Welcome back Janolidae and Antiopella: Improving the understanding of Janolidae and Madrellidae (Cladobranchia, Heterobranchia) with description of four new species
Abstract
Proctonotidae and Madrellidae are families that belong to the suborder Cladobranchia. Historically, both have been the subjects of taxonomic confusion. Thus, Proctonotidae Gray, 1853, was subsequently named as Zephyrinidae Iredale and O'Donoghue, 1923 and Janolidae Pruvot-Fol, 1933, but currently both are considered as synonyms of Proctonotidae. On the other hand, Alder and Hancock (1864) erected the genus Madrella in Proctonotidae. Here, we completed a detailed morphological and molecular study of four apparently undescribed species of Madrellidae and Proctonotidae from the Indo-Pacific. We performed a maximum likelihood and Bayesian inference phylogenetic analyses using two mitochondrial and one nuclear genes to improve the understanding of the families. Prompted by our results, Janolidae is removed from synonymy with Proctonotidae. Within Janolidae, there are two well-supported clades. One includes species with smooth cerata that are found in the Atlantic and eastern Pacific Oceans. The taxa in this clade include the type species of Antiopella and several other species. We resurrect Antiopella as the valid name for this clade. The sister clade to Antiopella includes a variety of taxa with species that have been traditionally included in Janolus Bergh, 1884 and Bonisa Gosliner, 1981. Further systematic revision requires more comprehensive taxon sampling. The new species discovered have clear morphological differences and strong molecular support. They include Madrella amphora Pola and Gosliner sp. nov., Janolus tricellariodes Pola and Gosliner sp. nov., Janolus flavoanulatus Pola and Gosliner sp. nov., and Janolus incrustans Pola and Gosliner sp. nov.
1 INTRODUCTION
Nudibranchs of the families Proctonotidae and Madrellidae belong to the suborder Cladobranchia and historically have been the subject of taxonomic confusion. Proctonotidae was described by Gray (1853). It was subsequently named as Zephyrinidae by Iredale and O'Donoghue (1923) and Janolidae by Pruvot-Fol (1933). Both of these names have been more recently considered as synonyms of Proctonotidae (Bouchet et al., 2017).
Presently, Proctonotidae is considered to be comprised of five genera: Proctonotus Alder & Hancock, 1844; Janolus Bergh, 1884; Caldukia Burn & Miller, 1969; Galeojanolus Miller, 1971; and Bonisa Gosliner, 1981 (Bouchet & Rocroi, 2005; Gosliner, 1981).
Alder and Hancock (1844) described the monospecific genus Proctonotus. Proctonotus mucroniferus is known from the British Isles and also has been reported for the Atlantic coast of France and Norway (Rudman, 2007; Skauge, 2007). Very little is known of its anatomy or its biology.
Bergh (1884) described the genus Janolus. To date, Janolus has three synonyms. Vérany (1844) proposed the genus Janus but this name was preoccupied by a junior homonym of Janus Stephen, 1835, which is a lepidopteran. Later, Alder and Hancock (1848) proposed Antiopa, with A. splendida Alder & Hancock, 1848; as type species (=Eolis cristata Delle Chiaje, 1841). Antiopa was preoccupied as a junior homonym of Antiopa Meigen, 1800, which is a dipteran. Hoyle (1902) proposed Antiopella as a replacement name for Antiopa. Yet, it is important to highlight that Bergh (1884) did not propose Janolus as a replacement name for Janus or Antiopa, but for a new exotic genus, primarily distinguished by the absence of denticulate jaws. In fact, Antiopella and Janolus have been considered as distinct genera by most workers who have studied the Janolidae (Baba & Abe, 1970; Burn & Miller, 1969; Eliot, 1910; Marcus, 1958; Miller, 1971; Pruvot-Fol, 1954) because most species of Janolus have pustules on the outside of the cerata, a single digestive diverticulum within each ceras and simple jaws, whereas Antiopella has smooth cerata, bifurcate diverticula and the jaw margins are denticulate. Several other workers have considered them as similar genera (Franc, 1968; Thiele, 1931; Thompson & Brown, 1976). Finally, Gosliner (1981), based on the presence of intermediate morphological characters in several species of Janolus, noted the necessity to synonymize Janolus Bergh, 1884; and Antiopella Hoyle, 1902.
More recently, the genus Caldukia Burn & Miller, 1969; was described and includes three species: C. affinis (Burn, 1958), C. albolineata (Miller, 1970) and C. rubiginosa (Miller, 1970). Caldukia affinis is found in southeast Australia (Burn, 1958, 1989; Nimbs & Smith, 2016), while the other two species are from New Zealand. Caldukia albolineata seems to be restricted to the southern half of South Island (Miller, 1970; Miller & Willan, 1986; Spencer, Marshall, & Willan, 2009). Yet, C. rubiginosa is found in the northern islands of Goat Island Bay and Poor Knights Islands (Gordon & Ballantine, 2013; Miller, 1970; Miller & Willan, 1986). Caldukia and Proctonotus share a common distinguishing feature: absence of the “caruncle” between the rhinophores. The histology and function of this structure were studied by Schrödl (1996). Yet, in Proctonotus mucroniferus, the morphology of the radula is not well known but appears to have about five lateral teeth on either side of the rachis (Alder & Hancock, 1844; as Venilia mucronifera), whereas Caldukia is characterized by having a narrow radular ribbon with six lateral teeth on either side of the rachidian row (Miller & Willan, 1986). Members of the other three genera possess a caruncle between the rhinophores (Gosliner, 1981).
Galeojanolus was added by Miller (1971) and is only found in New Zealand. Galeojanolus ionnae is characterized by having the head with a helmet-like extension and digestive gland extending into the notum and cerata.
Finally, Gosliner (1981) described the genus Bonisa to include a new species found only in South Africa. This new species, Bonisa nakaza, has a caruncle, short lobe anterior to the head, and several features considered diagnostic of the new genus: digestive gland surrounding the stomach and not entering into the notum and cerata, lateral radular teeth with elongate denticles, a thick, club-shaped penis, and a large receptaculum seminis situated next to the reduced bursa copulatrix near the genital opening. In this work, it was noted that some species of Proctonotidae have an androdiaulic reproductive system, whereas others had a triaulic system with three separate ducts. Also, there was determined to be variation in the arrangement of the receptaculum seminis. It was either a tubular (serial receptaculum) portion of the oviduct or emerged from the oviduct as a separate outpocketing or stalked structure (semi-serial receptaculum).
Madrellidae Preston, 1911; is a poorly known group of cladobranchs that includes only the single genus Madrella Alder & Hancock, 1864. Originally, Alder and Hancock (1864), in describing Madrella ferruginosa and erecting the genus, included Madrella in Proctonotidae. Angas (1864) described Janus sanguineus from Australia, and it was later transferred to Madrella (Burn, 1965). Vayssière (1902) described a third species, M. aurantiaca Vayssière, 1902; from the Gulf of Marseille, France. Preston (1911) considered that Madrella was different enough from proctonotids and erected a new family Madrellidae to include these three taxa. Baba (1949) described two new species of Madrella from Japan, M. gloriosa Baba, 1949; and M. granularis Baba, 1949. The latter has been inadvertently omitted from World Registry of Marine Species.
Prompted by the discovery of four apparently undescribed species of Madrellidae and Proctonotidae from the Indo-Pacific, the goal of this study was to perform a complete and detailed anatomical study of the new species as well as to attempt to understand their phylogenetic position among related taxa. Accepting or rejecting the monophyly of Proctonotidae is beyond the scope of this work until species of all genera included are available for study.
2 MATERIAL AND METHODS
2.1 Taxon sampling
The four new species (one species of Madrella and three species of Janolus) described in this study were collected on several field trips to the Philippines and Papua New Guinea, as well as material sent to us by a colleague and collaborator from the Marshall Islands. All the specimens here studied are currently located at the California Academy of Sciences Department of Invertebrate Zoology and Geology in San Francisco (CASIZ), the National Museum of the Philippines (NMP), and the Museu de Zoologia da Universidade de São Paulo (MZUSP), Brazil.
Specimens were collected by scuba diving. Features of living animals were recorded in the field and from photographs. The specimens were preserved in 75% or 95% EtOH. Some specimens were preserved in 10% formalin except for small tissue pieces from the foot and cerata which were directly preserved in 95% EtOH for molecular studies. In order to undertake the morphological descriptions, the specimens were dissected under a microscope, and external and internal features were drawn with the aid of a camera lucida. The buccal mass was removed and soaked in a 10% NaOH solution to dissolve the connective and muscle tissue, leaving only the radula, jaws, and the labial cuticle. The penis was critical point-dried. The coated radula, jaws, labial cuticle, and penis of each specimen were examined, and images were obtained using scanning electron microscope (Leo 1450 VP).
The Proctonotidae specimens used in this study for molecular purposes included 36 individuals representing three genera: the genus Caldukia (1 specimen of C. affinis), the monospecific genus Bonisa (4 specimens of B. nakaza), and 10 described species of the genus Janolus plus three undescribed species of the same genus. A total of 15 species of Cladobranchia from 13 genera were included in the analyses. Specimens of two species of Madrella were included to determine whether they were sister taxa with Proctonotidae. Berthella martensis was used to root the tree as outgroup taxa. In total, this study includes 52 specimens and a total of 137 sequences (73 novel sequences and 64 retrieved from GenBank; Table 1).
