Speciation of silverside Chirostoma attenuatum (Pisces: Atheriniformes) in Central Mexico
Abstract
The high speciation rate of Chirostoma in Central Mexico has been associated with allopatric speciation events promoted by the emergence of vicariant barriers in freshwater habitats, as well as by sympatric ecological segregation, common in those species inhabiting lacustrine ecosystems. Through nuclear and mitochondrial markers, this study revealed a speciation process within Chirostoma attenuatum resulting in two evolutionary independent units that coincide with their morphological differentiation, indicating that Chirostoma attenuatum and Chirostoma zirahuen may be considered separate species. This process was the result of vicariance associated with geological dynamics of the region. Phylogeographic findings indicated two speciation stages: early allopatric isolation, during which the isolated populations accumulated unique adaptations, and secondary contact with low migration rate and the maintenance of the evolutionary trajectory. Historical demographic analysis indicated that the two well-differentiated lineages underwent independent evolutionary histories in their respective lakes. Chirostoma zirahuen from Zirahuen and C. attenuatum from Patzcuaro represent unique and irreplaceable genetic diversity that must to be conserved.
1 INTRODUCTION
Atherinopsidae include marine and freshwater lineages. Studies have revealed that the transition from marine to freshwater habitats resulted in their accelerated speciation and extinction rates, yielding a remarkable disparity in species richness between continental and marine lineages (Bloom, Weir, Piller, & Lovejoy, 2013). In Atherinopsidae that inhabit continental habitats, high species richness has been attributed to the frequency of allopatric speciation events resulting from emergence of vicariant barriers, limiting gene flow and promoting genetic divergence (Barbour, 1973; Bloom et al., 2013; Echelle & Echelle, 1984; García-Martínez, Mejia, García -De León, & Barriga-Sosa, 2015; Miller, Minckley & Norris, 2005). For fish species distributed throughout Central Mexico, allopatric events related to geological activity in the Trans-Mexican Volcanic Belt (TMVB), together with the climate history, are regarded as the main source of diversification (Beltrán-López et al., 2017; Domínguez-Domínguez, Doadrio, & Pérez-Ponce de León, 2006; Pérez-Rodríguez, Domínguez-Domínguez, Doadrio, Cuevas-Gacía, & Pérez-Ponce de León, 2015). Diversification of the genus Chirostoma Swainson 1839 from Central Mexico has been associated with such allopatric speciation events as well as with sympatric ecological segregation, particularly in the species inhabiting lacustrine ecosystems (Barbour, 1973; Barbour & Chernoff, 1984; Echelle & Echelle, 1984).
The continuous activity of the TMVB from the Miocene to the present has been responsible for changes in the physiographic configuration of the region, including the formation and destruction of lake basins in the most important lacustrine region in Central Mexico (Ferrari, Orozco-Esquivel, Maena, & Maena, 2011; Gómez-Tuena, Orozco-Esquivel, & Ferrari, 2005), including the basins of Patzcuaro and Zirahuen lakes. Earlier hypotheses, based on faunistic studies, of connectivity between these lakes proposed the existence of a stream flowing from Zirahuen to Patzcuaro Lake that disappeared approximately 700,000 to 1 million years ago (Álvarez Del Villar, 1972; De Buen, 1943). Geological findings suggest a more recent stream connection between Zirahuen and Patzcuaro lakes ca. 30,000 years ago (Israde-Alcántara, Garduño-Monroy, Fisher, Pollard, & Rodríguez-Pascua, 2005). Evidence of a secondary paleoconnection, about 8,000 years ago (Garduño-Monroy et al., 2009; Israde-Alcántara et al., 2005), is supported by phylogeographic findings of the closely related goodeine species Allotoca meeki Álvarez, 1959 from Zirahuen and Allotoca diazi Meek, 1902 from Patzcuaro that suggest a recent isolation process ca. 7,000 years ago (Corona-Santiago, Doadrio, & Domínguez-Domínguez, 2015).
An important aspect in the process of speciation is the potential of migrants to adapt to new habitats and to move through new dispersion routes. Successful colonization of freshwater habitats is often associated with the emergence of key evolutionary innovations, providing the colonizing lineage with the ability to adapt to the new habitat (Seehausen & Wagner, 2014). Among the fish species inhabiting both Patzcuaro and Zirahuen lakes, only the silversides of the genus Chirostoma are restricted to lacustrine habitats (Barbour, 1973; Bloom et al., 2013), indicating a lake-adapted natural history of the species. Sympatric ecological radiation has been proposed as the major factor in the radiation of the silversides inhabiting large lacustrine ecosystems in Central Mexico, such as Chapala and Patzcuaro lakes (Barbour & Chernoff, 1984; Echelle & Echelle, 1974).
In the case of slender silverside Chirostoma attenuatum, two subspecies have been recognized, Chirostoma attenuatum attenuatum Meek 1902 and Chirostoma attenuatum zirahuen Meek 1902, endemics from Patzcuaro Lake and Zirahuen Lake, respectively. These subspecies are supported based on biochemical markers, as well as considerable differences in morphological and meristic characteristics (Barbour, 1973; Barriga-Sosa, 2001). Based on it, we present a phylogeographic study of populations of C. attenuatum using mitochondrial and nuclear markers to (i) test the independence of lineage evolution of the population and determine whether C. a. attenuatum from Patzcuaro and C. a. zirahuen from Zirahuen could be identified as independent evolutionary significant units and valid species; (ii) reveal the biogeographic processes implicated in the evolutionary history of C. attenuatum; and (iii) elucidate the demographic history and its link with climate events.
