Molecular phylogenetics of the predatory lineage of flower flies Eupeodes-Scaeva (Diptera: Syrphidae), with the description of the Neotropical genus Austroscaeva gen. nov.
Abstract
enPhylogenetic relationships among the genera and subgenera of the Scaeva-Eupeodes clade (Diptera: Syrphidae: Syrphinae) were analyzed based on molecular characters. Sequence data from three gene regions were analyzed using maximum likelihood and Bayesian inference: the mitochondrial protein-coding gene cytochrome c oxidase subunit I (COI) and the nuclear 28S and 18S ribosomal RNA genes. The genus Paragus (single representative of the tribe Paragini) was resolved as sister group of the monophyletic Scaeva-Eupeodes lineage. Inside this clade, a Neotropical radiation of genera was well supported comprising Notosyrphus, Austroscaeva gen. nov., and Dioprosopa. For the first time, the placement of Doros, Pseudodoros, and Betasyrphus was inferred using molecular data. Our results resolved the genus Pseudodoros as sister group of Ischiodon, corroborating the generic rank of Ischiodon, Simosyrphus, Dioprosopa, and Pseudodoros. The current subgeneric division of Eupeodes with three subgenera is not supported by our data. Present results and the study of adult morphological characters prompted us to erect a new genus, Austroscaeva gen. nov., which includes four Neotropical species, that is, Austroscaeva melanostoma (Macquart, 1842) comb. nov., Austroscaeva occidentalis (Shannon, 1927) comb. nov., Austroscaeva patagoniensis (Kassebeer, 1999) comb. nov., and Austroscaeva penai (Marnef in Dušek & Láska, 1985) comb. nov. We described and characterized Austroscaeva gen. nov., and provided the description of the male of Scaeva patagoniensis (=Austroscaeva patagoniensis) and Scaeva penai (=Austroscaeva penai), as well as an illustrated identification key for the Austroscaeva species. Moreover, we explained and stated that the correct name for Syrphus lunatus Wiedemann, 1830 is Scaeva opimia (Walker, 1852).
Resumen
frFilogenia molecular del linaje de sírfidos depredadores Eupeodes-Scaeva (Diptera: Syrphidae), con la descripción del género Neotropical Austroscaeva gen. nov.
Las relaciones filogenéticas entre los géneros y subgéneros del clado Scaeva-Eupeodes (Diptera: Syrphidae: Syrphinae) se analizaron en base a caracteres moleculares. Para los análisis basados en máxima verosimilitud e inferencia bayesiana, se usaron las secuencias de tres regiones génicas: el gen mitocondrial codificador de la proteína citocromo c oxidasa subunidad I (COI) y los genes nucleares 28S y 18S de ARN ribosómico. El género Paragus (representante único de la tribu Paragini) se resolvió como un grupo hermano del linaje monofilético Scaeva-Eupeodes. Dentro de este clado, se resolvió una radiación neotropical de géneros bien soportada, incluyendo Notosyrphus, Austroscaeva gen. nov. y Dioprosopa. Por primera vez, la ubicación de Doros, Pseudodoros y Betasyrphus se infirió utilizando datos moleculares. Nuestros resultados resolvieron el género Pseudodoros como grupo hermano de Ischiodon, corroborando el rango genérico de Ischiodon, Simosyrphus, Dioprosopa y Pseudodoros. La división subgenérica actual de Eupeodes con tres subgéneros no es compatible con nuestros datos. Los resultados actuales y el estudio de los caracteres morfológicos adultos nos llevaron a erigir un nuevo género, Austroscaeva gen. nov., que incluye cuatro especies neotropicales, a saber, Austroscaeva melanostoma (Macquart, 1842) comb. nov., Austroscaeva occidentalis (Shannon, 1927) comb. nov., Austroscaeva patagoniensis (Kassebeer, 1999) comb. nov., y Austroscaeva penai (Marnef in Dušek & Láska, 1985) comb. nov. A su vez, se describió y se caracterizó el género Austroscaeva gen. nov., y se proporcionó por primera vez la descripción del macho de Scaeva patagoniensis (=Austroscaeva patagoniensis) y Scaeva penai (=Austroscaeva penai), así como una clave de identificación ilustrada para las especies de Austroscaeva gen. nov. Además, se explica que el nombre correcto para Syrphus lunatus Wiedemann, 1830 es Scaeva opimia (Walker, 1852).