| Species | Voucher | Locality | H3 | COI | 16S |
|---|---|---|---|---|---|
| Berthella martensi | MZUCR6982 | Panama | HM162498 | HM162683 | HM162592 |
| Sakuraeolis enosimensis | CASIZ 178876 | California | HM162591 | HM162758 | HM162682 |
| Dendronotus regius | CASIZ 179492 | Philippines | HM162535 | HM162708 | HM162629 |
| Bornella stellifer | CASIZ 167989 | Hawaii | HM162529 | HM162703 | HM162623 |
| Hancockia californica | CASIZ 175722 | Costa Rica | HM162527 | HM162702 | HM162621 |
| Notobryon wardi | CASIZ 177537 | Philippines | HM162545 | HM162714 | HM162638 |
| Doto coronata | CASIZ 176278 | South Africa | HM162566 | HM162734 | HM162657 |
| Armina semperi | CASIZ 177534 | Philippines | HM162512 | HM162696 | HM162606 |
| Marionia arborescens | CASIZ 177578 | Philippines | HM162554 | HM162722 | HM162646 |
| Dirona albolineata | CASIZ 174466 | Washington | HM162577 | – | HM162668 |
| Doridomorpha gardineri | CASIZ 178233 | Malaysia | HM162511 | HM162695 | HM162605 |
| Leminda millecra | CASIZ 176348 | South Africa | HM162578 | HM162745 | HM162669 |
| Lomanotus sp. | LACM 174962 | Mexico | HM162547 | HM162715 | HM162640 |
| Madrella ferruginosa | CASIZ 182188 | Marshall Islands | MH781076 | – | MH781031 |
| Madrella ferruginosa | CASIZ 182798 | Philippines | MH781077 | MH781053 | MH781032 |
| Madrella amphora sp. nov. | CASIZ 191283 | Papua New Guinea | MH781078 | – | MH781033 |
| Caldukia affinis | NMSC513 | Australia | MH781079 | MH781054 | – |
| Bonisa nakaza | CASIZ 176146 | South Africa | HM162579 | HM162746 | HM162670 |
| Bonisa nakaza | CASIZ 176147 | South Africa | MH781080 | MH781055 | – |
| Bonisa nakaza | CASIZ 176279 | South Africa | MH781081 | MH781056 | – |
| Bonisa nakaza | CASIZ 176945 | South Africa | MH781082 | MH781057 | MH781034 |
| Janolus anulatus | CASIZ 189420 | Portugal | MH781083 | MH781058 | MH781035 |
| Janolus barbarensis | CASIZ 176833 | California | HM162580 | HM162747 | HM162671 |
| Janolus capensis | CASIZ 176129 | South Africa | HM162581 | HM162748 | HM162672 |
| Janolus capensis | CASIZ 176130 | South Africa | MH781084 | MH781059 | MH781036 |
| Janolus capensis | CASIZ 176131 | South Africa | – | MH781060 | MH781037 |
| Janolus capensis | CASIZ 176132 | South Africa | MH781085 | MH781061 | MH781038 |
| Janolus capensis | CASIZ 176133 | South Africa | MH781086 | MH781062 | MH781039 |
| Janolus capensis | CASIZ 176135 | South Africa | MH781087 | MH781063 | MH781040 |
| Janolus capensis | CASIZ 176137 | South Africa | MH781088 | MH781064 | MH781041 |
| Janolus capensis | CASIZ 176974 | South Africa | MH781089 | MH781065 | MH781042 |
| Janolus comis | MZUSP103312 | Brazil | MH781090 | MH781066 | MH781043 |
| Janolus cristatus | – | ? | – | AF249813 | – |
| Janolus fuscus | – | ? | – | GQ292048 | – |
| Janolus hyalinus | CASIZ 178727 | Portugal | MH781091 | – | – |
| Janolus longidentatus | CASIZ 176134 | South Africa | MH781092 | MH781067 | MH781044 |
| Janolus longidentatus | CASIZ 176136 | South Africa | MH781093 | MH781068 | MH781045 |
| Janolus longidentatus | CASIZ 176316 | South Africa | MH781094 | MH781069 | MH781046 |
| Janolus longidentatus | CASIZ 176320 | South Africa | HM162582 | HM162749 | HM162673 |
| Janolus mirabilis | CASIZ 179494 | Philippines | HM162583 | HM162750 | HM162674 |
| Janolus savinkini | CASIZ 177533 | Philippines | HM162585 | HM162752 | HM162676 |
| Janolus flavoanulatus sp. nov. | CASIZ 177562 | Philippines | HM162586 | HM162753 | HM162677 |
| Janolus flavoanulatus sp. nov. | CASIZ 177561 | Philippines | MH781095 | MH781070 | MH781047 |
| Janolus flavoanulatus sp. nov. | CASIZ 177367 | Philippines | MH781097 | MH781071 | MH781048 |
| Janolus flavoanulatus sp. nov. | NMP 041288 | Philippines | MH781096 | MH781072 | – |
| Janolus flavoanulatus sp. nov. | CASIZ 177588 | Philippines | MH781098 | MH781073 | – |
| Janolus flavoanulatus sp. nov. | CASIZ 177569 | Philippines | MH781099 | – | MH781049 |
| Janolus tricellariodes sp. nov. | CASIZ 177234 | Philippines | MH781100 | MH781074 | MH781050 |
| Janolus tricellariodes sp. nov. | CASIZ 177573 | Philippines | HM162584 | HM162751 | HM162675 |
| Janolus tricellariodes sp. nov. | NMP 041287 | Philippines | MH781101 | MH781075 | MH814722 |
| Janolus incrustans sp. nov. | CASIZ 182193 | Marshall Islands | MH781102 | – | MH781051 |
| Janolus incrustans sp. nov. | CASIZ 182191 | Marshall Islands | – | – | MH781052 |
2.2 DNA extraction, amplification, and sequencing
Genomic DNA was extracted from small pieces of foot and cerata tissue for most samples using Qiagen’ DNeasy Blood and Tissue Kits. Amplification of DNA was conducted on Bio-Rad MyCycler™ Thermocycler (software version 1.065, Bio-Rad Laboratories). Partial sequences of the mitochondrial genes for cytochrome c oxidase subunit I (658 bp) and 16S ribosomal RNA (451 bp) and the nuclear gene Histone 3 (328 bp) were amplified using pairs LCO1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′) and HCO2198 (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′; Folmer, Black, Hoeh, Lutz, & Vrijenhoek, 1994), 16Sar-L (5′-CGCCTGTTTATCAAAAACAT-3′) and 16Sbr-H (5′-CCGGTCTGAACTCAGATCACGT-3′; Palumbi et al., 1991) and H3a F (5′-ATGGCTCGTACCAAGCAGACVGC-3′) and H3a R (5′-ATATCCTTRGGCATRATRGTGAC-3′; Colgan, McLauchlan, Wilson, Livingston, & Edgecombe, 1998), respectively. PCR amplifications were carried out in a 25 μl reaction volume including 1 μl of 10× PCR buffer, 0.2 μl dNTPs (10 mM stock), 1.5 μl MgCl (25 mM stock), 0.025 μl Taq (1.25 units/μl)-Apex, 0.2 μl of each primer (25 μM stock), and 1 μl of genomic DNA. Standard PCRs for COI consisted of an initial denaturing step at 94°C for 3 min; 40 cycles of denaturing at 94°C for 30 s, annealing at 48–50°C for 30 s; and final extending at 72°C for 5 min. The partial 16S amplifications followed the following parameters: an initial denaturing step at 94°C for 3 min; 39 cycles of denaturing at 94°C for 30 s, annealing at 50–52°C for 30 s; and extension at 72°C for 2 min and 25°C for 2 min. Finally, the PCR conditions for the H3 amplification consisted of an initial denaturing step at 94°C for 3 min; 35 amplification cycles (94°C for 35 s, 50°C for 1 min, and 72°C for 1 min and 15 s), and a final step at 72°C for 2 min. Double-stranded amplified product was electrophoresed in a 0.5% TBE agarose gel stained with ethidium bromide. Amplified products were purified with ExoSAP-IT (USB Scientific). Cycle-sequencing reactions were performed using ABI Prism Big Dye Terminator (Applied Biosystems; total volume 10 μl) and analyzed using the automated sequencers ABI 3130 and 3730XL (Applied Biosystems). All new DNA sequences have been deposited in GenBank (Table 1).
2.3 Phylogenetic analyses
Geneious Pro 4.7.6. (Drummond, Ashton, Cheung, Heled, & Kearse, 2009) was used to inspect, edit, and assemble the chromatograms of the forward and reverse DNA strands. All sequences were blasted in GenBank to check for contamination. Geneious and MAFFT (Katoh, Asimenos, & Toh, 2009) were employed to align the sequences, using the default settings in both programs. Sequences of protein coding genes were translated into amino acids using the Geneious translation tool to confirm that no stop codons were present. Saturation was visually inspected in MEGA 5.0 (Tamura et al., 2011) by plotting for all specimens including outgroup the total number of pairwise differences (transitions and transversions) against uncorrected p-distances between sequences. For the COI and H3 genes, saturation was further examined separately for the first, second, and third codon positions.
The most variable regions from the 16S alignment were removed using both the default settings and the standard options for stringent and less stringent selection in Gblocks (Talavera & Castresana, 2007). Excluding “indel-rich” regions, the tree was in general the same. Therefore, final analyses were performed with all bases included. Sequences of COI, 16S, and H3 were trimmed to 658, 430, and 328 base pairs, respectively. Amplicon sizes were 709, 485, and 374 base pairs, respectively.
Individual gene analyses (COI, 16S, and H3) and two different concatenated analyses (COI+16S and H3+COI+16S) were performed. COI+16S dataset included four partitions (COI-1st, COI-2nd, COI-3rd, 16S). H3+COI+16S dataset included seven partitions (H3-1st, H3-2nd, H3-3rd, COI-1st, COI-2nd, COI-3rd, 16S). To test for conflicting phylogenetic signal between genes, the incongruence length difference test (ILD; Farris, Källersjö, Kluge, & Bult, 1994) was conducted as the partition homogeneity test in PAUP* 4.0b10 (Swofford, 2002). Test settings consisted of 10 random stepwise additions (100 replicates) with TBR branch swapping. Alignment of the concatenated H3+COI+16S is provided as Supporting information (Figure S1).
Sequence analysis was based on the maximum likelihood (ML) optimality criterium. ML analyses were performed using the software RAxML v.8.2.4. (Stamatakis, 2014), and node support was assessed via nonparametric bootstrapping with 50,000 replicates, random starting trees, and parameters estimated from each dataset under the model selected for the original dataset. Bayesian inference analyses (BI) were also conducted using MrBayes version 3.2.6 (Ronquist & Huelsenbeck, 2003) for 20 million generations and four chains. Markov chains were sampled every 1,000 generations. Of the resulting trees, 2,500 were discarded as “burn in”. The best-fit models of evolution for each gene were determined using the Akaike information criterion (Akaike, 1974) implemented in MrModeltest 2.3 (Nylander, 2004). The selected models by positions were as follow: GTR + I + G for COI-1st, COI-3rd, H3-2nd, H3-3rd, and 16S and GTR + I for COI-2nd, and GTR + G for H3-1st. The combined dataset was partitioned among genes, and the “unlink” command was used to allow all parameters to vary independently within each partition. Only nodes supported by bootstraps values (BS) ≥ 70 (Hillis & Bull, 1993) and posterior probabilities (PP) ≥ 0.95 were considered statistically significant (Alfaro, Zoller, & Lutzoni, 2003).
2.4 Species delimitation analyses
In order to compare the genetic distances among specimens of the traditional Proctonotidae included in this study, we calculated the pairwise uncorrected p-distances for COI and 16S using PAUP* 4.0 b 10.0. All codon positions were considered for the analysis. We also applied the Automatic Barcode Gap Discovery (ABGD) method (Puillandre, Lambert, Brouillet, & Achaz, 2012). ABGD analysis was run on the ingroup. ABGD is a distance-based method designed to detect the so-called barcode gap in the distribution of pairwise distances calculated in a COI alignment (Puillandre, Modica, & Zhang, 2012; Puillandre, Lambert, et al., 2012). The web-based ABGD program (available at http://wwwabi.snv.jussieu.fr/public/abgd/) was employed with the following settings: Pmin = 0.001, Pmax = 0.1, Steps = 10, X = 1.0, Nb bins = 20, and Jukes Cantor (JC69), Kimura (K80) and Simple Distance. Poisson Tree Processes (PTP; Zhang, Kapli, Pavlidis, & Stamatakis, 2013) was also used for species delimitation. This approach is based on phylogenetic species concept assumption, thus implements Bayesian MCMC methods to find the groups descent from a single ancestor (i.e., phylogenetic species) using previously inferred phylogenetic trees. The test was run using the bPTP server http://species.h-its.org/ptp/ with 100,000 generations and with other settings set as default for each dataset as well as the concatenated dataset. Convergence quality was checked using the ML convergence plot generated by bPTP server. Since the results were all similar, the maximum likelihood phylogenetic tree inferred using the COI (inferred as described above) was used as the input tree. The species delimitation analysis plugin (Masters, Fan, & Ross, 2011) for Geneious was also used to provide a statistical framework to help determine whether clades obtained in the phylogenetic analyses are identified as distinct species. The statistics implemented were the ratio between the mean distance within the members of the clade and the mean distance of those individuals to the nearest clade and the P ID, which represents the mean probability, 95% confidence interval, for a member of the putative species to fit inside (strict p ID), or at least to be the sister group (liberal p ID) of the clade made up by the other individuals belonging to this species.