2 MATERIALS AND METHODS
2.1 Fish sampling
Twenty-five specimens of C. attenuatum attenuatum from Patzcuaro and 26 C. attenuatum zirahuen from Zirahuen were obtained with the help of local fishermen (Table 1). A fragment of pectoral fin was removed from each specimen and stored in a microtube in 100% ethanol. The fish were fixed in 5% formalin, preserved in 70% ethanol, identified following Miller et al. (2005) and Barbour (1973), and deposited in the ichthyological collection of the Universidad Michoacana de San Nicolas de Hidalgo (CPUM) (Table 1).
| Species | Tissue collection | Locality | Basin | GB cytb | GB S7 | Fish collection |
|---|---|---|---|---|---|---|
| C. attenuatum | 10623 | Uranden | Patzcuaro | MG592205 | MG98478 | No voucher |
| C. attenuatum | 9720 | Ukasanatacua | Patzcuaro | MG592206 | MG98476 | No voucher |
| C. attenuatum | 34245 | Uranden | Patzcuaro | MG592207 | MG98480 | No voucher |
| C. attenuatum | 34244 | Uranden | Patzcuaro | MG592208 | MG98479 | No voucher |
| C. attenuatum | 10618 | Uranden | Patzcuaro | MG592209 | – | No voucher |
| C. attenuatum | 10621 | Uranden | Patzcuaro | MG592210 | MG98477 | No voucher |
| C. attenuatum | 35209 | Uranden | Patzcuaro | – | MG98481 | 14104 |
| C. attenuatum | 35212 | La Pacanda | Patzcuaro | MG592211 | MG98482 | 14104 |
| C. attenuatum | 35214 | La Pacanda | Patzcuaro | MG592212 | MG98483 | 14104 |
| C. attenuatum | 35217 | La Pacanda | Patzcuaro | MG592213 | – | 14104 |
| C. attenuatum | 35260 | La Pacanda | Patzcuaro | MG592214 | – | 14104 |
| C. attenuatum | 35254 | La Pacanda | Patzcuaro | – | MG98484 | 14104 |
| C. attenuatum | 35257 | La Pacanda | Patzcuaro | MG592215 | MG98485 | 14104 |
| C. attenuatum | 35269 | La Pacanda | Patzcuaro | MG592216 | MG98486 | 14104 |
| C. attenuatum | 35270 | La Pacanda | Patzcuaro | MG592217 | – | 14104 |
| C. attenuatum | 45322 | La Pacanda | Patzcuaro | MG592218 | – | 14104 |
| C. attenuatum | 45323 | La Pacanda | Patzcuaro | MG592219 | – | 14104 |
| C. attenuatum | 45324 | La Pacanda | Patzcuaro | MG592220 | – | 14104 |
| C. attenuatum | 45325 | La Pacanda | Patzcuaro | MG592221 | – | 14104 |
| C. attenuatum | 45326 | La Pacanda | Patzcuaro | MG592222 | – | 14104 |
| C. attenuatum | 45328 | La Pacanda | Patzcuaro | MG592223 | – | 14104 |
| C. attenuatum | 45329 | La Pacanda | Patzcuaro | MG592224 | – | 14104 |
| C. attenuatum | 45330 | La Pacanda | Patzcuaro | MG592225 | – | 14104 |
| C. attenuatum | 45333 | La Pacanda | Patzcuaro | MG592226 | – | 14104 |
| C. attenuatum | 45334 | La Pacanda | Patzcuaro | MG592227 | – | 14104 |
| C. attenuatum | 35519 | Zirahuen | Zirahuen | MG592228 | MG598465 | 14103 |
| C. attenuatum | 35512 | Zirahuen | Zirahuen | MG592229 | – | 14103 |
| C. attenuatum | 35514 | Zirahuen | Zirahuen | MG592230 | MG598463 | 14103 |
| C. attenuatum | 35515 | Zirahuen | Zirahuen | MG592231 | – | 14103 |
| C. attenuatum | 35516 | Zirahuen | Zirahuen | MG592232 | – | 14103 |
| C. attenuatum | 35517 | Zirahuen | Zirahuen | MG592233 | MG598464 | 14103 |
| C. attenuatum | 35518 | Zirahuen | Zirahuen | MG592234 | MG598462 | 14103 |
| C. attenuatum | 35520 | Zirahuen | Zirahuen | – | MG598466 | 14103 |
| C. attenuatum | 35523 | Zirahuen | Zirahuen | MG592235 | – | 14103 |
| C. attenuatum | 35524 | Zirahuen | Zirahuen | MG592236 | MG598467 | 14103 |
| C. attenuatum | 35525 | Zirahuen | Zirahuen | MG592237 | MG598468 | 14103 |
| C. attenuatum | 35527 | Zirahuen | Zirahuen | MG592238 | – | 14103 |
| C. attenuatum | 35529 | Zirahuen | Zirahuen | MG592239 | MG598469 | 14103 |
| C. attenuatum | 35531 | Zirahuen | Zirahuen | MG592240 | – | 14103 |
| C. attenuatum | 35532 | Zirahuen | Zirahuen | MG592241 | MG598470 | 14103 |
| C. attenuatum | 35533 | Zirahuen | Zirahuen | MG592242 | – | 14103 |
| C. attenuatum | 35534 | Zirahuen | Zirahuen | MG592243 | – | 14103 |
| C. attenuatum | 35535 | Zirahuen | Zirahuen | MG592244 | MG598471 | 14103 |
| C. attenuatum | 35536 | Zirahuen | Zirahuen | MG592245 | – | 14103 |
| C. attenuatum | 35537 | Zirahuen | Zirahuen | MG592246 | – | 14103 |
| C. attenuatum | 35538 | Zirahuen | Zirahuen | MG592247 | – | 14103 |
| C. attenuatum | 35539 | Zirahuen | Zirahuen | MG592248 | MG598472 | 14103 |
| C. attenuatum | 35540 | Zirahuen | Zirahuen | MG592249 | MG598473 | 14103 |