1 INTRODUCTION
The genus Scaeva Fabricius, 1805 includes medium to large species of flower flies (Diptera: Syrphidae, Syrphinae) with very clear wings, pilose eyes, oblique and often lunulate abdominal maculae, and usually with strongly swollen frons in male (Vockeroth, 1969). Adults are common in forests and grasslands, where they visit flowers of several plant families. The widespread species Scaeva pyrastri (Linnaeus, 1758) is highly mobile and migratory (Speight, 2016), and it has been used as a species model to study the genetic base of the antennal olfaction (Li et al., 2016). Larvae, on the other hand, are predators of aphids, psyllids, adelgids, scale insects (Hemiptera), and thrips (Thysanoptera) (see Rojo, Gilbert, Marcos-García, Nieto, & Mier, 2003 for a review). A total of 16 species of Scaeva are known (Dušek & Láska, 1985; Peck, 1988; Thompson, 2013), mostly from the Palaearctic region, while four taxa have been described from the Neotropics (Kassebeer, 1999). Five species reach the Oriental region, that is, S. albomaculata (Macquart, 1842), S. latimaculata (Brunetti, 1923), S. opimia (Walker, 1852), S. pyrastri, and Scaeva selenitica (Meigen, 1822) (Sengupta et al., 2016). One species, S. pyrastri, occurs in the Nearctic region along the West coast (from Alaska to New Mexico), and another, S. selenitica, was introduced into North Carolina although there is no indication that the species has become established (Thompson, 2013).
A revision of the Palaearctic species of the genus Scaeva was carried out firstly by Violovitsh (1975). Later, Kuznetzov (1985) again reviewed the Palaearctic taxa of Scaeva, while Dušek and Láska (1985) did the same for all Scaeva species. In addition to improving the systematics of the genus, these two works created a bit of confusion as they did not refer to each other, and different species concepts were employed in the two papers (Speight, 2016). Based on morphological characters, Kuznetzov (1985) divided Scaeva into three subgenera, while Dušek and Láska (1985) characterized three species groups with phylogenetic significance within Scaeva. Unfortunately, the species groups of Dušek and Láska (1985) were not equivalent to the subgenera of Kuznetzov (1985). One of the Scaeva species groups of Dušek and Láska (1985) comprised the Neotropical taxa of this genus that were not treated in Kuznetsov's work.
The Neotropical species of Scaeva were reviewed by Kassebeer (1999), who described a new species and provided an identification key and diagnoses for all four species, that is, S. melanostoma (Macquart, 1842), S. occidentalis Shannon, 1927, S. penai Marnef in Dušek & Láska, 1985, and S. patagoniensis Kassebeer, 1999. These Neotropical taxa occur in the Andean region and differ remarkably from the Palaearctic species in some morphological characters, such as a more forward produced face and an almost straight vein R4+5 (Dušek & Láska, 1985; Kassebeer, 1999). Two of these species, S. penai and S. patagoniensis, are only known from females.
