Phylogenomics of the bumblebee catfishes (Siluriformes: Pseudopimelodidae) using ultraconserved elements
Contributing authors: Bruno F. Melo ([email protected], [email protected]), Fábio F. Roxo ([email protected]), Luz E. Ochoa ([email protected]), Oscar A. Shibatta ([email protected]), Mark H. Sabaj ([email protected]), Claudio Oliveira ([email protected])
Zoobank link: LSID: urn:lsid:zoobank.org:pub:EF11973E-91D7-4267-9D29-86457B34706A
Online ISSN: 1439-0469
Abstract
Neotropical catfishes of the family Pseudopimelodidae comprise 53 species allocated to seven genera widely distributed in South America from northwestern Colombia and Venezuela to Argentina and Uruguay. Intergeneric relationships based on morphology-based phylogenies are conflicting, and the interspecific relationships remain incipient. We conducted the first molecular phylogeny of the family by analyzing sequence data from ultraconserved elements (UCEs) of the genome for 33 specimens of Pseudopimelodidae and 19 related taxa. Phylogenetic relationships were accessed by concatenated matrices using Bayesian inference and, maximum likelihood, and the coalescent approach by a species tree analysis. The phylogeny with 868 UCE loci and 906,689 bp strongly support the monophyly of Pseudopimelodidae, and the arrangement of two major subclades herein classified as subfamilies Pseudopimelodinae and the newly proposed Batrochoglaninae. Pseudopimelodinae is composed by Cruciglanis sister to Pseudopimelodus and Rhyacoglanis, whereas the new subfamily Batrochoglaninae is composed by Cephalosilurus and Lophiosilurus as sister to Batrochoglanis and Microglanis. Pseudopimelodinae is supported by five morphological synapomorphies and Batrochoglaninae supported by three such synapomorphies. The results of this study will surely guide future research aiming to delimit and describe species within the monophyletic groups.
1 INTRODUCTION
Catfishes, order Siluriformes, occur in freshwater habitats on all continents, and a few groups are found in estuarine and saltwater environments (Hardman, 2005). The recent diversity of the order is composed of an estimated 3915 species in 500 genera and 39 families (Fricke et al., 2021). In the freshwaters of South America, catfishes are represented by over 2500 species (Fricke et al., 2021) allocated to 15 families in five major monophyletic lineages: Loricarioidei, Diplomystidae, Cetopsidae, Aspredinoidea, and Pimelodoidea (Sullivan et al., 2006). One of the most species-rich clades of siluriforms is the superfamily Pimelodoidea containing Conorhynchos (monotypic), Heptapteridae (228 species), Phreatobiidae (three species), Pimelodidae (114 species), and Pseudopimelodidae (53 species) (Fricke et al., 2021; Sullivan et al., 2013).
Pseudopimelodidae, known as bumblebee catfishes, are characterized by small eyes covered by skin, short maxillary and mental barbels, wide mouth, relatively small head, dorsal- and pectoral-fin spines serrate, and color patterns often including dark blotches over the head and body (Shibatta, 1998, 2003) (Figure 1). They are widely distributed throughout South America, from the La Plata basin in Argentina to Lago Maracaibo in Venezuela and trans-Andean rivers of northwestern Colombia (Shibatta & Vari, 2017). Some species of Batrochoglanis and Microglanis are popular in the aquarium trade (Shibatta, 2003) and Lophiosilurus, a Rio São Francisco endemic, is used in aquaculture (Costa et al., 2015; Sato et al., 2003). In addition, pseudopimelodids have been in the subjects of various studies on development (Guimarães-Cruz et al., 2009), cytogenetics (Gouveia et al., 2018; Martinez et al., 2004), mitogenomics (Carvalho et al., 2016), internal anatomy (Abrahão et al., 2018, 2021; Abrahão & Shibatta, 2015; Birindelli & Shibatta, 2011), and biogeography (Rangel-Medrano et al., 2020; Souza-Shibatta et al., 2018).

Pseudopimelodidae is currently divided into seven genera: Batrochoglanis Gill, 1858; Cephalosilurus Haseman, 1911; Cruciglanis Ortega-Lara & Lehmann, 2006; Lophiosilurus Steindachner, 1889; Microglanis Eigenmann, 1912; Pseudopimelodus Bleeker, 1858, and Rhyacoglanis Shibatta & Vari, 2017. The systematic understanding of the family was recently improved with the descriptions of two genera (Ortega-Lara & Lehmann, 2006; Shibatta & Vari, 2017), and multiple species, especially in Batrochoglanis (Shibatta, 2019; Shibatta & Pavanelli, 2005), Microglanis (e.g., Sarmento-Soares et al., 2006; Ruiz, 2016; Shibatta, 2016; Terán et al., 2016; Souza-Shibatta et al., 2018), and Pseudopimelodus (Restrepo-Gómez et al., 2020). Sixteen out of the 53 valid species of Pseudopimelodidae were described in the last 10 years (Fricke et al., 2021).