| C. attenuatum | 35541 | Zirahuen | Zirahuen | MG592250 | – | 14103 |
| C. attenuatum | 35543 | Zirahuen | Zirahuen | MG592251 | MG598474 | 14103 |
| C. attenuatum | 35546 | Zirahuen | Zirahuen | – | MG598475 | 14103 |
| C. attenuatum | nd | Patzcuaro | Patzcuaro | KM400699 | – | SLU nd |
| C. attenuatum | nd | Patzcuaro | Patzcuaro | KC736405 | – | SLU 5036a |
| C. attenuatum | nd | Zirahuen | Zirahuen | KC736404 | – | SLU 5036a |
| C. estor | 3320 | Patzcuaro | Patzcuaro | – | MG747647 | No voucher |
| C. estor | nd | Patzcuaro | Patzcuaro | KC736403 | – | SLU 5114a |
| C. grandocule | nd | Patzcuaro | Patzcuaro | KC736369 | – | SLU 5118a |
| C. patzcuaro | nd | Patzcuaro | Patzcuaro | JQ282029 | – | SLU 5117a |
| C. humboldtianum | nd | Zacapu | Zacapu | JQ282026 | – | SLU 5117a |
| C. sphyraena | nd | Chapala | Chapala | KC736400 | – | SLU 5025a |
| C. jordani | 5090 | Cajititlán | Chapala | – | MG747648 | No voucher |
| C. jordani | nd | Chapala | Chapala | KC736407 | – | SLU 5033a |
- nd, no data (sequence obtained of GenBank database); GB, GenBank accession number; –, no sequence; SLU, code voucher of each sequence retrieved from GenBank (see Bloom et al., 2013).
- Collection numbers of tissues and specimens deposited in Tissue bank and/or Fish Collection of the Universidad Michoacana de San Nicolas de Hidalgo (CPUM) are indicated in columns 2 and 7, respectively.
- a Data were obtained of GenBank database.
2.2 DNA sequences
DNA extraction was carried out using the proteinase K and phenol–chloroform protocol (Sambrook, Fritsch, & Maniatis, 1989). The mitochondrial cytochrome b gene (cytb) and the first intron of S7 ribosomal protein gene (S7) were amplified. The PCR consisted of a 25 μl volume reaction with a final concentration of 50–100 ng DNA, 1× buffer, 1.5 mM MgCl2, 2.5 mM dNTP mix (mM 10), 0.25 μM of each primer, and 1 unit of Taq DNA polymerase (Invitrogen).
For the cytb gene (length 1140 bp), the primers were Glud-G (5′-TGACTTGAARAACCAYCGTTG-3′; Palumbi et al., 1991) and H16460 (5′-CGAYCTTCGGATTAACAAGACCG-3′; Perdices, Bermingham, Montilla, & Doadrio, 2002). Amplification was performed with the following conditions: denaturation at 94°C for 45 s, annealing at 48°C for 1 min, extension at 72°C for 90 s, and a final extension at 72°C for 5 min. For the S7, the primers were S7RPEX1F (5′-TGGCCTCTTCCTTGGCCGTC-3′) and S7RPEX2R (5′-AACTCGTCTGGCTTTTCGCC-3′) described for Chow and Hazama (1998). The amplification was performed using denaturation at 94°C for 30 s, annealing at 56°C for 45 s, extension at 72°C for 2 min, and a final extension at 72°C for 7 min. PCR products were submitted to the sequencing service at the High-throughput Genomics Center, Washington University, Seattle, WA, USA.
2.3 Sequence analyses
Sequences obtained in both directions were edited using MEGA v5 (Tamura et al., 2011), and the alignment of cytb was conducted manually; additionally, two sequences of C. attenuatum from Patzcuaro Lake and one sequence of Zirahuen Lake were included (for GenBank accession numbers, see Table 1). For the S7 sequences, the phase of heterozygous single nucleotide polymorphisms (SNPs) was resolved using DnaSP v. 5.10 (Librado & Rozas, 2009) and performed with the algorithm provided by PHASE 2.0 (Stephens, Smith, & Donnelly, 2001) with default parameters. Posterior sequence alignment was carried out using Clustal_X 1.83 (Thompson, Gibson, Plewniak, Jeanmougin, & Higgins, 1997) and revised manually. To test the monophyly of C. attenuatum samples, the following closely related species (sensu Bloom, Piller, Lyons, Mercado-Silva, & Medina-Nava, 2009) were included as ingroup (Table 1): Chirostoma estor Jordan 1897, Chirostoma grandocule, Steindeachner 1894, and Chirostoma patzcuaro Meek 1902 from Patzcuaro Lake; Chirostoma humboldtianum Valenciennes 1835 from Zacapu Lake; and Chirostoma sphyraena Boulenger 1900 from Chapala Lake. Chirostoma jordani Woolman 1984 from Chapala were used as outgroup in phylogenetic assays (Bloom et al., 2009, 2013). Alignment for each locus was used to estimate and select the substitution model that best fit the datasets using jModelTest v. 1.7 (Posada, 2008) considering the Akaike information criterion (AIC).