The systematics of Scaeva and its phylogenetic relationships have been addressed several times in combination with the genus Eupeodes Osten Sacken, 1877, and these studies are considerably uninformative without the other genera that together form a distinct “natural group” (sensu Dušek & Láska, 1967, 1985). These genera, subgenera, or species groups related to Scaeva are Eupeodes, Ischiodon Sack, 1913, Lapposyrphus Dušek and Láska, 1967, Macrosyrphus Matsumura, 1917, Metasyrphus Matsumura, 1917 (a junior synonym of Eupeodes; see Vockeroth, 1986), and Simosyrphus Bigot, 1882. The ranking of each of them with regard to the others has changed many times based on the perception of previous authors, and difficulties to reach a consensus among taxonomists about their ranking and inter-relationships still prevail. The close relationship among these taxa has been reported several times based on preimaginal morphological characters (Láska et al., 2006; Rotheray, 1987; Rotheray & Gilbert, 1989, 1999), molecular characters (Mengual, 2015a; Mengual, Ståhls, & Rojo, 2008a), or a combination of molecular and adult morphological characters (Mengual, Ståhls, & Rojo, 2015).
On the other hand, three major contributions published in the 1960s in three consecutive years are the base of the current systematics of the tribe Syrphini (Syrphidae: Syrphinae), where all these taxa belong. Dušek and Láska (1967) tried to create a “natural system” for European genera of the subfamily Syrphinae, and one of their “natural groups” comprised Scaeva and Eupeodes (as Metasyrphus, as Posthosyrphus Enderlein, 1938, and as Scaevosyrphus Dušek & Láska, 1967; all three junior synonyms of Eupeodes). One year later, Hippa (1968) reviewed the Palaearctic genera related to Syrphus Fabricius, 1775 using male genitalia characters. Hippa 1968 divided Scaeva into two subgenera: Scaeva s. str., whose male genitalia were similar to Eupeodes (as Posthosyrphus), and his new subgenus Beszella Hippa, 1968 (a junior synonym of Lapposyrphus), with male genitalia similar to Epistrophe Walker, 1852 and comprising the single species Scaeva lapponica Zetterstedt, 1838.
Lastly, the revisionary work of Vockeroth (1969) had a much broader taxonomic scope, the Syrphini genera of the World. He pointed out the close taxonomic relationship between Metasyrphus, Lapposyrphus, Eupeodes, and Scaeva, and at some extent with Notosyrphus Vockeroth, 1969. In his work, Vockeroth (1969) recognized two subgenera for Metasyrphus, namely Lapposyrphus and Metasyrphus s. str., comprising three different species groups (corollae, confrater, and luniger species groups). Moreover, Vockeroth (1969) mentioned the possibility to consider Eupeodes and Metasyrphus synonyms, a synonymy that the same Vockeroth published later (Vockeroth, 1986). With the establishment of Metasyrphus as a junior synonym of Eupeodes, Vockeroth (1986) only recognized two subgenera in Eupeodes: Lapposyrphus and Eupeodes s. str. (see Vockeroth, 1992). Earlier, Vockeroth (1973) synonymized Macrosyrphus (originally described as a subgenus of Syrphus) with Metasyrphus. The type species of Macrosyrphus belongs to the confrater group sensu Vockeroth (1969).
The concept of the genus Eupeodes changed again years later, when Thompson and Vockeroth (1989) considered Macrosyrphus and Metasyrphus as valid subgenera of Eupeodes, establishing a system of four subgenera that was also adopted by Thompson and Rotheray (1998). These subgenera, namely Eupeodes, Macrosyrphus, Metasyrphus, and Lapposyrphus, reflect the species grouping of Vockeroth (1969). Thus, the current concept of Eupeodes follows Thompson and Vockeroth (1989). In this contemporary view, the subgenus Eupeodes comprises only the type species of the genus, Eupeodes volucris Osten Sacken, 1877, while the subgenus Macrosyrphus contains at least, or at best, three species (i.e., Syrphus confrater Wiedemann, 1830, Syrphus horishanus Matsumura, 1917, and Syrphus okinawensis Matsumura, 1916), but there are no detailed studies on this subgenus and its composition may vary in the future. The subgenus Lapposyrphus comprises only two species, Syrphus aberrantis Curran, 1925 and Scaeva lapponica, and all the other species belong to the subgenus Metasyrphus (Thompson, 2013).
