Deep Downunder: Integrative taxonomy of Austrobela, Spergo, Theta and Austrotheta (Gastropoda: Conoidea: Raphitomidae) from the deep sea of Australia
Online ISSN: 1439–0469
Contributing authors: Anders Hallan ([email protected]), Alexander Fedosov ([email protected]), Nicolas Puillandre ([email protected])
Abstract
enRecent sampling efforts in the deep seas of southern and eastern Australia have generated a wealth of DNA-suitable material of neogastropods of the family Raphitomidae. Based on this material, a molecular phylogeny of the family has revealed a considerable amount of genus and species level lineages previously unknown to science. These taxa are now the focus of current integrative taxonomic research. As part of this ongoing investigation, this study focuses on the genera Austrobela, Austrotheta (both Criscione, Hallan, Puillandre & Fedosov, 2020), Spergo Dall, 1895 and Theta Clarke, 1959. We subjected a comprehensive mitochondrial DNA dataset of representative deep-sea raphitomids to Automatic Barcode Gap Discovery, which recognized 24 primary species hypotheses (PSHs). Following additional evaluation of shell and radular features, as well as examination of geographic and bathymetric ranges, 18 of these PSHs were converted to secondary species hypotheses (SSHs). Based on the evidence available, the most likely speciation mechanisms involved were evaluated for each pair of sister SSHs, including niche partitioning. Eleven SSHs were recognized as new and their systematic descriptions are provided herein. Of these, four were attributed to Austrobela, one to Austrotheta, four to Spergo and two to Theta. While all new species are endemic to Australian waters, other species studied herein exhibit wide Indo-Pacific distributions, adding to the growing body of evidence suggesting that wide geographic ranges in deep-sea Raphitomidae are more common than previously assumed.
Résumé
frUn récent effort d'échantillonnage dans les eaux profondes du sud et de l'est de l'Australie ont généré une grande quantité de néogastropodes de la famille des Raphitomidae, conservés pour des analyses moléculaires. Sur la base de ce matériel, une phylogénie moléculaire de la famille a révélé plusieurs lignées de rangs génériques et spécifiques auparavant inconnues de la science. Ces taxons sont en cours d’étude via une approche de taxonomie intégrative. Dans ce contexte, cette étude se concentre sur les genres Austrobela, Austrotheta (Criscione, Hallan, Puillandre & Fedosov, 2020), Spergo Dall, 1895 et Theta Clarke, 1959. Un jeu de données de séquences mitochondriales de Raphitomidae a été analysé à l’aide de l’outil Automatic Barcode Gap Discovery (ABGD), qui a délimité 24 hypothèses d'espèces primaires (PSH). Suite à une analyse de la morphologie de la coquille et de la radula, ainsi qu'à l'examen des aires de répartition géographiques et bathymétriques, 18 de ces PSH ont été convertis en hypothèses d'espèces secondaires (SSH). Sur la base des indices disponibles, les mécanismes de spéciation, y compris le partitionnement de niche, probablement en jeu ont été identifiés pour chaque paire de SSH sœurs. Onze SSH ont été reconnues comme nouvelles pour la science et leurs descriptions systématiques sont proposées. Parmi elles, quatre ont été attribués à Austrobela, une à Austrotheta, quatre à Spergo et deux à Theta. Alors que toutes les nouvelles espèces étaient endémiques des eaux australiennes, d'autres espèces étudiées ici présentaient de larges distributions indo-pacifiques, ce qui s'ajoute à la liste toujours croissante des Raphitomidae des grands fonds avec de vastes aires de distribution.
1 INTRODUCTION
“Turriform conoideans” (Caenogastropoda: Neogastropoda) (Abdelkrim et al., 2018; Bouchet et al., 2011; Puillandre et al., 2011) are well-known for their extensive shell homoplasy (Criscione et al., 2021; Kantor et al., 2008, 2018), although challenges associated with their systematics extend beyond shell morphology. For instance, turriform conoideans do generally not occur in readily accessible intertidal habitats and are typically rare, with many species known from single individuals only (Bouchet et al., 2009; Criscione et al., 2021; Hallan et al., 2021). While already alluded to by Bouchet and Warén (1980), recent findings also suggest that several deep-sea species may be unusually widespread geographically, with some taxa also occupying considerable bathymetric and geographic ranges (e.g. Criscione et al., 2021; Hallan et al., 2021; Sánchez & Pastorino, 2020; Zaharias et al., 2020). With such complicating factors to their taxonomy, the notion that purely shell-based morphology can resolve the systematics of deep-sea turriform conoideans has therefore been largely abandoned in recent years, in favor of integrative approaches combining morpho-anatomical, genetic and distribution data (Criscione et al., 2021; Hallan et al., 2020, 2021; Puillandre et al., 2009, 2017; Zaharias et al., 2020). Owing to their unique venom apparatus, an apomorphic character to the Conoidea (Puillandre et al., 2015), there is significant impetus to characterize turriform conoidean diversity in order to facilitate further studies on their venom diversity (Criscione et al., 2021; Gonzales & Saloma, 2014; Lopez-Vera et al., 2004; Puillandre, Koua, et al., 2012). However, due to the taxonomic challenges of the group as outlined above, the understanding of their diversity lags far behind that of the related Conidae (Puillandre et al., 2014) and Terebridae (Modica et al., 2019).
In the deep sea, notably in the Australasian region, recent research suggests that the family Raphitomidae is the most diversified conoidean family (Criscione et al., 2021; MacIntosh et al., 2018; O'Hara et al., 2020). Criscione et al. (2021) showed that widespread shell homoplasy among this fauna had led taxonomists to incorrectly attribute a considerable number of unrelated species to very few raphitomid genera (such as Pleurotomella Verril, 1872 and Gymnobela Verrill, 1884), some of which were shown to be polyphyletic. In constraining these genera, based on their support as clades and on diagnostic morphological characters, Criscione et al. (2021) introduced a number of new genus-level taxa and described their type species. The same study also revealed a multitude of putatively undescribed species remaining to be tested through integrative taxonomy. Two subsequent studies commenced that task, describing a total of 11 species of the genera Gladiobela Criscione, Hallan, Puillandre & Fedosov, 2020 and Pagodibela Criscione, Hallan, Puillandre & Fedosov, 2020 (Hallan et al., 2021) and Famelica Bouchet & Warén, 1980 and Rimosodaphnella Cossmann, 1916 (Criscione et al., in press)
Based on a larger sampling size and on an integrative taxonomic approach, this study aims to test putative species as reported in Criscione et al. (2021) for four genera: Austrobela Criscione, Hallan, Puillandre & Fedosov, 2020, Austrotheta Criscione, Hallan, Puillandre & Fedosov 2020, Spergo Dall, 1895 and Theta Clarke, 1959. In the analysis of that study, these genera formed a monophyletic group (Criscione et al., 2021). Formal descriptions are here presented for newly recognized species. Furthermore, revised genus diagnoses and new anatomical and morphological data are introduced for both established and new taxa, which are discussed in terms of their diagnostic utility at the genus level. Finally, geographic and bathymetric distributions are presented for the taxa treated herein, and their biogeography is briefly discussed.
2 MATERIALS AND METHODS
2.1 Taxon sampling
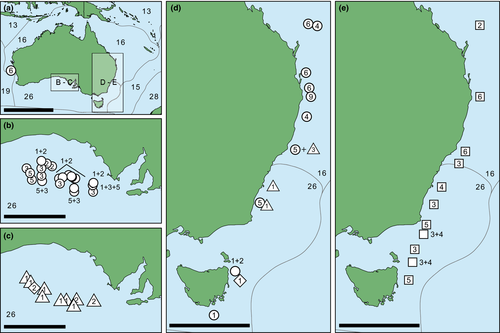
The samples studied herein were selected among all deep-sea Raphitomidae ethanol-preserved material from the malacological collections of the Australian Museum, Sydney (AMS), the South Australian Museum, Adelaide (SAMA), the Tasmanian Museum and Art Gallery, Hobart, Australia (TMAG), the Western Australian Museum, Perth (WAM) and the Muséum national d'Histoire naturelle, Paris (MNHN). Most of the studied material has been collected off Australia during the expeditions IN2015_C01, IN2015_C02 (in the Great Australian Bight, GAB), IN2017_V03 (Tasman and Coral Seas) and IN2018_V06 (Tasmanian seamounts), targeting several Commonwealth Marine Reserves (CMR) among other sites. The remaining material has been sampled from other localities (mainly of the tropical and temperate Indian and Pacific Oceans), during a number of voyages that formed part of the Tropical Deep-sea Benthos program of MNHN (https://expeditions.mnhn.fr/; Figure 1, Table S1).
As a result of ongoing systematic research on the Conoidea at the AMS and MNHN, several hundred of (mostly unpublished) sequences of two mitochondrial genes, cytochrome c oxidase subunit I gene (cox1 – 1053 sequences) and 16S ribosomal RNA gene (16S – 336 sequences) were obtained (see methodology below) from a considerable number of largely undescribed representative raphitomid taxa. In order to assist with the selection of the study material, two pilot analyses were performed separately on two datasets including, respectively, all raphitomid cox1 and 16S sequences, using the neighbor-joining method (NJ) (Saitou & Nei, 1987) implemented in MEGA 7 (Kumar et al., 2016). In particular, the datasets included cox1 and 16S sequences of the holotypes for the type species of several deep-sea raphitomid genera, including Austrobela rufa Criscione, Hallan, Puillandre & Fedosov, 2020 (GenBank ANs: MN983272 for cox1, MT395563 for 16S) and Austrotheta crassidentata Criscione, Hallan, Puillandre & Fedosov, 2020 (MT260886 for cox1, MN985768 for 16S), as well as sequences of non-topotypic specimens of Theta lyronuclea (Clarke, 1959) (type species of Theta) and sequences of well-recognizable species of Spergo [other than the type species, Spergo glandiniformis (Dall, 1895)].
- Sequences of any of the species of Austrobela, Austrotheta, Theta, or Spergo listed above.
- All sequences that were more closely related to the sequences of any of the species in 1 than to sequences of any other raphitomid genus in the larger dataset.
Sequences representing 37 deep-sea raphitomid species of 13 different genera were selected to serve as outgroup (Table S1). Their choice was based on the phylogeny of Criscione et al. (2021), containing many southern and southeastern Australian Raphitomidae, which established the phylogenetic framework for subsequent systematic studies on the family (Hallan et al., 2021; Criscione et al., in press).
Among the ingroup specimens, morphological examination was only conducted on those collected in Australian waters and some of those collected outside Australia (see section 3). However, for samples outside Australia, examination of shell photographs was possible and thus utilized when necessary and appropriate. Geographic and bathymetric data were available for all ingroup specimens. Geographic distributions were assessed with reference to marine biogeographic realms as delimited in Costello et al. (2017). According to Bouchet et al. (2008), when inferring bathymetric distributions of species from sampling depth intervals, only shallower depth values were considered, as there is no evidence that the species collected occurs beyond that value. A comprehensive list of the material studied and of the type of analysis carried out on each sample is provided as a Table S1.
2.2 Molecular genetic methods
Molecular genetic work was performed in laboratories at two different institutions (AMS and MNHN). Unless otherwise stated, the same methodology was followed by both laboratories. DNA was extracted from small pieces of foot muscle by use of a Bioline Isolate II Genomic DNA extraction kit for animal tissue, following the standard procedure of the manual (AMS) or using the Epmotion 5075 robot (Eppendorf), following the recommendations by the manufacturer (MNHN). Fragments of cox1 and 16S were amplified using the primer pairs LCO1490 (GGTCAACAAATCATAAAGATATTGG)/HCO2198 (TAAACTTCAGGGTGACCAAAAAATCA) for cox1 (Folmer et al., 1994) and 16SH (CCGGTCTGAACTCAGATCACG)/16LC (GTTTACCAAAAACATGGCTTC) for 16S (Palumbi, 1996). PCRs were performed in volumes of 25 μl, containing 3 ng DNA, 1X Qiagen CoralLoad PCR Buffer, 2.5 mM MgCl2, 0.25 mM dNTP, 0.5 mM of each primer, 0.5 μg/μl of BSA and 0.2 μl of Bioline MyTaq DNA polymerase. Amplification consisted of an initial denaturation step at 94°C for 4 min, followed by 37 cycles of denaturation at 94°C for 30 s, annealing at 50°C (cox1) and 55°C (16S) for 30 s, followed by extension at 72°C for 1 min. The final extension was at 72°C for 5 min. PCR products were purified and sequenced by the Macrogen (AMS) and Eurofins (MNHN) sequencing facilities. When necessary, chromatograms were manually corrected for misreads and forward and reverse strands were merged into one sequence file using CodonCode Aligner v. 9.0.1 (CodonCode Corporation). Sequence alignments were generated using MUSCLE as implemented in MEGA7 (Kumar et al., 2016). Sequences were deposited in GenBank (Table S1). Phylogenetic trees were generated using maximum likelihood (ML) and Bayesian inference (BI) methods. ML was performed using the program MEGA7 with Nearest Neighbour Interchange (NNI) as heuristic method and automatic generation of the initial tree. One thousand bootstrap replicates (BTSP) were performed to assess the topology support. The BI analysis was performed in MrBayes 3.2.6 (Ronquist & Huelsenbeck, 2003) and included two runs of 107 generations, with four chains each and a sampling frequency of one tree per 1000 generations. Other parameters were set to default. After checking for convergence (ESS >200) with Tracer (Rambaut et al., 2018), a consensus tree was calculated after discarding the first 25% trees as burn-in. Nodal support was assessed by values of Bayesian posterior clade probabilities (BPP). Prior to the model-based ML phylogenetic analyses, the TN93 model (Tamura & Nei, 1993) with gamma distribution and proportions of invariable sites (TN93+Γ+I) was identified as the best-fit model of sequence evolution for both gene fragments by means of the Bayesian information criterion as implemented in MEGA 7 (Kumar et al., 2016). According to MrBayes manual (p. 94), a priori model testing was not performed, and the GTR+G+I model was applied to the BI analysis. Uncorrected pairwise genetic distances were calculated using MEGA7 with the option “pair-wise deletion of gaps.”
2.3 Morphological examinations
All studied samples consisted of bodies and their shells, from which they had been removed following the methodology described in Criscione et al. (2021). We studied shell morphology and (when possible) internal anatomy, including radular morphology. Shells of sequenced specimens were affixed to plasticine and positioned with their vertical axis parallel to the observation plane. Each shell was then photographed from above (frontal and lateral views) using a digital SLR camera. Maximum shell length (SL) and width (SW) were measured on digitized images using the calibrated ruler tool in Adobe Photoshop CC v.20.0.6. Measurements were rounded to the nearest 0.1 mm. The number of shell whorls was counted under a Leica MZ8 stereomicroscope, in accordance with Bouchet and Kantor (2004). While it was possible to obtain the number of teleoconch whorls (Wt) for almost all studied specimens, protoconch whorls could only be counted occasionally due to widespread erosion of the apex. When sufficient samples were available, morpho-spaces of individual species were compared through scatterplots of SW and SL.