2.4 Phylogenetic, species trees and haplotype network
Phylogenetic trees were constructed for the cytb and S7 genes based on maximum likelihood (ML) and Bayesian inference (BI). The ML was run in RAxML (Silvestro & Michalak, 2012; Stamatakis, Hoover, & Rougemont, 2008). Bayesian analyses were run in MrBayes 3.2.2 (Ronquist et al., 2012). Two independent runs were implemented with four-chain MCMC and 10,000,000 generations, sampling every 100 generations. Chain convergence was verified by suitable effective sample size for all parameters in Tracer 1.5 (Rambaut & Drummond, 2007), discarding 10% of generations as burn-in.
To deal with incomplete lineage sorting, the common source of discrepancy between mtDNA and nDNA (Tows & Brelsford, 2012), especially in recently radiated lineages (Niemiller, Near, & Fitzpatrick, 2012), a species tree under a multispecies coalescent model was created using *BEAST v. 2.0 (Bouckeart et al., 2014; Heled & Drummond, 2010) through the CIPRES Science Gateway v. 3.3 (Miller et al., 2015). Patzcuaro and Zirahuen populations were designated as distinct groups. The best fit models of nucleotide evolution for cytb (TN93+G) and the S7 intron (GTR+G) were used with four gamma categories. Due to the lack of fossil data for Chirostoma, a lognormal relaxed clock model was chosen for cytb, using the mutation rate of 1% estimated for Atheriniformes (Campanella et al., 2015). For the S7, the mutation rate was estimated relative to the mitochondrial mutation rate, choosing a lognormal relaxed clock using a normal prior (0.005 ± 0.01). For species tree and population size models, a Yule model species tree prior and a constant species tree population size were chosen. Two independent MCMC runs were performed for 30,000,000 generations sampling every 1,000 generations. Chain convergence was assessed by visualizing the sampled parameter values in Tracer. Runs were pooled using LogCombiner in the BEAST 2 package, with 10% of the generations discarded as burn-in. The maximum clade credibility species tree was created by TreeAnnotator in the BEAST 2 package.
We constructed a haplotype network to describe the intraspecific relationships of C. attenuatum in HaploView v. 4.2 (Barrett, Fry, Maller, & Daly, 2005).
2.5 Divergence times
To estimate the time of the cladogenetic event separating the Patzcuaro and Zirahuen populations, divergence times of C. a. attenuatum and C. a. zirahuen based on the cytb and S7 were obtained. The mutation rate of 1% estimated for Atheriniformes (Campanella et al., 2015) was used for cytb; for S7, the mutation rate was estimated relative to the mitochondrial mutation rate, using the normal prior (0.05 ± 0.01). The best fit models of nucleotide evolution for cytb (TN93+G) and the S7 (GTR+G) were used with four gamma categories. We used a prior coalescent model of Bayesian skyline plot. The assay was performed with BEAST 2 implemented on the webserver CIPRES Science Gateway v. 3.3 (Miller et al., 2015). Two independent runs of 50,000,000 generations, sampling every 500 generations, were implemented with 10% discarded as burn-in.
2.6 Genetic diversity and structure
A conventional diversity index was obtained for both genes. Haplotype diversity (h), nucleotide diversity (π), and segregating sites (S) were calculated using DnaSP v5. Differences between C. a. attenuatum and C. a. zirahuen were calculated by the uncorrected genetic distances (Dp) with 1,000 bootstrap replicates, using MEGA v5. To analyze the structure of populations, an analysis of molecular variance (AMOVA; Excoffier, Smouse, & Quattro, 1992) was performed, with significance levels set at α = .05 and 10,000 random permutations, implemented in ARLEQUIN 3.5.1.2 (Excoffier & Lischer, 2010). The degree of genetic variation between C. a. attenuatum and C. a. zirahuen was calculated using the fixation index Φst. The assay was run in ARLEQUIN with a significance level of α = .05 and 10,000 random permutations.
2.7 Historical demography and gene flow
To detect signatures of demographic changes in the two populations, Tajima's D (Tajima, 1989) and Fu's Fs (Fu, 1997; Fu & Li, 1993) were implemented in ARLEQUIN 3.5.1.2 (Excoffier & Lischer, 2010) with significance levels set at α = .05 and 10,000 random permutations. To infer population size through time, we created Bayesian skyline plots (Drummond, Rambaut, Shapiro, & Pybus, 2005) in BEAST 2 for the subspecies based on the cytb gene. This coalescent-based method provided a robust framework to infer population dynamics through time and to deduce historical change in populations from DNA sequences. The molecular clock was calibrated with a mutation rate of 1% estimated for atherinopsids (Campanella et al., 2015). The assay was conducted in BEAST, implemented on the webserver CIPRES Science Gateway v. 3.3 (Miller et al., 2015) for 30,000,000 generations, sampling every 1,000 generations. The Bayesian reconstruction of each population was performed in Tracer v. 4.
