2021; McKenna et al., 2015, 2019; Zhang et al., 2018), and it has been recently divided into several families in the most comprehensive analysis by Gimmel et al. (2019). Under the traditional broad definition, the family comprises about 600 extant species in over 50 genera distributed worldwide (Kolibáč & Leschen, 2010; Kolibáč, 2013). The modern Trogossitidae sensu Gimmel et al. (2019) includes approximately 400 species in 25 genera distributed worldwide, however, with the distinctly largest species richness in North and South America (Kolibáč, 2013).
Numerous cleroid fossils classified within the former broadly defined Trogossitidae, that is, the present Lophocateridae, Peltidae, and Trogossitidae, have been reported from various Mesozoic deposits (e.g., Kolibáč & Peris, 2021; Peris et al., 2014; Yu et al., 2014, 2015). Three fossil genera, Cretocateres, Eotenebroides and Thoracotes, have been placed into the tribe Trogossitini (Kolibáč, 2013). However, these compression fossils are generally poorly preserved, making their systematic placement dubious in some cases. Here, we report the first certain member of Trogossitidae (the family name used in its modern sense following Gimmel et al., 2019, hereafter) from mid-Cretaceous Burmese amber (ca. 99 Ma) with astonishing fine details preserved. This newly discovered genus implies that the family was already diverse by the Cretaceous. The phylogenetic position of the fossil is investigated with a formal morphological phylogenetic analysis. We furthermore address the phylogeny of the recently revised Trogossitidae by re-analyzing a previously published four-gene molecular dataset (Gimmel et al., 2019).
2 RESULTS
2.1 Systematic paleontology
Order Coleoptera Linnaeus, 1758
Suborder Polyphaga Emery, 1886
Superfamily Cleroidea Latreille, 1802
Family Trogossitidae Latreille, 1802
Microtrogossita Li & Cai gen. nov.
- Type species. Microtrogossita qizhihaoi sp. nov.
- Etymology. The generic name is derived from the Latin “micro-”, meaning small, and the generic name “Trogossita”, the type genus of Trogossitidae (= Temnoscheila Westwood, 1830). The name is feminine in gender.
- Diagnosis. Body minute. Frons without a longitudinal groove in center or horns at anterior corners. Eyes moderately large. Antennal grooves absent. Antennae with 10 antennomeres; club weakly asymmetrical. Pronotal disk as wide as long, flattened in center, with coarse sculpture composed of deep punctures. Prosternal process in its mid-length as wide as transverse coxal diameter. Procoxal cavities externally closed. Mesocoxal cavities open. Mesocoxal cavities comparatively widely separated; space between mesocoxae approximately as wide as half of transverse coxal diameter. Elytra with eight longitudinal striae of large punctures.
Microtrogossita qizhihaoi Li & Cai sp. nov.
http://zoobank.org/urn:lsid:zoobank.org:act:D7A3BC9B-AD3B-4A39-A800-E6C55479BF89
- Etymology. The species is named after Mr. Zhihao Qi, who kindly donated many fossil specimens for our research.
- Material. Holotype, NIGP173910, sex unknown, mid-Cretaceous (upper Albian to lower Cenomanian; Shi et al., 2012; Mao et al., 2018; but also see Balashov, 2021), from amber mine near Noije Bum Village, Tanai Township, Myitkyina District, Kachin State, Myanmar.
- Diagnosis. As for the genus.
-
Description. Body elongate, coarsely punctate and sparsely covered with setae. Sculpture of head composed of deep but scarce punctures; pronotum with deep punctures along lateral sides (interspaces approximately as wide as puncture diameters), middle part with sparse punctation (interspaces wider than puncture diameters); elytral sculpture composed of eight distinct regular striae with rounded deep punctures, interspaces among them as wide as puncture diameter or wider. Ventral face of thorax with deep sparse punctation in middle parts (namely in prosternum, meso-, and metaventrite).