Anatomical studies were conducted on animals removed from ethanol and briefly rehydrated in distilled water. Using standard dissection tools, the venom apparatus was excised and the radular sac isolated and placed on a glass slide; during this dissection process, head-foot, mantle, genital, and (non-radula) foregut characters were examined where possible. After dissolution in diluted commercial bleach, clusters of hypodermic teeth were rinsed repeatedly in distilled water, then separated into individuals and ligament-connected pairs or smaller clusters. Subsequently, the glass stub was affixed to a carbon adhesive placed on a 12-mm-diameter aluminum mount. All samples were imaged at Macquarie University, Sydney, using a Phenom XL Scanning Electron Microscope. For radular descriptions, we followed the terminology accepted and discussed by Kantor and Taylor (2000).
2.4 Species delimitation
The Automatic Barcode Gap Discovery (ABGD) (Puillandre, Lambert, et al., 2012) was applied for primary species delimitation to a dataset including all cox1 sequences. The web-based version of ABGD (https://bioinfo.mnhn.fr/abi/public/abgd/abgdweb.html) was used with a p-distance model. The relative gap width (X) was set to 1 and the other parameters left to default. Resulting ABGD groups were considered primary species hypotheses, henceforth referred to as PSHs. Following Puillandre, Modica, et al. (2012), conversion of PSHs to secondary species hypotheses (SSHs) was conducted through comparative examination of morphological characters as well as through evaluation of geographic and bathymetric distributions.
In particular, when converting individual PSHs to SSHs, the occurrence of the following conditions was assessed: (a) the PSH is a highly supported clade (BPP >0.98 and BS >90%), (b) all its constituent specimens share at least one distinctive morphological feature deemed not to be polymorphic or ecophenotypic, and without exhibiting intermediate forms, (c) the PSH maintains genetic or morphological divergence and/or bathymetric partitioning when occurring in sympatry with another PSH. When available, species names were assigned to SSHs based on the current taxonomy. New species names were introduced when no names were available, and formal descriptions for these taxa are given in the systematic section below.
3 RESULTS
3.1 Molecular studies
The size of the PCR products was 711 bp for cox1 and ranged between 538 and 541 bp for 16S. Molecular analyses were based on a total of 190 cox1 sequences (158 new and 32 GenBank-sourced) and 148 16S sequences (112 new and 36 GenBank-sourced). Of the total sequences employed, 283 (153 cox1 + 132 16S) constituted the ingroup and the remaining 55 (37 cox1 and 18 16S) were used as outgroup.
In the vicinity of the barcode gap, the ABGD analysis of the cox1 ingroup dataset consistently returned an initial partition with 23 PSHs. Among all PSHs, fourteen (A1–A5, S3–S6, U1–U2, T1–T3) contained exclusively Australian samples, two (A6 and S2) included samples from Australian seas and beyond, while the remaining seven (A7–A8, AA–AD and S1) encompassed sequences from outside Australian waters only.
Molecular analyses (BI and ML) were conducted on both single-gene datasets and on a dataset formed by concatenating all 190 cox1 sequences and 128 16S sequences obtained from samples of the cox1 dataset. The final size of the aligned gene fragments was 658 bp for cox1 and 502 for 16S, the concatenated cox1 + 16S dataset (Alignment S1) had a total length of 1160 bp. In all analyses, one sequence was used for each cluster of identical sequences (CIS, Tables S2 and S3) and identical haplotypes are labeled accordingly in the resulting trees (Figures 2 and 3, Figure S1).
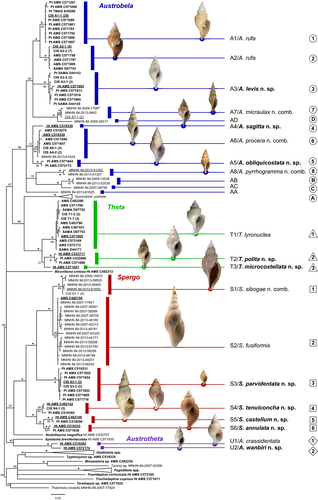
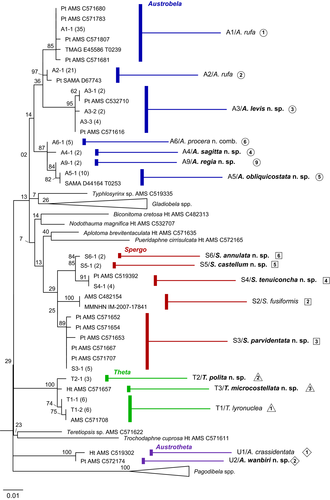
The trees generated with the cox1 dataset (not shown) were very similar to that of the combined dataset (Figure 2, Figure S1). As these latter trees provided higher support to the PSH clades, we refer to them in the below section detailing species delimitation.
The BI and ML analyses of the cox1 + 16S dataset generated trees with comparable topologies (Figure 2, Figure S1). While deeper nodes were unstable across trees and typically lacked support, only minor differences were observed in the relative position of individual sequences within some of the clades representing PSH-level relationships. In both analyses, four major genus-level clades were retrieved among the ingroup sequences, namely Austrobela (BPP = 0.96, BTSP = 56%), Theta (BPP = 1, BTSP = 99%), Austrotheta (BPP = 1, BTSP = 99%) and Spergo (BPP = 1, BTSP = 66%). These four generic clades included 12, three, two and six PSHs, respectively, mostly well-differentiated (in terms of branch lengths) and exhibiting moderate (BPP = 0.95–0.98; BTSP = 75–90%) to high (BPP >0.98; BTSP = >90%) values of nodal support.
No supported conflicting topologies were found between BI and ML trees obtained analyzing the 16S dataset; hence, only the ML tree is shown here (Figure 3). Based on a dataset of rather different composition (i.e., missing sequences of outgroup and of samples of A7, A8 and S1 as well as presence of 17 additional samples with no corresponding cox1 sequence), this tree (Figure 3) differed to some extent from the cox1 + 16S trees. In particular, clades corresponding to only 20 of the total PSHs were retrieved (although generally well-supported), with an additional clade (A9) recovered, formed by two identical 16S sequences and for which no corresponding cox1 sequence was available. Given the substantial topological congruence between 16S- and cox1-based trees with respect to the PSH-level clades (Figures 2 and 3), A9 is considered an additional PSH to undergo further testing for conversion to SSH.
In the Austrobela clade, the intra-PSH pairwise distances in cox1 ranged from 0% to 0.5% (average = 0.2%) with inter-PSH distances ranging from 2.8% to 9.8% (average = 6.9%; Table 1). The lowest inter-PSH distances were observed between A1 and A2 and the highest intra-PSH distances were found within A5. In the Spergo clade, the intra-PSH pairwise distances in cox1 ranged from 0.2% to 0.8% (average = 0.4%) with inter-PSH distances ranging from 2.8% to 8.0% (average = 6.2%; Table 2). The lowest inter-PSH distances were observed between the pair S5/S6 and the highest intra-PSH distances were found within S2. In the Theta clade, the intra-PSH pairwise distances ranged from 0.3% to 0.6% (average = 0.5%), whereas inter-PSH distances ranged from 3.3% to 4.9% (average = 4.1%). The lowest inter-PSH distances were observed between T2 and T3 and the highest intra-PSH distances were found within T2. The distance between the two PSHs (one sequence each) in the Austrotheta clade was 3.1%.
| A1/ruf | A2/ruf | A3/lev | A4/sag | A5/obl | A6/pro | A7/mic | A8/pyr | AA | AB | AC | AD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1/ruf | 0.001 | Min | Max | Mean | ||||||||
| A2/ruf | 0.028 | 0.002 | Within | 0.000 | 0.005 | 0.002 | ||||||
| A3/lev | 0.044 | 0.053 | 0.004 | Between | 0.028 | 0.098 | 0.069 | |||||
| A4/sag | 0.060 | 0.068 | 0.057 | 0.002 | ||||||||
| A5/obl | 0.053 | 0.061 | 0.043 | 0.033 | 0.005 | |||||||
| A6/pro | 0.053 | 0.060 | 0.046 | 0.042 | 0.029 | 0.003 | ||||||
| A7/mic | 0.059 | 0.060 | 0.057 | 0.076 | 0.060 | 0.061 | 0.003 | |||||
| A8/pyr | 0.086 | 0.082 | 0.071 | 0.086 | 0.075 | 0.075 | 0.086 | 0.000 | ||||
| AA | 0.073 | 0.067 | 0.068 | 0.070 | 0.067 | 0.061 | 0.073 | 0.081 | – | |||
| AB | 0.085 | 0.098 | 0.071 | 0.082 | 0.075 | 0.080 | 0.082 | 0.077 | 0.082 | 0.002 | ||
| AC | 0.089 | 0.095 | 0.075 | 0.079 | 0.075 | 0.074 | 0.083 | 0.084 | 0.084 | 0.036 | – | |
| AD | 0.071 | 0.072 | 0.070 | 0.081 | 0.066 | 0.069 | 0.030 | 0.096 | 0.073 | 0.085 | 0.089 | – |
Note
- Intra-PSH/specific distances shaded. Maximum and minimum values of inter-PSHs/specific distance in bold. Inset: minimum, maximum and average intra- and inter-PSHs/specific p-distances within Austrobela. Species codes: lev, A. levis.; mic, A. micraulax n. sp; obl, A. obliquicostata n. sp; pro, A. procera n. sp; pyr, A. pyrrogramma n. sp.; ruf, A.rufa; sag, A. sagitta n. sp; codes of species described herein in bold.
| S1/sib | S2/fus | S3/par | S4/ten | S5/cas | S6/ann | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1/sib | 0.003 | Min | Max | Mean | |||||||
| S2/fus | 0.029 | 0.008 | Within | 0.002 | 0.008 | 0.004 | |||||
| S3/par | 0.075 | 0.075 | 0.002 | Between | 0.028 | 0.080 | 0.062 | ||||
| S4/ten | 0.070 | 0.065 | 0.067 | 0.006 | |||||||
| S5/cas | 0.075 | 0.072 | 0.069 | 0.042 | 0.003 | ||||||
| S6/ann | 0.080 | 0.072 | 0.072 | 0.034 | 0.028 | 0.005 |
Note
- Intra-PSH/specific distances shaded. Inset: minimum, maximum and average intra- and inter-PSHs/specific p-distances within Spergo. Maximum and minimum values of inter-PSHs/specific distance are in bold underlined. Species codes: ann, S. annulata n. sp.; cas, S. castellum n. sp; fus, S. fusiformis; par, S. parvidentata n. sp; sib, S. sibogae; ten, S. tenuiconcha n. sp. Codes of species described herein in bold.
Genetic distances in 16S within clades of Austrobela, corresponding to PSHs (Figure 3), ranged from 0% to 0.2%, while distances between clades ranged from 0.2% to 3.1%. The lowest value of inter-PSH distance was recorded between A9 and A5 and the highest intra-PSH distance was measured for A3.
3.2 Morphological studies
Morphological observations refer to PSHs that are examined herein and do not include PSHs assigned with a letter suffix (i.e., AB, AC, etc.). Considerable shell erosion affected the protoconchs of most specimen studied. As a consequence, protoconch sculpture could not be studied for Spergo. For Theta and Austrotheta, some sculptural detail could be inferred from heavily eroded protoconchs by careful examination using a microscope. However, these are not figured herein owing to their very poor quality. Due to the limited number of adult samples available for the other genera (see Table S1), the extent of intraspecific variability in shell features could be assessed in Austrobela only, albeit for just five PSHs, namely A1, A2, A3, A5 and A6. For the same reason, SW/SL scatterplots (Figure 4) could be generated for three of these PSHs only (A1–A3).
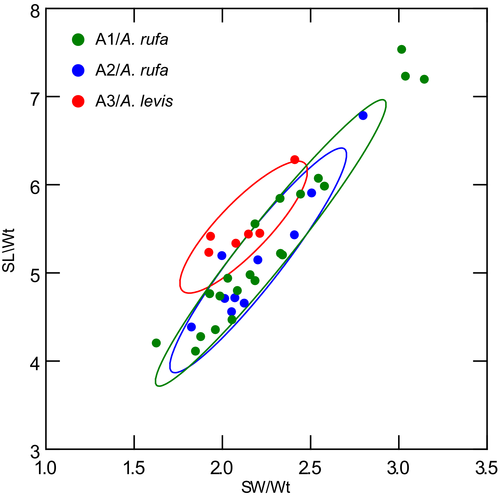
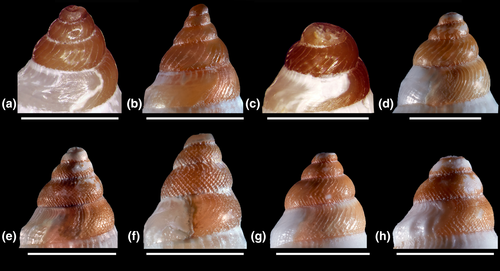
Differences in shell morphology among Austrobela species largely relate to sculptural elements, with the gross morphology in most PSHs consisting of a fusiform-biconical shape (Figures 5-7). When compared to the other PSHs, A6 and A7 (Figure 7b and f) exhibit tall-spired shells with 2–3 additional whorls. While shells of A6 were morphologically homogeneous, there was considerable intra-PSH variability for A1, A2, A3 and A5 (Figures 5, 6a–g—see section 3.6 for details on individual species). The protoconch (Figure 8) is multispiral in all Austrobela PSHs, exhibiting sculpture of arcuate riblets in A1–A3 (Figure 8a–d), diagonally cancellate sculpture in A4 and A6 (Figure 8e,f) and with diagonally cancellate abapical portion with arcuate riblets on the adapical portion in A5 (Figure 8g). No material of A7 and A8 was available for protoconch examination. The general radular morphology observed in all examined members of Austrobela consists of hypodermic teeth with two large, sharp distal barbs, a somewhat inflated lower portion of the shaft and a thick ligament (Figure 9). While virtually indistinguishable among most PSHs, radular teeth are somewhat different in A3 and A4. The tooth of A3 has a rather marked excavation of the ventral barb (when viewed laterally; Figures 9f and 10a), whereas the tooth of A4 is far longer than that of other PSHs (Figure 9g).