To estimate the level and direction of historical gene flow between populations from Patzcuaro and Zirahuen lakes, migration rates were computed using MIGRATE-N 3.6 (Berreli, 2009). MCMC simulations were performed as follows: one long chain and 12 short parallel chains with initial temperatures of 1.0, 1.5, 3.0, and 100,000.0 and a static heating scheme. Final MCMC searches used 100,000 steps in 50-step increments, discarding the first 20,000 as burn-in. Initial uniform priors were Θ (0.0–0.1) and M (0.0–25,000.0).
3 RESULTS
3.1 Sequence data
Twenty-five sequences of the mitochondrial cytb gene (length of alignment 1,121 bp; Dataset S1) of C. a. attenuatum and 25 of C. a. zirahuen, along with 11 sequences of S7 (length of alignment of 671 bp; Dataset S2) of C. a. attenuatum and 14 of C. a. zirahuen, were included in the analyses (Table 1). For S7, all heterozygous sites were successfully resolved for SNP variation (phase threshold value >85%), resulting in 50 genotypes, 22 for C. a. attenuatum and 28 for C. a. zirahuen. Obtained sequences were deposited in GenBank (Table 1). The best fit evolutionary models for the cytb and S7 datasets were TrN+G and GTR+I+G, respectively.
3.2 Phylogenetic relationships and species trees
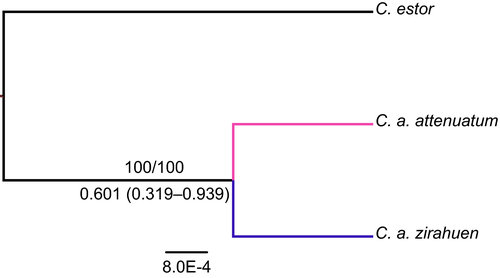
The phylogenetic trees based on the cytb gene and S7 recovered C. attenuatum as a monophyletic assemblage with both BI and ML. For the tree based on the cytb, BI and ML produced two highly supported well-differentiated clades, one corresponding to specimens collected in Patzcuaro Lake and the other comprising the specimens from Zirahuen Lake (Figure S1). In contrast, the tree based on the S7 intron, although supporting the monophyly of C. attenuatum, showed unresolved polytomy in within-species relationships with both BI and ML (Figure S2). The species tree based on the two loci showed two well-supported lineages (Figure 1) as the a priori assignments of specimens. The time of separation of the lineages was estimated at 600,000 years ago (HPD 95% 319,000–939,000; Figure 1).
3.3 Haplotype network
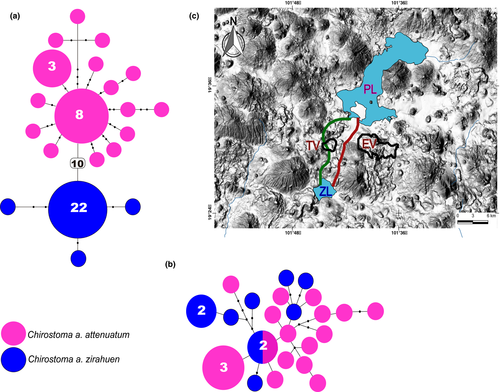
The cytb haplotype network showed well-differentiated haplogroups corresponding to C. a. attenuatum and C. a. zirahuen, separated by 10 mutation steps. The Patzcuaro population showed a network with a central haplotype (frequency = 7) and several peripheral unique haplotypes. The Zirahuen population showed one haplotype with high frequency (n = 22) and three unique peripheral haplotypes (Figure 2A). The S7 haplotype network displayed separation of the C. a. attenuatum haplotypes from C. a. zirahuen, with only one of the central haplotype shared between subspecies. Fifteen unique haplotypes were found for the Patzcuaro population and seven for the Zirahuen population.
3.4 Genetic diversity
For the mitochondrial cytb gene, the complete C. attenuatum dataset revealed high haplotype diversity (0.785) and low nucleotide diversity (0.00619), with 35 segregating sites. Chirostoma a. attenuatum showed high haplotype diversity (0.8933) and low nucleotide diversity (0.00210) with 21 segregating sites, whereas C. a. zirahuen exhibited low haplotype diversity (0.18), low nucleotide diversity (0.00025), and three segregating sites (Table 2).
| n | nH | π | h | S | DT | FS | |
|---|---|---|---|---|---|---|---|
| Mitochondrial (cytb) | |||||||
| C. a. attenuatum | 25 | 16 | 0.00210 | 0.8933 | 21 | −2.22923a | −10.06747a |
| C. a. zirahuen | 25 | 4 | 0.00025 | 0.18 | 3 | −1.82093a | −0.06249 |
| Nuclear (S7) | |||||||
| C. a. attenuatum | 11 | 20 | 0.00698 | 0.987 | 18 | −0.19231 | −16.47126a |
| C. a. zirahuen | 14 | 24 | 0.00852 | 0.989 | 21 | 0.22923 | −17.59861a |
- nH, number of haplotypes; π, nucleotide diversity; h, haplotype diversity; S, polymorphic sites; DT, Tajima's D; FS, Fu's Fs
- a Significance value p < .05
For S7, a similar diversity pattern was obtained. The complete dataset of C. attenuatum showed high haplotype diversity (0.9935) and low nucleotide diversity (0.00882) with 29 segregating sites. Chirostoma a. attenuatum presented high haplotype diversity (0.987) and low nucleotide diversity (0.00698) with 18 segregating sites, whereas C. a. zirahuen showed high haplotype diversity (0.989) and low nucleotide diversity (0.00852) with 21 segregating sites (Table 2).