2.5 Nomenclatural acts
This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix “http://zoobank.org/”. The LSID for this publication is: urn:lsid:zoobank.org:pub:9C86BB6B-1CE2-4D2D-BE84-75F57A7E9532.
3 RESULTS
3.1 Phylogenetic analyses
The results of the ILD test show no significant conflicting signal between the three genes (p = 0.1). No saturation is observed across genes and codon positions (data not shown). In addition to the individual gene analyses, two concatenated datasets were tested; combined mitochondrial genes (COI+16S), and all-gene markers combined (see Table 1 for sequences included on each analysis). The combined H3+COI+16S dataset yields a sequence alignment of 1,417 positions.
Figure 1 illustrates the maximum likelihood tree of the H3+COI+16S genes concatenated and includes posterior probabilities (PP) and bootstraps support values from the ML analysis. Analysis of the combined molecular dataset resulted in a tree where Janolus and Bonisa formed a monophyletic group (PP = 1, ML = 94; Figure 1). Figure 1 also showed that the three included specimens of Madrellidae clustered together (PP = 1, ML = 92) and are separated from Janolus and Bonisa. Within Janolus and Bonisa, we obtained two clearly distinct well-supported clades by BI and ML analyses. One clade including Janolus cristatus, J. fuscus, J. barbarensis, J. longidentatus, and J. capensis (PP = 1, ML = 95), and a second clade (PP = 0.99, ML = 89) including B. nakaza, Janolus hyalinus, J. mirabilis, J. anulatus, J. comis, J. savinkini, and the three species of Janolus described here. Within the Janolus cristatus clade, J. longidentatus and J. capensis are clearly well-supported sister species (PP = 1, ML = 97) but the relationship with the other taxa is not resolved. This clade includes the traditional members of the genus Antiopella (Marcus, 1958) including the type species. Within the second major clade, there is a polytomy including Bonisa nakaza, Janolus mirabilis, J. hyalinus, J. comis, J. anulatus and a well-supported clade including J. savinkini, J. incrustans sp. nov., J. tricellariodes sp. nov., and J. flavoanulatus sp. nov. (PP = 1, ML = 94), but the relationship between these taxa is not resolved.
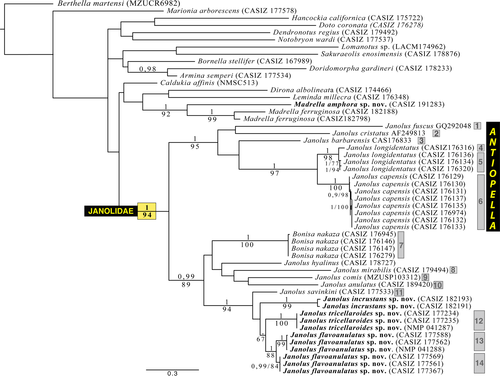
3.2 Species delimitation analysis and uncorrected p-distances
The bPTP analysis of the COI for Caldukia, Janolus, and Bonisa here studied identified 14 lineages. The species delimitation analysis plugin for Geneious and the ABGD analysis showed the same result (Figure 1). Representatives of these groups constitute 10 previously described species of Caldukia, Janolus, and Bonisa and substantiate the validity of these species. Also recognized was the distinctness of the three species of Janolus described here.
The minimum pairwise uncorrected p-distances for COI among key taxa are presented in Table 2. Within the specimens that were identified as Janolus longidentatus, one specimen (CASIZ 176316) was sufficiently distinct in its molecular sequences that all species delimitation analyses considered it to represent a species distinct from the other members of J. longidentatus. This is despite the fact that it was sympatric with the other members of this species studied here. Similarly, three specimens of J. flavoanulatus formed a distinct clade from the other three specimens of that species. The species delimitation analysis concluded that they should be regarded as distinct species, again with about 4.5% (4.7%) difference in COI sequences (Table 2), despite the fact that representatives of both clades were collected from precisely the same locality during March of 2008.
| B. martensi | N. wardi | L. millecra | M. ferruginosa | C. affinis | B. nakaza | J. cristatus | J. barbarensis | J. capensis | |
|---|---|---|---|---|---|---|---|---|---|
| Berthella martensi | |||||||||
| Notobryon wardi | 25.5 | ||||||||
| Leminda millecra | 24.2 | 20.7 | |||||||
| Madrella ferruginosa | 23.6 | 18.5 | 18.2 | ||||||
| Caldukia affinis | 21.1 | 20.4 | 19.9 | 18.8 | |||||
| Bonisa nakaza | 24 | 22 | 21.3 | 21.4 | 18.4 | ||||
| Janolus cristatus | 24.5 | 23.4 | 22.9 | 23.2 | 22.9 | 22 | |||
| Janolus barbarensis | 24.2 | 23 | 23.9 | 23.5 | 20.2 | 20.4 | 20.8 | ||
| Janolus capensis | 22.5–23.1 | 23.6–23.9 | 19.6–19.9 | 22.6–22.9 | 19.6–20.2 | 19.1–19.9 | 20.1–20.5 | 18.1–18.6 | 0–1.5 |
| Janolus fuscus | 24 | 23.4 | 22.8 | 22 | 21.2 | 20.4 | 20.8 | 20.6 | 18.6–18.9 |
| Janolus longidentatus | 22.5–22.8 | 23.1–23.6 | 20.8–21.3 | 21.4–22.3 | 19.3–19.8 | 18.7–19 | 20.2–20.6 | 18.6–18.8 | 11.4–12.3 |
| Janolus anulatus | 23.7 | 23.6 | 22.5 | 22.1 | 20.4 | 20.7 | 22.8 | 21.2 | 18.4–18.8 |
| Janolus comis | 24.9 | 21.7 | 19.5 | 21.3 | 19.1 | 18.4 | 20.3 | 21.2 | 21.1–21.6 |
| Janolus mirabilis | 24.2 | 24.1 | 21.5 | 23 | 20.9 | 18.5 | 21.5 | 23.6 | 20.1–20.3 |
| Janolus savinkini | 21.9 | 21 | 18.4 | 21 | 15.7 | 18.2 | 20.4 | 19.6 | 19.1–19.9 |
| Janolus flavoanulatus sp. nov. | 22.6–23.3 | 20–20.8 | 21.3–21.7 | 21.1–23.4 | 15.8–16.4 | 18.1–18.5 | 19.7–20.6 | 17.6–19.4 | 19.6–19.8 |
| Janolus tricellariodes sp. nov. | 23.4 | 21.3 | 21.4 | 20.4 | 15.5 | 17.9 | 20.3 | 18.9 | 19.1–19.5 |
| J. fuscus | J. longidentatus | J. anulatus | J. comis | J. mirabilis | J. savinkini | Janolus flavoanulatus sp. nov. | |
|---|---|---|---|---|---|---|---|
| Janolus fuscus | |||||||
| Janolus longidentatus | 18.1–18.7 | 0–4.4 | |||||
| Janolus anulatus | 21.5 | 18.8–18.9 | |||||
| Janolus comis | 18.1–18.7 | 20.2–20.5 | 17.8 | ||||
| Janolus mirabilis | 21.3 | 19.1–19.5 | 21.7 | 19.6 | |||
| Janolus savinkini | 21.2 | 19.8–19.9 | 18.2 | 18.1 | 20.5 | ||
| Janolus flavoanulatus sp. nov. | 18.1–21.9 | 18.7–19.3 | 17.8–19.9 | 17.8–18.5 | 20–21 | 10.5–11.1 | 0–4.7 |
| Janolus tricellariodes sp. nov. | 22.5 | 19.3–20 | 20.1 | 17.6 | 21.2 | 11.9 | 9.7–10.2 |
- Distances for Janolus capensis, Janolus longidentatus, and Janolus flavoanulatus sp. nov. take into account multiple specimens sampled for our analyses.
4 SYSTEMATICS FAMILY MADRELLIDAE PRESTON, 1911 MADRELLA ALDER & HANCOCK, 1864
4.1 Type species
Madrella ferruginosa Alder & Hancock, 1864: 141–142, pl 33, figs. 10–12, by monotypy.
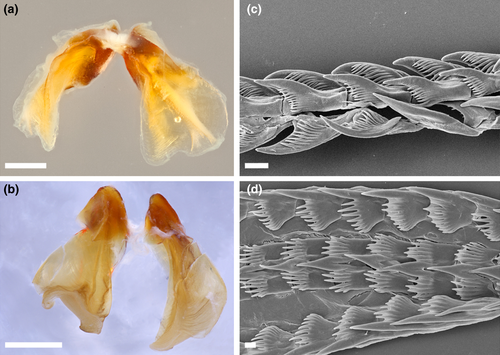
4.2 MADRELLA AMPHORA POLA & GOSLINER SP. NOV. (FIGURES 2a–d, 3, 4a)

LSID urn: lsid:zoobank.org:act:2911F1F3-BF9B-4322-AB3A-BEAC2ACE6AD1
Galeojanolus sp. Gosliner, Behrens, & Valdés, 2008: 320 upper right photo.
Madrella sp. 1 Gosliner, Valdés, & Behrens, 2015: 305, upper left photo.
4.2.1 Type material
Holotype: CASIZ 191283, one specimen, subsampled for molecular study, dissected. Malmal Passage, 5.1204°S 145.8232°E, Madang Lagoon, Madang, Papua New Guinea, 17 November, 2012, A. Berberian. Paratype: CASIZ 086413, s. side Rasch Passage, 5.1550°S 145.8299°E, Madang Lagoon, Madang, Papua New Guinea, 16 June, 1992, T. Gosliner.
4.2.2 Comparative material
Madrella ferruginosa CASIZ 182902, one specimen, dissected. Anilao, Balayan Bay, Batangas Province, Luzon, Philippines, May 2010, P. Paleracio. CASIZ 182188, two specimens, on Halimeda stalks, Bigej-Meck Reef, Kwajalein Atoll, Marshal Islands, 19 February 2007, J. Johnson. CASIZ 182798, one specimen, Kirby's Rock, Caban Island, Tingloy, Balayan Bay, Batangas, Luzon, Philippines, 19 May 2010, A. Hermosillo.