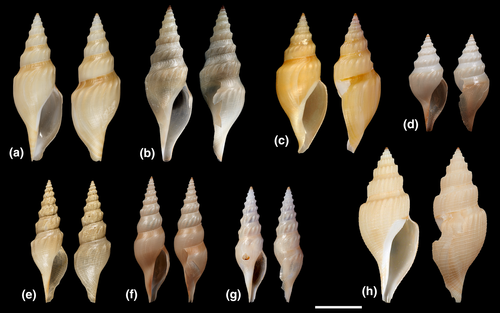
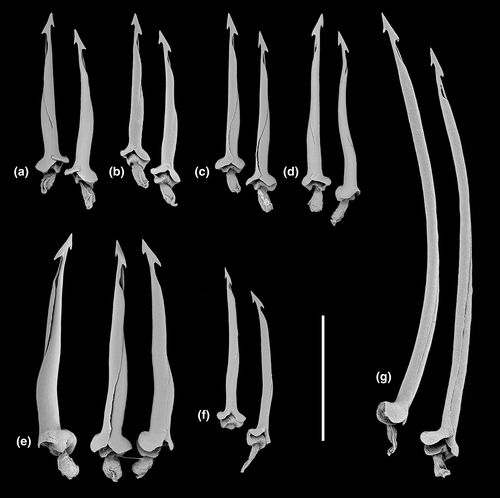
The Spergo clade is comparatively heterogenous based on shell morphology, and all PSHs can readily be differentiated based on their shell features (Figures 11 and 12). Spergo PSHs exhibit shells ranging from large, elongate-fusiform with tall, cylindrical whorls in S5 (Figure 11c), to rather small and fusiform-biconical in S3 (Figure 11d). There are significant differences among PSHs in the presence and relative position of the shoulder, and, while shells of all PSHs exhibit axial and spiral sculpture, there are notable differences in the arrangement and prominence of sculptural elements. The radulae in all but one studied PSH (S2, S4–S6; Figure 13) consist of awl-shaped hypodermic teeth with a comparatively short, simple dorsal blade, a lateral process and a large, wide ligament. In S3, the tooth is significantly smaller, without a blade and a lateral process and with a highly inflated base (Figure 13c). In S5, there is considerable variability of tooth formation, ranging from straight and comparatively tightly rolled (e.g., Figure 14a,e) to entirely unrolled (Figure 14b).



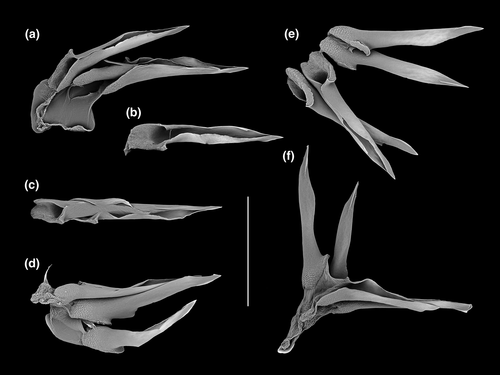
In the Theta clade, all PSHs can be readily differentiated based on teleoconch morphology (Figure 15e–g), ranging from distinctly shouldered with prominent axial tubercles in T1 to comparatively smooth with very weak to absent shoulder and rather convex teleoconch whorls in T2, with T3 exhibiting somewhat intermediate morphology. Two types of protoconch sculpture were exhibited by PSHs of Theta: arcuate riblets were present in T1 and T2, while T3 exhibited a (at least partly) diagonally cancellate pattern. In terms of the radula (Figure 16a–c), the hypodermic teeth with two comparatively weak barbs are arguably indistinguishable between T1 and T3, with somewhat weaker barbs in T2.

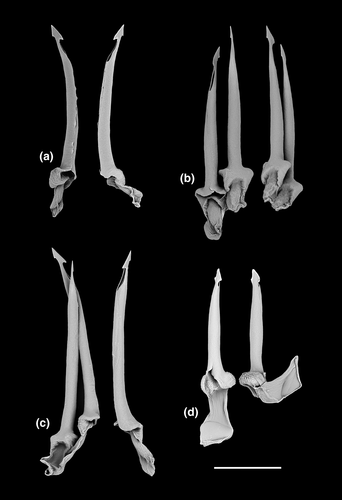
The two PSHs comprising the Austrotheta clade can be readily differentiated based on shell morphology (Figure 15h,i), in that U1 exhibits long, sharp, weakly opisthocline axial ribs on early to mid-teleoconch whorls, with U2 possessing a more distinct shoulder with tuberculate axial elements. The hypodermic teeth have only successfully been extracted for U1 (Figure 16d), which possesses very thick and weakly double-barbed teeth with extremely coarse basal texture and a very large ligament.
3.3 Geographic and bathymetric distributions
The recorded bathymetric range for Austrobela spans from 372 to 3235 m, with Spergo exhibiting a very wide range from 318 to 4750 m (Figure 17). Theta is recorded between 2474 and 4890 m and Austrotheta between 2751 and 3389 m.
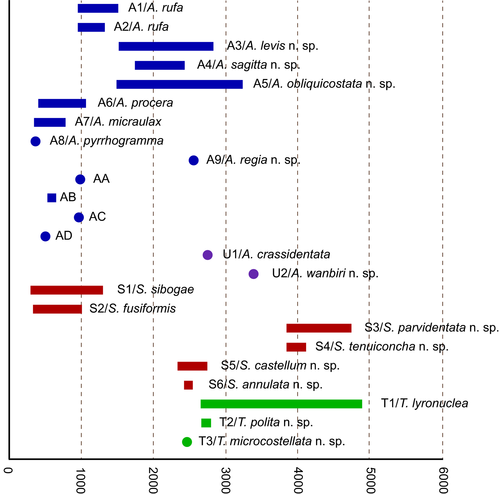
In Austrobela, the sister PSHs A1 and A2 (Figure 3) are known only from southern Australia, with the majority of records occurring in the GAB where they exhibit considerable geographic and bathymetric overlap (Figures 1 and 17) between 965 and 1321 m. Their sister taxon A3 (Figure 3) is exclusively recorded from the GAB with no bathymetric overlap with A1 and A2, with a reported range of 1535–2831 m. In the GAB, A3 occurs in partial micro-sympatry with A5, the latter occupying a depth range between 1509 and 3235 m, also extending eastward and up the eastern Australian coast to the Hunter Commonwealth Reserve (Figures 1 and 17). Records of A4 and A9 are restricted to the eastern Australian coast, with A4 occurring between 1761 and 2429 m depth, with records from the Central Eastern Marine Commonwealth Reserve and the Coral Sea Commonwealth Reserve. A9 is recorded at 2562 m depth off Byron Bay, northern New South Wales (NSW).
With the exception of S2, all PSHs of the Spergo clade treated herein are recorded exclusively from the southeast Australian coast, from east of Tasmania northward to northern NSW. In terms of the bathymetric distribution, PSHs of Spergo can be divided into three groups: S1 and S2 occur above 2000 m, S5 and S6 are found between 2000 and 3000 m, and S3 and S4 occur at depths below 3750 m (Figure 17). Only two PSHs, S3 and S4, have been recorded in micro-sympatry in the Bass Strait (Figure 1e).
For the Theta clade, no clear bathymetric partitioning can be inferred due to the small sample size of T2 and T3, with all three PSHs recorded below 2500 m (Figure 17). T1 exhibits a wide bathymetric range between 2649 and 4890 m, and with T2 and T3 recorded in 2677 to 2800 m and at 2474 m, respectively.
For the Austrotheta clade, both PSHs have been collected from single localities only: U1 from 2751 meters off eastern Tasmania and U2 from 3389 m in the GAB (Figures 1c,d and 17).
3.4 PSH to SSH conversion
Comparative examination of the morphological, geographic, and bathymetric data available was employed to attempt the conversion of PSHs to SSHs. As generating morphological data for most species with distribution outside Australian waters was beyond the scope of this study, testing of four PSHs (i.e., AA–AD), out of the total of 23 retrieved by ABGD, was not attempted and these are pending further sampling and taxonomic investigation. Of the remaining 19 PSHs, 17 (16 retrieved by ABGD—namely A3–A8 S1–S6, T1–T3 and U1–U2, and one inferred from 16S data—A9), satisfied the conditions described in the methodological section, while two PSHs (A1 and A2) did not. The evidence for PSHs to SSHs conversion is detailed below, where congeneric PSHs are compared with each other according to their relationships as resolved by the molecular analysis (Figures 2 and 3 and Figure S1).
In Austrobela, A1 and A2 corresponded to highly supported clades (BPP = 100%, BTSP = 99%; Figure 2 and Figure S1) in a sister relationship. Both exhibited very low intra-PSH genetic distance (average 0.01% and 0.02%, respectively; Table 1) and moderate reciprocal genetic distance (average 2.8%; Table 1). Although both A1 and A2 could typically be distinguished from all other PSHs by their combined dark orange, broad, distinctly shouldered teleoconch whorls with few wide axial ribs (Figure 5) and protoconch with arcuate riblets (Figure 8a–c), no morphological features could be used to readily differentiate between the two. Furthermore, their bathymetric (Figure 17) and geographic ranges overlap extensively, with numerous occurrences of micro-sympatry (i.e., the two PSHs were found in the same trawl haul; Figure 1b,d). Rather than supporting the conversion into separate SSHs, the evidence produced indicates that A1 and A2 may be two mitochondrial lineages within the same SSH (A1/2).
Clade A3 was highly supported (BPP = 1 and BTSP = 99%), exhibiting values of intra-PSH genetic distances (average 0.4%; Table 1) well below values of reciprocal between-PSH genetic distance with its most closely related PSHs (4.4% with A1 and 5.3% with A2; Table 1). The distinctive, virtually unsculptured white teleoconch (Figure 6a-e), the protoconch sculpture of very closely set arcuate ribs (Figure 8d) and the excavated adapical opening of the hypodermic teeth (Figures 9f and 10a), shared by its constituent samples, set A3 apart from all other PSHs, including the microsympatric A1 and A5 (Figure 1b).
In the ML analysis of the combined dataset (Figure S1), samples of A5 are sister to the only sample of A4 (AMS C.519338), from which they exhibited values of genetic differentiation (average = 3.3%; Table 1) that were notably higher than values measured between themselves (average = 0.5%). They both occurred in the same marine realm (Coral Sea, #16 of Costello et al., 2017; Figure 1d) and at a comparable depth (Figure 17). However, A4 can be readily differentiated from A5 by its much more shouldered and sculptured shell (Figure 6h), by its diagonally cancellate protoconch and by its distinctively more elongate hypodermic teeth (Figure 8e). This latter feature is not found in any of the other congeneric PSHs.
In the Coral Sea (Figure 1d), the sister pair A4/A5 co-occurred and were closely related with A6. This latter PSH received low BPP support (0.94) but exhibited low values of intra-PSH genetic distance (average = 0.3%; Table 1) and moderately high values of genetic differentiation from both A4 and A5 (4.2% and 2.9%, respectively; Table 1). The shell of A6 (Figure 7b) is markedly more elongate than shells of both A4 and A5 (Figure 6f–h), and it is found at shallower depth than the latter two PSHs (Figure 17).
The analysis of the 16S dataset (Figure 3) revealed a sister relationship between A5 and A9, that occur at the same depth range. However, despite being sympatric in the Coral Sea realm, these two PSHs maintain considerable morphological differentiation. In particular, A9 differs from A5 in overall shell shape and color (Figure 6f,g,i) and protoconch sculpture (diagonally cancellate vs. diagonally cancellate and with arcuate ribs; Figure 8g,h).
Two further PSHs, A7 and A8, were recorded at much shallower depths (Figure 17) outside Australian waters (Table S1). Although A7 received phylogenetic statistical support in the ML analysis only (BTSP = 88%; Figure S1), it exhibited low intra-PSH genetic distance (0.3%, Table 1) and moderate values of inter-PSH distance (3.0%) with its closely related PSHs, AD (Table 1). The geographic and bathymetric ranges of the two PSHs overlap in the South Pacific (Table S1, Figure 17), but their morphological distinctiveness could not be assessed, due to the lack of shell or radular data for AD. Although A7 is here tentatively regarded as a distinct SSH from AD, it is not unlikely that the two PSHs would prove to be conspecific, once further data are available. A8 was highly supported (BPP = 1 and BTSP = 100%) and exhibited high levels of genetic differentiation (>7.1%, Table 1) from all other PSHs in the Austrobela clade. It is found in the Caribbean Sea, well outside the focus area of this paper, and its shell exhibits a characteristic “speckled” coloration, not found in other congeners. These elements are considered sufficient to warrant its conversion to SSH.
In Spergo, two well-supported PSHs, S1 (BPP = 1; BTSP = 99%) and S2 (BPP = 0.98; BTSP = 91%), forming a sister relationship, exhibited low values of intra-PSH genetic distance (average = 0.3% for S1 and 0.8% for S2; Table 2) but were separated by moderately high inter-PSH distance (average = 2.8%; Table 2). They occur at much shallower depths than all other congeneric PSHs, from which they can be differentiated by a more prominent axial sculpture. Samples of S2 were collected in the Coral and northern Tasman Sea (Figure 1e) and in the South China Sea, where they co-occur with samples of S1 (Table S1). Although the radulae of S1 and S2 have not been studied here, their shells are markedly distinct (i.e., more elongate and with less pronounced axial ribs in S2, Figure 12b).
A strongly supported PSH clade (BPP=1 and BTSP = 100%), S3, was sister to the S1/S2 pair in the cox1 + 16S tree (Figure 2). It exhibited low values of intra-PSH genetic distance (0.2%; Table 2) and was separated from all other congeneric PSHs by comparatively high values (>6.7%; Table 2) of genetic distance. Along with its genetic distinctiveness, S3 could be readily separated from other PSHs in Spergo mainly by its extremely reduced venom apparatus and extremely small teeth, bearing neither barbs nor a blade (Figure 13c).
Despite its low BPP support (0.90), S4 exhibited low intra-PSH genetic distance (0.6%; Table 2) and was separated from the closely related S5 and S6 by relatively high genetic distance (4.2% and 3.4%, respectively; Table 2). In the South Australia realm (#26 of Costello et al., 2017), S4 co-occurs with S5 (Figure 1e), where it occupies a clearly distinct bathymetric range (Figure 17). Furthermore, S4 can be readily differentiated from all congeneric PSHs by a combination of its distinctively thin shell with a curved siphonal canal (Figure 11b). The sister pair S5/S6 were both highly supported (BPP = 1, BTSP = 99%) and exhibited little intra-PSH genetic distance (0.3% and 0.5%, respectively; Table 2). These were separated by the lowest inter-PSHs genetic distance (2.8%; Table 2) of all PSHs in Spergo. Their distribution is geographically disjunct (Figure 1e) and bathymetrically overlapping (Figure 17). However, S5 and S6 differ considerably in both shell color and shape (white and elongate vs. red and broad, respectively) and whorl profile (cylindrical vs. convex; Figure 11c,e). In addition, these two PSHs also differ in radula features, with S5 having loosely rolled teeth (Figure 13a) and S6 exhibiting more tightly rolled ones (Figure 13e). Given their low genetic divergence, S5 and S6 could be (in theory) considered geographically distinct populations of a single species. However, the morphological differentiation observed was higher than that expected between potential ecophenotypes. Hence, S5 and S6 are considered distinct SSHs.
Within Theta, T1 was highly supported (BPP = 1; BTSP = 98%), exhibited low values of intra-PSH genetic distance (average = 0.3%) and comparatively high levels of genetic differentiation from both T2 (4.9%) and T3 (4.2%). This PSH occurs in deeper waters than both T2 and T3 (Figure 17) and can be distinguished from these by its much broader shell bearing coarse, prominent axial ribs on all whorls (Figure 15e). In particular, T1 possesses a different protoconch sculpture (of arcuate riblets) from that of T3 (at least partly diagonally cancellate). The difference in shell morphology between T1 and T2 (Figure 15e,g) is maintained in spite of their co-occurrence in the South Australia realm (Figure 1c).