Pseudopimelodids were first recognized as monophyletic by Lundberg et al. (1991) and corroborated as such by morphological and molecular studies (Arcila et al., 2017; Hardman, 2005; de Pinna, 1998; Shibatta & Vari, 2017; Sullivan et al., 2006, 2013). In their descriptions of Cruciglanis (Ortega-Lara & Lehmann, 2006) and Rhyacoglanis (Shibatta & Vari, 2017), the authors used morphological data to hypothesize phylogenetic relationships among pseudopimelodid genera (Figure 2a,b). The major conflict between those phylogenies involved the placement of Cephalosilurus + Lophiosilurus either as the sister lineage of all members of the family (Ortega-Lara & Lehmann, 2006) or the sister group to Batrochoglanis + Microglanis (Shibatta & Vari, 2017). Furthermore, Cruciglanis was supported either as the sister group to Batrochoglanis + Microglanis (Ortega-Lara & Lehmann, 2006) or Pseudopimelodus (Shibatta & Vari, 2017). However, these studies included few ingroup species and did not aim to test their interspecific relationships.
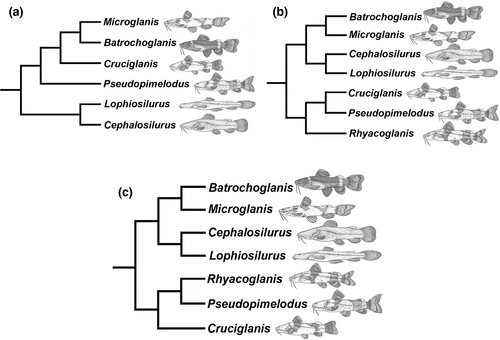
New and informative morphological characters were developed in comparative studies of the gas bladder (Birindelli & Shibatta, 2011) and brain (Abrahão et al., 2018). Although phylogenetic analyses of gas bladder morphology resulted in two distinct hypotheses based on choice of outgroup, both topologies supported a sister group relationship between Cruciglanis and Pseudopimelodus. This relationship is similarly supported by two putative synapomorphies associated with brain morphology (Abrahão et al., 2018). In the most recent phylogenetic study of pseudopimelodids, Rangel-Medrano et al. (2020) used partial sequences from two molecular markers (cytochrome c oxidase subunit I and recombination activation subunit 2) to analyze Pseudopimelodus from trans- and cis-Andean basins in Colombia. Their phylogenetic analysis included all valid genera of Pseudopimelodidae but with few species aside from Pseudopimelodus, and supported the same topology proposed by Shibatta and Vari (2017).
Considering the conflicting hypotheses over intergeneric relationships and the incomplete understanding of interspecific relationships, we conducted a phylogenetic analysis of Pseudopimelodidae based on all seven genera and 36% of the species diversity, and the largest DNA dataset assembled for the family. We used the newly designed ostariophysan probe set to capture 2708 nuclear loci of ultraconserved elements (UCEs) (Faircloth et al., 2012, 2020) to test previous hypotheses of intergeneric and interspecific relationships within Pseudopimelodidae (Ortega-Lara & Lehmann, 2006; Rangel-Medrano et al., 2020; Shibatta & Vari, 2017).
2 MATERIALS AND METHODS
2.1 Taxon sampling
Ingroup sampling included 33 specimens spanning all seven genera and 21 species, (19 valid plus two undescribed), representing 36% of the valid species diversity of Pseudopimelodidae (Table 1). Related taxa included 18 species of the siluriform families Pimelodidae (six species), Heptapteridae (six species), Ariidae (one species), Aspredinidae (one species), Callichthyidae (one species), Doradidae (two species), and Ictaluridae (one species; Table 1). Related taxa were chosen based on a previous phylogenetic hypothesis within Siluriformes (Sullivan et al., 2006). Trees were rooted in the most distant taxon Charax metae (Characiformes: Characidae).