Type locality: Malmal Passage, Madang Lagoon, Madang, Papua New Guinea.
Etymology: This species is named Madrella amphora since the shape of the dorsal appendages resembles an amphora, a Greek vessel for storing liquids.
Distribution: This species is known only from Madang, Papua New Guinea (Gosliner et al., 2015).
Natural history: This species found on the under sides of large pieces of coral rubble and closely resemble the egg cases of neogastropods (Figure 2c).
External morphology (Figure 2a–d): Living specimens 7–25 mm in length (Gosliner et al., 2015). Body broadest in middle with long tapering posterior end of foot. Rhinophores perfoliate with 10–12 complete, transverse lamellae. Inter-rhinophoral caruncle absent between the rhinophores. Eyes appearing as two small black dots situated immediately behind rhinophores. Cardiac area located near the middle of the notum, not markedly elevated from remainder of notum. Pair of short, triangular oral tentacles extending from either side of head. Dorsal appendages elongate, very globose centrally, and smooth, but with much narrower apical region, resembling the neck of bottle or amphora. Cerata distributed over the entire body, but with a distinct gap between cardiac region and head. Appendages are arranged in 1–2 rows on either side of the body. Digestive gland inserting into base of cerata but not extending into most of cerata length. Anteriormost cerata appearing to lack extensions of digestive gland. Anus located mid-dorsally, near the posterior end of notum. Gonopore located on right side in the middle of body.
Coloration (Figure 2a–d): Background coloration translucent orange. One juvenile specimen (CASIZ 086413) with network of opaque white on notum (Figure 2d). Irregular markings of opaque white also found on cerata. Apices of the cerata usually bright orange. Posterior end of foot uniformly orange.
Internal anatomy (Figure 3): Buccal mass visible through translucent body. Buccal mass large and muscular with oval opening. Pair of large, highly dendritic salivary glands extending anteriorly from stomach and entering buccal mass near anterior limit via glandular ducts. Salivary glands thick at point of insertion, tapering sharply, and again expanding into the dendritic portion. Paired jaws large, strong, and thick with entirely smooth masticatory border (Figure 3a,b). Radula broad and well-developed. Radular formula is 18 × 1.1.1 in holotype (CASIZ 191283). Rachidian teeth narrow with pointed cusp bearing 8–11 elongate denticles. Hook-shaped lateral teeth having long base, bearing eight elongate denticles on inner side of tooth. Teeth smooth and sharply arched (Figure 3c,d).
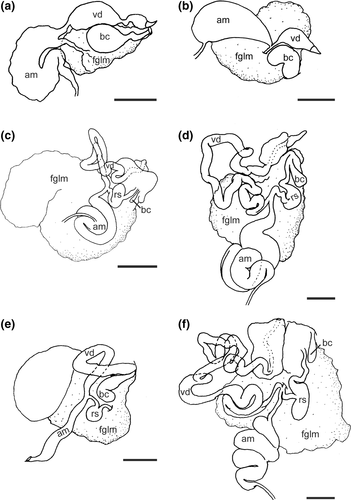
Reproductive system (Figure 4a): Androdiaulic system. Ampulla thick and curved, narrowing into bifurcation of vas deferens and oviduct. Vas deferens widening immediately into wide, thick prostatic portion. Prostate narrowing into a short ejaculatory portion, again widening into penial sac. Unarmed penial papilla narrow and curved apically. Oviduct long, narrow, and straight, entering female gland mass near albumen gland. Bursa copulatrix large, rounded, entering female atrium by means of narrow duct, opening adjacent to penis. Female glands very well developed.
4.2.3 Remarks
Madrella is a small genus that was described by Alder & Hancock in 1864. It is composed of only four species, with M. ferruginosa, the type species by monotypy. Madrella ferruginosa was originally found in southern India (Alder & Hancock, 1864) but it can be found in both Indian and western Pacific oceans (Eliot, 1902; Gosliner et al., 2015; Nimbs & Smith, 2016). Three other species are considered as valid: Madrella sanguinea (Angas, 1864), recorded from temperate Australia (Baker et al., 2013; Burn, 2006; Nimbs & Smith, 2016), Madrella aurantiaca Vayssière, 1902 from the Mediterranean Sea and Portugal (Calado & Silva, 2012; Cervera et al., 2004; Pruvot-Fol, 1954; Vayssière, 1902), Madrella gloriosa Baba, 1949 from Japan, Tanzania (Gosliner et al., 2015), and the Red Sea (Yonow, 2015) and Madrella granularis Baba, 1949; also known only from Japan. The new species clearly differs from all the described species of Madrella in coloration since it is the only one that does not have a predominantly reddish body color. Madrella ferruginosa, M. granularis, and M. sanguinea have a bright orange-reddish or reddish-brown coloration with opaque white pigment between the rhinophores. Madrella aurantiaca has a yellow or yellow-orange color (Vayssière, 1902), while M. gloriosa is brown with white spots. Also, M. amphora is the only species with short blunt rather than thin, elongate cerata with curved apices. Internally, M. amphora is the only species with a narrow rachidian tooth and has the denticles on the lateral tooth right on the large cusp rather than as separate wing. In contrast, M. ferruginosa (Figure 3d; CASIZ 182902) and the other species have a much broader rachidian and the laterals have their denticles on a separate wing.
The reproductive anatomy has been previously described only for M. ferruginosa (Odhner, 1917: fig. 23). The arrangement of organs described by Odhner, from Western Australia, differs from that described here for M. ferruginosa (Figure 4b) and M. amphora (Figure 4a). Odhner's specimen has a seminal vesicle separated by a long duct that is not evident in our specimens of both species and has a much more elongate penis. It is unclear whether this system was depicted correctly or whether it may represent a species distinct from our specimens of M. ferruginosa. In any case, our specimen of M. ferruginosa lacks the large, distinctly widened prostate found in M. amphora and has a much shorter bursa copulatrix duct than found in M. amphora. The reproductive anatomy of the remaining species of Madrella remains undescribed.
Madrella differs from Janolus in that Madrella has short, triangular jaws, a triseriate radula, and the reproductive system with only a bursa copulatrix while Janolus has elongate jaws, a multidentate radula, and the reproductive system having a receptaculum seminis in addition to a bursa copulatrix. In our phylogenetic analyses, we only included three specimens of the genus Madrella, since no other specimens were available for molecular study; one initially identified as M. ferruginosa and two unidentified specimens. Although we were not able to obtain sequences for COI for two of the specimens of Madrella, our results clearly show that we have two species; M. ferruginosa and the new species here described Madrella amphora sp. nov. (Figure 1).
4.3 FAMILY JANOLIDAE PRUVOT-FOL, 1933 JANOLUS BERGH, 1884
4.3.1 Type species
Janolus australis Bergh, 1884: 19, pl. 8, figs. 15–22, pl. 9, figs. 6–8, by monotypy.
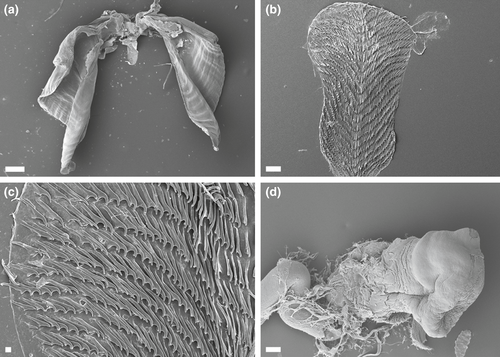
4.4 JANOLUS TRICELLARIODES POLA & GOSLINER SP. NOV. (FIGURES 2e–h, 4c, 5)
LSID urn: lsid:zoobank.org:act:C7DB62CF-B843-4079-AFFF-01B92C9B303C
Janolus sp.1 Gosliner et al., 2008: 317. lower photo.
Janolus sp. 8 Gosliner et al., 2015: 308. top two photos.
4.4.1 Type material
Holotype: NMP 041287 (ex, CASIZ 177235), subsampled for molecular study, dissected, 10 mm in length (preserved), Kirby's Rock, 13.6911°N, 120.8414°E, Caban Island, Tingloy, Batangas Province, Luzon, Philippines, 16 March 2008, 31.2 m depth, T. Gosliner, A. Valdes, M. Pola, L. Witzel, B. Moore, A. Alejandrino. Paratypes: CASIZ 177234, subsampled for molecular study, dissected, 10 mm in length (preserved), Kirby's Rock, 13.6911°N, 120.8414°E, Caban Island, Tingloy, Batangas Province, Luzon, Philippines, 31.2 m depth, 16 March 2008, T.M. Gosliner, A. Valdes, M. Pola, L. Witzel, B. Moore, A. Alejandrino. CASIZ 177573, subsampled for molecular study, dissected, 10 mm in length (preserved), Red Palm (Pulanbuli) Tingloy, Batangas, Philippines, 16.9 m depth among coral rubble, 22 March 2008, T. Gosliner, A. Valdes, M. Pola, L. Witzel, B. Moore, A. Alejandrino. CASIZ 083649, s. side of Ligpo Island, Bauan, Batangas, Luzon, Philippines, on steep wall at edge of reef, 27 m depth, 21 February 1992, T. Gosliner. CASIZ 083716, one specimen, Kirby's Rock, 13.6911°N, 120.8414°E, Caban Island, Tingloy, Batangas Province Luzon Philippines, 18 February 1992, T. Gosliner. CASIZ 083725, five specimens of 7, 9, 10 (2) and 15 mm in length, 15 mm adult specimen dissected, Kirby's Rock, 13.6911°N, 120.8414°E Caban Island, Tingloy, Batangas Province Luzon Philippines, 18 February 1992, 21 m depth, T. Gosliner. CASIZ 083781, dissected, 10 mm in length (preserved), Batangas Province, 17–26 February 1992, T. Gosliner. CASIZ 086003, Kirby's Rock, 13.6911°N, 120.8414°E Caban Island, Tingloy, Batangas Province, Luzon, Philippines, 1 March 1993, T. Gosliner. CASIZ 096339, Kirby's Rock, 13.6911°N, 120.8414°E, Caban Island, Tingloy, Batangas Province, Luzon, Philippines, 1 March 1994, 23 m depth, T. Gosliner. CASIZ 103720, Escarcia Point (Sinandigan), Puerto Galera, Mindoro Oriental, Philippines, 1 March, 1995, T. Gosliner. CASIZ 105748, two specimens, Kirby's Rock, 13.6911°N, 120.8414°E Caban Island, Tingloy, Batangas Province, Luzon, Philippines, 23 February 1995, T. Gosliner. CASIZ 106485, Kirby's Rock, 13.6911°N, 120.8414°E, Caban Island, Tingloy, Batangas Province, Luzon, Philippines, 15 April 1996, 24.7 m depth, T. Gosliner. CASIZ 208363, Pinnacle dive site, east end Verde Island, Batangas, Philippines, 27 April 2015, 40 m depth, T. Gosliner. CASIZ 208582, three specimens, wreck of Alma Jane, Puerto Galera, Mindoro Oriental, Philippines, 29 March 2015, T. Gosliner.