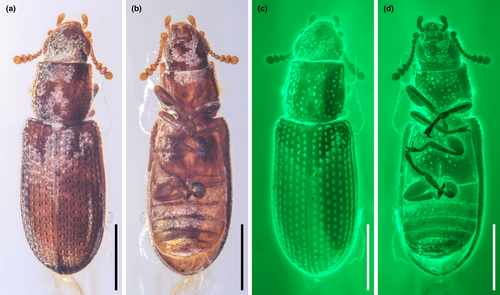
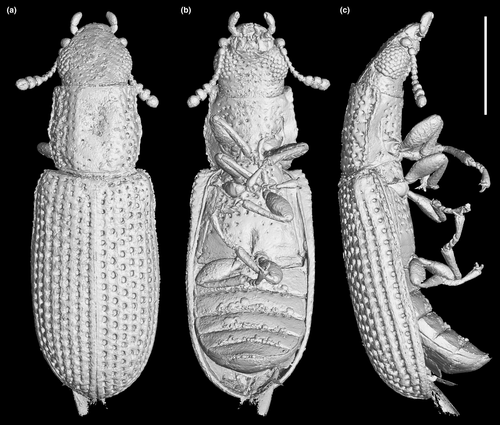
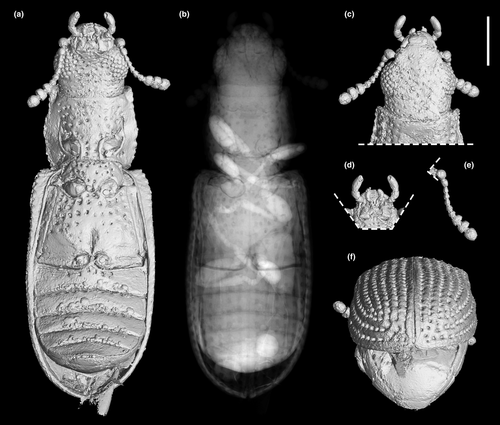
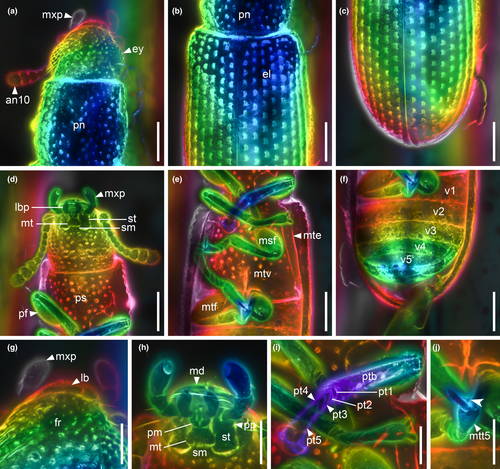
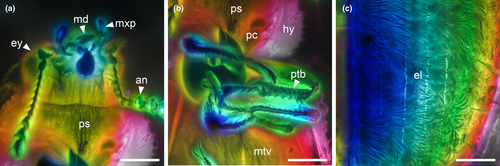
Head (Figures 3c, and 4a,d) prognathous, including eyes almost as wide as anterior margin of pronotum. Frons (Figure 4g) flat, without a longitudinal groove in center or horn-like processes at lateral corners. Frontoclypeal suture absent; anterior margin of cranium along base of labrum straight, without emargination; and lateral corners angulate but without extending horns. Compound eyes moderately large, not emarginate, laterally situated, without interfacetal setae, coarsely facetted. Antennal insertions widely separated. Well-defined antennal grooves absent. Antennae (Figures 3e and 4d) with 10 antennomeres; antennomere 1 (scape) robust, antennomere 2 (pedicel) smaller than antennomere 1 but longer and wider than antennomere 3, antennomeres 3–7 mutually similar in size; antennomeres 8–10 enlarged, weakly asymmetrical, forming a distinct club. Labrum (Figure 3c) free, broadly oval. Mandibles (Figure 4h) possibly with two vertically situated apical teeth. Maxillary palps (Figures 3d and 4h) 4-segmented; palpomere 1 small; palpomeres 2–3 similar in size; palpomere 4 about twice as long as palpomere 3, with a large sensorial cavity at apex. Labial palps (Figures 3d and 4h) 3-segmented; apical palpomere about twice as long as palpomere 2. Mentum (Figure 4h) trapezoidal. Prementum seemingly deeply emarginate anteriorly. Submentum (Figure 4h) without ctenidium. Gular sutures inconspicuous.
Prothorax along basal margin 0.8 times as wide as humeral part of elytra. Pronotum with distinct lateral carina; carina not bordered from dorsal side; pronotal disk (Figure 4a) flattened in center, almost as wide as long, widest at middle, distinctly narrowed forwards and backwards with anterior portion approximately as wide as posterior (basal) one; anterior margin nearly straight; anterior corners not projected. Prosternum (Figures 3a and 4d) in front of coxae transverse, twice as long as diameter of procoxal cavity; prosternal process weakly dilated at apex; apex truncate. Procoxal cavities externally closed (Figure 3a).