The other two genera related with the Scaeva group, that is, Ischiodon and Simosyrphus, are rather small, but they have probably caused more confusion than any other group of very distinct species in Syrphinae (Vockeroth, 1969). Ischiodon has three species with quite distinct distributions: I. aegyptius (Wiedemann, 1830) is mainly present in Africa, I. feae (Bezzi, 1912) is endemic of the Cape Verde Islands, and I. scutellaris (Fabricius, 1805) occurs in the Oriental and Australasian regions. On the other side, Simosyrphus comprises a single species, S. grandicornis (Macquart, 1842), widely distributed throughout Oceania, New Zealand and Australia, reaching Hawaiian Islands (Mengual, 2015b). Both genera have been previously synonymized with one another several times, despite some important differences in the male genitalia (Vockeroth, 1969). Recently, Láska et al. (2006) synonymized Ischiodon under Simosyrphus based on larval and pupal morphology, supported by a molecular study of a very limited taxon sampling using only part of the gene cytochrome c oxidase subunit 1 (COI).
In the same paper, Láska et al. (2006) rearranged the genus Scaeva, dividing it into two subgenera that corresponded to two of the species groups defined by Dušek and Láska (1985). Láska et al. (2006) used the subgenus names erected by Kuznetzov (1985), but these names were applied to completely different taxa: the subgenus Semiscaeva Kuznetzov, 1985 comprised the selenitica species group, and the subgenus Scaeva s. str. referred to the pyrastri species group. The third subgenus erected by Kuznetzov (1985), Mecoscaeva Kuznetzov, 1985, was synonymized under Semiscaeva (Láska et al., 2006). Both Scaeva subgenera were characterized by Dušek and Láska (1985) and Láska et al. (2006) using adult and immature morphological characters. Láska et al. (2006), however, did not studied the third species group defined by Dušek and Láska (1985) comprising the Neotropical species, but they mentioned that these Neotropical Scaeva species form a separate monophyletic group, probably sister group to all Palearctic Scaeva species and that these Neotropical taxa should be classified as a separate taxon.
More recently, Láska, Mazánek, and Bičík (2013) pointed out a neglected adult morphological character for the genera of the Scaeva group, the broad and undulating wing membrane posterior to veins dm-cu and M1. This character was already mentioned by Dušek and Láska (1985) together with the reduction in the wing microtrichia. Furthermore, Láska et al. (2013) stated that they kept Semiscaeva as subgenus of Scaeva for practical purposes but they would have preferred to consider it as a valid genus.
The aim of this study was to re-explore the phylogenetic relationships among the genera of the Eupeodes-Scaeva clade based on significantly increased taxon sampling within the Eupeodes-Scaeva clade as compared to Mengual et al. (2008a) and Mengual (2015a). Moreover, we provide the description of the male of Scaeva penai and Scaeva patagoniensis, and an identification key for the Neotropical species of Scaeva, improved with images and based on a translation from Kassebeer (1999). To perform this study, we analyzed the sequences of the mitochondrial gene cytochrome c oxidase subunit I (COI), a fragment of the nuclear 18S rRNA, and the region D2–D3 of the nuclear 28S rRNA genes. We used the secondary structure of the ribosomal genes 28S and 18S to align the obtained DNA sequences.
2 MATERIAL AND METHODS
2.1 Taxonomy
Males of Scaeva penai and Scaeva patagoniensis are described in full, with terminology following Thompson (1999) and Mengual (2012). The abbreviations used for collections follow the standard of the Systema Dipterorum (Thompson, 2013), and their equivalents are given below:
- CAS: California Academy of Sciences, San Francisco, USA.
- ZFMK: Zoologisches Forschungsmuseum Alexander Koenig, Bonn, Germany.