3.5 Genetic differentiation and structure
For cytb, the mean genetic distance between C. a. attenuatum and C. a. zirahuen was calculated as 1.1%. For S7, it was 0.4%. The AMOVA showed significant values for both markers. While for the cytb marker (cytb = 0.88, α = .000), most variation was observed between populations, for the S7 intron, the primary source of variation was within-population, with lower, but significant, between-population variation (S7 intron = 0.01, α = .023; Table S1).
3.6 Historical demography and gene flow
In the neutrality tests using the cytb marker, Tajima's D and Fu's Fs showed significant negative values, −2.22923 and −10.06747, respectively, for C. a. attenuatum. Chirostoma a. zirahuen showed significant negative Tajima's D value (-1.82093) and a negative, non-significant, Fu's Fs value (-0.06249). For S7 in C. a. attenuatum, Tajima's D was negative but not significant (-0.19231), and a significant negative Fu's Fs value (-17.59861) was obtained (Table 2).
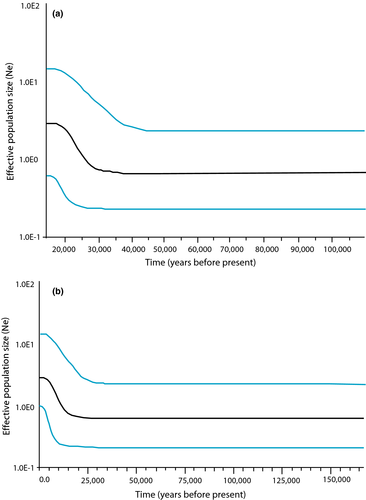
The Bayesian skyline plot indicated expansion of C. a. attenuatum ca. 25,000 years ago (Figure 3A) and of C. a. zirahuen ca. 15,000 years ago (Figure 3B). Migration rates for cytb were lower (mZirahuen-Patzcuaro = 0.01 and mPatzcuaro-Zirahuen = 0.012) than those for S7 (mZirahuen-Patzcuaro = 0.13 and mPatzcuaro-Zirahuen = 0.06); while cytb indicates symmetry in magnitude and direction between the two populations, the rates recorded for S7 show an asymmetrical rate from Zirahuen to Patzcuaro.
4 DISCUSSION
4.1 Chirostoma a. attenuatum and C. a. zirahuen, incipient or complete speciation?
Results show significant structure in the paired Φst and AMOVA (Table S1), as well as genetic distance between C. a. attenuatum and C. a. zirahuen (cytb = 1.1% and S7 = 0.4%). The genetic distance is relatively high for population level compared with the cyprinid Algansea lacustris endemic to Patzcuaro Lake, which presents a divergence of 1.5% from A. tincella, its sister species inhabiting other areas (Pérez-Rodríguez, Domínguez-Domínguez, Pérez Ponce de León, & Doadrio, 2009), and the 0.03% found between the Goodeinae Allotoca meeki endemic to Zirahuen and its sister species A. diazi endemic to Patzcuaro Lake (Corona-Santiago et al., 2015), suggesting isolation of the ancestors of the subspecies in their respective lakes. In contrast, whereas the cytb showed reciprocal monophyly at the subspecies level, S7 demonstrated unresolved relationships and a mixture of haplotypes in the genetic tree and haplotype network (Figure 2A and B; Figures S1 and S2). Disparities between mitochondrial and nuclear trees can be associated with horizontal transfer, lineage sorting, introgression, gene duplication/extinction (Goncalves, Martínez-Solano, Ferrand, & García-París, 2007; Holland, Benthin, Lockhart, Moulton, & Huber, 2008), and the differing mutation rates in nuclear and mitochondrial loci (Egge, Nicholson, & Stark, 2015). In this case, the lack of resolution and mixed haplotypes in the S7 genetic tree reflected the combined effects of low variation and incomplete lineage sorting that is consistent with the low evolutionary rate and high coalescence time for nuclear loci (Egge et al., 2015). These variation patterns are usually demonstrated by species complexes that have undergone relatively recent divergence (Beltrán-López et al., 2017; Buj, Šanda, Marčić, Ćaleta, & Mrakovčić, 2014; Buj et al., 2015), which is consistent with the most recent common ancestor of C. a. attenuatum and C. a. zirahuen populations dated ca. 600,000 years ago (Figure 1), considered a recent isolation event compared to divergence of the Patzcuaro endemic A. lacustris dated 1.9 Ma ago.