Scutellar shield (Figure 4b) small, transversely oval. Elytra (Figures 3f and 4b,c) completely covering abdomen, without humeral denticles, widest at second third, rounded at apex, 1.8 times as long as width combined, 2.6 times as long as pronotal length; surface with eight rows of large punctures. Prepectus not observed. Mesoventrite transverse, shortened; intercoxal process with apex divided into two tips. Mesocoxal cavities relatively widely separated, externally narrowly open (Figure 3a). Metaventrite (Figures 3a, and 4e) wide; discriminal line (discrimen) conspicuous in posterior third of metaventrite.
Legs as shown in Figure 4e. Procoxae not projecting, transverse; mesocoxae nearly spherical; metacoxae reach almost elytral epipleura. All trochanters small, triangular. All tibiae without conspicuous spines along the outer margin. Tarsal formula 5–5–5; tarsomere 1 short in all legs; tarsomeres 2–4 approximately same in size; and tarsomere 5 as long as 1–4 combined (Figure 4i). Claws (Figure 4j) without denticles; empodium projected, bisetose.
Abdomen (Figure 4f) with five ventrites. Ventrite 1 with distinct intercoxal process; ventrite 5 about 1.1 times as long as ventrite 4. Sternites III–VII with two pairs of parasternites (Figure 2c).
- Measurements of holotype. Body length, 1.93 mm; body width, 0.66 mm; head length, 0.46 mm; head width, 0.39 mm; pronotal length, 0.47 mm; pronotal width, 0.48 mm; and elytral length, 1.21 mm.
2.2 Morphological phylogenetic analysis
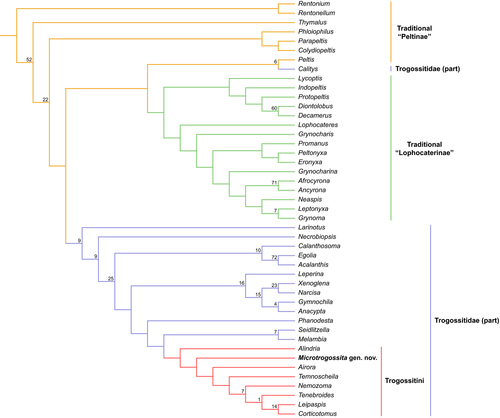
The morphological character matrix was modified based on Kolibáč (2006, 2008), who conducted a phylogenetic analysis of 44 genera of the former “Trogossitidae.” Recent molecular studies have demonstrated that the traditional “Trogossitidae” is actually polyphyletic (Gimmel et al., 2019; Kolibáč et al., 2021; McKenna et al., 2019; Zhang et al., 2018). Thus, this morphological matrix cannot appropriately resolve the higher-level relationship between traditional trogossitid taxa. Because of above-mentioned progress in a study of Cleroidea, the goal of our analysis is merely to assess a placement of our fossil within the modern Trogossitidae.
Parsimony analyses using implied weighting (K = 12) yielded a single most parsimonious tree (Figure 5). The overall support values of key nodes of the tree were quite low, suggesting morphological information alone is not sufficient to solve the phylogeny of Trogossitidae. The traditional “Peltinae” was resolved as a basal paraphyletic grade, and the traditional “Lophocaterinae” appeared to be a monophyletic clade. The majority of modern Trogossitidae (not including Calityini) formed a monophyletic clade. The newly discovered fossil, M. qizhihaoi, was nested within the extant tribe Trogossitini (Trogossitidae), and sister to all other trogossitids except Alindria, though with low statistical support.
2.3 Molecular phylogenetic analysis
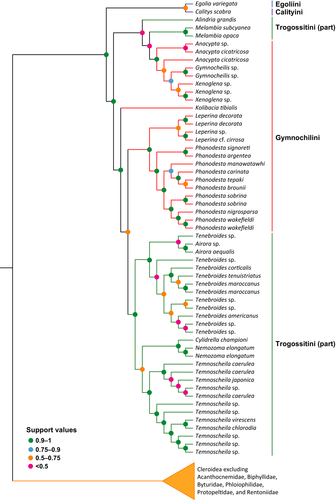
We focused on quantifying uncertainty in intertribal relationships in Trogossitidae (Trogossitinae sensu Kolibáč, 2013) by testing the robustness of Gimmel et al.'s (2019) recovered topology to different analytical methods. Specifically, we tested the effect of using a site-heterogeneous model to analyze the four-gene dataset. While more computationally intensive than the site-homogeneous models applied to the study of cleroid phylogeny in the past, site-heterogeneous models account for the unequal rate of evolution in sequences and yield phylogenies more robust to phylogenetic artifacts such as long branch attraction (Cai et al., 2020; Lartillot et al., 2007; Pisani et al., 2015).