At the end of each record, between square brackets ([]) and separated by commas, the number of specimens and sex, and the holding institution are given. All measurements are in millimeters and were taken using a reticule in a Leica® M165 C microscope. Photographs were composed using the software Zerene Stacker® 1.04 (Richland, Washington, USA), based on images of pinned specimens taken with a Canon EOS 7D® mounted on a P–51 Cam-Lift (Dun Inc., VA, USA) and with the help of Adobe Lightroom® (version 5.6). Body length was measured from the anterior oral margin to the posterior end of the abdomen in lateral view. Wing length was measured from the wing tip to the basicosta.
2.2 Taxon sampling
The taxon sampling covered as much taxonomic diversity as possible for the Scaeva-Eupeodes clade, as well as most genera and subgenera of the tribe Syrphini. Members of other tribes of Syrphinae were also included (Bacchini and Paragini). Table 1 lists the species included in the analysis, the collection data and the GenBank accession numbers. A total of 108 taxa were included in this study. We sequenced and analyzed for the first time 43 specimens representing 34 different species. Additionally, we obtained new sequences for 10 already sequenced voucher specimens of 10 different species (Table 1, GenBank accession numbers starting with MF; MF446421–MF446565). Members of the subfamily Eristalinae were used as outgroups as the monophyly of Syrphinae is well supported (Hippa & Ståhls, 2005; Mengual et al., 2015; Ståhls, Hippa, Rotheray, Muona, & Gilbert, 2003; Young et al., 2016). We also included representatives of Pipizinae, that is, Neocnemodon vitripennis (Meigen, 1822) and Pipiza quadrimaculata (Panzer, 1804). Merodon equestris (Fabricius, 1794) (Syrphidae: Eristalinae) was constrained as outgroup. DNA sequences for the genera Doros Meigen, 1803, Pseudodoros Becker, 1903, Notosyrphus, and Austroscaeva gen. nov. were obtained for the first time for molecular phylogenetic study.
| Species | Label information | DNA voucher code | COI | 28S | 18S |
|---|---|---|---|---|---|
| Allograpta neotropica Curran, 1936 | Colombia: Dpto Valle del Cauca, Cali, Cerro San Antonio, 2175 m., 15.ii.2006. Leg.: X. Mengual. Det.: X. Mengual. | MZH_XP59 | EU241733 | EU241780 | EU241831 |
| Asiobaccha marissae Mengual, 2016 | Indonesia: SE Sulawesi, North Kolaka, Mekongga Mt., nr Tinukari, 1000 m., 03°38′23.244′’S 121°08′56.76′’E, 30.ix.2010. Leg.: R.B. Kimsey. Det.: X. Mengual. | ZFMK_XM127 | KM270854 | KM270823 | KM270771 |
| Austroscaeva melanostoma (Macquart, 1842) | Chile: Limari prov., Fray Jorge Natl. Park, 30.ix–4.x.1997. Leg.: M. E. Irwin & D.K. Yeates. Det.: G. Ståhls. | MZH_S185 | MF446558 | MF446505 | – |