4.4.2 Type locality
Kirby's Rock, 13.6911°N, 120.8414°E, Caban Island, Tingloy, Batangas Province, Luzon, Philippines.
4.4.3 Etymology
The specific name refers to Tricellaria sp., the bryozoan on which it feeds.
4.4.4 Distribution
Western Pacific Ocean (Gosliner et al., 2015). This species is found in the Philippines (Debelius & Kuiter, 2007; Gosliner et al., 2008; present study), Okinawa, Japan and Indonesia (Gosliner et al., 2008).
4.4.5 Natural history
This species feeds on arborescent bryozoans of the genus Tricellaria. It is usually found on steep reef walls and pinnacles in areas where strong currents are present in 20–40 m.
4.4.6 Description
External morphology (Figure 2e–h): Living specimens reaching 25 mm in length (Gosliner et al., 2008, 2015). Body broadest anteriorly, tapering to elongate posterior end of the foot extending well beyond notum. Rhinophores elongate with about 15 complete or incomplete transverse lamellae. Well-developed, convoluted caruncle (Figure 2e–g) present between rhinophores. Eyes appearing as two small black dots just behind rhinophores. Large, swollen cardiac area located near middle of notum. Pair of short, digitiform oral tentacles extending from either side of head. Cerata elongate, very globose centrally. Most cerata not entirely smooth, but with some tubercles. Cerata distributed over whole body, in 13–16 rows, with 4–5 cerata per row. Digestive gland inserting into most of the cerata at the base and branching within the papillae. Anteriormost cerata lacking extensions of digestive gland. Anus located mid-dorsally near posterior end of notum. Gonopore located on right side, in middle of body.
Coloration (Figure 2e–h): Background coloration translucent white. Caruncle opaque white. Opaque white also appearing in middle part of head, notum, posterior rachis of rhinophores and also coloring many of ceratal tubercles. Bases of cerata translucent white. Near middle of ceratal length, coinciding with more globose part, translucent orange color beginning to appear. Well-defined band of purple color present above orange coloration, extending toward ceratal tip. Purple coloration becoming weaker and ending in translucent white tip. Branches of digestive gland within cerata chocolate brown with many black dots. Rhinophores almost of the same color as cerata, with a darker orange color, varying to brown. Posterior end of foot purple with median line of opaque white.
Internal anatomy (Figure 5a–c): Buccal mass large, muscular with oval opening. Numerous small, simple, oral glands present near opening of the mouth. Pair of large, highly dendritic salivary glands extending anteriorly from stomach and entering buccal mass near anterior limit, via glandular ducts. Salivary glands thick at their insertion, tapering sharply and again expanding into dendritic portion. Three major branches of the digestive gland emerging from the stomach. Intestine curving to right and continuing posteriorly at posterior limit of stomach. Intestine terminating at medial anus. Anal glands absent. Paired jaws large, strong, and thick with entirely smooth masticatory border. Pair of elongate curved flanges present where two jaws articulate (Figure 5a). Radula broad and well-developed, consisting of 22–32 rows of teeth (Figure 5b). Twenty-five to 32 lateral teeth present on each side of rachidian tooth (Figure 5c). Radular formula of holotype 22 × 25.1.25 (NMP 041287), 26 × 25.1.25 (CASIZ 0837255, 27 × 30.1.30 (CASIZ 083781), and 33 × 26.1.26 (CASIZ 177573). Rachidian teeth narrow and elongate with pointed cusp and lacking denticles. Hook-shaped lateral teeth with long base, smooth, and sharply arched (Figure 5c). Base of lateral teeth proportionately shorter toward outer sides of radula.
Reproductive system (Figure 4c): Androdiaulic system. Ovotestis consisting of numerous lobes and giving rise to thick and convoluted ampulla. Ampulla narrowing into bifurcation of vas deferens and oviduct. Vas deferens long, highly convoluted, and prostatic throughout its length. Vas deferens terminating at thick, wide, unarmed penis (Figure 5d). Oviduct short and narrowing and expanding into elongate receptaculum seminis. Oviduct narrows into a short vagina. Very small, but distinct digitiform bursa copulatrix entering female atrium near its junction with vagina. Female gland very well developed, indicating full maturity of specimen.
4.4.7 Remarks
Janolus tricellariodes sp. nov. is easily distinguishable from any other described species of the genus by its orange coloration with opaque white pigment. The most similar species is Janolus savinkini Martynov & Korshunova, 2012; but this later species lacks the opaque white pigment of the bulbous cerata (Martynov & Korshunova, 2012). Internally, the radular teeth are very similar. Yet, the reproductive systems, described later in this manuscript for the first time for J. savinkini, have several differences. For instance, the vas deferens and the ampulla of J. tricellariodes are shorter than in J. savinkini (Figure 4f). Both species have a stalked semi-serial receptaculum seminis (as differentiated by Gosliner, 1981) and a distinct bursa copulatrix but the tubular bursa is smaller in J. tricellariodes sp. nov. than in J. savinkini. The penis of J. tricellariodes (Figure 5d) appears to be much broader than the conical penis of J. savinkini (Figure 9e,f). Our phylogenetic analyses clearly separate J. tricellariodes from its apparent sister species, J. savinkini. The uncorrected p-distance for COI between both species is 11.9% (Table 2). Species delimitation analyses bPTP and ABGD also differentiate both species (Figure 1).
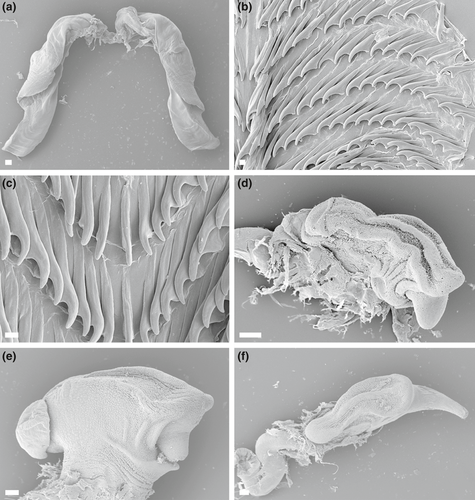
4.5 JANOLUS FLAVOANULATUS POLA & GOSLINER SP. NOV. (FIGURES 4d, 6a–c, 7)

LSID urn: lsid:zoobank.org:act:E55DA95F-6D62-471B-B1D2-3FB8F348F9AE
Janolus sp. 1 Debelius & Kuiter, 2007: 283, lower left photo of Janolus sp. 1.
Janolus sp. 7 in Gosliner et al., 2008: 319, middle three photos.
Janolus sp. 7 Gosliner et al., 2015: 307, lower three photos.
4.5.1 Type material
Holotype: NMP 041288 (ex. CASIZ 177363), subsampled for molecular study, Bethlehem, 13.6728°N, 120.8413°E, Maricaban Island, Tingloy, Batangas, Luzon, Philippines, 18 March 2008, 20.7 m depth between coral rubble, T. Gosliner, A. Valdes, M. Pola, L. Witzel, B. Moore, A. Alejandrino. Paratypes: CASIZ 177569, subsampled for molecular study, dissected, 13 mm in length (preserved), Bethlehem, 13.6728°N, 120.8413°E, Maricaban Island, Tingloy, Batangas, Luzon, Philippines, 22 March 2008, 20.9 m depth among coral rubble, T. Gosliner, A. Valdes, M. Pola, L. Witzel, B. Moore, A. Alejandrino. CASIZ 177561, subsampled for molecular study, dissected, 10 mm in length (preserved), Bethlehem, 13.6728°N, 120.8413°E, Maricaban Island, Tingloy, Batangas, Luzon, Philippines, Bethlehem, 22 March 2008, 20.9 m depth, among coral rubble, T. Gosliner, A. Valdes, M. Pola, L. Witzel, B. Moore, A. Alejandrino. CASIZ 177367, subsampled for molecular study, dissected, 16 mm in length (preserved), Bethlehem, 13.6728°N, 120.8413°E, Maricaban Island, Tingloy, Batangas, Luzon, Philippines, 18 March 2008, 20.7 m depth among coral rubble, T. Gosliner, A. Valdes, M. Pola, L. Witzel, B. Moore, A. Alejandrino. CASIZ 177562, one specimen, one juvenile specimen, 6 mm in length preserved, Bethlehem, 13.6728°N, 120.8413°E, Maricaban Island, Tingloy, Batangas, Luzon, Philippines, 22 March 2008, 20.9 m depth among coral rubble, T. Gosliner. CASIZ 177372, subsampled for molecular study, dissected, juvenile, 6 mm in length (preserved), Bethlehem, 13.6728°N, 120.8413°E, Maricaban Island, Tingloy, Batangas, Luzon, Philippines, 18 March 2008, 20.7 m depth among coral rubble, T. Gosliner, A. Valdes, M. Pola, L. Witzel, B. Moore, A. Alejandrino. CASIZ 177588, subsampled for molecular study, Matotonngil Point, 13.755572°N, 120.90685°E, Mabini, Calumpan Peninsula, Balayan Bay, Batangas, Luzon, Philippines, 16 April 2008, 21 m depth among coral rubble, T. Gosliner.
4.5.2 Type locality
Bethlehem, 13.6728°N, 120.8413°E, Maricaban Island, Tingloy, Batangas, Luzon, Philippines.
4.5.3 Etymology
The specific name, flavoanulatus refers to the yellow subapical ring of the cerata (from the Latin words flavus, yellow and anulatus, ringed).
4.5.4 Distribution
Indian and western Pacific oceans (Gosliner et al., 2015). This species has been found in the Philippines (Gosliner et al., 2008; present study), Papua New Guinea (Coleman, 2008), Japan, Solomon Islands, Red Sea, Indonesia, Vanuatu (Gosliner et al., 2008), Indonesia (Debelius & Kuiter, 2007) and perhaps Australia (www.nudipixel.net).
4.5.5 Natural history
It is found in 20–30 m, on rubble fields and slopes where it feeds on arborescent bryozoans, likely Tricellariodes sp.
4.5.6 Description
External morphology (Figure 6a–c): Living specimens reaching 50 mm in length (Gosliner et al., 2008, 2015). Body broadest anteriorly, tapering to posterior end of foot extending well beyond notum. Rhinophores elongate with about 20–25 complete or incomplete transverse lamellae. Well-developed convoluted caruncle present between rhinophores (Figure 6a–c). Two eyes appearing as small black dots, appearing by transparency just behind rhinophores. Large, swollen cardiac area located near middle of notum. Pair of short and digitiform oral tentacles extending from either side of head. Cerata elongate, globose centrally, ending with acutely pointed apex. Most of cerata are covered with many prominent tubercles near middle. Cerata arranged in 8–12 closely-packed longitudinal rows with 4–5 cerata per row on each side which are irregularly arranged. Digestive gland inserting into most cerata at base, splitting into numerous branches, and terminating at globose portion of cerata. Anteriormost cerata often lacking extensions of digestive gland. Anus located mid-dorsally near posterior end of notum on elevated papilla. Anal glands absent. Gonopore located on right side in middle of body.