The inter-PSHs genetic distance separating T2 (BPP = 1; BTSP = 99%) and T3 (one sample only) was the lowest of all PSHs in Theta (3.3%; Table 2). Their distribution is geographically disjunct (Figure 1c) and bathymetrically overlapping (Figure 17). However, T2 and T3 differ considerably in the sculpture of both teleoconch (respectively unsculptured vs. bearing axial ribs; Figure 15f,g) and protoconch (with arcuate ribs vs. at least partly diagonally cancellate), and these differences are deemed sufficient for their conversion to SSH.
Despite their moderate inter-PSH genetic distance (3.3%), both PSHs of Austrotheta (U1 and U2, each represented by one specimen only) were converted into SSHs, based on their difference in shell features (such as relative size, teleoconch whorl profile and sculpture; Figure 15h,i) and their disjunct bathymetric distributions (Figure 17). The observed divergence was present in spite of their co-occurrence in the South Australia realm (Figure 1c,d).
3.5 Assigning names to SSHs
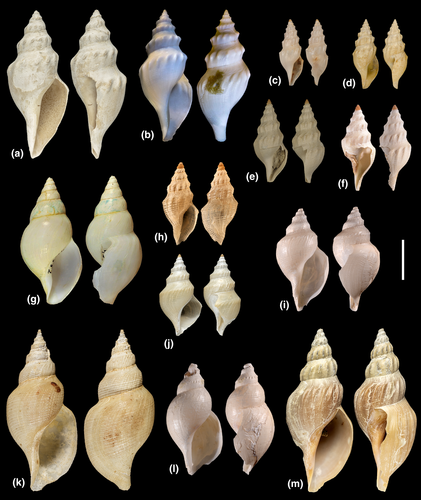
A search was conducted for all names available and potentially applicable to the eighteen SSHs resulting from the conversion process described above. By consulting the relevant literature on Raphitomidae (Bouchet & Sysoev, 2001; Bouchet & Warén, 1980; Clarke, 1959; Criscione et al., 2021; Kantor & Taylor, 2002; Sánchez & Pastorino, 2020; Sysoev, 1997; Sysoev & Bouchet, 2001) and by comparison of molecular and morphological data available on type specimens with the data generated on sequenced specimens, we found eight names applicable to eight SSHs. Two of these SSHs, A1/2 and U1, comprised the type material of, respectively, Austrobela rufa (Figure 5a) and Austrotheta crassidentata (Figure 15i) and could therefore be, respectively, assigned to these species. The remaining six SSH, namely A6, A7, A8, S1, S2 and T1, included specimens whose shells closely resembled the holotypes of, respectively, Gymnobela procera Sysoev & Bouchet, 2001 (Figure 7a), Gymnobela micraulax Sysoev, 1997 (Figure 7e), Gymnobela pyrrhogramma (Dautzenberg & Fischer, 1896) (Figure 6j), Gymnobela sibogae (Schepman, 1913) (Figure 11f), Spergo fusiformis (Kuroda & Habe, 1961) (Figure 12a) and Theta lyronuclea (Figure 15a). Shells of specimens of all five SSH also exhibited patterns of morphological variation which were consistent with those reported in the literature (see Bouchet & Warén, 1980; Criscione et al., 2021; Kantor & Taylor, 2002; Sánchez & Pastorino, 2020; Sysoev & Bouchet, 2001). Therefore, these SSHs were attributed to these species. This required the formal transfer (as hereby proposed) of the first three species to Austrobela as Austrobela procera n. comb., Austrobela micraulax n. comb. and Austrobela pyrrhogramma n. comb and of the fourth species to Spergo as Spergo sibogae orig. comb. As no available names could be found for the remaining eleven SSHs, new taxon names were assigned, namely Austrobela levis n. sp. (A3), Austrobela sagitta n. sp. (A4), Austrobela obliquicostata n. sp. (A5) and Austrobela regia n. sp. (A9; for Austrobela); Spergo parvidentata n. sp. (S3), Spergo tenuiconcha n. sp. (S4) and Spergo castellum n. sp. (S5) and Spergo annulata n. sp. (S5; for Spergo); Theta polita n. sp. (T2) and Theta microcostellata n. sp. (T3; for Theta) as well as Austrotheta wanbiri n. sp. (U2; for Austrotheta). Formal taxonomic descriptions of these newly recognized species are provided below.
3.6 Systematics
If not stated otherwise, holotypes are dissected ethanol-preserved specimens on which all systematic descriptions are based. Shell whorls counts (approximated to one decimal unit) are reported with reference to intact whorls only. When applicable, the expression “at least” is used in combination with the whorl count to indicate potential additional missing whorls that could not be counted. Shell and head-foot coloration reported in the descriptions are based on observations performed prior to fixation and thus may not be fully reflected in the illustrations provided (Figures 5-8, 11, 12 and 15).
- Superfamily Conoidea Fleming, 1822
- Family Raphitomidae Bellardi, 1875
- Genus Austrobela Criscione, Hallan, Puillandre and Fedosov, 2020 (Criscione et al., 2021; p. 983)
Type species: Austrobela rufa Criscione, Hallan, Puillandre and Fedosov, 2020 by original designation (PSHs A1–A2).
Other species: Austrobela levis n. sp., A. micraulax (Sysoev, 1997) (Figure 7e; Sysoev, 1997; p. 338–339, figures 47–48), A. obliquicostata n. sp., A. procera (Sysoev & Bouchet, 2001) (Figure 7a; Sysoev & Bouchet, 2001; p. 312–313; figsures 131–133, 172), A. pyrrhogramma (Dautzenberg & Fischer, 1896) (Figure 6j; Dautzenberg & Fischer, 1896; p. 415–416; pl. 17, figures 6–8), A. regia n. sp., A. sagitta n. sp.
Diagnosis
Shell fusiform, thin. Protoconch multispiral, light to reddish orange. Protoconch sculpture varying from diagonally cancellate to bearing widely distanced to closely set arcuate ribs, or combination of diagonally cancellate (abapical) and arcuate (adapical). Teleoconch red orange, cream or white, suture impressed. Whorl profile medium- to very broad, with wide, concave to oblique subsutural ramp, clearly demarcated from whorl periphery. Lower portion of whorl cylindrical or convex. Subsutural ramp sculpture of dense arcuate growth lines, reflecting shape of anal sinus. Teleoconch axial sculpture absent or of ribs below subsutural ramp; spiral sculpture of fine, sometimes flattened cords or shallow grooves; microsculpture of growth lines. Last adult whorl evenly convex, clearly to very clearly demarcated from rather straight, subcylindrical to tapering siphonal canal.
Aperture elongate, from about 2/5 to half of total shell length; outer lip thin, unsculptured. Inner lip with distinct callus and with or without spiral cords extending onto rather straight columella; callus whitish, red orange with or without a darker transversal band. Anal sinus wide, moderately deep to deep, L- or U-shaped.
Cephalic tentacles muscular, subcylindrical to cylindrical; eyes large. Rhynchodeal introvert rather thin-walled, densely folded. Venom apparatus extremely large, occupying majority of rhynchocoel. Radula of hypodermic teeth with two large, sharp distal barbs; lower portion of shaft somewhat inflated; base broad; ligament thick.
Remarks
Prior this study, the type species A. rufa was the only described Austrobela species. Here, the total number of species is increased to eight, following the description of four new species and the transfer to Austrobela of further three species previously included in different genera. The current genus distribution appears disjunct, with most species occurring in the Indo-Pacific (three realms, Figure 1a,b,d) and one (A. pyrrhogramma) in the Caribbean Sea (Figure S1). However, the picture of the genus diversity and distribution emerging here is far from complete, due to the narrow geographic focus of this study. It is almost certain that a comprehensive revision of Austrobela (with access to data from taxa not treated here) would result in an increase of its species number and in a considerable expansion of its geographic range. Our results indicate that up to four additional species from outside Australian waters could be added to Austrobela once morphological data are available for PSHs AA–AD (Figure 2). Further molecular data would be also necessary to evaluate the genus placement of further 9 deep-sea raphitomid species (currently included in Gymnobela, Xanthodaphne Powell, 1942 and Theta) that exhibit conchological and (when available) radular features very similar to those observed in Austrobela.
One of these species, Gymnobela nivea Sysoev, 1990 (Figure 18f; Sysoev, 1990, figure 3.7), occurs in the Nazca and Salas y Gomez Ridges off the coast of Chile. Another species, Gymnobela gypsata (Watson, 1881) (Figure 18a; Dell, 1963, figures 10–11), was described from (off) New Zealand. Two more species, Gymnobela ceramensis (Schepman, 1913) (Figure 18h; Schepman, 1913, pl. 30, figure 3) and Gymnobela dubia (Schepman, 1913) (Figure 18c; Schepman, 1913, pl. 30, figure 8), were described for the Ceram Sea (off E Indonesia). One species, Xanthodaphne pyrropelex (Barnard, 1963) (Barnard, 1963, figure 2c; Sysoev, 1996, figures 16–18, 20), is found off the South African Cape region. Of the further four species described for the Atlantic, two are from the NE, namely Gymnobela fulvotincta (Dautzenberg & H. Fischer, 1896) (Figure 18b; Bouchet & Warén, 1980, figures 109, 251) and Theta chariessa (Watson, 1881) (Figure 15c; Bouchet & Warén, 1980, figure 129—but not 130) and two are from the NW, Atlantic namely Gymnobela filifera (Dall, 1881) (Figure 18d; Dall, 1889, pl. 12, figure 9) and Gymnobela petiti Garcia, 2005 (Figure 18e; Garcia, 2005, figures 17–19).
Material examined
Holotype: Australia, GAB, (−35.15, 134.11), IN2015_C02_131, 965–1077 m, AMS C.571709.
Paratypes: Australia, Tasmania, Flat area south of Brians, (−44.24, 147.29), IN2018_V06_169, 1443–1422 m, 1 wet (TMAG E59197); St Helens flat, (−41.21, 148.8), IN2018_V06_184, 1221–1202 m, 1 wet (AMS C.271201), 1 wet (AMS C.574588), (TMAG E45585), 1 wet (TMAG E45586), 1 wet (TMAG E59223). GAB, (−35.34, 134.05), IN2015_C02_134, 1509–1544 m, 1 wet (AMS C.532691), 1 wet (AMS C.571699); (−35.15, 134.11); IN2015_C02_131, 965–1077 m, 1 wet (AMS C.571786), 1 wet (AMS C.571787); (−34.82, 132.69), IN2015_C02_167, 1015–998 m, 1 wet (AMS C.532677); (−34.78, 131.73), IN2015_C01_099, 1323–1340 m, 1 wet (AMS C.483801), 1 wet (AMS C.483802), 1 wet (AMS C.571668); (−34.74, 131.84), IN2015_C01_108, 1350–1321 m, 1 wet (SAMA D44253), 1 wet (SAMA D67742), 1 wet (SAMA D67745); (−34.71, 132.53), IN2015_C01_114, 994–980 m, 1 wet (AMS C.571679), 1 wet (AMS C.571781); (−34.67, 132.48), IN2015_C01_117, 1016–1014 m, 1 wet (AMS C.571681), 1 wet (AMS C.571784), 1 wet (AMS C.483817); (−33.52, 130.27), IN2015_C02_382, 978–1013 m, 1 wet (AMS C.571680), 1 wet (AMS C.571790), 1 wet (AMS C.571791); (−33.52, 130.27), 1 wet (AMS C.571792), 1 wet (AMS C.571793), 1 wet (AMS C.571796), 1 wet (AMS C.571799), 1 wet (AMS C.571801), 1 wet (AMS C.571803), 1 wet (AMS C.571804), 1 wet (AMS C.571805), 1 wet (AMS C.571806), 1 wet (AMS C.571807), 1 wet (AMS C.571808).
Other material.
Australia, Tasmania, St Helens flat, (−41.21, 148.8), IN2018_V06_184, 1221–1202 m, 1 wet (AMS C.271202), 1 wet (AMS C.557076), 1 wet (TMAG E45587); GAB, (−35.15, 134.11), IN2015_C02_131, 965–1077 m, 1 wet (AMS C.532684); 1 wet (AMS C.571664), 1 wet (AMS C.571788), 1 wet (AMS C.571789), 1 wet (AMS C.571756), 1 wet (AMS C.575584); (−34.82, 132.69), IN2015_C02_167, 1015–998 m, 1 wet (AMS C.571670); (−34.74, 131.84), IN2015_C01_108, 1350–1321 m, 1 wet (SAMA D67743), 1 wet (SAMA D67744); (−34.71, 132.53), IN2015_C01_114, 994–980 m, 1 wet (AMS C.483826); (−34.67, 132.48), IN2015_C01_117, 1016–1014 m, 1 wet (AMS C.571785); (−33.93, 131.06), IN2015_C02_196, 1021–1033 m, 1 wet (AMS C.532702); (−33.72, 130.67), IN2015_C02_292, 1010–1011 m; (−33.52, 130.27), IN2015_C02_382, 978–1013 m, 1 wet (AMS C.532874), 1 wet (AMS C.571794), 1 wet (AMS C.571795), 1 wet (AMS C.571797), 1 wet (AMS C.571798), 1 wet (AMS C.571800), 1 wet (AMS C.571802), 1 wet (AMS C.571809).
Remarks
Material examined
Holotype: Australia, GAB, (−34.074, 129.182), IN2015_C01_064, 2649–2803 m (AMS C.571693).
Paratypes: Australia, GAB, (−35.54, 132.676), IN2015_C02_155, 1942–1926 m, 1 wet (= ethanol-preserved specimen); (AMS C.532671); (−35.009, 130.317), IN2015_C02_227, 2848–2831 m, 1 wet (AMS C.532710); (−34.625, 130.28), IN2015_C02_449, 2007–2067 m, 1 wet (AMS C.532883), 1 wet (AMS C.571695); (−35.798, 132.693), IN2015_C02_151, 2773–2677 m, 1 wet (AMS C.571616); (−35.558, 134.083), IN2015_C02_137, 1927–1995 m, 1 wet (AMS C.571694); 1 wet (AMS C.571813); (−34.072, 130.267), IN2015_C02_435, 1570–1535 m, 1 wet (SAMA D44145); (−35.202, 131.629), IN2015_C01_054, 1912–1836 m, 1 wet (SAMA D44143).
ZooBank registration: http://zoobank.org/urn:lsid:zoobank.org:act:EB55708D-8C49-411F-90E5-3952BDF26ABF
Etymology
In reference to its unsculptured shell, derived from “levis” (Latin=smooth). Adjective of feminine gender.
Distribution
This new species is known from bathyal depths in the GAB (Figure 1b).
Description
Shell (SL = 29.4, SW = 12.0 mm) fusiform, chalky-white, with polished surface. Protoconch cyrthoconoid, multispiral, of at least three evenly convex orange whorls, with sculpture of dense arcuate riblets. Teleoconch of 5.4 uniformly whitish whorls. Early teleoconch whorls with clear angulation at about mid-height of whorl, separating wide straight subsutural ramp from whorl periphery. Late whorls with gradual transition from subsutural ramp to more convex periphery. Sculpture of shallow striae and very fine collabral growth lines. Shell base convex, clearly demarcated from long, tapering siphonal canal. Striae becoming denser toward siphonal canal, resulting in finely lyrate sculpture. Aperture elongate, about half length of shell. Outer lip thin, unsculptured, evenly convex below subsutural ramp and attenuated toward tip of siphonal canal in its lower portion; inner lip smooth, with thin callus. Siphonal canal wide and deep.