| Austroscaeva occidentalis (Shannon, 1827) | Chile: Limari prov., Fray Jorge Natl. Park, 30.ix–4.x.1997. Leg.: M. E. Irwin & D.K. Yeates. Det.: G. Ståhls. | MZH_S184 | MF446559 | MF446506 | – |
| Baccha elongata (Fabricius, 1775) | Finland: Ta, Vesijako, vii.2004, malaise trap. Leg.: J. Jakovlev. Det.: G. Ståhls. | MZH_Y242 | EF127326 | EF127407 | EU431540 |
| Betasyrphus aff. adligatus | South Africa: Botanical Royal Garden, Pitermaritzburg. 12.xii.2012. Leg.: S. Rojo. Det.: A. Ssymank. | ZFMK_D081 | MF446532 | MF446480 | MF446436 |
| Betasyrphus intersectus (Wiedemann, 1824) | South Africa: Drakensberg (Sentinel Peak), 8.xii.2012. Leg.: C. Pérez-Bañón & S. Rojo. Det.: A. Ssymank. | ZFMK_D079 | MF446530 | MF446478 | MF446435 |
| Betasyrphus intersectus (Wiedemann, 1824) | South Africa: Kwazulu-Natal, near Howick, meadow near exit 125 of N3 to Balgowan, 1280 m., 29°21′45.9″S 30°05′52.5″E, 18.x.2015. Leg. X. Mengual. Det.: X. Mengual. | ZFMK_D232 | MF446539 | MF446487 | MF446443 |
| Betasyrphus keiseri Ssymank, 2010 | Madagascar: Fianarantsoa Prov., Ranomafana N.P., open area in front of ValBio, 19.xi.2004. Leg.: X. Mengual. Det.: A. Ssymank. | MZH_XP101 | MF446549 | – | MF446453 |
| Betasyrphus keiseri Ssymank, 2010 | Madagascar: Fianarantsoa Prov., Ranomafana N.P.Open area in front of Valbio, 21.xi.2004. Leg.: X. Mengual. Det.: A. Ssymank. | MZH_XP55 | MF446548 | MF446496 | – |
| Chrysotoxum cautum (Harris, 1776) | Greece: Lesvos Island, Agiasos, 08.v.2007. Leg.: G. Ståhls. Det.: G. Ståhls. | MZH_XP166 | KM270857 | KM270826 | KM270774 |
| Chrysotoxum continuum Bezzi, 1915 | Tanzania: Kilimanjaro N.P., Horombo to Mandara campament, 3200 m., 09.vi.2007. Leg.: A. Menargues. Det.: X. Mengual. | MZH_XP216 | MF446553 | MF446500 | MF446457 |
| Chrysotoxum intermedium Meigen, 1822 | Spain: Alicante, Ibi, E.B. Torretes, 18.v.2007. Leg.: X. Mengual. Det.: X. Mengual. | MZH_XP154 | EU431498 | EU431466 | EU431541 |
| Dasysyrphus albostriatus (Fallén, 1817) | The Netherlands: Leiden, Meijendel dune area, 5.ix.2005. Leg.: excursion participants. Det.: G. Ståhls. | MZH_S565 | EF127323 | EF127402 | EU431542 |
| Dasysyrphus creper (Snow, 1895) | USA: Arizona, San Doval Co. Det.: L. Mazánek. | MZH_S359 | EF127368 | EF127448 | MF446464 |
| Dasysyrphus hilaris (Zetterstedt, 1843) | Finland: N: Vantaa, Keimola, 05.06.2003. Leg.: G. Ståhls. Det.: G. Ståhls. | MZH_Y7 | MF446554 | MF446501 | MF446458 |
| Dasysyrphus lenensis Bagatshanova, 1980 | Germany: Nordrhein-Westfalen, TK: 5403, R 2521143, H 5596510, Döppeskaul 2009, Malaise-Falle, Bachtal, Nebenbach des Fuhrtsbachtals, FFH DE-5403-301. NP Eifel, 18.v–01.vi.2009. Leg.: J. Esser. Det.: A. Ssymank. | ZFMK_D006 | KM270861 | KM270829 | KM270777 |