We recovered a strongly supported clade (Bayesian Posterior Probabilities [BPP] = 0.91) consisting of Egoliini, Calityini, Gymnochilini, and Trogossitini (Figure 6). The Egolia + Calitys clade was well supported as sister to Gymnochilini and Trogossitini. In line with Gimmel et al. (2019), we recovered Gymnochilini nested within Trogossitini. The phylogenetic position of the fifth tribe traditionally associated with Trogossitinae, Larinotini, was poorly supported in the present analysis (BPP = 0.59) and recovered as sister to the genus Peltis (Peltidae).
3 DISCUSSION
3.1 Systematic position of Microtrogossita and comparison with extant relatives
A classification of the new genus within Trogossitidae is chiefly based on combination of (a) general habitus (elongate body), (b) pronotum with lateral carina, (c) asymmetrical antennal club, (d) all pairs of legs with ultimate tarsomeres as long as previous tarsomeres together, (e) projected bisetose empodium situated between simple tarsal claws, and (f) procoxal cavities externally closed (Kolibáč, 2013; Lawrence et al., 2014).
Microtrogossita gen. nov. further shares some morphological similarities with the tribe Trogossitini in (a) elongate and moderately convex body, (b) the absence of scales or tufts of setae on dorsal surface, (c) elytra with regular rows of punctures ordered in striae, (d) the absence of frontoclypeal suture and structure of anterior margin of head with reduced clypeal area, and (e) pronotum without projected anterior corners. This affinity between Microtrogossita and Trogossitini was also supported by our cladistic analysis (Figure 5).
On the other hand, Microtrogossita differs from all known trogossitid genera in (a) weakly asymmetrical club (in contrast to the distinctly asymmetrical club: figure 9C, D in Kolibáč et al., 2021; figure 14G in Kolibáč, 2013), (b) relatively wide separation of meso- and procoxal cavities (in contrast to the narrower separation: figure 1A in Kolibáč, 2013), (c) extremely coarse sculpture of dorsal and even ventral surface. Also, the absence of spines along outer margin of tibiae is rare within Trogossitidae. Considering its unique character combination in Trogossitidae, a more basal position for Microtrogossita cannot be confidently ruled out.
Currently, there are 13 extant genera in the tribe Trogossitini (Kolibáč, 2013). Microtrogossita shares shape of head (especially structure of anterior margin of clypeus; Figure 3c) with the most of trogossitids excepting Nemozoma, Corticotomus, and partly also Euschaefferia. The latter genera possess frons with pair of distinctly projecting horns which is believed to assist movement in narrow galleries inside wood (Hinson & Buss, 2016; Kolibáč, 2014a). The antennae of Microtrogossita only have 10 antennomeres (Figures 3e and 4d), which are rare in Trogossitini. The majority of Trogossitini (except for some species in Nemozoma) all possess 11-segmented antennae. Pronotum without distinctly extended anterior corners differs Microtrogossita from majority of trogossitids but the feature is shared with several genera including Airora, Parallelodera, Nemozoma, and Corticotomus. However, widened mid-part with narrowed anterior and posterior parts is exceptional (on contrary to cordate pronotum of, e.g., Tenebroides or some Temnoscheila). Coarsely facetted eyes of Microtrogossita may refer to hidden way of life, for example, under bark, in wood-borers galleries or even in litter as documented in various Cleroidea including Afrocyrona (Kolibáč, 2014b), Parapeltis, and Colydiopeltis (Ślipiński, 1992). In contrary, rapid flyers and surface dwellers among Trogossitidae (e.g., Anacypta, Xenoglena, Elestora, Parallelodera) and Lophocateridae (Ancyrona) often hunt for other insects in full sunlight on tree bark, branches, or leaves—such species mostly have eyes finely facetted (Kolibáč, 2013). Deep punctures in both dorsal and ventral surfaces as well as distinct striae in elytra also differ Microtrogossita from the extant trogossitids.