Animal (based on AMS C.532671) uniform cream. Cephalic tentacles cylindrical, with blunt tips; large eyes situated at their outer base. Penis large, thick, evenly tapering toward pointed tip.
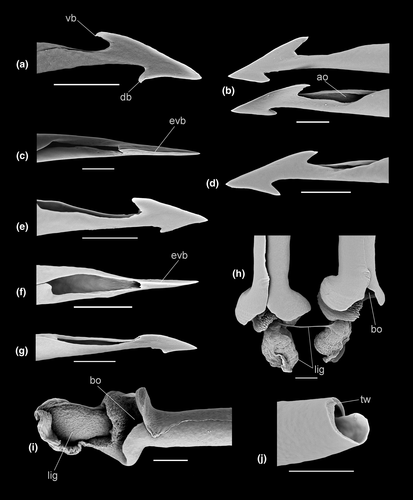
Radula (Figures 9f and 10a) of hypodermic, relatively straight, somewhat loosely rolled marginal teeth, attaining 300 µm in length. Tip with two barbs of roughly equal size, of which barb anterior to adapical opening occurs slightly more distal from tip, strongly excavated, commonly curved in profile (Figure 10a); adapical opening subterminal posterior to ventral barb, elongate, approximately 1/12–1/15 of shaft length, somewhat depressed in profile. Base swollen, texture somewhat coarse; ligament narrow, small.
Remarks
This species is very similar to A. rufa in overall shell morphology but can be readily differentiated based on its comparatively smooth, whitish shell. The hypodermic tooth has a somewhat shorter adapical opening than the two former species and with the barb on the side of the adapical opening more excavated and curved in profile and with a notable depression where the opening is situated (Figure 9f).
This species bears notable similarity to the South African X. pyrropelex, in that both taxa exhibit comparatively smooth, elongate shells with a moderately steep, wide subsutural ramp and a protoconch sculpture of arcuate riblets (Barnard, 1963; Sysoev, 1996). Furthermore, Barnard (1963, figure 2c) illustrated the radula which shows a double-barbed hypodermic tooth with a somewhat inflated lower shaft, which is similar to that of Austrobela (Figure 9). As Barnard (1963) provided a relatively simple line illustration, there is limited detail upon which to make further comparison with Austrobela, such as the morphology of the adapical and basal openings, basal texture and ligament. We also note that Barnard (1963) reported that the eyes are very small or absent in X. pyrropelex, whereas A. levis n. sp., as all other Austrobela spp. examined herein, possess large eyes. Criscione et al. (2021) suggested that the presence and/or relative size of the eyes appeared comparatively consistent at the genus-level in deep-sea Raphitomidae. Owing to the lack of detail in the line drawing by Barnard (1963), the radula cannot be readily compared to that of Austrobela apart from noting that they are at least superficially similar. The divergent eye morphology, however, does suggest that X. pyrropelex and A. levis n. sp. are not conspecific.
Material examined
Holotype: Australia, GAB, (−35.54, 132.67), IN2015_C02_155, 1942 m, 1 wet (SAMA D67741).
Paratypes: Australia, GAB, (−35.798, 132.69), IN2015_C02_151, 2773–2677 m, 1 wet (AMS C.571645), 1 wet (AMS C.532869); (−35.345, 134.045), IN2015_C02_134, 1509–1544 m, 1 wet (AMS C.571710); (−35.54, 132.67), IN2015_C02_155, 1942–1926 m, 1 wet (SAMA D44141); (−35.009, 130.317), IN2015_C02_227, 2848–2831 m, 1 wet (SAMA D44164); (−34.452, 129.492), IN2017_C01_197, 3350–3235 m, 1 wet (AMS C.571728), 1 wet (AMS C.571729), 1 wet (AMS C.572173); NSW, off Bermagui, (−36.355, 150.644), IN2017_V03_044, 2821–2687 m, 1 wet (AMS C.482317); NSW, Hunter CMR, (−32.575, 153.162), IN2017_V03_070, 2595–2474 m, 1 wet (AMS C.571644).
ZooBank registration: http://zoobank.org/urn:lsid:zoobank.org:act:95629EDD-2DBC-46DC-A74F-FADF8D05FBE5
Etymology
In reference to its shell sculpture of opisthocline ribs, derived from “obliquus” (Latin = oblique) and “costatus” (Latin = bearing ribs). Composite adjective of feminine gender.
Distribution
Known for the GAB and for off the southeastern coast of Australia.
Description
Shell (Figure 6f; SL = 27.7, SW = 10.5 mm) thin-walled, semi-translucent, broadly fusiform, with strongly shouldered whorls. Protoconch (Figure 8g) multispiral of four light to reddish orange whorls. Protoconch II whorls evenly convex, with arcuate riblets on adapical half to two-thirds of whorl, with diagonally cancellate sculpture below. Teleoconch of 5.8 whitish whorls; first whorl nearly cylindrical; subsequent whorls strongly shouldered, with wide weakly concave subsutural ramp. Sculpture of prominent, evenly interspaced, notably opisthocline ribs on whorl periphery, more pronounced at shoulder and barely reaching lower suture. Spiral sculpture of regular, slightly undulate striae, indistinct on subsutural ramp and well-pronounced on whorl periphery. Subsutural ramp with fine, densely set collabral growth lines, some forming short regularly spaced raised riblets bordering upper suture. Last adult whorl with approximately 20 ribs of regularly decreasing prominence below shoulder. Shell base gently convex, continued into long, straight and slender siphonal canal. Aperture elongate, about half of length of shell. Outer lip thin, unsculptured; inner lip smooth, with thin callus on columella. Anal sinus wide, moderately deep, L-shaped.
Animal uniform whitish cream. Cephalic tentacles moderately short, stubby, broad; eyes situated on outer side of tentacles, approximately 1/3–1/4 from their bases.
Proboscis yellowish, cylindrical, blunt, with latitudinal folds in wall; radular sac large, elongate; venom gland extremely long and convoluted, colorless, filled with whitish substance; muscular bulb lustrous, yellowish, large.
Radula (based on paratype AMS C.571644, Figure 9d) of relatively straight to slightly undulating, loosely rolled hypodermic teeth, attaining 200 µm in length. Tip with two barbs of approximately equal size, ventral barb situated more distal from tip than dorsal barb; adapical opening posterior to ventral barb, elongate, approximately 1/8 of length of shaft. Base swollen, with somewhat coarse texture; ligament thick.
Remarks
This species overlaps geographically and bathymetrically with A. levis throughout much of its range. However, the latter has not been recorded outside of the GAB. Of the Austrobela species, these are among the two taxa most readily distinguished based on their shell morphology; A. levis n. sp. possesses a comparatively smooth shell with a rounded shoulder, whereas A. obliquicostata n. sp. has marked axial sculpture and a prominent, angulated shoulder. Furthermore, the protoconch of A. levis n. sp. possesses a sculpture of arcuate riblets (Figure 8d), as opposed to the combination of these elements with more typical diagonally cancellate sculpture observed in A. obliquicostata n. sp. (Figure 8g).
Material examined
Holotype: Australia, NSW, off Byron Bay, (−28.677, 154.203), IN2017_V03_090, 2587–2562 m, (AMS C.571682).
Paratype: As per holotype, 1 wet (AMS C.519374).
ZooBank registration: http://zoobank.org/urn:lsid:zoobank.org:act:F9B67DF8-D865-4ADD-8FDA-4B259637DFC1
Etymology
In reference to its elegant spiral sculpture, resembling a crown, derived from “regius” (Latin=regal). Adjective of feminine gender.
Distribution
Known for the type locality only, off Byron Bay, NSW, Australia.
Description
Shell (Figure 6i; SL = 28.9, SW = 12.6 mm) thin, broadly fusiform, with strongly shouldered whorls. Protoconch (Figure 8h) multispiral, light orange, eroded, with at least 3.5 whorls, with dense diagonally cancellate sculpture. Teleoconch of 5.9 whitish to amber whorls, spiral sculpture of many wavy spiral grooves, about half width of their interspaces, and extending across axial sculpture, consisting of distinct ribs, forming starkly angulated periphery and extending to base in early whorls, then gradually becoming subobsolete to obsolete at base of mature whorls. Microsculpture of bi-sinuose growth lines, forming regularly placed cordlets with finer striae in their interspaces, most distinct at sinus then graduating toward subobsolete at periphery. Suture deep, adpressed; subsutural ramp moderately steep (approx. 55–60°), concave on early whorls and rather straight on last whorl; sinus wide, subsutural, broadly U-shaped, widely arcuate, deep. Aperture elongate-pyriform, about equal in length to spire, outer lip thin, rounded anterior to subsutural ramp and evenly tapering toward moderately long siphonal canal; inner lip smooth, whitish posteriorly, graduating to orange to reddish brown anteriorly.
Animal uniform cream; cephalic tentacles of medium length, broad at base, evenly tapering toward blunt tip; eyes situated on outer side, approximately 1/4 from base.
Radula (Figures 9e and 10b,h) consisting of long, rather thick, loosely rolled, relatively straight to lightly curved hypodermic teeth exceeding 300 µm in length; tip with two prominent barbs (Figure 8b) of which the dorsal is somewhat larger and more distal from tip; adapical opening situated immediately posterior to dorsal barb, somewhat elongate; base swollen, texture rather coarse. Ligament thick, rather short (Figure 8h).
Remarks
Material examined
Holotype: Australia, Central Eastern CMR, (−30.098, 153.899), IN2017_V03_086, 2429–2518 m, (AMS C.519338).
Paratype: Australia, Coral Sea CMR, (−23.631, 154.66), IN2017_V03_128, 1770–1761 m, 1 wet (AMS C.519400).
ZooBank registration: http://zoobank.org/urn:lsid:zoobank.org:act:FF4F714B-6C7D-4072-BCB7-426D07F4A0EF
Etymology
In reference to its very long and straight hypodermic teeth, derived from “sagitta” (Latin = arrow). Noun in apposition.
Distribution
This species is recorded from Coral Sea, Queensland (1770 m) and off the coast of northern NSW (2429 m).
Description
Shell (Figure 6h; SL = 25.0, SW = 11.3 mm) broadly fusiform, thin-walled, with glossy surface. Protoconch (Figure 8e) multispiral, cyrthoconoid, of four orange whorls. PII whorls evenly convex, with fine, diagonally cancellate sculpture throughout height of whorl. Protoconch-teleoconch transition well-defined, with opisthocline boundary. Teleoconch of 5.2 whorls, uniformly white, with distinct suture. Whorls rather broad, with wide, distinctly to slightly concave subsutural ramp and well-pronounced shoulder situated slightly below mid-height of whorl. Subsutural ramp sculptured with regular collabral riblets. Below, axial sculpture of strong, sharp, densely set, weakly opisthocline ribs, thicker at whorl periphery; clearly arcuate on penultimate and last whorls and obsolete at base. Spiral sculpture of regular fine, wavy grooves, about half width of their interspaces, extending across axial sculpture. Siphonal canal long, slender, tapering. Aperture large, elongate-pyriform, about half length of shell, outer lip thin, opisthocline; inner lip white, smooth except for extensions of few spiral cords of siphonal canal inside aperture. Anal sinus wide, deep, u-shaped.
Animal uniform cream, cephalic tentacles of medium length, broad, blunt; eyes rather large, situated at outer base of cephalic tentacles.
Proboscis broad, blunt; radular sac extremely large; venom gland very long, convoluted; muscular bulb large, bean-shaped, lustrous.
Radula (Figure 9g) consisting of very long, relatively straight hypodermic teeth, exceeding 600 µm in length. Tip with two barbs of approximately equal size, of which ventral barb more distal from tip; adapical opening posterior to ventral barb, elongate, approximately 1/17 length of shaft; base swollen, texture rather indistinct; basal opening large. Ligament comparatively long, moderately thick.
Remarks
This species can be differentiated from other Australian Austrobela spp. by its significantly longer hypodermic tooth (Figure 9g). A comparison of A. sagitta n. sp. with the very similar A. regia n. sp. is provided under the remarks to this latter species. Kantor and Taylor (2002) figured a similarly elongate tooth for the Atlantic A. pyrrhogramma: however, our molecular analyses (Figures 2 and 3) suggest these two taxa are not closely related. In terms of shell morphology, A. sagitta n. sp. can be differentiated from A. pyrrhogramma by its significantly broader shell and from the other Australian spp. by its multiple prominent axial ribs that extend across the periphery to the lower suture and about half-way across the base of last adult whorl (Figure 6h). Austrobela sagitta n. sp. can also readily be separated from A. rufa, A. levis and A. obliquicostata by its fine, diagonally cancellate protoconch, which in the two former species bears arcuate riblets, whereas in the latter one the abapical portion is coarsely diagonally cancellate, above which arcuate riblets are present. Austrobela sagitta n. sp. differs from T. lyronuclea in its more pronounced and numerous axials, which remain distinctive throughout the height of whorl periphery, while vanishing quickly below shoulder on late whorls of T. lyronuclea.
Distribution
New Caledonia, Norfolk Ridge, Loyalty Ridge, Wallis and Futuna and East and West Tropical Australia.
Remarks
This species was previously known for its shell only and all specimens studied herein for this species exhibit typical features (Sysoev & Bouchet, 2001). The radula of A. procera is illustrated for the first time in the present study (Figure 9c) and is typical of the genus. The penis (based on AMS C.571647) is very long, narrow with no obvious glands or swellings, with a small distal seminal papilla. Large eyes are situated at the outer lower bases of moderately long, cylindrical cephalic tentacles. The protoconch differs from A. rufa, A. levis n. sp. and A. obliquicostata n. sp. in its fine, diagonally cancellate sculpture throughout the height of the whorls (Figure 8f) but it is not readily differentiated from that of A. sagitta n. sp. However, A. procera differs from A. sagitta n. sp. by its notably more elongate shell (Figure 7a,b).
Genus Spergo Dall, 1895 (Dall, 1895; p. 680)
Type species Mangilia glandiniformis Dall, 1895 (Figure 11a; Dall, 1895, p. 681–683, pl. 24, figures 1–2) by subsequent designation (Dall, 1918, p. 331).
Other species: Spergo aithorrhis Sysoev & Bouchet, 2001 (Figure 12c; Sysoev & Bouchet, 2001, p. 303–305, figures 9, 121–124, 170), S. annulata n. sp., S. castellum n. sp., S. fusiformis (Kuroda & Habe, 1961) (Figure 12a; Habe, 1961, p. 81, pl. 40, figure 9, app. 30; Sysoev & Bouchet, 2001, p. 302–303, figures 8, 115–120), Spergo parunculis Stahlschmidt et al., 2015 (Figure 12d; Stahlschmidt et al., 2015, p. 9–10, figures 10–20), S. parvidentata n. sp., S. sibogae Schepman, 1913 (Figure 11f; Schepman, 1913, p. 448–449, pl. 30, figure 9; Sysoev & Bouchet, 2001, p. 306, figure 125–128), S. tenuiconcha n. sp.