Coloration (Figure 6a–c): Background coloration translucent white. Caruncle and tubercles of the cerata yellowish white. Opaque white coloration also apparent at midline immediately posterior to caruncle and as spots irregularly scattered surrounding rhinophoral area. Opaque white midline outlined by purple spots and markings, similarly distributed as opaque white spots on notum. Basal half of each ceras translucent white. Next, third toward apex of cerata with purple with pigment and prominent white tubercles. Above this area, narrow brown-reddish ring followed by a yellow one. Apically from yellow ring, translucent band and broad purple area extending toward small, acutely pointed translucent-yellow tip. Branches of digestive gland within cerata chocolate brown-reddish with many small black dots. Rhinophores reddish-brown with white tips. Posterior end of foot purple with outer translucent white outline. Foot with irregular scattered purple spots.
Internal anatomy (Figure 7a–c): Buccal mass large, muscular with oval opening. Numerous small, simple, oral glands present near opening of mouth. Pair of elongate salivary glands present on ventral side of stomach, posterior to buccal mass. Three major branches of digestive gland emerging from stomach. At posterior limit of stomach, intestine curving to right and continuing posteriorly, terminating at the medial anus. Large paired jaws strong and thick with entirely smooth masticatory border. Pair of elongate curved flanges present where jaws articulate (Figure 7a). Radula broad, elongate, well developed. The radular formulae: 30 × 28.1.28 (CASIZ 177367; 16 mm preserved length specimen), 28 × 18.1.18 (CASIZ 177569; 13 mm preserved length specimen) and 15 × 16.1.16 (CASIZ 177561; 10 mm preserved length specimen). Rachidian teeth narrow with pointed cusp, without lateral denticles. Hook-shaped lateral teeth have a long base. Laterals are smooth and sharply arched, progressively smaller toward outer margin (Figure 7b,c).
Reproductive system (Figure 4d): Androdiaulic system. Ovotestis consisting of numerous lobes and giving rise to long, thick, convoluted ampulla. Ampulla narrowing into bifurcation of vas deferens and oviduct. Vas deferens very long, highly convoluted, prostatic throughout length. Vas deferens terminating at thick, wide, unarmed penis with conical apex (Figure 7d–f). Oviduct short, expanding into a semi-spherical receptaculum seminis. Receptaculum duct narrowing into short vagina. Relatively small elongate bursa copulatrix entering the female atrium near junction with vagina. Female gland very well developed, indicating fully maturity of specimens.
4.5.7 Remarks
Janolus flavoanulatus is similar to J. tricellariodes sp. nov. in many aspects of its morphology. Yet, one of the most consistent features to distinguish both species in the field is the coloration of the rhinophores. In J. flavoanulatus, the rhinophores are brown-reddish with white tips, whereas the rhinophores in J. tricellariodes sp. nov. as well as in J. savinkini, have the same sequence of colors as is found on the cerata of those species. Other differences in color are also very obvious, since the cerata of J. flavoanulatus sp. nov. have purple pigmentation with white prominent tubercles and a very obvious yellow ring, which are missing in J. tricellariodes and J. savinkini. Additionally, J. tricellariodes has prominent opaque white pigment anterior and posterior to the rhinophores and caruncle, which is not as pronounced or entirely absent in the other two species. The radula of all these species is very similar but there are also a few consistent differences in the reproductive system. In J. flavoanulatus, the length of the vas deferens and the ampulla are more similar to J. savinkini, but in J. flavoanulatus, the size of the bursa copulatrix is clearly larger than in the other two species. Mature specimens of J. tricellariodes are smaller and reach a maximum of 25 mm in length, whereas J. flavoanulatus and J. savinkini may reach twice that size.
Our phylogenetic analyses also separate J. flavoanulatus from J. tricellariodes sp. nov. and J. savinkini (Figure 1) . The minimum uncorrected p-distance for COI between J. flavoanulatus sp. nov. and J. tricellariodes sp. nov. is 9.7%, and between J. flavoanulatus sp. nov. and J. savinkini is 10.5% different. Species delimitation analyses and ABGD also differentiate both species (Figure 1).
There is clearly some genetic structure within the single population of J. flavoanulatus studied here. Our species delimitation analyses both consider the individuals of J. flavoanulatus as representing two distinct species (Figure 1) that have a maximum uncorrected p-distance for COI of 4.7%. Yet, we can find no morphological differences between the individuals that comprise these two subgroups. These different individuals were collected from the same locality during the same collecting event. Owing to the lack of features to differentiate individuals of these two lineages, we retain them here as a single species, pending a more thorough population genetic study of a larger sample of individuals.
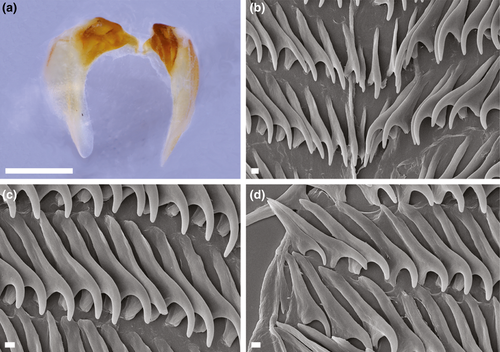
4.6 JANOLUS INCRUSTANS POLA & GOSLINER SP. NOV. (FIGURES 4e, 6d–f, 8a–d)
LSID urn: lsid:zoobank.org:act:D5664A43-9124-4E41-92D9-59DA2D0216F2
Janolus sp. 11 in Gosliner et al., 2015: 308, bottom right photo.
4.6.1 Type material
Holotype: CASIZ 193493, Bigej-Meck Reef, Kwajalein Atoll, Marshall Islands, 23 January 2012, 8 m depth, Scott Johnson. Paratypes: CASIZ 182191, subsampled for molecular study, Bigej-Meck Reef, Kwajalein Atoll, Marshall Islands, 27 May 2008, 8 m depth, S. Johnson. CASIZ 182193, subsampled for molecular study, dissected, South Lol sand spit, South Lol Island, Kwajalein Atoll, Marshall Islands, 5 July 2008, 8 m depth, S. Johnson.
4.6.2 Etymology
This species is named from the Latin incrustans, meaning encrusted, and referring to the opaque white pigment that appears to encrust the body.
4.6.3 Distribution
This species is known only from the Marshall Islands and Indonesia (Gosliner et al., 2015).
4.6.4 Natural history
This species has been found under coral rubble and in Halimeda beds in 2–10 m depth. It has not been found in association with a specific prey source.
4.6.5 Description
External morphology (Figure 6d–f): Living specimens reaching 18 mm in length (Gosliner et al., 2015). Body broadest anteriorly, tapering to elongate posterior end of the foot extending well beyond notum. Rhinophores elongate without distinct lamellae, covered by series of irregular tubercles over most of length. Well-developed caruncle present between rhinophores consisting of series of irregular papillae. Eyes, appearing as two small black dots immediately behind rhinophores. Swollen cardiac area located near middle of notum, immediately posterior to rhinophores. Pair of short, digitiform oral tentacles extending from either side of head. Cerata elongate, very globose apically, and densely covered with numerous white papillae. Each ceras with pointed tubercle apically. Cerata distributed over entire body in 11–13 rows, with 2–3 cerata per row. Digestive gland inserting into most of cerata at base. Digestive gland duct very narrow and elongate, extending to tip of each ceras. Each duct bifurcating in upper half of ceras. Anteriormost cerata also containing extensions of digestive gland. Anus located mid-dorsally near posterior end of notum. Anal glands absent. Gonopore located on right side middle of body.
Coloration (Figure 6d–f): Background translucent white. Entire body covered with opaque white markings. Markings especially prominent on rhinophores and cerata. Caruncle opaque white. Opaque white also appears on middle part of head and notum. Posterior rachis of rhinophores and many of the ceratal tubercles also opaque white. Branches of digestive gland within cerata orange to brown. Rhinophores almost same color as cerata. Posterior end of the foot opaque white.
Internal anatomy (Figure 8a–d): Buccal mass large and muscular with oval opening. Pair of large, highly dendritic salivary glands extending anteriorly from stomach and entering buccal mass near anterior limit via glandular ducts. Paired jaws large, strong, and thick, with entirely smooth masticatory border (Figure 8a). Pair of elongate curved flanges present where two jaws articulate. Radula broad and well developed with formula of 17 × 20.1.20 in one specimen (CASIZ 182193). Rachidian teeth narrow and elongate, without denticles (Figure 8b). Inner lateral teeth on each side of rachidian tooth evenly curved, without denticles. The middle lateral teeth (Figure 8c) with broad base with curved apex, also lacking denticles. Hook-shaped outer lateral teeth have long base, but shorter cusp than middle laterals. Outer lateral smooth and sharply arched (Figure 8d), without denticles.
Reproductive system (Figure 4e): Androdiaulic system. Ampulla elongate. narrow with few curves along its length. Ampulla narrowing into bifurcation of vas deferens and oviduct. Oviduct short and connecting small rounded receptaculum seminis. Oviduct continuing to enter female gland mass near albumen gland. Vas deferens short and with single loop, prostatic throughout length. Vas deferens terminating at thick, wide, unarmed penial sac. Distinct bursa copulatrix entering female atrium near junction with vagina. Bursa curved near distal end. Female gland very well developed, indicating maturity of specimen.
4.6.6 Remarks
Externally, this species is clearly different from any other described species of the genus. The cerata and rhinophores are intensely papillate with white opaque pigment covering the whole body. Another distinguishing character is their long rhinophores. Initially, this species appears similar to Janolus mirabilis Baba & Abe, 1970; but Janolus incrustans lacks the distinct gap between anterior and posterior clusters of cerata (Baba & Abe, 1970). The reproductive system of Janolus incrustans sp. nov. has clear differences with the other species described in this paper. In J. incrustans, the ampulla and vas deferens are clearly shorter and less convoluted than in J. flavoanulatus sp. nov., J. tricellariodes sp. nov., and J. savinkini. Furthermore, it is the only species having the bursa curved near the distal end. Also, the receptaculum seminis is rounded and not elongate as in the remaining species.
Figure 1 shows that J. incrustans sp. nov. is a different species including in the same highly supported clade as J. flavoanulatus sp. nov., J. tricellariodes sp. nov., and J. savinkini. Janolus incrustans is sister to the clade containing the other two species of Janolus described here. Unfortunately, we could not amplify the COI sequences for these individuals so the uncorrected p-distances for COI for this species with J. flavoanulatus, J. tricellariodes, and J. savinkini remain unknown.