3.2 Notes on the intertribal relationships in Trogossitidae
Traditionally, five tribes were included in the subfamily Trogossitinae (or Trogossitidae in the modern sense): Calityini, Egoliini, Larinotini, Gymnochilini, and Trogossitini (e.g., Kolibáč, 2013). This arrangement was supported by the morphological phylogenetic analyses of Kolibáč (2006, 2008). However, in the molecular phylogeny of Gimmel et al. (2019), Calityini, Egoliini, and Larinotini did not form a clade together with Gymnochilini and Trogossitini, but rather occupied a position closer to Peltidae. Though Calityini indeed display a mixture of both peltid and trogossitid characters, the larvae of Egoliini clearly show an affinity to trogossitids (Kolibáč, 2013). In our re-analysis of Gimmel et al.'s (2019) data with the site-heterogeneous model CAT-GTR+G4, Calityini and Egoliini clustered with Gymnochilini and Trogossitini (Figure 6). Thus, the placement of Calityini and Egoliini remains yet to be convincingly resolved. Both Gymnochilini and Trogossitini were recovered as non-monophyletic in both the original analysis by Gimmel et al. (2019) and our re-analysis, indicating that their taxonomic status requires further attention.
3.3 Mesolophocateres Yu, Leschen & Ślipiński as a junior synonym of Burmacateres Kolibáč & Peris
Kolibáč and Peris (2021) reported the first Lophocateridae species from the mid-Cretaceous Burmese amber, Burmacateres longicoxa Kolibáč & Peris, 2020 (Figure 7, Figures S1 and S2). Burmacateres is characterized by 11-segmented antennae (Figure 7a), extremely enlarged procoxae (Figure 7b), and dorsal surface with tufts of hairs (Figure 7c). A few weeks later, Yu et al. (2021) described another two lophocaterids from Burmese amber, Mesolophocateres pengweii Yu, Leschen & Ślipiński, 2020 and Parayixianteres parvus Yu, Leschen & Ślipiński, 2020, in the same journal. However, the description and photographs of M. pengweii match well with those of B. longicoxa. Actually, we are unable to detect any significant differences between the two species. Thus, we herein suggest that Mesolophocateres syn. nov. is a junior synonym of Burmacateres, and M. pengweii syn. nov. is a junior synonym of B. longicoxa.
4 MATERIALS AND METHODS
4.1 Materials
The Burmese amber specimens studied herein originated from amber mines near Noije Bum (26°20′N, 96°36′E), Hukawng Valley, Kachin State, northern Myanmar. The specimens are deposited in the Nanjing Institute of Geology and Palaeontology (NIGP), Chinese Academy of Sciences, Nanjing, China. The amber pieces were trimmed with a small table saw, ground with emery papers of different grit sizes, and finally polished with polishing powder.
4.2 Fossil imaging
Photographs under incident light were taken with a Zeiss Discovery V20 stereo microscope. Widefield fluorescence images were captured with a Zeiss Axio Imager 2 light microscope combined with a fluorescence imaging system. Confocal images were obtained with a Zeiss LSM710 confocal laser scanning microscope, using the 488-nm Argon laser excitation line (Fu et al., 2021). Images under incident light and widefield fluorescence were stacked in Helicon Focus 7.0.2 or Zerene Stacker 1.04. Confocal images were stacked with color coding for depth in ZEN 2.3 (Blue Edition). The holotype of M. qizhihaoi was also imaged using high-resolution X-ray microtomography (Zeiss Xradia 520 Versa) at the micro-CT laboratory of NIGP. Due to the comparatively small size of the fossil specimen, a CCD-based 4× objective was used, providing isotropic voxel sizes of 2.4165 μm with the help of geometric magnification. During the scanning, the acceleration voltage for the X-ray source was 50 kV. To improve the signal-to-noise ratio, 3001 projections over 360° were collected, and the exposure time for each projection was 1.5 s. The tomographic data were analyzed using VGStudio MAX 3.0. Images were further processed in Adobe Photoshop CC to enhance contrast.
The original confocal and micro-CT data are available in Zenodo repository (https://doi.org/10.5281/zenodo.3994902).