| Matrices | Trimming | Total UCE loci | Total bp | Analysis | Figures | |
|---|---|---|---|---|---|---|
| 1 | 70% with data-partitioning schemes | Edge | 868 | 906,689 | RAxML | Figure S1 |
| 2 | 80% with data-partitioning schemes | Edge | 781 | 811,025 | RAxML | Figure S2 |
| 3 | 90% with data-partitioning schemes | Edge | 634 | 603,209 | RAxML | Figure S3 |
| 4 | 70% with data-partitioning schemes | Edge | 868 | 906,689 | Exabayes | Figure S4 |
| 5 | 80% with data-partitioning schemes | Edge | 781 | 811,025 | Exabayes | Figure S5 |
| 6 | 90% with data-partitioning schemes | Edge | 634 | 603,209 | Exabayes | Figure S6 |
| 7 | 70% with data-partitioning schemes | Edge | 868 | 906,689 | ASTRAL | Figure S7 |
| 8 | 80% with data-partitioning schemes | Edge | 781 | 811,025 | ASTRAL | Figure S8 |
| 9 | 90% with data-partitioning schemes | Edge | 634 | 603,209 | ASTRAL | Figure S9 |
2.2 DNA extraction and sequencing
Tissue samples were taken from fresh voucher specimens and preserved in 95% ethanol. Vouchers were then fixed in 10% formaldehyde and transferred to 70% ethanol for permanent storage (see Table 1 for catalog and locality data). Institutional codes follow Sabaj (2020). Whole genomic DNA was extracted from tissue samples with the DNeasy Tissue Kit (Qiagen) and quantified using the Qubit® dsDNA broad range (BR) Assay Kit (Invitrogen, Life Technologies) following manufacturer's instructions. We used a newly developed probeset for Ostariophysi to capture sequence data for 2708 UCE loci (Faircloth et al., 2020). Library preparation, sequencing, and data pipelining were performed at Arbor Biosciences (AB) using the following protocol: DNA libraries were prepared for the 52 specimens (33 ingroup, 18 related taxa, and one outgroup taxon) by modifying the Nextera (Epicentre Biotechnologies) library preparation protocol for solution-based target enrichment following Faircloth et al. (2012) and increasing the number of PCR cycles following the tagmentation reaction to 20 as recommended by Faircloth et al. (2013). AB staff used the Nextera library preparation protocol of in vitro transposition followed by PCR to prune the DNA and attach sequencing adapters, then used the Epicentre Nextera kit to prepare transposase-mediated libraries with insert sizes averaging 100 bp (95% CI: 45 bp) following Adey et al. (2010).
Whole genomic DNA (40 ng/µl) was first sheared with a QSonica Q800R instrument and selected to modal lengths of approximately 500 nt using a dual-step SPRI bead cleanup to prepare the libraries. AB staff then converted the DNA to Illumina sequencing libraries with a slightly modified version of the NEBNext(R) Ultra(TM) DNA Library Prep Kit for Illumina(R). After ligation of sequencing primers, libraries were amplified using KAPA HiFi HotStart ReadyMix (Kapa Biosystems) for six cycles using the manufacturer's recommended thermal profile and dual P5 and P7 indexed primers (Kircher et al., 2012). After purification with SPRI beads, libraries were quantified with the Quant-iT (TM) Picogreen (R) dsDNA Assay kit (ThermoFisher). AB staff then enriched pools comprising 100 ng each of eight libraries (800 ng total) using the MYbaits(R) Target Enrichment system (MYcroarray) following manual version 3.0. After capture cleanup, the bead-bound library was re-suspended in the recommended solution and amplified for 10 cycles using a universal P5/P7 primer pair and KAPA HiFi reagents. After purification, each captured library pool was quantified with PicoGreen and combined with all other pools in projected equimolar ratios prior to sequencing. Sequencing was performed across two Illumina HiSeq paired-end 100 bp lanes using v4 chemistry.
2.3 Raw data analysis
After sequencing, adapter contamination, low-quality bases, and sequences containing ambiguous base calls were trimmed using the Illumiprocessor software pipeline developed by Faircloth et al. (2013); https://github.com/faircloth-lab/illumiprocessor). After trimming, we assembled Illumina reads into contigs on a species-by-species basis using Abyss pipeline (Simpson et al., 2009; https://github.com/bcgsc/abyss). We then used a custom Python program (match_contigs_to_probes.py) implemented in PHYLUCE (Faircloth, 2016) integrating LASTZ (Harris, 2007) to align species-specific contigs to the probe-UCE set. This last program creates a relational database of matches to UCE loci by taxon. We then used the get_match_counts.py program (also included in PHYLUCE) to query the database and generate fasta files for UCE loci that were identified across all taxa. A custom Python program (seqcap_align_2.py) was then used to align contigs using the MUSCLE algorithm (Edgar, 2004) and to perform edge trimmings (i.e., cutting edges of each alignment, eliminating highly variable and saturated regions). We also performed phylogenetic analyses on three matrices with varying amounts of data (70%, 80%, and 90% of UCEs present in the complete alignment matrices) to explore the potential effects of missing data on tree reconstructions (Hosner et al., 2016; Streicher et al. 2016). All matrices are available in Figshare [https://doi.org/10.6084/m9.figshare.14182865]. Information about data for each matrix is summarized in Table 1; species read information is presented in Table S1. All sequences are available at NCBI Sequence Read Archive (SRA) submissions: SAMN18849693-SAMN18849744. Details on UCE sequence analyses are available online in the PHYLUCE documentation (Faircloth, 2016).