Diagnosis
Shell large, fusiform to elongate-fusiform, walls solid, opaque to moderately thin. Protoconch multispiral. Teleoconch white, cream or dark orange; whorl profile slender to medium broad, evenly convex or with well-defined shoulder; whorl portion below subsutural ramp short to very tall, cylindrical to convex. Subsutural ramp varying from indistinct to wide, concave; suture impressed. Spiral sculpture evenly developed throughout whorl height, or below subsutural ramp; of cords, often regularly spaced. Axial sculpture of opisthocline ribs, usually weak and/or confined to early whorls. Microsculpture of growth lines, most prominent on subsutural ramp with slightly to moderately raised cordlets present at regular to uneven intervals, reflecting shape of anal sinus. Last adult whorl evenly convex to distinctly shouldered below subsutural ramp, not clearly demarcated from moderately long, evenly tapering siphonal canal. Aperture elongate-pyriform, from about one third to over half of shell length; outer lip thin, unsculptured; inner lip with distinct, rather wide whitish (with or without dark orange stain), cream or yellowish callus. Anal sinus wide, shallow, u-shaped.
Animal color variable (grayish, pink, whitish). Head broad to very broad, blunt; cephalic tentacles broad, short, tapering, with medium to large eyes situated at outer basal part. Rhynchocoel capacious. Proboscis short, broad to very broad. Venom apparatus well-formed to greatly reduced; venom gland moderately long to short; muscular bulb elongate to very elongate. Radula of hypodermic teeth. Teeth rolled, loosely rolled to entirely unrolled, straight to curved or bent, barbs absent; no blade or with short dorsal blade; adapical opening elongate of variable length; base swollen, lateral process distinct to absent; external base with medium coarse sculpture; basal opening subcircular, large to very large. Ligament broad.
Remarks
Prior to this study, the genus included five species, namely the type species S. glandiniformis (from off Hawaii), S. aithorrhis (Norfolk Ridge), S. parunculis (Mozambique Channel), S. fusiformis (West Pacific) and Spergo nipponensis Okutani & Iwahori, 1992 (Japan Sea) (Okutani & Iwahori, 1992, figures 65–66). However, the description of the holotype of the latter species (Okutani & Iwahori, 1992, p. 264) mentions the presence of an operculum, which is absent in all Raphitomidae. The combination of shell and radula traits shown by this specimen (Okutani & Iwahori, 1992, figures 65 and 66) are more typical of the Borsonidae, supporting the tentative transfer of S. nipponensis to Borsonia Bellardi, 1839. The number of remaining species of Spergo is here doubled and the genus distribution further extended to cover six different realms (#9, 13, 15, 16, 26 and 29 of Costello et al., 2017) across the Indian (not shown) and the Pacific Oceans (Figure 1e partim). In order to confirm the boundaries of the genus Spergo, molecular data need to be generated for species not studied here, in particular, for the type species. There is a plethora of species, currently placed in different genera, but sharing some shell and radular features with the species of Spergo and whose affinities to Spergo should be evaluated. Among them are the Antarctic Xanthodaphne pastorinoi Kantor et al., 2016 (Kantor et al., 2016, figure 16), Gymnobela africana Sysoev, 1996 from (off) E Africa (Figure 18m; Sysoev, 1996, figures 109–111), the N Pacific Gymnobela oculifera Kantor & Sysoev, 1986 (Figure 18l; Kantor & Sysoev, 1986, figures 1A, 2A, 3), the NE Atlantic Bathybela nudator (Locard, 1897) (Figure 18k; Bouchet & Warén, 1980, figures 16, 133) and the NW Atlantic Gymnobela emertoni (Verrill & S. Smith, 1884) (see below remarks under Spergo tenuicostata).
Spergo was traditionally regarded as part of an informal “complex” or “group” of nominal genera (Stahlschmidt et al., 2015; Sysoev & Bouchet, 2001), which were considered evolutionarily related based on their shell similarities. However, analysis of molecular data has revealed considerable homoplasy in shell features and resolved this artificial group into a number of unrelated genus-level lineages (Criscione et al., 2021). Among these lineages, Spergo is characterized by species with large shells featuring a shallow anal sinus, weak spiral cords and with short awl-shaped radular teeth. While the combination of shell characters is sufficient to differentiate Spergo from most raphitomid genera, the examination of the radula is necessary to distinguish this genus from the conchologically similar Pontiothauma E. A. Smith, 1895, Nodothauma Criscione, Hallan, Puillandre & Fedosov, 2020 and Abyssobela Kantor & Sysoev, 1986.
Material examined
Holotype: Australia, Victoria, East Gippsland CMR, (−37.792, 150.382), IN2017_V03_035, 2338–2581 m, (AMS C.482148).
Paratype: Australia, Tasmania, Freycinet CMR, (−41.731, 149.12), IN2017_V03_004, 2820–2751 m, 1 wet (AMS C.519290).
ZooBank registration: http://zoobank.org/urn:lsid:zoobank.org:act:5BACDBB2-0A37-4097-BAFB-67D1BFA93EBC
Etymology: In reference to the cylindrical, high wall-like appearance of the whorl periphery, derived from “castellum” (Latin = castle). Noun in apposition.
Distribution
Known only from two localities; East Gippsland Commonwealth Marine Reserve, Victoria and Freycinet Commonwealth Marine Reserve (Figure 1e).
Description.
Shell (Figure 11c; SL = 66.4 mm, SW = 24.8 mm) elongate-fusiform, with high spire; walls solid, opaque. Protoconch eroded. Teleoconch of 7.7 uniform white whorls; whorl profile slender, with well-defined shoulder at approximately adapical third of whorl, whorl base very tall, cylindrical to weakly convex. Subsutural ramp wide; suture impressed. Spiral sculpture below subsutural ramp of cords, rather regularly spaced on early whorls, more irregularly placed on mature whorls. Axial sculpture of twenty or more weak opisthocline ribs, largely confined to shoulder area and rapidly becoming obsolete below shoulder and on last whorl. Microsculpture of growth lines, most prominent on subsutural ramp with slightly raised cordlets present at uneven intervals, reflecting shape of anal sinus. Last adult whorl evenly convex below subsutural ramp, not clearly demarcated from long, evenly tapering siphonal canal. Aperture elongate, approximately 40% of shell length; outer lip thin, unsculptured; inner lip with distinct, rather wide yellowish callus. Anal sinus wide, moderately deep, u-shaped.
Animal grayish; head broad, blunt; penis narrow, moderately large, with seminal papilla. Cephalic tentacles broad, stubby, somewhat tapering to blunt tip. Large eyes situated at outer basal part.
Rhynchocoel walls covered in thick layer of dark red matter. Inside of oesophagus covered in thick layer of charcoal-coloured matter. Proboscis very broad (retracted); venom gland moderately long, convoluted; muscular bulb elongate, semi-transparent.
Radula (Figures, 13a and 14) of hypodermic, somewhat loosely rolled to entirely unrolled, rather straight to curved or bent, teeth attaining 140 µm in length; barbs absent; dorsal blade approximately 1/5 of length of shaft; adapical opening rather elongate, highly variable in length; base lightly swollen, distinct lateral process; external base with coarse sculpture; basal opening subcircular, very large. Ligament broad, rather large.
Remarks
The distinct, cylindrical whorls with high periphery of this species make it rather distinct among its congeners. It bears some resemblance to Nodothauma magnifica Criscione, Hallan, Fedosov & Puillandre, 2020; however, it can be separated from the latter by its distinctly shouldered, cylindrical whorls, taller spire, a comparatively lower aperture (as a ratio of its total length), a less-defined siphonal canal and in its white coloration in contrast to the orange-brown N. magnifica. Furthermore, these taxa can readily be differentiated anatomically, as N. magnifica does not possess a radula and venom apparatus. A high proportion of the hypodermic teeth encountered in the holotype exhibit unusual characteristics for Raphitomidae, with some entirely unrolled and trough-shaped (Figure 14), similar to members of the Mangeliidae (see Bouchet et al., 2011), whereas other exhibit various degrees of unrolling, or where one tooth is contained by another.
Material examined
Holotype: Australia, Victoria, East Gippsland CMR, (−38.479, 150.185), IN2017_V03_032, 3850–3853 m, (AMS C.482142).
Paratypes: As per holotype, 1 wet (AMS C.571636), 1 wet (AMS C.571658); Australia, Tasmania, Flinders CMR, (−40.473, 149.397), IN2017_V03_015, 4114–4139 m, 1 wet (AMS C.519392); NSW, Jervis CMR, (−35.114, 151.469), IN2017_V03_053, 3952–4011 m, 1 wet (AMS C.482310).
ZooBank registration: http://zoobank.org/urn:lsid:zoobank.org:act:2DC536C6-AB42-4C93-989C-A8FA3BBC4EF0
Etymology
In reference to its thin shell, derived from “tenuis” (Latin = thin) and “concha” (Latin = shell). Noun in apposition.
Distribution
Known for off the southeastern coast of Australia (Figure 1e).
Description
Shell (Figure 11b; SL = 42.4, SW = 20.3) fusiform, thin, opaque. Protoconch eroded. Teleoconch of at least 5.5 yellowish whorls; whorl profile medium broad, with well-defined shoulder approximately at mid-height in spire whorls, becoming rounded on penultimate- and subobsolete on last adult whorl. Subsutural ramp very wide, deeply concave in early teleoconch whorls, becoming straight to somewhat convex in mature whorls; suture impressed. Axial sculpture of about 15 low ribs, largely confined to shoulder area, on early teleoconch whorls, becoming subobsolete to absent in later whorls. Spiral sculpture below subsutural ramp of about 8 grooves (30+ on last adult whorl), forming dense pairs on mature whorls; each groove or pair of grooves separated by wide interspace, becoming weaker and less regularly set toward base of last adult whorl. Microsculpture of collabral growth lines, most prominent on subsutural ramp with slightly raised cordlets at regular intervals (rather strong on early teleoconch whorls), reflecting shape of anal sinus. Last adult whorl evenly convex below subsutural ramp, tapering evenly toward long siphonal canal. Aperture elongate-pyriform, approximately 60% of total shell length; outer lip thin, unsculptured; columella recurved with distinct whitish callus, pinkish in upper third (pink area also on base of last whorl). Anal sinus wide, moderately deep, u-shaped.
Anatomy (based on AMS C.571636):
Animal uniform pink; head very broad, with thick, muscular walls, blunt; cephalic tentacles thick, muscular, rather short, tapering, with large eyes on outer base. Penis situated well-posterior of cephalic tentacle, muscular, coiling clockwise, bearing gland-like swellings on distal quarter.
Proboscis pink, of moderate size, broad, rather conical; radular sac long, opening into buccal mass posterior to right side of proboscis; venom gland moderately large, whitish, convoluted, muscular bulb moderately large, very long, lustrous pink, bending abruptly at middle, with one half pointing posteriorly.
Radula (Figure 13b) of hypodermic type, teeth attaining approximately 140 µm in length, mostly straight but somewhat curved distally, rather broad; barbs absent; dorsal blade approximately 1/5 of length of shaft; adapical opening elongate, about 1/5 of length of shaft; base slightly inflated, with distinct lateral process; base texture rather coarse; basal opening large, subcircular. Ligament wide, rather long.
Remarks
This species can be differentiated from its congeners and other raphitomids by its distinctly recurved columella, and with the last adult whorl being rather cylindrical below the shoulder (Figure 11b) until it tapers toward the siphonal canal.
Material examined
Holotype: Australia, Tasmania, Flinders CMR, (−40.473, 149.397), IN2017_V03_015, 4114–4139 m, 1 wet (AMS C.519401).
Paratypes: As per holotype, 1 wet, (AMS C.571654); Australia, Tasmania, Bass Strait, (−39.552, 149.553), IN2017_V03_030, 4197–4133 m, 1 wet, (AMS C.519331), 1 wet, (AMS C.571669); Victoria, East Gippsland CMR, (−38.479, 150.185), IN2017_V03_032, 3850–3853 m, 1 wet, (AMS C.571652); NSW, off Bermagui, (−36.351, 150.914), IN2017_V03_043, 4753–4750 m, 1 wet, (AMS C.571707); off Newcastle, (−33.441, 152.702), IN2017_V03_065, 4280–4173 m, 1 wet, (AMS C.519367), 1 wet, (AMS C.571667), 1 wet, (AMS C.571716).
ZooBank registration: http://zoobank.org/urn:lsid:zoobank.org:act:5EB9BD05-6A52-4429-B455-78B3B01357E9
Etymology
In reference to the small size of its hypodermic teeth, derived from “parvus” (Latin = small) and “dentatus” (Latin = bearing teeth). Composite adjective of feminine gender.
Distribution
Known for off the southeastern coast of Australia (Figure 1e).
Description
Shell (Figure 11d; SL = 20.4, SW = 9.4 mm) fusiform, moderately thin, semi-translucent. Protoconch eroded. Teleoconch of 4.8 light yellow whorls; whorl profile medium broad, with well-defined shoulder approximately at mid-height to abapical third. Subsutural ramp very wide, concave to rather straight; suture impressed. Axial elements of about 15 low ribs, most prominent on shoulder, gradually weakening toward suture on early teleoconch whorls, becoming subobsolete to absent in later whorls. Spiral sculpture below subsutural ramp of five regularly set cords, separated by rather deep grooves. On last adult whorl first spiral cord after shoulder slightly swollen; in total 24 cords, rounded adapically and becoming progressively wider, flat and oblique toward siphonal canal. Microsculpture of collabral growth lines, most prominent on subsutural ramp with slightly raised cordlets at regular intervals (rather strong on early teleoconch whorls), reflecting shape of anal sinus. Last adult whorl evenly convex below shoulder, not clearly demarcated from long siphonal canal. Aperture elongate-pyriform, approximately 60% of total shell length; outer lip thin, unsculptured; inner lip recurved with whitish callus. Anal sinus wide, medium deep, broadly u-shaped.
Anatomy (based on paratypes AMS C.571707, AMS C.571716 and AMS C.571652): animal whitish to pinkish; head very broad; cephalic tentacles medium long, broad, subcylindrical to cylindrical, tip blunt; small eyes situated at their outer lower bases. Penis large, muscular. Venom apparatus extremely small; venom gland thin, short; muscular bulb very elongate.
Radula (based on paratype AMS C.571667, Figure 13c) of hypodermic type, short, attaining 30 µm in length, loosely rolled, gradually tapering from base of shaft toward sharpened tip; adapical opening elongate, narrow. No apparent blade, barbs absent. Base swollen, somewhat broader than basal portion of shaft, lateral process or spur absent. Basal opening large, subcircular, situated obliquely relative to orientation of tooth; basal texture coarse. Ligament moderately large.
Remarks
Material examined
Holotype: Australia, NSW, off Byron Bay, (−28.677, 154.203), IN2017_V03_090, 2587–2562 m, (AMS C.519333).