| Dasysyrphus lotus (Williston, 1887) | Colombia: Cali, Cerro San Antonio, 2200 m, 25.viii.2004. Leg. C. Prieto. Det. F.C. Thompson. | MZH_XP23 | EF127298 | EF127377 | MF446451 |
| Didea fuscipes Loew, 1863 | USA: New Mexico, San Doval Co., 4 km NW La Cueva FR 144, 9.vii–17.viii.2002, malaise trap. Leg. M. Hauser. Det.: G. Ståhls. | MZH_XP1 | MF446545 | MF446493 | MF446449 |
| Didea intermedia Loew, 1854 | Finland: Espoo, vii.2001. Leg.: G. Ståhls. Det.: G. Ståhls. | MZH_S90 | EF127336 | EF127418 | EU431543 |
| Dideoides coquilletti (Goot, 1964) | South Korea: Gangweon-do, Weonju-si, Maeji-ri, Yonsei Univ. Campus, 4.x.1999. Leg.: C.H. Park. Det.: H.Y. Han & D.S. Choi. | MZH_XP8 | EF127293 | EF127373 | KM270778 |
| Dideoides latus (Coquillett, 1898) | South Korea: kangwon-do, Wonju, panbumyon, Seogok-ri. Mt. Paekun from Yongsu-gol, 5.vii.1998. Leg.: H.Y. Han, K.E. Ro & H.W. Byun. Det.: H.Y. Han & D.S. Choi. | MZH_XP7 | MF446546 | MF446494 | MF446450 |
| Dideopsis aegrota (Fabricius, 1805) | Australia: Queensland, Daintree National Park, Cafe Tribulation, 16°05′S 145°29′E, 4.vi.1997, rainforest opening. Leg.: J. & A. Skevington. J95#1241. Det.: J. Skevington. | MZH_S92 | EF127333 | EF127414 | MF446463 |
| Dideopsis aegrota (Fabricius, 1805) | Malaysia: Sabah (Borneo), Penampang Distr., Crocker Range, Kipandi Butterfly Park, 720 m., 5°52′20″N 116°14′53″E, 15.x.2011. Leg.: M. Hauser & S. Gaimari. Det.: X. Mengual. | ZFMK_XM225 | KM270862 | KM270830 | KM270779 |
| Dioprosopa clavata (Fabricius, 1794) | Cuba: La Habana, ii.2001. Leg.: M.A. Marcos-García. Det.: G. Ståhls. | MZH_S84 | EF127332 | EF127413 | MF446462 |
| Dioprosopa clavata (Fabricius, 1794) | Mexico: Villa de Álvarez, Crta. Minatitlán, Colonia Burócratas, 23.viii.2006. Leg.: X. Mengual. Det.: X. Mengual. | MZH_XP116 | KM270873 | KM270841 | KM270807 |
| Dioprosopa clavata (Fabricius, 1794) | Peru: Valle del Cañete, Huaral trap, 17.04.2008. Leg.: S. Rojo. Det.: X. Mengual. | MZH_Y914 | MF446555 | MF446502 | – |
| Dioprosopa vockerothi Kassebeer, 2000 | Peru: Valle del Cañete, 4.iv.2008. Det.: X. Mengual. | MZH_Y915 | MF446563 | MF446514 | – |
| Dioprosopa vockerothi Kassebeer, 2000 | Peru: Dpto. Lima, Cañete, point 4, 04.iv.2008. AECID 013484–07. Leg. X. Mengual. Det.: X. Mengual. | ZFMK_D068 | MF446531 | MF446479 | MF446433 |
| Doros destillatorius Mik, 1885 | France: Dpt. Haute-Garonne, Castelnau-Picampeau, 07–22.vi.2005. Leg.: J.P. Sarthou. Det.: J.P. Sarthou. | MZH_XP167 | MF446551 | MF446498 | MF446455 |
| Doros profuges (Harris, 1780) | France, Dpt. Haute-Garonne, Montégut-Bourjac, 10–25.vi.2004. Leg.: J.P. Sarthou. Det.: J.P. Sarthou. | MZH_XP168 | MF446552 | MF446499 | MF446456 |
| Epistrophe eligans (Harris, 1780) | The Netherlands: Limburg, Epen, Bovenste bos. 50°45′24.6″N 5°53′55.7″E, 19.v.2012. Leg. X. Mengual. Det. X. Mengual. | ZFMK_D010 | MF446525 | MF446473 | MF446428 |