Since the *BEAST coalescent-based approach provides the ability to model incomplete lineage sorting and ancestral polymorphism, the species tree produced strongly supported two reciprocally monophyletic lineages (Figure 1), implicating defined barriers to gene flow (Heled & Drummond, 2010). The differentiation pattern of the subspecies C. a. attenuatum from C. a. zirahuen lineages is consistent with their considerable reported morphological differentiation, including the number of lateral predorsal scales, caudal peduncle length, snout-to-first dorsal fin distance, and mouth shape (Barbour 1973; Barriga-Sosa, 2001; Meek, 1902; Miller et al., 2005). Based on the forgoing evidence, along with the geographic isolation of the lakes, we suggest that these subspecies be considered independent evolutionary units, and argue that a species level of Chirostoma attenuatum from Patzcuaro Lake and Chirostoma zirahuen from Zirahuen Lake must be recognized.
4.2 Persistence of a barrier versus ecological isolation
The date of the most recent common ancestor of C. attenuatum and C. zirahuen was estimated from 319,000 to 939,000 years ago. The most plausible biogeographic hypothesis of the early cladogenetic event separating the species is the existence of a paleoriver connecting the lakes that disappeared ~700,000 years ago (Figure 2C). A similar biogeographic hypothesis has been proposed based on taxa of freshwater fishes codistributed in Patzcuaro and Zirahuen lakes, arguing that a paleotributary connected the Lerma River with Cuitzeo, Patzcuaro, and Zirahuen lakes 700,000–1,000,000 years ago (Álvarez Del Villar, 1972; De Buen, 1943). This is supported by stratigraphic information (Israde-Alcántara, Wade, Garduño-Monroy, & Barron, 2008), limnologic data (Bradbury, 2000; Israde-Alcántara, Garduño-Monroy, & Ortega, 2002), and geological evidence (Israde-Alcántara & Garduño-Monroy, 1999). The Patzcuaro and Zirahuen lakes are located in an area of high volcanic activity known as the “Tarasco Corridor,” where more than 1,000 active volcanic cones have been recorded since the Pliocene (Corona-Santiago et al., 2015). The volcanic dynamism was produced by tectonic activity that increased in the area ~700,000 years ago (Johnson & Harrison, 1990; Luhr & Simkin, 1993), shaping the physiography of the Zirahuen and Patzcuaro basins, and corresponding to the time of the early cladogenetic event.
A hypothesis of a more recent connection between Zirahuen and Patzcuaro lakes, from 30,000 to 8,000 years ago (Garduño-Monroy et al., 2009; Israde-Alcántara et al., 2005, 2010), proposes a paleostream flowing from Zirahuen to Patzcuaro until tecto-volcanic events, such as activity in the Tazas volcano, disrupted the connection (Figure 2C). These dates are supported by the genetic flow demonstrated in the sister goodeid species A. diazi (Patzcuaro) and A. meeki (Zirahuen; Corona-Santiago et al., 2015). In this scenario, the symmetrical migration rates in mtDNA = 0.01 migrants per generation and the asymmetrical migration rates in nDNAZirahuen-Patzcuaro = 0.013 versus nDNAPatzcuaro-Zirahuen = 0.06% migrants per generation between lineages, indicating that the recent connection of the lakes allowed migration but was sufficiently low to maintain genetic differentiation, as is supported by the significant values in Φst and AMOVA, as well as the haplotype network and species trees. These results support the suggestion that the speciation process is better explained by ecological speciation than by the existence of a barrier, as has been proposed in A. meeki with respect to A. diazi, in which migration was found, but was not significant and was low enough to maintain the genetic differentiation of the species (Corona-Santiago et al., 2015).
Evolutionary, biogeographic, and phylogenetic studies of Chirostoma demonstrate that C. attenuatum is member of a clade of species restricted to lake habitats (Bloom et al., 2009, 2013). The environmental conditions of lotic and lentic systems exert unique ecological constraints on aquatic organisms (Letsch, Gottsberger, & Ware, 2016). Studies of speciation focusing on transition between streams and lakes are scarce. They include characterization of the divergence of lake and stream ecotypes in the three-spined stickleback Gasterosteus aculeatus, in which differing predation regimes, dietary resources, light conditions, and parasite communities led to substantial morphological differences (Seehausen & Wagner, 2014). For Chirostoma from Patzcuaro, the diversity of food resources, including zooplankton, fish, insects, and microcrustaceans, can be associated with interspecific morphological differences (Soria-Barreto & Paulo-Maya, 2005), suggesting food resources as a potential ecological constraint. The disparate environmental conditions of Zirahuen and Patzcuaro lakes, the former deep, monomictic, and oligotrophic (Bernal-Brook & MacCrimmon, 2000) and the latter shallow, polymictic, and eutrophic (Bernal-Brook, Gomez-Tagle, & Alcocer, 2002), may have acted to spur rapid differentiation in C. attenuatum and C. zirahuen traits during the first allopatric stage ca. 600,000 years ago, as supported by our genetic and previous morphologic evidence (Barbour 1973; Meek, 1902; Miller et al., 2005). The natural history of a species adapted to lake ecosystems suggests that a recent connection via a paleostream (Figure 2C) may have represented an environmental constraint resulting in a low dispersal rate between lakes. In this scenario, the unfavorable lotic habitat represented a barrier sufficient to maintain a low genetic flow and prevent the establishment of migrants, maintaining the genetic differentiation of C. attenuatum and C. zirahuen during a secondary drainage connection. This corresponds to a classic scenario of ecological speciation (Schluter, 2001; Seehausen & Wagner, 2014), in which reproductive isolation of two populations has its source in allopatry, during which a population accumulates adaptations to unique aspects of its environment, preventing genetic homogenization in a secondary contact (Schluter, 2001; Domínguez-Domínguez, 2008).