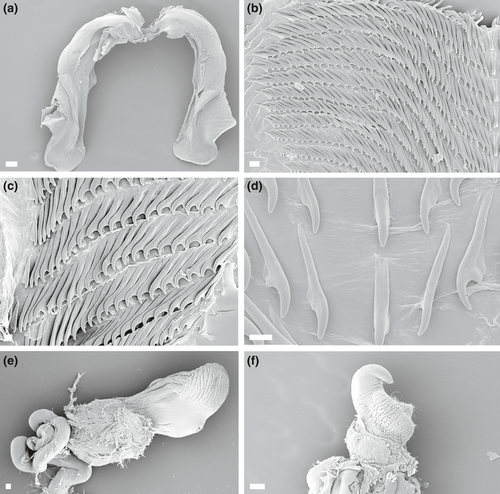
4.7 JANOLUS SAVINKINI MARTYNOV & KORSHUNOVA, 2012; (FIGURES 4f, 6g–h, 9)
4.7.1 Material examined
CASIZ 177533, subsampled for molecular study, dissected, 8 mm in length (preserved), Mainit Point, 13.6864°N, 120.8954°E, Mabini, Calumpan Peninsula, Batangas, Luzon, Philippines, 21 March 2008, 22.7 m depth among sand and rocks, T. Gosliner, A. Valdes, M. Pola, L. Witzel, B. Moore, A. Alejandrino. CASIZ 096347, dissected, 12 mm in length (preserved), Bethlehem 13.6864°N, 120.8954°E, Tingloy, Maricaban Island, Luzon, Batangas, Philippines, 14 March 1994, 20.4 m depth, T.M. Gosliner. CASIZ 085942, dissected, 25 mm in length (preserved), Bethlehem 13.6864°N, 120.8954°E, Tingloy, Maricaban Island, Luzon, Batangas, Philippines, 21 m depth, 27 March 1993, T. Gosliner. CASIZ 186188, Matontongil Point, 13.755572°N, 120.90685°E, Mabini, Batangas, Luzon, Philippines, 21 m depth, Alicia Hermosillo.
4.7.2 Distribution
Originally described from Vietnam (Nhatrang Bay), this species is present in the western Pacific Ocean (Gosliner et al., 2015) and has been found from Australia, Indonesia and Japan (Gosliner et al., 2008).
4.7.3 Description
External anatomy (Figure 6g–h): See Martynov and Korshunova (2012).
Internal anatomy (Figure 9a–f): Buccal mass large and muscular with oval opening. Numerous small, simple, oral glands present near the opening of the mouth. Pair of large, highly dendritic salivary glands extending anteriorly from stomach and entering buccal mass near anterior limit via glandular ducts. Salivary glands thick at point of insertion, tapering sharply, and again expanding into dendritic portion. Three major branches of digestive gland emerging from stomach. At posterior limit of stomach, intestine curving to right and continuing posteriorly, terminating at medial anus. Anal glands absent. Paired jaws large, strong, and thick. Masticatory border entirely smooth (Figure 9a). Radula broad, elongate, and well developed. Radular formulae: 28 × 40.1.40 (CASIZ 085942; 25 mm preserved length specimen), 16 × 25.1.25 (CASIZ 096347; 12 mm preserved length specimen), and 18 × 20.1.20 (CASIZ 177533; 8 mm preserved length specimen). Rachidian teeth narrow, elongate, without denticles. Hook-shaped lateral teeth smooth and sharply arched with long base (Figure 9b–d).
Reproductive system (Figure 4f): Androdiaulic system. Ovotestis consisting of numerous lobes and giving rise to long, thick, convoluted ampulla. Ampulla narrowing into bifurcation of vas deferens and oviduct. Vas deferens very long, highly convoluted, and prostatic throughout length. Vas deferens terminating at thick, wide, unarmed penis (Figure 9e,f). Oviduct short, expanding into large elongate semi-serial receptaculum seminis (Gosliner, 1981). Oviduct narrowing into short vagina. Small, but distinct tubular bursa copulatrix entering female atrium near junction with smooth and sharply arched vagina.
4.7.4 Remarks
This species was described based on a single studied specimen from Nhatrang Bay, Vietnam, and several photographic records (Martynov & Korshunova, 2012). Thus, in the original description there is no mention to the reproductive system or other important structures, such as oral and salivary. Since our specimens, collected in the Philippines, perfectly match the external description by Martynov and Korshunova (2012), in this paper, we complete the anatomical description and add molecular genetic sequences for this species. Differences in the external morphology and anatomy of the reproductive system of J. savinkini with the new species have already been discussed under the remarks of the different species. Uncorrected p-distances for COI are shown in Table 2. The species delimitation analyses support the hypothesis that J. savinkini is clearly a distinct species from J. tricellariodes (Figure 1).
5 DISCUSSION
Morphological and molecular genetic studies of the Cladobranchia (Goodheart, Bazinet, Collins, & Cummings, 2015; Goodheart, Bazinet, Valdés, Collins, & Cummings, 2017; Pola & Gosliner, 2010; Wägele & Willan, 2000) have shown that the traditional Arminina and Dendronotina are not monophyletic. Within the Cladobranchia, detailed relationships of taxa are beginning to be resolved, but the relationships of families and genera still require additional study. This is certainly true within several of the cerata-bearing clades, Dironidae, Madrellidae, and Proctonotidae. In fact, Madrella was described as a genus within the family Proctonotidae (Alder & Hancock, 1864). They stated that the anatomy of Madrella proved that it was closely related to Antiopa Alder & Hancock, 1848; and that Madrella “evidently belongs to the family Proctonotidae” (Alder & Hancock, 1864). Alder and Hancock (1864) also extended the previous characters of the family to include Madrella, since it differed from Proctonotus Alder & Hancock, 1844; and Antiopa “in the lateral position of the anus, and the absence of oral tentacles, unless the veil-like expansion of the head be so considered.” (Alder & Hancock, 1864).
Madrella differs from Janolus by lacking a caruncle, having short and triangular jaws, a triseriate radula and the reproductive system with only a bursa copulatrix. Regarding the molecular results, the minimum p-uncorrected distance for COI between Madrella and other genera here included is 18.2% (between M. ferruginosa CAS182798 and Leminda millecra CAS176348) and the maximum p-uncorrected distance is 23.6% (between M. ferruginosa CAS182798 and Berthella martensi MZUCR6982; Table 2).
As we previously clarify, in the objectives of this study, addressing the monophyly of Proctonotidae is beyond our scope since no specimens of the genera Proctonotus or Galeojanolus could be included due to the lack of material for molecular analyses. Yet, one surprising result of this study was that members of the genera Janolus and Bonisa formed a clade with strong support, but the other member of the traditional Proctonotidae, Caldukia affinis (Burn, 1958) fell outside of this clade, Presently, this result could simply be explained by the missing taxa, and thus, we are aware that more taxa and more genes are necessary to address the phylogeny of the family Proctonotidae and its relationship with Madrellidae.
We did include specimens of the single species of Bonisa, B. nakaza Gosliner, 1981; and 10 of the 23 species of Janolus (Bouchet & Gofas, 2016). Yet, we have not included the type species of the genus, Janolus australis Bergh, 1884; because this species has not been reported since its original description in the Arafura Sea (Bergh, 1884). Bergh described the single specimen as “yellowish, but brownish or reddish along the middle line of the back; the region between the rhinophoria and the inter-rhinophoral ‘comb’ covered with a black spot, and the extremities of the rhinophoria also blackish”. Although the author presented two plates (Pl. VIII. Figs. 15–22, Pl. IX. Figs. 6–8) showing drawings of different parts of the masticatory and the central nervous systems, there is no drawing of the entire animal.
The fact that in this study, members of the genera Janolus and Bonisa form a well-supported clade, whereas Caldukia affinis was not a member of this clade, has led us to reevaluate the morphological features of the traditional genera included in the family. Members of the genera Janolus, Galeojanolus, and Bonisa have a well-developed caruncle, whereas a caruncle is absent in both Proctonotus and Caldukia. The radula of Proctonotus remains largely unknown, but appears to have five lateral teeth on either side of the rachis. What is unclear is whether a rachidian row of teeth is present (Alder & Hancock, 1844: fig. 6). Madrella (present study) has only a single rachidian tooth and a single row of lateral teeth on either side of the rachidian tooth, whereas Caldukia has six lateral teeth on either side of the rachidian tooth. In contrast, species of Janolus, Galeojanolus, and Bonisa all have a broad radula with many more lateral teeth than do species of Madrella, Proctonotus, and Caldukia. With the exclusion of Proctonotus and Caldukia neither Proctonotidae nor Zephyrinidae can be used as the family name for Janolus, Galeojanolus, and Bonisa, since both of these families have Proctonotus and Zephyrina (a synonym of Proctonotus), as type genera, which are excluded from this clade. Thus, the only available name for members of this clade is Janolidae, which has been regarded as a synonym of Proctonotidae (Bouchet & Gofas, 2016). Thus, we reinstate the name Janolidae for this clade and restrict Proctonotidae for Caldukia and Proctonotus, since Caldukia appears as a distinct lineage in our analysis.
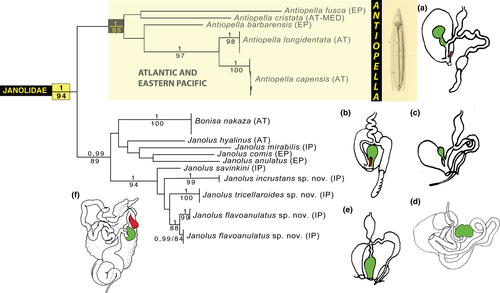
The results of our analyses, summarized in Figure 1, clearly showed two distinct well-supported clades within the Janolidae. The first clade included Janolus cristatus (Delle Chiaje, 1841), J. fuscus O'Donoghue, 1924, J. barbarensis (J. C. Cooper, 1863), J. longidentatus Gosliner, 1981; and J. capensis Bergh, 1907. The second clade included Janolus hyalinus (Alder & Hancock, 1854), Bonisa nakaza, Janolus mirabilis Baba & Abe, 1970; J. anulatus Camacho-García & Gosliner, 2006, J. comis Er. Marcus, 1955, and also a well-supported clade, which includes the recently described J. savinkini and the here described J. tricellariodes sp. nov., J. flavoanulatus sp. nov., and J. incrustans sp. nov. We thus refrain from synonymyzing Janolus with Bonisa until greater resolution within this clade has clarified the relationships of these taxa.
We looked closely at the anatomy of members of these clades in an attempt to integrate molecular phylogeny and morphology. It is noteworthy that most of the reproductive systems of the species (Figure 10) have distinctive anatomical features. All the species in the Janolus cristatus clade have a receptaculum seminis with an elongate duct and a bursa copulatrix. The Janolus cristatus clade forms a distinct clade. Janolus cristatus is the type species of Antiopella, originally described as Eolis cristata Delle Chiaje, 1841. Based on this study, we suggest that Antiopella be reinstated for this clade that includes species with smooth cerata, digestive gland duct thin and extending to the end of the cerata as well as the reproductive system having a receptaculum seminis with a long duct and distinct bursa copulatrix. Also, the cerata are tipped with opaque white or yellow. Certainly, the denticulation of the jaws varies within Antiopella, as does the presence or absence of anal glands (Gosliner, 1981, 1982). It appears that Janolus gelidus Millen, 2016; should also be placed in Antiopella. Yet, the reproductive systems of the species included in the second clade are more variable in their configuration: J. hyalinus has only a receptaculum seminis with an elongate duct, J. comis has a tubular, serial receptaculum seminis (Gosliner, 1981), and no bursa copulatrix, J. anulatus (not shown) has a serial receptaculum seminis and a bursa copulatrix, Bonisa has a basal receptaculum seminis and a bursa copulatrix, and J. savinkini as well as all the new species have a stalked semi-serial receptaculum seminis and a small but distinct bursa copulatrix (Gosliner, 1981). Also, it is remarkable that the species included in the A. cristata clade have smooth cerata and single digestive diverticulum within cerata, which may divide (Figure 10).