2.4 Phylogenetic analyses
We analyzed the Pseudopimelodidae dataset, with 52 specimens, using maximum likelihood (ML; RAxML v8; Stamatakis, 2014), Bayesian (BI; ExaBayes v1.4; Aberer et al., 2014), and coalescent tree (ASTRAL-II; Mirarab & Warnow, 2015) approaches. For all analyses, we partitioned the UCE data using the Partition-UCE (Tagliacollo & Lanfear, 2018) and performed model selection in PartitionFinder (Lanfear et al., 2012). The RAxML analysis was performed on 70%, 80%, and 90% complete matrices using partitions with edge-trimming alignment (Table 2). Five alternative runs using GTRGAMMA model and distinct parsimony-starting trees were performed to find the best ML tree. Pseudo-replicates applied the autoMRE function for the extended majority-rule consensus tree criterion available in RAxML v8 (Stamatakis, 2014) to assess bootstrap support for branches. This option allows tests for bootstrap convergence, determining if pseudo-replicates are getting stable support values (Pattengale et al., 2010).
| Species | Collection | Specimen number | Locality (river basin/city/state/country) | Geographic coordinates | |
|---|---|---|---|---|---|
| 1 | Amaralia hypsiura | LBP 10891 | 50203 | Madeira basin/Porto Velho/RO/Brazil | – |
| 2 | Aspistor luniscutis | LBP 4583 | 19083 | Atlantic coastal drainage/São Vicente/SP/Brazil | – |
| 3 | Batrochoglanis melanurus | LBP 8501 | 41749 | Paraguay basin/Cáceres/MT/Brazil | S 15°19′53.5″ W 57°11′31.1″ |
| 4 | Batrochoglanis raninus | LBP 17990 | 72464 | Amazon basin/Itacoatiara/AM/Brazil | S 03°07′07.0″ W58°27′14.7″ |
| 5 | Batrochoglanis villosus | LBP 7312 | 32725 | Negro basin/São Gabriel da Cachoeira/AM/Brazil | N 00°04′66.5″ W 66°49′54.6″ |
| 6 | Batrochoglanis villosus | LBP 9157 | 42523 | Guamá basin/Capitão Poço/PA/Brazil | S 01°34′28.3″ W 47°02′03.5″ |
| 7 | Batrochoglanis villosus | LBP 12121 | 51810 | Madeira basin/Porto Velho/AM/Brazil | S 9°12′42.1″ W 64°20′08.6″ |
| 8 | Batrochoglanis villosus | LBP 12127 | 51823 | Madeira basin/Porto Velho/AM/Brazil | S 9°12′42.1″ W 64°20′08.6″ |
| 9 | Batrochoglanis villosus | LBP 15681 | 64479 | Xingu basin/Ribeirão Cascalheira/MT/Brazil | S 13°09′13.6″ W 51°55′18.7″ |
| 10 | Brachyglanis microphtalmus | LBP 7106 | 34644 | Negro basin/São Gabriel da Cachoeira/AM/Brazil | N 00°03′38.1″ W 66°51′00.7″ |
| 11 | Brachyplatystoma filamentosum | LBP 5171 | 26646 | Amazon basin/Belém/PA/Brazil | S 01°18′20″ W 48°36′28″ |
| 12 | Cephalosilurus apurensis | LBP 3034 | 19182 | Orinoco basin/Caicara del Orinoco/Venezuela | N 07°38′11.6″ W 66°19′04.2″ |
| 13 | Cephalosilurus fowleri | LBP 11275 | 48788 | São Francisco basin/Gararu/SE/Brazil | S 09°51′23.0″ W037°06′30.3″ |
| 14 | Cetopsorhamdia iheringi | LBP 8053 | 37803 | Paraná basin/Delfim Moreira/MG/Brazil | S 22°26′56.2″ W 45°20′47.2″ |
| 15 | Charax metae | LBP 18653 | 61598 | Orinoco basin/Granada/Colombia | N 03º29′26.62″ W 73º44′34.10″ |
| 16 | Cheirocerus goeldii | LBP 21845 | 83779 | Negro basin/Iranduba/AM/Brazil | S 03°08′41″ W 59°54′38″ |
| 17 | Corydoras nattereri | LBP 1266 | 11102 | Ribeira do Iguape basin/Miracatu/SP/Brazil | – |
| 18 | Cruciglanis pacifici | LBP 24323 | 91524 | Cauca basin/Buenaventura/Colombia | S 03º44′30.0″ W76º58′11.0″ |
| 19 | Heptapterus mustelinus | LBP 13129 | 55150 | Uruguai basin/Augusto Pestana/RS/Brazil | S 28°32′03.2″ W 53°58′03.9″ |
| 20 | Ictalurus punctatus | LBP 2165 | 15148 | Pisciculture/Brazil | – |
| 21 | Leiarius marmoratus | LBP 9787 | 53207 | Amazon basin/Iquitos/Peru | S 03°42′ W 73°13′ |
| 22 | Lophiosilurus alexandri | LBP 276 | 4235 | São Francisco basin/Três Marias/MG/Brazil | S 18°13′66.1″ W 45°14′85.7″ |
| 23 | Microglanis n.sp.1 | LBP 9944 | 46688 | Orinoco basin/Cabruta/Venezuela | N 7°52′04.1″ W 66°12′40.1″ |
| 24 | Microglanis n.sp.1 | LBP 2260 | 15738 | Orinoco basin/Caicara del orinoco/Venezuela | N 07º32′22.4″ W 66º08′29.2″ |
| 25 | Microglanis cf. parahybae | LBP 3471 | 16096 | Macaé basin/Macaé/RJ/Brazil | S 22°14′07.0″ W 41°51′44.6″ |
| 26 | Microglanis cottoides LP | LBP 14459 | 60608 | Laguna dos Patos basin/Caraá/RS/Brazil | S 29°46′34.1″ W 50°26′34.1″ |