4.3 Measurements and description
The measurements were generally based on the micro-CT reconstruction. The measurements were taken as follows: body length as distance from mandibular apex to elytral apex when body fully stretched; body width as maximum width of body (at the middle part of elytra); head length as distance from mandibular apex to anterior margin of pronotum (from dorsal view); head width as maximum width of head across eyes; pronotal length as maximum length of pronotum; pronotal width as maximum width of pronotum; and elytral length as distance from anterior margin to apex.
The morphological terminology follows Lawrence and Ślipiński (2013) and Kolibáč (2005, 2013).
4.4 Morphological phylogenetic analysis
To evaluate the systematic placement of the new species, a morphological phylogenetic analysis was performed. The data matrix (Data S1 and S2) was mainly derived from a previously published dataset (Kolibáč, 2008). The coding of character 36 (shape of pronotum) in Kolibáč (2008) was problematic. The states “transverse” and “elongate” refer to the ratio of length and width, while the state “cordate” means the pronotum is narrowed toward base. A species may match the criteria of both “transverse” (or “elongate”) and “cordate” at the same time. Thus, this character was split into two characters in our analysis. The character 32 (shape of antennal club) in Kolibáč (2008) was also split into two characters. In addition, a new informative character, the shape of pronotal carina, was added. All characters were treated as non-additive. Rentonium and Rentonellum were selected as the out-group taxa, based on previous molecular results (Gimmel et al., 2019; McKenna et al., 2019). Parsimony analysis was performed under implied weights using the program TNT 1.5 (Goloboff & Catalano, 2016; Goloboff et al., 2008). Parsimony analysis has been shown to achieve the highest accuracy under a moderate weighting scheme (e.g., when concavity constants K are between 5 and 20) (Goloboff et al., 2018; Smith, 2019). Therefore, the concavity constant was set to 12 here, as suggested by Goloboff et al. (2018). Standard bootstrap analysis was implemented by 1000 pseudoreplicates, where the support values were shown as frequency differences (Goloboff et al., 2003). The tree was drawn with the online tool iTOL 5.7 (Letunic & Bork, 2021) and graphically edited with Adobe Illustrator CC 2017.
4.5 Molecular phylogenetic analysis
To test the relationships among the tribes of Trogossitidae, we used the most comprehensive dataset available for the group published to date, including all the currently recognized cleroid families, compiled by Gimmel et al. (2019). Four genes were included, namely the nuclear genes 18S and 28S, and the mitochondrial CYTB and COX1. All sequences were obtained from GenBank using the Batch Entrez tool. The accession numbers were the same as provided by Gimmel et al. (2019). Mitochondrial genes were aligned in MEGA X 10.0.5 with the “MUSCLE” tool (Kumar et al., 2018), while 18S and 28S were aligned in the MAFFT 7.313 plug-in in PhyloSuite 1.2.1 (Zhang et al., 2020) using the Q-INS-I algorithm (Katoh et al., 2002; Katoh & Toh, 2008). The CYTB sequence for Chaetosoma scaritides could not be unambiguously aligned and was excluded. The site-heterogeneous mixture model CAT-GTR+G4 was run in PhyloBayes mpi 1.7 (Lartillot et al., 2009). Two independent Markov chain Monte Carlo (MCMC) chains were run until convergence (maxdiff <0.3). Convergence was assessed by using the bpcomp program to generate output of the largest (maxdiff) and mean (meandiff) discrepancies observed across all bipartitions.
All data needed to run the molecular analysis and the output files are available in Zenodo repository (https://doi.org/10.5281/zenodo.4737037).
4.6 Nomenclatural acts
This published work and the nomenclatural acts it contains have been registered in ZooBank. The LSID for this publication is http://zoobank.org/urn:lsid:zoobank.org:pub:3694913C-FA40-4520-88F8-3C42E441EDC6.
ACKNOWLEDGMENTS
We are grateful to Su-Ping Wu for technical help in micro-CT reconstruction, Yan Fang for technical help in confocal imaging, and Christopher Grinter and Rachel Diaz-Bastin for providing information regarding the holotype of Euschaefferia mclay. Financial support was provided by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB26000000 and XDB18000000), the National Natural Science Foundation of China (41688103, 31702039, and 32070471), and the Second Tibetan Plateau Scientific Expedition and Research project (2019QZKK0706). Richard Leschen was funded by Strategic Science Investment Funding for Crown Research Institutes from the Ministry of Business, Innovation and Employment's Science and Innovation Group. Jiří Kolibáč was supported through long-term conceptual development program for research institutions (MK000094862) by the Ministry of Culture of the Czech Republic.