Paratype: Australia, NSW, Hunter CMR, (−32.575, 153.162), IN2017_V03_070, 2595–2474 m, 1 wet (AMS C.571638).
ZooBank registration: http://zoobank.org/urn:lsid:zoobank.org:act:7B600648-C2C8-406F-B8FA-560B36B73AB9
Etymology
In reference to its shell sculpture of deep spiral grooves, derived from “annulatus” (Latin = “bearing rings”). Adjective of feminine gender.
Distribution
Known from northern NSW, Australia.
Description
Shell (Figure 11e; SL = 20.7, SW = 9.8 mm) fusiform, rather thin-walled, orange, semi-translucent. Protoconch eroded. Teleoconch of at least six orange-brown whorls; whorl profile medium broad, with weakly defined shoulder approximately at mid-height in early teleoconch whorls, becoming obsolete on subsequent whorls. Subsutural ramp on early spire whorls very wide, concave to straight, subobsolete to absent in subsequent whorls; suture impressed. Axial elements of low, rather indistinct, densely set ribs on early teleoconch whorls, spanning height of whorl below subsutural ramp, becoming subobsolete to absent in later whorls. Spiral sculpture below subsutural ramp of deep grooves, evenly interspaced on early spire whorls subsequently paired; each pair separated by wide rounded spiral cord, single on penultimate whorl and bipartite on last whorl. Spiral cords becoming weaker, flatter and less regularly set toward base of last adult whorl. Microsculpture of collabral growth lines, traceable throughout whorl height, but most prominent on subsutural ramp, forming slightly raised cordlets at regular intervals (very prominent on early teleoconch whorls). Last adult whorl evenly convex below very weak subsutural ramp, weakly demarcated from long siphonal canal. Aperture elongate-pyriform, more than 60% of total shell length; outer lip thin, unsculptured; columella rather straight, with wide whitish callus, distinct burnt-orange vertical stain on lower half. Anal sinus wide, rather shallow, weakly u-shaped.
Cephalic tentacles short, stubby; large eyes situated at their outer lower bases. Rhynchocoel large, capacious, lined with porous dark reddish brown epithelium. Venom apparatus small, far retracted into posterior rhynchocoel; muscular bulb small, elongate; venom gland thin, rather small; radular sac small, filled with reddish teeth. Proboscis broad, short. Oesophagus (based on AMS C.571638) very thick.
Radula (based on paratype AMS C.571638, Figure 13e) of hypodermic, somewhat loosely rolled to semi-unrolled, rather straight teeth attaining 100 µm in length; barbs absent; dorsal blade approximately 1/5 of length of shaft; adapical opening rather elongate, highly variable in length); base lightly swollen, with distinct lateral process; external base with coarse sculpture; basal opening subcircular, very large. Ligament broad, rather large.
Remarks
Remarks
As no published radular or other anatomical data are available for this species, details are provided herein based on specimen AMS C.482154: Animal grayish white, head broad, blunt; cylindrical, medium length cephalic tentacles, with eyes on outer lower base. Rhynchocoel capacious, internal walls covered in dark red matter. Muscular bulb extremely small, elongate, semi-transparent; venom gland thin, small; radular sac small; proboscis broad, sphincter surrounded by green filamentous/lamellate structure. Internal oesophagus also lined with dark matter.
Radula (Figure 13d) of hypodermic, somewhat loosely rolled, rather straight teeth attaining 100 µm in length; barbs absent; dorsal blade approximately 1/4 of length of shaft; adapical opening elongate; base lightly swollen, distinct lateral process; external base with coarse sculpture; basal opening subcircular, very large. Ligament broad, rather large.
Genus Theta Clarke, 1959 (Clarke, 1959; p. 234)
Type species Pleurotomella (Theta) lyronuclea Clarke, 1959 by original designation (Figure 15a; Clarke, 1959, p. 234, pl. 13, figures 1–2).
Other species. Theta baiocchii Nappo & Pellegrini, 2021 (Figure 15d; Nappo & Pellegrini, 2021, p. 72-75, fig. 1A-N), Theta chariessa (R. B. Watson, 1881) (Figure 15c; Watson, 1881, p. 458–460, figure 2; 1886, p. 352–353, pl. 20, figure 6; Bouchet & Warén, 1980, p. 59–61, figures 14, 129–130, 254–255), T. microcostellata n. sp., T. polita n. sp., Theta vayssierei (Dautzenberg, 1925) (Figure 15b; Dautzenberg, 1925, p. 1, figure 2; Bouchet & Warén, 1980, p. 59, figures 126–127, 253).
Diagnosis
Shell biconical- to elongate-fusiform, semi-translucent to opaque. Protoconch multispiral (moderately to heavily eroded in observed material); sculpture of arcuate riblets throughout, or riblets limited to upper portion of whorls and diagonally cancellate below. Teleoconch with distinctly shouldered to rounded whorls; axial sculpture ranging from weak, present largely in mid-whorls, to bearing sharply opisthocline or orthocline axial ribs in most or all whorls, vanishing below shoulder or present more or less from shoulder to suture; spiral sculpture of weak, indistinct cords; last whorl cylindrical to evenly convex below indistinct to wide subsutural ramp. Siphonal canal moderately long to long, rather straight. Aperture large, pyriform, moderately broad to narrow, about half of shell length. Anal sinus rather shallow to comparatively deep, wide, u-shaped. Radula of hypodermic, slightly curved teeth with two blunt to moderately sharp distal barbs; adapical opening rather long; base broad, angular, lateral process indistinct; basal opening large; ligament broad.
Remarks
Prior to this work, Theta consisted of four accepted species: the type species T. lyronuclea [(from off the Bermuda Islands (Clarke, 1959) and possibly from S Australia (this study but also Criscione et al., 2021)], T. vayssierei, T. chariessa [both from the N Atlantic (Bouchet & Warén, 1980)] and T. baiocchii (from off the Philippines). With the addition of the two species described here from Australia, Theta currently encompasses a total of six species. However, the genus placement of T. vayssierei, T. chariessa (exhibiting Austrobela-like features – see above) and of the morphologically highly divergent T. baiocchii remains to be tested molecularly. Thus delimited, the genus exhibits a disjunct Atlantic/Pacific distribution. Arguably, a complete picture of the genus diversity and distribution depends on the availability of molecular data on additional species and it is beyond the scope of this study. Taxa such as Mangilia argeta Dall, 1890 from (off) the Galapagos Islands (Figure 18g; Dall, 1890, pl. 6, figure 5), Gymnobela latistriata Kantor & Sysoev, 1986 from the NW Pacific (Figure 18i; Kantor & Sysoev, 1986, figures 1b–e, 2b, 4), Typhlosyrinx chrysopelex Barnard, 1963 from (off) the Cape Point region, S Africa (Barnard, 1963, figure 3g–h; Sysoev, 1996, Figure 6), “Gymnobela” camerunensis Thiele, 1925 from (off) W Africa (Thiele, 1925, pl. 28, figure 20) and Gymnobela homoeotata (Watson, 1886) from the mid-Atlantic (Figure 18j; Watson, 1886, pl. 26, figure 12; Bouchet & Warén, 1980, figures 18, 199, 240), exhibit shell and (when available) radular characters that are close to Theta, and so affinity of these species to Theta needs further evaluation.
- Theta lyronuclea (Clarke, 1959) (PSH T1)
- (Figures 10e,f, 15a,e and 16a)
- Pleurotomella (Theta) lyronuclea Clarke, 1959: p. 233–235, pl. 13, figures 1–2)
- Gymnobela lyroniclea [sic] (misspelling)—Sysoev (2014, p. 148)
Material examined
Australia, GAB, (−36.069, 132.637), IN2015_C01_016, 4602–4612 m, 1 wet, (AMS C.487451), 1 wet, (AMS C.571655), 1 wet, (AMS C.571708); (−35.794, 131.711), IN2015_C01_026, 4576–4459 m, 1 wet, (AMS C.487453); (−34.074, 129.182), IN2015_C01_064, 2649–2803 m, 1 wet, (AMS C.483790); (−35.009, 130.317), IN2015_C02_227, 2848–2831 m, 1 wet, (SAMA D44171); (−35.852, 131.977), IN2017_C01_175, 3930–4250 m, 1 wet, (AMS C.572169; (−35.811, 131.71), IN2017_C01_179, 4741–4618 m, 1 wet, (SAMA D67752), (SAMA D67753); (−35.523, 130.351), IN2017_C01_182, 4890–5032 m, 1 wet, (AMS C.571733), 1 wet, (AMS C.572171); (−34.452, 129.492), IN2017_C01_197, 3235–3350 m, 1 wet, (AMS C.572172); NSW, off Bermagui, (−36.351, 150.914), IN2017_V03_043, 4763–4750 m, 1 wet, (AMS C.571718); Jervis CMR, (−35.114, 151.469), IN2017_V03_053, 3952–4011 m, 1 wet, (AMS C.482290).
Remarks
This species was described based on a single shell collected off the Bermuda Islands by the M/V Theta of the Lamont Geological Observatory and its description was accompanied by the illustration of the shell (Clarke, 1959, pl. 13, figures 1–2). A photograph of the shell and a line drawing of the radula were figured for an additional specimen from the NE Atlantic (Bouchet & Warén, 1980, figures 13, 128). Illustrations of shell, radula and penis were provided for samples collected in the SW Atlantic (Sánchez and Pastorino, 2020, figures 1-3, 6-21). Sequences obtained from specimens of T. lyronuclea from Australia are included in the phylogenies of Criscione et al. (2021) as well as in that of this study. The description of the radula and the anatomy of this species, reported below, is based on these specimens.
Radula (based on AMS C.571733; Figures 10e,f and 16a) of hypodermic, slightly curved teeth exceeding 200 µm, with two moderately sharp distal barbs (Figure 10e), dorsal barb smaller; adapical opening moderately long (Figure 10f), about 1/5 of length of shaft; base broad, angular, indistinct lateral process; basal opening large; ligament broad.
Anatomy (based on AMS C.487453 and AMS C.482290): males with extremely large, muscular penis. Eyes moderately large, albeit may be in part covered by epidermis, situated at outer lower base of moderately long, cylindrical tentacles which may bear a longitudinal furrow.
- Theta polita n. sp. (PSH T2)
- (Figures 10g,i, 15f and 16b)
Material examined
Holotype: Australia, GAB, (−35.818, 134.109), IN2015_C02_141, 2852–2800 m (AMS C.532711).
Paratypes: Australia, GAB, (−35.798, 132.693), IN2015_C02_151, 2773–2677 m, 1 wet (AMS C.532868); 1 wet (AMS C.571696).
ZooBank registration: http://zoobank.org/urn:lsid:zoobank.org:act:ADD9935E-63BE-4C18-80BA-4097EF19D685
Etymology
In reference to its unsculptured shell, derived from “politus” (Latin = smooth). Adjective of feminine gender.
Distribution
Known for the GAB (Figure 1c).
Description
Shell (Figure 15f; SL = 23.1, SW = 10.1) fusiform, thin-walled, with glossy surface. Protoconch orange, cyrtoconoid, of at least three evenly convex orange whorls, with sculpture of arcuate riblets. Teleoconch of five uniformly whitish whorls. Whorl profile moderate to rather broad, evenly convex to somewhat angulated; subsutural ramp approximately 60°, straight to lightly concave; sinus wide, rounded, subsutural, rather deep. Spiral sculpture absent; axial sculpture obsolete or of weak arcuate ribs, appearing as a series of nodules about mid-height of second whorl; microsculpture of bi-sinuose growth lines throughout whorl height, short and deeply convex at sinus, in places regularly arranged low riblets, long and slightly concave below. Last whorl evenly convex, clearly demarcated from the straight, moderately long, tapering siphonal canal. Aperture large, about half of shell length, ovate. Outer lip thin, convex along most of its length, with anterior portion attenuated toward tip of siphonal canal; inner lip smooth, with thin, narrow, white callus.
Radula [based on holotype (not figured) and paratype AMS C.532868 (Figure 16b)] of hypodermic, rather straight teeth exceeding 200 µm in length, with two rather blunt distal barbs (Figure 10g); adapical opening moderately long, about 1/5 of length of shaft; base broad, angular; lateral process indistinct; basal opening large; ligament broad, very thick (Figure 16b).
Venom gland long, convoluted; muscular bulb large, bean-shaped.
Remarks
Material examined
Holotype: Australia, NSW, Hunter CMR, (−32.575, 153.162), IN2017_V03_070, 2595–2474 m, (AMS C.571657).
ZooBank registration: http://zoobank.org/urn:lsid:zoobank.org:act:9DB5C008-9796-45F0-BE1C-2F032F3DA269
Etymology
In reference to the finely ribbed pattern created by its prominent growth lines, derived from “micros” (ancient Greek = small) and “costellatus” (scientific Latin = finely ribbed or ridged). Adjective of feminine gender.
Distribution
Known only from the type locality.
Description
Shell (Figure 15g; SL = 19.9, SW = 8.8 mm) fusiform, rather thin-walled. Protoconch of at least two orange whorls, with diagonally cancellate sculpture remaining on lower part of last whorl. Teleoconch of 4.8 uniformly yellowish-white whorls. Whorl profile subcylindrical to rather broad, with distinct shoulder in all early to median teleoconch whorls and rounded shoulder in last adult whorl; suture deep; subsutural ramp lightly concave; sinus rather narrow, rounded, subsutural, rather shallow. Spiral sculpture represented by indistinct cords on the shell base; axial sculpture of strong sharp orthocline ribs on spire whorls (about 20 on penultimate whorl); microsculpture of marked bi-sinuose growth lines throughout whorl height, forming distinct, raised riblets on subsutural ramp. Aperture large, pyriform, about half of shell length, opening posteriorly to medium length, rather broad, siphonal canal; outer lip thin, simple, orthocline; inner lip smooth, with rather thick, broad, white callus.
Radula (Figure 16c) of hypodermic, slightly curved teeth of approximately 240 µm in length, with two barbs, dorsal barb smaller, rather blunt; adapical opening moderately long, about 1/5–1/6 of length of shaft; base medium broad, with gentle slope, lateral process indistinct; basal opening large; ligament broad.
Animal uniform cream. Eyes large, situated about 1/4 dorsal to outer base of cephalic tentacles. Cephalic tentacles broad, blunt, of medium length. Venom apparatus large; venom glad long, coiled; muscular bulb elongate, lustrous, extremely large; proboscis short, blunt, large.
Remarks
- Genus Austrotheta Criscione, Hallan, Fedosov and Puillandre, 2020 (Criscione et al., 2021; p. 985–986)
Type species Austrotheta crassidentata Criscione, Hallan, Fedosov and Puillandre, 2020 by original designation. (PSH U1).
Other species. Austrotheta wanbiri n. sp.
Diagnosis
Shell fusiform, semi-translucent to opaque. Protoconch multispiral; sculpture of arcuate cordlets on upper portion of whorls and diagonally cancellate below. Teleoconch with distinctly shouldered to rounded whorls, bearing sharp opisthocline or orthocline axial ribs in most or all whorls; last whorl cylindrical to evenly convex below narrow subsutural ramp, with or without undulating striae throughout its height. Siphonal canal straight, moderately long to long. Aperture elongate to wide, about half of shell length. Anal sinus rather shallow, u-shaped. Radula (based on type species only) of very thick, cylindrical hypodermic teeth, bearing two weak barbs and with very short adapical opening. Base very broad, with extremely coarse external sculpture. Ligament very large.