| 27 | Microglanis cottoides PS | LBP 3659 | 21728 | Passa Sete basin/Morretes/PR/Brazil | S 25°31′14.9″ W 48°47′52.7″ |
| 28 | Microglanis cottoides RI | LBP 7393 | 35386 | Ribeira do Iguape basin/Itapeúna/SP/Brazil | S 24°35′41.1″ W 48°12′53.3″ |
| 29 | Microglanis garavelloi | LBP 3907 | 22539 | Paraná basin/Avaré/SP/Brazil | S 23°01′27.4″ W 48°49′41.0″ |
| 30 | Microglanis leptostriatus | LBP 23996 | – | São Francisco basin/Jaíba/MG/Brazil | S 15º19′31.61″ W 43º39′51.11″ |
| 31 | Microglanis leptostriatus | LBP 19492 | 76356 | São Francisco basin/Pirapóra/MG/Brazil | S 17º20′56.6″ W 44º57′08.7″ |
| 32 | Microglanis malabarbai | LBP 13151 | 55106 | Uruguai basin/Uruguaiana/RS/Brazil | S 29°30′49.2″ W 56°43′28.7″ |
| 33 | Microglanis oliveirai | LBP 1852 | 13269 | Araguaia basin/Barra do Garça/MT/Brazil | S 15°32′54.2″ W 52°12′17.7″ |
| 34 | Microglanis poecilus | LBP 4083 | 23505 | Juruá basin/Mâncio Lima/AC/Brazil | S 07°34′28.8″ W 72°55′24.9″ |
| 35 | Microglanis poecilus | LBP 8612 | 43447 | Arinos basin/Diamantino/MT/Brazil | S 14°08′39.8″ W 56°05′48.6″ |
| 36 | Microglanis poecilus | LBP 14085 | 58490 | Tapajós basin/Itaituba/PA/Brazil | S 04°55′58.8″ W 56°51′51.6″ |
| 37 | Microglanis poecilus | LBP 15497 | 63828 | Branco basin/Mucajaí/RR/Brazil | N 02°54′49.9″ W 60°52′15.7″ |
| 38 | Microglanis sparsus | LBP 15909 | 65598 | Xingu basin/Canarana/MT/Brazil | S 13°31′34.1″ W 52°43′52.5″ |
| 39 | Nemuroglanis pauciradiatus | LBP 7001 | 34054 | Negro basin/São Gabriel da Cachoeira/AM/Brazil | N 00°01′19.9″ W 67°10′19.2″ |
| 40 | Phenacorhamdia roxoi | LBP 8247 | 38276 | Paraná basin/São Miguel Arcanjo/SP/Brazil | S 23°59′49″ W 48°00′57″ |
| 41 | Phractocephalus hemioliopterus | LBP 12883 | 54064 | Tapajós basin/Itaituba/PA/Brazil | S 04°33′09.7″ W 56°17′59.6″ |
| 42 | Pseudopimelodus mangurus | LBP 6560 | 31516 | Paraguay basin/Barra do Bugre/MT/Brazil | S 15°04′37″ W 57°10′51″ |
| 43 | Pseudoplatystoma punctifer | LBP 12822 | 54009 | Tapajós basin/Itaituba/PA/Brazil | S 04°29′11.1″ W 56°17′22.1″ |
| 44 | Rhamdia quelen | LBP 6515 | 31616 | São Francisco basin/Santana do Riacho/MG/Brazil | S 19°23′06.0″ W 43°39′33.3″ |
| 45 | Rhinodoras dorbignyi | LBP 21876 | 84491 | Paraná basin/Ipameri/GO/Brazil | S 17°18′41″ W 47°30′09″ |
| 46 | Rhyacoglanis paranensis | LBP 8083 | 37227 | Paraná basin/Campo Mourão/PR/Brazil | S 23°40′29.0″ W 52°07′08.0″ |
| 47 | Rhyacoglanis paranensis | LBP 11743 | 60260 | Paraná basin/Rio Paranaíba/MG/Brazil | S 19°11′58.0″ W 46°21′49.0″ |
| 48 | Rhyacoglanis pulcher | AUFT 4072 | 46789 | Amazon basin/Rio Maranõn/Peru | – |
| 49 | Rhyacoglanis seminiger | LBP 13260 | 69410 | Tapajos basin/Diamantino/MT/Brazil | S 13°59′04.1″ W 57°04′01.7″ |
| 50 | Rhyacoglanis n.sp.1 | LBP 15906 | 65589 | Xingu basin/Canarana/MT/Brazil | S 13°31′34.1″ W 52°43′52.5″ |
| 51 | Sorubimichthys planiceps | LBP 22301 | 86401 | Solimões basin/Tabatinga/AM/Brazil | S 04°17′32.8″ W 69°54′55.5″ |
| 52 | Trachydoras microstomus | LBP 6898 | 33244 | Negro basin/São Gabriel da Cachoeira/AM/Brazil | S 00°08′15.6″ W 67°05′05.7″ |
BI of the concatenated alignment was performed using ExaBayes (Aberer et al., 2014) on two independent runs, each with two chains, (one cold and one hot), of 1,000,000 generations using the partition schemes for all three complete matrices (Table 2). Tree space was sampled every 100 generations to yield 10,001 trees. Parameter estimates and ESS values were visualized in Tracer v1.6 (Rambaut et al., 2018), and the last 7500 trees were sampled after checking results for convergence (25% burn-in). This procedure allowed us to visualize the posterior probability log within and between independent runs and ensure that the average standard deviation of split frequencies was <1%. The effective sample sizes (ESS) were >200, and the potential scale reduction factor for estimated parameters was approximately 1.0. We generated the 50% most credible set of trees from the posterior distribution of possible topologies using the consensus algorithm of ExaBayes (burn-in 25%; thinning 500).