Gosliner (1981: 28–29) reviewed the anatomy of various species of Janolus and discussed the characters proposed by Pruvot-Fol (1954) to separate them: the denticulate (Antiopella) or smooth masticatory border of the jaw (Janolus), the smooth versus papillate cerata, the branched instead of undivided ducts of the digestive glands within the cerata, as well as his own observations of the division of the genital ducts into a diaulic or triaulic configuration (Gosliner, 1981). Gosliner decided there was not enough information to clearly separate Janolus Bergh, 1884 from Antiopella Hoyle, 1902; and thus, he regarded Antiopella as a junior synonym of Janolus, on the basis of priority (Gosliner, 1981). Since then, the following species have been described: Janolus anulatus Camacho-García & Gosliner, 2006; J. chilensis Fischer, Cervera, & Ortea, 1997; J. costacubensis Ortea and Espinosa, 2000; J. eximius Miller & Willan, 1986; J. faustoi Ortea & Llera, 1988; J. gelidus Millen, 2016; J. ignus Miller & Willan, 1986; J. kinoi Edmunds & Carmona, 2017; J. mokohinau Miller & Willan, 1986; J. rebeccae Schrödl, 1996 and J. savinkini Martynov & Korshunova, 2012. Subsequently, J. costacubensis has been regarded as a synonym of J. comis Marcus, 1955. Yet, there has not been any molecular analysis on this family.
In our analyses, Janolus is paraphyletic because Bonisa is nested within the clade that includes Janolus. Most of the species in this clade have tuberculate or papillate cerata. Also, there are some of these species that are from the Atlantic and eastern Pacific, while the remaining species are from the Indo-Pacific. In addition, most of the species in this second clade lack any denticles on the radular teeth. But, at this stage, several species from the Indo-Pacific, temperate Australia and New Zealand have not been studied by genetic methods. These include the type species of Janolus as well as members of the genera Galeojanolus and Proctonotus. Thus, it would be premature to revise any of the remaining genera of Proctonotidae until a more complete picture of their relationships can be undertaken. It is interesting to note that within the Bonisa/Janolus clade there are some apparent differences. In the clade that includes the Indo-Pacific species, J. incrustans, J. savinkini, J. tricellariodies, and J. flavoanulatus, all have a proximal receptaculum seminis that is serial and a distal bursa copulatrix near the gonopore, whereas the Atlantic and eastern species, J. comis, J. hyalinus, J. anulatus, and Bonisa nakaza all have a receptaculum seminis situated near the gonopore and only B. nakaza also has a distal bursa copulatrix. The Indo-Pacific Janolus mirabilis, which is sister to the other members of this clade, has only a proximal receptaculum seminis.
The species delimitation analyses generally have not proven to be problematic within the Madrellidae and Janolidae. In two instances, one within Antiopella and the other in Janolus, distinct species suggested by the ABGD analysis cannot be recognized morphologically. In Antiopella longidentata, one specimen was found to be about 4.5% different in its COI gene from the other specimens studied here. All the species delimitation analyses considered this specimen distinct from the other A. longidentata. Yet, there were no discernable morphological differences observed that permitted its differentiation from the other specimens of A. longidentata and we prefer to retain this as the same species until additional specimens can be studied.
The same situation was found in the specimens of Janolus flavoanulatus examined here. In this instance, three specimens were found to be about 4.5% different from the other three specimens sequenced. Similarly, no morphological differences were observed between these individuals. In both A. longidentata and J. flavoanulatus, the individuals that were genetically distinct were all found in close geographical proximity to other members of the same species (generally within 5 km of each other). In addition to conducting phylogenetic analyses and species delimitation, it is advised to formally name and describe cryptic and pseudo-cryptic species, so they are further taken into consideration in subsequent biological investigations or biodiversity projects (Nygren, 2014). Yet, in the absence of morphological and ecological differential features, we prefer to consider all members of these two species as members of that nominal species until more studies can be conducted.
Based on the taxonomic changes made here, we include the following diagnoses for the genera included in the revised Janolidae:
5.1 Janolidae Pruvot-Fol, 1933
Body containing many smooth or tuberculate cerata, with or without branches of digestive gland. Rhinophores perfoliate. Caruncle present between rhinophores. Head broad with short oral tentacles. Anus dorsal, with or without glands. Jaws massive with or without denticles. Radula broad. Rachidian tooth denticulate or smooth. Lateral teeth denticulate or smooth. Reproductive system androdiaulic or triaulic with or without receptaculum seminis and bursa copulatrix. Receptaculum seminis, when present either serial or semi-serial. Penis simple or wrinkled.
5.2 Antiopella Hoyle, 1902
Body containing smooth cerata with branches of the digestive gland extending to tips of cerata. Digestive branches unbranched or branching near apex. Rhinophores perfoliate. Caruncle present. Head broad with short oral tentacles. Anus dorsal, with or without glands. Jaws massive with or without denticles. Radula broad. Rachidian tooth denticulate or smooth. Lateral teeth smooth. Reproductive system androdiaulic with elongate receptaculum seminis and small basal bursa copulatrix. Receptaculum seminis elongate semi-serial. Penis simple, conical.
Type species: Antiopella cristata (Delle Chiaje, 1841; by typification of replaced name).
5.3 Bonisa Gosliner, 1981
Body containing many smooth cerata, lacking branches of digestive gland in cerata. Digestive gland surrounding stomach and visceral mass. Rhinophores perfoliate. Caruncle present between rhinophores. Head broad with short oral tentacles. Anus dorsal, without glands. Jaws massive without denticles. Radula broad. Rachidian and lateral teeth with elongate denticles. Reproductive system androdiaulic. Large receptaculum seminis and smaller bursa copulatrix proximal to genital opening. Receptaculum seminis semi-serial. Penis club-shaped, wrinkled.
Type species: Bonisa nakaza Gosliner, 1981 (by original designation).
5.4 Galeojanolus Miller, 1971
Body containing many smooth or tuberculate cerata, with short branches of digestive gland. Digestive gland branching basally in cerata. Rhinophores perfoliate. Caruncle present between rhinophores. Head broad with short oral tentacles. Anus dorsal, without glands. Jaws massive without denticles. Radula broad. Rachidian and lateral teeth with elongate denticles. Reproductive system androdiaulic with serial receptaculum seminis and basal bursa copulatrix. Penis simple, conical.
Type species: Galeojanolus ionnae Miller, 1971 (by original designation).
5.5 Janolus Bergh, 1884
Body containing many smooth or tuberculate cerata, with branches of digestive gland. Digestive gland branching with cerata, usually near base. Rhinophores perfoliate. Caruncle present between rhinophores. Head broad with short oral tentacles. Anus dorsal, without glands. Jaws massive without denticles. Radula broad. Rachidian and lateral teeth smooth. Reproductive system androdiaulic or triaulic with or without receptaculum seminis and bursa copulatrix. Receptaculum seminis, when present serial. Penis simple, conical or wrinkled. Currently, appears polyphyletic in present analysis.
Type species: Janolus australis Bergh, 1884 (type by monotypy).
A strong biogeographical component can also be found in the taxa included in the phylogenetic tree (Figure 10). In the first clade, all species included are from the Atlantic (including the Mediterranean Sea) and the eastern Pacific (Ballesteros, Madrenas, & Pontes, 2012; Behrens, 1991; Camacho-García, Gosliner, & Valdés, 2005; Cervera et al., 2004; Goddard & Foster, 2002; Gosliner, 1981, 1982). Yet, the biogeography of the second clade is complex: J. hyalinus, B. nakaza, and J. comis are also from the Atlantic Pacific (Gosliner, 1981; Pontes, Ballesteros, & Madrenas, 2012; Rosenberg, Moretzsohn, & García, 2009) and J. anulatus from the eastern Pacific (Camacho-García et al., 2005), and J. mirabilis and the remaining species are all from the Indo-Pacific (Gosliner et al., 2015). Janolus australis, the type species of Janolus, is also known from the Indo-Pacific. What is evident from this study is that Atlantic and eastern Pacific janolids have at least two separate origins, based on the facts that both regions have members from the two major clades of Janolidae.
ACKNOWLEDGEMENTS
This research was also supported by a grant from National Science Foundation: DEB 12576304 grant to Terrence Gosliner, Richard Mooi, Luis Rocha, and Gary Williams. This collaborative research involved the following partners in the Philippines: former Secretary of Agriculture Proceso J. Alcala; former Philippine Consul General Marciano Paynor and the Consular staff in San Francisco; former Bureau of Fisheries and Aquatic Resources (BFAR) Director Attorney Asis G. Perez; BFAR colleagues, especially Attorney Analiza Vitug, Ludivina Labe; National Fisheries and Research Development Institute (NFRDI) colleagues, especially Director Drusila Bayate and November Romena; US Embassy staff, especially Heath Bailey, Richard Bakewell, and Maria Theresa N. Villa; staff of the Department of Foreign Affairs; University of the Philippines (UP) administrators and colleagues including UP President Alfredo Pascual, Vice President Giselle Concepción, Dr. Annette Meñez; the staff of the National Museum of the Philippines, especially Dr. Jeremy Barns, Anna Labrador, and Marivene Manuel Santos. We also thank Jessie de los Reyes, Marites Pastorfide, Sol Solleza, Boy Venus, Joy Napeñas, Peri Paleracio, Alexis Principe, the staff of Atlantis Dive Resort Puerto Galera (especially Gordon Strahan, Andy Pope, Marco Inocencio, Stephen Lamont, P.J Aristorenas), the staff of Lago de Oro Beach Club and Protacio Guest House, May Pagsinohin, Susan Po-Rufino, Ipat Luna, Enrique Nuñez, Jen Edrial, Anne Hazel Javier, Jay-o Castilla, Arvel Malubag, and Mary Lou Salcedo. Lastly, our sincere thanks are extended to our fellow Academy and Filipino teammates on the expeditions as well as two anonymous reviewers who have helped greatly improve the manuscript. All the specimens from the Philippines were collected under our Gratuitous Permits (GP-0077-14, GP-0085-15) from the shallow waters of the municipalities of Mabini, Tingloy, Calatagan, and Puerto Galera. This is part of the joint Department of Agriculture—NFRDI-California Academy of Sciences Memorandum of Agreement for the ongoing implementation of the National Science Foundation—funded biodiversity expedition in the Verde Island Passage. The specimens were collected in accordance with the terms and conditions of the gratuitous permit and under the supervision of our partners from BFAR Fisheries Regulatory and Quarantine Division and NFRDI. This work was also supported by the Hope for Reefs initiative of the California Academy of Sciences, and the support of numerous donors to this initiative is greatly appreciated.