4.3 Demography and its conservation implications
Since the first allopatric stage 600,000 years ago, the studied lineages were exposed to different environmental conditions, as well as to different evolutionary forces and demographic processes. With respect to historical demography, the high genetic diversity and polymorphism of C. attenuatum suggests the persistence of a high effective population size (Buj et al., 2015), whereas the Fu's Fs, Tajima's D statistics, and the Bayesian skyline plot imply a population expansion dated ca. 25,000 years ago (Figure 3A), consistent with the early glacial stage (Caballero, Lozano-García, Vázquez-Selem, & Ortega, 2010). Paleoclimatic information from Patzcuaro indicates that during this period ca. 25,000 to 13,000 years ago, the basin was dominated by high humidity and precipitation. The lake was turbid with high productivity and deep with an extended shallow littoral zone, as evidenced by the abundance of fern Isoetes sp. (Bradbury, 2000; Caballero et al., 2010), facilitating the reproduction of Chirostoma and growth of larvae (Rojas-Carrillo, 2006). The deep open areas would support high populations of mature fish (García-De León, Ramírez-Herrejón, García-Ortega, & Hendrickson, 2014; Soria-Barreto & Paulo-Maya, 2005), promoting the population expansion shown by the Bayesian skyline plot for that period.
The demographic history of C. zirahuen is more complex, as indicated by the lack of consensus in the two molecular markers, with cytb showing low genetic diversity (H = 0.18; π = 0.00025) and a bottleneck effect, while the nuclear gene showed high diversity (H = 0.989; π = 0.00852) and population expansion. This inconsistency could be explained by the formation of Zirahuen Lake via damming of a stream that flowed to Patzcuaro Lake, producing a biogeographic scenario of peripheral isolation. Accordingly, the founder population was low, allowing stochastic processes, such as genetic drift and selection, to occur more slowly in nDNA than in mtDNA (Funk & Omland, 2003; Zink & Barrowclough, 2008), influencing the C. zirahuen demographic history (Lacy, 1987), as was proposed for the A. diazi complex (Corona-Santiago et al., 2015). The Bayesian skyline plot showed a population expansion ca. 15,000 years ago (Figure 3B), during the late glacial stage (Caballero et al., 2010; Clark et al., 2009). Paleoclimatic information demonstrates that 15,000 years ago, Zirahuen Lake underwent considerable increase in surface area, the littoral zone, and productivity (Caballero et al., 2010; Metcalfe, O'Hara, Caballero, & Davies, 2000; Ortega et al., 2010; Torres-Rodríguez, Lozano-García, Figueroa-Rangel, Ortega-Guerrero, & Vázquez-Castro, 2012; Vázquez, Ortega, Davies, & Aston, 2010). As with the C. attenuatum population, this paleoclimate scenario may have facilitated the population expansion of the C. zirahuen in Zirahuen Lake 15,000 years ago.
From a conservation perspective, the mitochondrial structure, the considerable morphological differentiation, and the historical isolation of the lake populations are sufficient evidence to consider the lineages as separate species, one endemic to Patzcuaro Lake (C. attenuatum) and the other endemic to Zirahuen Lake (C. zirahuen), representing a unique and irreplaceable genetic pool (Crandall, Bininda-Edmonds, Mace, & Wayne, 2000). This is especially important when considering (i) the restricted geographic range of each species, since for freshwater organisms in general (Ribera, 2008), and fishes in particular (Rosenfield, 2002), the likelihood of the extinction increases as geographic range is reduced; (ii) that Chirostoma have represented the most important fishery in Patzcuaro and Zirahuen lakes since pre-Hispanic times (Barbour, 1973; Berlanga-Robles, Luna, Nepita, & Vera, 1997; Berlanga-Robles, Madrid-Vera, & Ruiz-Luna, 2002; Hernández-Batista, Ramírez-Torrez, Azaola-Espinoza, Mayorga-Reyes, & Monroy-Dosta, 2015; Miller, 2005) and it is considered overfished (Chacón-Torres, 1993; Hernández-Batista et al., 2015; Rojas-Carrillo, 2006); and 3) that habitat degradation has been widely documented since the pre-Hispanic time (Guzmán, Polaco, & Pollard, 2001; Nichols & Pool, 2012; Williams & Weigand, 1996) and continues to the present (Lyons, González-Hernández, Soto-Galera, & Guzmán-Arroyo, 1998; Ramírez-Herrejón et al., 2014; Zambrano et al., 2001).
ACKNOWLEDGEMENTS
IBR would like to thank the Consejo Nacional de Ciencia y Tecnología (CONACYT) for granting a scholarship. We thank the Ministerio de Economía y Competitividad from Spain (CGL-2013 41375-P) and Coordinación de la Investigación Científica-UMSNH, México, for funding the project. We thank the División de estudios de Posgrado de la Universidad Michoacana de San Nicolás de Hidalgo for providing economic resources for English editing of the manuscript. We thank D.K. Santiago Corona, F. Anguiano Rodríguez, O. Valencia, B. Andrade-García, and A. González Alejo for their laboratory and field support. I.B.R. thanks Dr. I. Barriga-Sosa, Dr. I. Doadrio, and two anonymous reviewers for helpful comments.