We inferred a coalescent tree analysis from individual gene trees using a two-step process accounting for coalescent stochasticity among individual UCE loci and addressing the related problem where concatenated analyses can return highly supported but incorrect trees process. First, we used PHYLUCE to resample the 70%, 80%, and 90% complete matrices by loci and generated a best tree using RAxML bootstraped for each locus in each of those matrices. Then, we used ASTRAL-II (Mirarab & Warnow, 2015) to infer species trees from each of the best tree subsets of loci and generated a majority-rule consensus tree of the results (minimum clade frequency 0.7). Although ASTRAL is not strictly a coalescent method, it is statistically consistent with the multispecies coalescent model (Nute et al., 2018) and scales well for a high number of loci.
3 RESULTS AND DISCUSSION
Sequencing and data filtering yielded a 70% complete matrix with 868 loci and 906,689 bp; an 80% complete matrix with 781 loci and 811,025 bp; and a 90% complete matrix with 634 loci and 603,209 bp (Table 2). Phylogenetic resolution inferred from the concatenated datasets was strongly supported regardless of matrix completeness (70%, 80%, or 90%) or method of analysis (ML or BI; Figures S1–S8). The results of the concatenated ML trees and BI analyses showed identical topologies. Disagreements involve the ASTRAL analysis with two distinct nodes compared to the main topology chosen for discussion (70% complete matrix with data partitioning of UCEs and ML analysis). Details of differences among each analysis can be observed in Figures S1–S9.
Results support the monophyly of Pimelodoidea (Figure 3) with Heptapteridae sister to Pimelodidae + Pseudopimelodidae as supported by previous morphological and genetic studies (Arcila et al., 2017; Hardman, 2005; Sullivan et al., 2006, 2013); however, our analyses did not include pimelodoids Conorhynchos and Phreatobiidae. Importantly, all phylogenetic reconstructions strongly support the monophyly of Pseudopimelodidae (Figure 2) again corroborating previous morphological (Lundberg et al., 1991; Ortega-Lara & Lehmann, 2006; de Pinna, 1998; Shibatta & Vari, 2017) and genetic studies (Arcila et al., 2017; Hardman, 2005; Silva et al., 2021; Sullivan et al., 2006, 2013).
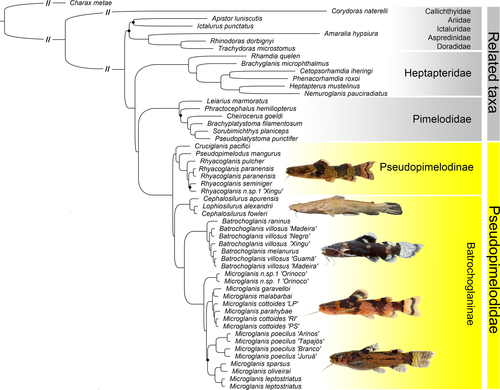
Internally, our phylogeny revealed two major clades of Pseudopimelodidae classified here as Pseudopimelodinae Lundberg et al. 1991, and Batrochoglaninae new subfamily. This two-clade arrangement agrees exactly with those recently based on morphology (Shibatta & Vari, 2017) and molecules (Rangel-Medrano et al., 2020), but provide a new hypothesis of interspecific relationships (Figure 2).
3.1 Relationships within Pseudopimelodinae Lundberg et al. 1991
Included genera: Cruciglanis Ortega-Lara, 2006; Pseudopimelodus Bleeker, 1858 (type genus); Rhyacoglanis Shibatta & Vari, 2017.