Remarks
- Austrotheta wanbiri n. sp. (PSH U2)
- (Figure 15h)
Material examined
Holotype: Australia, GAB, (−34.574, 129.572), IN2017_C01_198, 3389–3540 m, (AMS C.572174).
ZooBank registration: http://zoobank.org/urn:lsid:zoobank.org:act:B2ABF2BF-4A7C-47D9-B714-A82118BF3AFD
Etymology
In reference to its occurrence in the GAB, derived from “wanbiri” (Aboriginal Australian language Mirning = sea coast).
Distribution
Known only from the type locality in the GAB.
Description
Shell (Figure 15h; SL = 15.9, SW = 6.5) fusiform, rather thick-walled, opaque. Protoconch broken, orange, multispiral (at least 1.5 whorls), with arcuate cordlets on adapical half to two-thirds of whorl, with diagonally cancellate sculpture below. Teleoconch of 4.3 whorls; subsutural ramp distinctly concave; teleoconch whorls with prominent shoulder on early whorls, situated at adapical third of whorl, more rounded in last whorl; whorl periphery subcylindrical, slightly more convex in last whorl. Early teleoconch whorls with 14 rounded, orthocline axials, reaching lower suture, and producing prominent nodules at shoulder. Later whorls only with nodules becoming weaker on last whorl. Spiral sculpture absent. Microsculpture of collabral growth lines, forming distinct, raised riblets on subsutural ramp. Last adult whorl subcylindrical below subsutural ramp, with long, slender siphonal canal. Aperture rather narrow, pyriform, about half of shell length. Inner lip with whitish callus, rather straight. Outer lip thin, unsculptured. Anal sinus rather shallow, weakly u-shaped.
Anatomy and radula unknown.
Remarks
This species can be readily differentiated from Austrotheta crassidentata by its smaller and more slender shell, bearing more numerous and more prominent axial ribs. For a comparison with T. microcostellata n. sp. (Figure 15g), see remarks to this latter species.
4 DISCUSSION
4.1 Phylogenetic relationships and genus-level systematics
The five-gene phylogeny of Criscione et al. (2021) established the phylogenetic framework upon which the new genera Austrobela and Austrotheta were recognized and described, and it is shown herein that there is strong support in both BI and ML analyses for their monophyly, as is the case for Spergo and Theta (Figures 2 and 3).
The integrity of these genera is corroborated by morpho-anatomical, notably radular, features diagnostic for each genus. The radula of Austrobela is characterized by hypodermic teeth with two large, sharp distal barbs, commonly with a thickened cylindrical basal half of the shaft and with a rather solid, thick ligament (Figure 9). While double-barbed teeth appear to be less common than awl-shaped teeth in Australian deep-sea raphitomids (see Criscione et al., 2021), this configuration is not unique to Austrobela. Members of the closely related Theta also exhibit double-barbed teeth (see below), but they are encountered also, among others, in the more distantly related Typhlosyrinx Thiele, 1925 (Bouchet & Sysoev, 2001) and Pontiothauma (Pace, 1903). However, the barbs are particularly prominent in Austrobela, also when compared to Theta (Figures 9, 10 and 16). Furthermore, all PSHs of the Austrobela clade here examined possess an extremely large venom apparatus that occupies the majority of the body hemocoel, and all possess large eyes. Criscione et al. (2021) reported the presence and size of eyes to be a useful diagnostic character at the genus level for deep-sea raphitomids. In terms of shell morphology, members of Austrobela can be characterized by their primarily fusiform shells with a prominent shoulder and subcylindrical whorl periphery, a straight columella and a large aperture, which in most PSHs is about equal in length to that of the spire (Figures 5-7). Three discrete types of protoconch sculpture are found in the clade: a sculpture of arcuate ribs (A. rufa and A. levis), the typical raphitomid diagonally cancellate sculpture (A. sagitta, A. procera and A. micraulax) and a combination of the two former types, where arcuate ribs on the adapical portion are changed by cancellate sculpture on lower whorl portion (A. obliquicostata; Figure 8). While the sculpture of arcuate ribs is unusual among the Raphitomidae, it is not unique to members of Austrobela and it is also seen in T. lyronuclea. While Clarke (1959) considered this sculpture a distinctive feature of the newly erected (then) monotypic subgenus Theta, our results show that the protoconch of at least one species of this taxon (T. microcostellata n. sp.) possesses traces of a diagonally cancellated sculpture. The species of Austrobela with arcuate ribs on the protoconch (A. rufa and A. levis) formed a strongly supported clade in Criscione et al. (2021) (there labeled A. rufa n. gen. n. sp., A. n. gen. sp. 2 and A. n. gen. sp. 3), suggesting some phylogenetic signal to this sculptural feature.
Members of the Spergo clade primarily exhibit loosely rolled, awl-shaped hypodermic teeth with a short distal blade and a rather narrow base with a distinct lateral process (Figures 13 and 14). The very loosely rolled teeth encountered in some Spergo, notably in S. castellum (Figure 14), are unusual within the Raphitomidae. One individual, in particular, exhibited teeth unrolled to a variable extent, including an entirely unrolled tooth, similar to those seen in species of the Hemilienardia ocellata (Jousseaume, 1883) complex (Fedosov et al., 2017) as well as in the mangeliid Benthomangelia Thiele, 1925 (see Bouchet et al., 2011) where the outer margins do not overlap at any point (Figure 14b). Examples of teeth entirely contained within others were also observed (Figure 14a,c), as were distinctly bent teeth (Figure 14d). This same individual also exhibited teeth that are more typical, albeit loosely rolled (Figure 14e).
Our phylogenetic analyses (Figure 2, Figure S1) indicated the inclusion within the ingroup of one outgroup taxon, “Gymnobela” yoshidai (Kuroda & Habe, 1962), type species of Speoides Kuroda & Habe, 1962. This nominal genus was synonymized with Gymnobela, when a broader concept was adopted for this latter taxon (Sysoev & Bouchet, 2001). With the boundaries of Gymnobela currently restricted by combined morphology and genetics (Criscione et al., 2021), the current placement of Speoides yoshidai is untenable. A synonymy of Speoides with Theta would be supported by their strong phylogenetic relationship and their morphological similarity (see Bouchet & Warén, 1980, p. 59). However, the genetic distance between Speoides and Theta is comparable to that separating other genera in the trees (Figure 2, Figure S1). In addition, some of these genera (particularly Austrobela) exhibit a degree of morphological similarity with Speoides comparable to that observed between this latter taxon and Theta. For these reasons, a definite answer about the taxonomic status of Speoides must await evaluation of morphological and combined mitochondrial and nuclear molecular data.
4.2 Bathymetric and geographic patterns
Most species of Austrobela treated herein occur within an area corresponding approximately to the South Australia marine realm of Costello et al. (2017). The highest species diversity is recorded in the GAB, where three species are recorded: A. rufa, A. levis and A. obliquicostata, the latter also occurring on the east Australian coast (Figure 1b–d).
The evidence produced indicated that two distinct mitochondrial haplotypes (corresponding to A1 and A2), sharing virtually identical morphology, coexist within A. rufa, without showing any apparent geographic or bathymetric partitioning. This pattern may have been generated by a hypothetical scenario of transitory vicariance and subsequent contact of the diverged populations as observed in another deep-water neogastropod Amalda hilgendorfi (Martens, 1897) (Kantor et al., 2020). However, this scenario also is rather speculative, as, in the deep sea, the rapid rise and fall of a geographic barrier is an extremely rare event. The genetic divergence observed may also be the ongoing result of niche partitioning, following (for instance) exploitation of different resources (prey, microhabitat, etc.) as observed in Zvonareva et al. (2020). However, this will remain untested until more information is available on the ecology of Austrobela species and on the exact microhabitat composition of the portion of seafloor where the two divergent lineages occur. Whatever the underlying mechanism responsible, assessing whether A1 and A2 indeed represent a pair of cryptic species requires testing the occurrence of recombination by sequencing a suitable nuclear marker.
There is clear bathymetric partitioning between A. rufa and the closely related A. levis (Figure 14) and speciation as a result of partitioning into separate bathymetric niches could therefore explain their genetic distinction. This is in agreement with the bathymetric separation observed for some other turriform conoidean sister taxa, such as for members of Gladiobela Criscione, Hallan, Puillandre and Fedosov, 2020 (Hallan et al., 2021), Lophiotoma T. L. Casey, 1904 (Puillandre et al., 2017) and Cryptogemma Dall, 1918 (Turridae) (Zaharias et al., 2020). While the radulae are slightly different in these species (Figure 9a,b,f), nothing is known of their prey preference, so whether this is a potential factor in their differentiation remains unknown.
Only two of the new Australian Austrobela species do not occur in the GAB, namely A. sagitta and A. regia, neither of which is recorded outside of Australia. However, as these two taxa occur further north, with A. sagitta extending into the Coral Sea (Figure 1d), future deep-sea sampling may reveal distributions beyond Australian waters (particularly for the latter). This study reports a range extension for A. procera, not previously recorded in Australia [with records from both (off) Western Australia and the east coast (Figure 1a,d)] and in Taiwan and the Tuamotu archipelago (Table S1). The type locality of A. procera is the Loyalty Ridge, and it is recorded as far east as Wallis and Futuna (Sysoev & Bouchet, 2001). While it remains to be proven by means of molecular data that the material throughout this range is conspecific, the new records indicate that this may be a widespread species. There is growing evidence of wide distributions in several deep-sea conoideans species (Hallan et al., 2021; Zaharias et al., 2020) and further sampling and systematics work will likely reveal additional widespread taxa. Criscione et al. (2021) reported T. lyronuclea from Australia, although the topotypic Caribbean population has never been sequenced (Clarke, 1959). A record of T. lyronuclea from (off) Argentina by Sánchez and Pastorino (2020) (albeit not molecularly confirmed) lends more confidence to that species assignment, as their provision of both radular and penial anatomy reveals considerable similarity to the Australian material. Pending molecular confirmation linking the Caribbean, South Atlantic and Australian populations, there is therefore compelling morphological and anatomical evidence of T. lyronuclea as a widespread, transoceanic species. Due to the limited material of T. microcostellata and T. polita, we cannot infer much about any potential bathymetric zonation among Theta species, nor discuss the biogeography of the two latter. This is also the case for the species of Austrotheta treated herein. While any inference of rarity will inevitably, to some extent, be an artifact of sampling, there is evidence that many turriform conoidean species are comparatively rare based on their scarcity in reasonably well-sampled areas (Castelin et al., 2011). Bouchet et al. (2009) noted this for the New Caledonian fauna and Hallan et al. (2021) for some species of the deep-sea genus Gladiobela. Theta microcostellata, T. polita, Austrotheta crassidentata and Austrotheta wanbiri may therefore represent additional relatively rare species, as the regions in which they have been collected are comparatively well-sampled (MacIntosh et al., 2018; O’Hara et al., 2020), with a diverse raphitomid fauna (Criscione et al., 2021). Conversely, most Austrobela species studied here, notably A. rufa, A. levis and A. obliquicostata, can be considered relatively common, as is the case for T. lyronuclea.
In Australian waters, species of Spergo have only been recorded from the east coast (Figure 1e), with none recorded in the GAB despite comparable sampling efforts (MacIntosh et al., 2018). This notable pattern requires further study to be explained.
ACKNOWLEDGMENTS
This work has been made possible through financial support from the Australian Government (ABRS grant RF217-57, Principal Investigator FC). The participation of AF was supported by the Russian Science Foundation (grant 19-74-10020 to AF). The participation of NP was also supported by funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement No.865101).
Voyages in the GAB were part of (a) the GAB Research Program [GABRP—a collaboration between BP, CSIRO, the South Australian Research and Development Institute (SARDI), the University of Adelaide and Flinders University] and (b) the GAB Deepwater Marine Program (GABDMP—a CSIRO led research program sponsored by Chevron Australia]. Funding for the “Eastern Abyss” voyage (IN2017_V03) was provided by the Marine Biodiversity Hub (MBH), supported through the Australian Government’s National Environmental Science Program (NESP). The “Tasmanian seamounts” voyage (IN2018_V06) was sponsored by the CSIRO Marine National Facility (MNF), the NESP MBH and Parks Australia. The authors wish to thank the CSIRO MNF for its support in the form of sea time onboard, support personnel, scientific equipment and data management. We also thank the scientific staff and crew who participated in all voyages generating the samples studied herein.
The MNHN samples used in this study originates from shore-based expeditions (KARUBENTHOS 2015, PAPUA NIUGINI; Principal Investigator Philippe Bouchet) and deep-sea cruises (AURORA 2007, BIOMAGLO, BIOPAPUA, CONCALIS, EBISCO, KANADEEP, NANHAI 2014, NORFOLK 2, SALOMON 2, TAIWAN 2014, TARASOC, TERRASSES, ZHONGSHA 2015; Principal Investigators: Philippe Bouchet, Tin-Yam Chan, Laure Corbari, Nicolas Puillandre, Sarah Samadi, Wei-Jen Chen, Bertrand Richer de Forges) conducted by MNHN, Pro-Natura International (PNI) and Institut de Recherche pour le Développement as part of the Our Planet Reviewed and the Tropical Deep-Sea Benthos programs. Funders and sponsors included a bilateral cooperation research funding from the Taiwan Ministry of Science and Technology (MOST 102-2923-B-002-001-MY3, PI Wei-Jen Chen) and the French National Research Agency (ANR 12-ISV7-0005-01, PI Sarah Samadi), the Total Foundation, Prince Albert II of Monaco Foundation, Stavros Niarchos Foundation and Richard Lounsbery Foundation. All expeditions operated under the regulations then in force in the countries in question and satisfy the conditions set by the Nagoya Protocol for access to genetic resources (expeditions.mnhn.fr). We would like to express our gratitude to Mandy Reid, Alison Miller and Jennifer Caiza (AMS) for assistance with registration and databasing of material, to Barbara Buge (MNHN for the sample preparation and to Andrea Crowther (SAMA, Adelaide), Simon Grove and Kirrily Moore (TMAG, Hobart), Lisa Kirkendale and Corey Whisson (WAM, Perth). Adam Baldinger (MCZ, Cambridge MA), Michel Dagnino and Michèle Bruni (MOM, Monaco), Jeroen Goud and Bram van der Bijl (NCBN, Leiden), Kazunori Hasegawa (NSMT, Tokyo), Andreia Salvador and Kevin Webb (NHMUK, London), Ellen Strong (USNM, Washington) and Alexander Sysoev (ZMMU, Moscow) are thanked for providing photographs of the types of previously described species. Thanks are also due to Sue Lindsay and Chao Shen (Macquarie University, Sydney) for assisting with SEM work. Finally, Ben and Janine Travaglini (Mornington, Vic) are thanked for producing photographs of some of the specimens studied here.