Diagnosis. Pseudopimelodinae is distinguished within Pseudopimelodidae by five morphological synapomorphies: (a) pectoral-fin spine covered with thick (vs. thin) skin (Shibatta & Vari, 2017: character 3, state 0); (b) tip of pectoral-fin spine bifurcate (vs. pointed) (Shibatta & Vari, 2017: character 29, state 1); (c) gas bladder small, dumbbell-shaped (vs. large, heart-shaped) (Birindelli & Shibatta, 2011; Shibatta & Vari, 2017, character 35, state 1); (d) pseudotympanum opening small (vs. large) (Birindelli & Shibatta, 2011; Shibatta & Vari, 2017, character 38, state 1); (e) anterior portion of the cerebellum extremely prolonged, extending beyond mesencephalon, by less than half the length of telencephalon (vs. moderately prolonged, extending only as far as boundary between the mesencephalon and telencephalon and not contacting the latter) (Abrahão et al., 2018).
Pseudopimelodinae is herein restricted to the genera Cruciglanis, Pseudopimelodus, and Rhyacoglanis (Figures 2 and 3). In our results, the monotypic Cruciglanis is sister to Pseudopimelodus + Rhyacoglanis. Morphological (Shibatta & Vari, 2017) and genetic (Rangel-Medrano et al., 2020) analyses support a monophyletic group composed of the same three genera, but with Rhyacoglanis sister to Cruciglanis + Pseudopimelodus. According to Shibatta and Vari (2017), the sister group relationship between Cruciglanis and Pseudopimelodus is supported by the shared presence of a dark band on the predorsal region (vs. predorsal region light in Rhyacoglanis) and pectoral fin with seven branched rays (vs. usually six in Rhyacoglanis). However, Shibatta and Vari (2017) also noted some variation in the number of branched pectoral-fin rays in Rhyacoglanis with some specimens from the Rio Madeira having seven branched pectoral-fin rays. Give the UCEs phylogeny, the absence of a dark band on the predorsal region in Rhyacoglanis might be interpreted as a secondary loss within Pseudopimelodidae.
In the phylogeny of Shibatta and Vari (2017), Rhyacoglanis paranensis (upper Paraná basin) was sister to a clade of species from the Amazon and Orinoco basins, with Rhyacoglanis epiblepsis sister to Rhyacoglanis annulatus, and Rhyacoglanis seminiger sister to Rhyacoglanis pulcher. In our molecular genetic analysis, R. pulcher was the first species to diverge in the genus, and R. paranensis was sister to R. seminiger plus an undescribed species from Xingu basin (R. epiblepsis and R. annulatus not analyzed).
3.2 Relationships within Batrochoglaninae Shibatta & Silva, new subfamily
LSID: urn:lsid:zoobank.org:pub:EF11973E-91D7-4267-9D29-86457B34706A
Included genera: Lophiosilurus Steindachner, 1877; Cephalosilurus Haseman, 1911; Batrochoglanis Gill, 1858 (type genus); Microglanis Eigenmann, 1912.
Diagnosis. Batrochoglaninae is distinguished within Pseudopimelodidae by three morphological synapomorphies: (a) anterior nostril near (vs. distant from) margin of mouth (Shibatta & Vari, 2017: character 15, state 1); (b) vomer absent (vs. present) (Shibatta & Vari, 2017, character 16, state 1); and (c) anterior and posterior margins of pectoral-fin spine with serrae of approximately the same length (vs. anterior serrae shorter than posterior ones) (Shibatta & Vari, 2017: character 27, state 1).
One highly supported clade within Batrochoglaninae includes the monotypic Lophiosilurus (endemic to the São Francisco basin) and Cephalosilurus with four species distributed in the São Francisco and Orinoco basins and coastal drainages of the Guianas. Based on our results, Cephalosilurus is paraphyletic with Cephalosilurus fowleri (type species) more closely related to Lophiosilurus alexandri than to Cephalosilurus apurensis (Figure 2). Lophiosilurus alexandri is quite distinct morphologically, and all species in Cephalosilurus are more similar to each other than any are to Lophiosilurus. The L. alexandri phenotype emerges from an ancestral Cephalosilurus phenotype, and the close resemblance of C. fowleri to other Cephalosilurus is not convergent. According to Shibatta and Vari (2017), Lophiosilurus and Cephalosilurus share four synapomorphies: (a) well-developed unculiferous epidermal structures (vs. little developed; character 2, state 1); (b) seven branched pectoral-fin rays (vs. five, six, eight or more; character 26, state 1); (c) presence of a constrictor muscle of gas bladder (vs. absent; character 36, state 1); and (d) lateral trabeculae on internal T-shaped gas bladder septum (vs. absence of lateral trabeculae; character 37, state 1). Abrahão et al. (2018) also pointed out the reduced lobus facialis, comprising less than half length of the lateral line lobe (vs. elongated; state 1). Our results strongly support and suggest Cephalosilurus Haseman, 1911 as a junior synonym of Lophiosilurus Steindachner, 1877. A study assessing the synonymy of these genera has been conducted by Shibatta et al. (in review), including all species of Cephalosilurus.
Our analyses included three out of the six valid species of Batrochoglanis: Batrochoglanis raninus, Batrochoglanis melanurus and specimens of Batrochoglanis villosus (from Guamá, Madeira, Xingu, and Negro river basins). Batrochoglanis raninus is the sister of a major clade with B. melanurus nesting deeply within a paraphyletic B. villosus (Figure 3). Batrochoglanis villosus is a widely distributed species known from the Orinoco and Amazonas basins as well as coastal drainages of the Guianas, whereas B. melanurus is restricted to the upper Paraguay in the La Plata basin (Shibatta & Pavanelli, 2005). In their description of B. melanurus, Shibatta and Pavanelli (2005) noted its morphological similarity to B. villosus. They also identified B. villosus from the upper Tapajós (Amazon basin) which shares a low divide with the upper Paraguay basin. Based on our phylogeny, B. melanurus might represents a junior synonym of B. villosus; however, the lack of DNA sequence data of B. villosus from the type-locality (Potaro river, Guyana) prevents a formal proposal of synonymy.
Microglanis is the most diverse genus of Pseudopimelodidae, with 29 valid species (Tobes et al., 2020) broadly distributed in the Orinoco, Amazon, and La Plata basins as well as Atlantic coastal drainages from Guyana to Uruguay. A couple species occur west of the Andes in Pacific coastal drainages of Ecuador (Tobes et al., 2020). Despite its substantial diversity, no phylogenetic hypothesis has been proposed for the genus. In our topology, Microglanis can be divided into four large subclades. The first is represented by an undescribed species from the Orinoco basin. The second group consists of two subclades: species from the Atlantic coastal drainages (Microglanis cottoides, Microglanis cf. parahybae) and the other with two species from La Plata basin Microglanis garavelloi (upper Paraná River) and Microglanis malabarbai (Uruguay River). Souza-Shibatta et al. (2018) using genetic characters supported a clade composed by the same species, with M. cottoides + M. parahybae sister to M. garavelloi + M. malabarbai. Alternatively, our results nest M. parahybae within a paraphyletic M. cottoides (Figure 3).
Microglanis poecilus is the most widely distributed species in the genus (Ruiz & Shibatta, 2011) confirmed to be monophyletic based on our sampling from four distinct Amazonian sub-basins (Arinos, middle Tapajós, Juruá, and Branco). Ruiz and Shibatta (2011) noted that M. poecilus shares 11 features with Microglanis oliveirai, a species they described from the upper-middle Araguaia basin with drains into the Tocantins River. Our results, however, did not support a close relationship between M. poecilus and M. oliveirai and placed the latter species as sister Microglanis sparsus, a species described from the upper Xingu basin (Ruiz, 2016). Furthermore, our analyses revealed deep splits among lineages of M. poecilus suggestive of undescribed taxa.
Finally, our analyses supported a close relationship between Microglanis leptostriatus, M. oliveirai, and M. sparsus in a clade sister to M. poecilus. Mori and Shibatta (2006) considered M. leptostriatus to be a member of the M. parahybae species complex along with M. garavelloi and M. parahybae. Alcaraz et al. (2008) expanded this group to include Microglanis pataxo and Microglanis carlae. Our results placed M. leptostriatus closer to the Amazonian species M. sparsus and M. oliveirai than M. garavelloi and M. parahybae calling into question the cohesiveness of the M. parahybae species complex.
4 CONCLUSIONS
This study represents the most taxon-rich phylogenetic analysis of Pseudopimelodidae to date with representatives of all genera and 36% of the species diversity. Our results support the recognition of two major clades at the subfamilial level, Pseudopimelodinae and Batrochoglaninae. Although our analysis provides strong support for intergeneric relationships, an increased species-level coverage is needed to test the monophyly of Pseudopimelodus and to better delimit widely distributed taxa such as B. villosus, M. cottoides, and M. poecilus. The relationships will guide future research aiming to delimit species in well-resolved monophyletic groups as defined herein.
ACKNOWLEDGEMENTS
We thank Jorge E. García-Melo and Juan Albornoz-Garzón for providing necessary samples of Cruciglanis from Pacific rivers of Colombia. We also thank Benjamin Lee for the photograph of Lophiosilurus. Ricardo C. Benine contributed with comments and revision of the manuscript. Analyses were performed on Zungaro and Brycon servers at LBP/UNESP funded by FAPESP proc. 2014/26508-3, and the Center for Scientific Computing (NCC/GridUNESP). This project received support from Brazilian agencies FAPESP grants #2015/00691-9 (FFR), #2016/11313-8 (BFM), #2016/19075-9, 2018/23883 (LEO), and #2014/26508-3 (CO) and Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq proc. 306054/2006-0 (CO). The research received support from Capes/PNPD grants (GSCS and FFR).