| Epistrophe melanostoma (Zetterstedt, 1843) | Finland: N: Keimola, 10.vi.2000. Leg.: G. Ståhls. Det.: G. Ståhls. | MZH_S60 | EF127324 | EF127405 | MF446461 |
| Epistrophe nitidicollis (Meigen, 1822) | Finland: Liesjärvi, 11.vi.2000. Leg.: G. Ståhls. Det.: G. Ståhls. | MZH_S61 | EF127325 | EF127406 | KM270780 |
| Epistrophella euchroma (Kowarz, 1885) | Czech Republic: Bohemia, PLA distr., Chrudim Hermanuv mestec, park, 3.vi.2005. Leg.: L. Mazánek. Det.: L. Mazánek. | MZH_S559 | EF127315 | EF501964 | KM270781 |
| Episyrphus balteatus (De Geer, 1776) | Spain: Alicante, P.N. Marjal Pego-Oliva, Muntanyeta Verda, 19.v.2007. Leg.: X. Mengual. Det.: X. Mengual. | MZH_XP153 | EU241740 | EU241788 | EU241840 |
| Eriozona syrphoides (Fallén, 1817) | Russia: Gornyi Altai, Turotshakskii r-kordon obogo, 950 m, 30.vi.2003. Leg. Krolatscheva. Det.: G. Ståhls. | MZH_Y184 | EF127358 | EF127439 | EU431544 |
| Eupeodes (Eupeodes) volucris Osten Sacken, 1877 | USA: NE, Cass Co., Louisville Platte River Sp., 19.v.2005. Leg. W. van Steenis. Det. W. van Steenis. | MZH_XP43 | KM270868 | KM270836 | KM270787 |
| Eupeodes (Macrosyrphus) confrater (Wiedemann, 1830) | South Korea: Gyeongsangbuk-do, Yeongju Sunheung-myeon, 8.vi.2002. Leg.: D.S. Choi. Det.: G. Ståhls. | MZH_Y101 | EF127355 | EF127436 | KM270788 |
| Eupeodes (Metasyrphus) corollae (Fabricius, 1794) | Spain: Alicante, Aspe, Partida Tolomó, 07.ii.2006. Leg.: P. Hurtado. Det.: X. Mengual. | MZH_XP141 | EU431499 | EU431467 | EU431546 |
| Eupeodes (Metasyrphus) fumipennis (Thomson, 1869) | Canada: BC, Vancouver Island, Nanaimo, Morrel Sanct., 49°08′49″N 123°58′31″W, 140 m., 05.v.2005. Leg. W. van Steenis. Det. W. van Steenis. | MZH_XP46 | EF127313 | EF127392 | MF446452 |
| Eupeodes (Metasyrphus) latifasciatus (Macquart, 1829) | Germany: Nordrhein-Westfalen, Bonn, ZFMK garden, 50°43′18.6″N 07°06′48.8″E, 67 m., 18.v.2013. Leg.: X. Mengual. Det.: X. Mengual. | ZFMK_D208 (ZFMK-TIS-22072_Diptera) | MF446536 | MF446484 | MF446440 |
| Eupeodes (Metasyrphus) lucasi (Marcos-García & Láaska, 1983) | Spain, 2003. Leg. S. Rojo. | CEUA_L13 | EF127337 | EF127419 | – |
| Eupeodes (Metasyrphus) luniger (Meigen, 1822) | Germany: Nordrhein-Westfalen, Monschau, Döppeskaul, 50°30′14.4″N 06°17′50.6″E, 560–580 m., 5.x.2009. Leg.: J. Esser. Det.: A. Ssymank. | ZFMK_D209 (ZFMK-TIS-2903_Diptera) | MF446537 | MF446485 | MF446441 |
| Eupeodes (Metasyrphus) nitens (Zetterstedt, 1843) | Germany: Nordrhein-Westfalen. TK: 5404, R 2529538, H 5605439. Helingsberg 2009, Malaise-Falle, ehemaliger Sprengplatz, Dreiborner Hochfläche, ehem. Truppenübungsplatz Vogelsang. NP Eifel, 500 m., 29.vi-13.vii.2009. Leg. J. Esser. Det. A. Ssymank. | ZFMK_D013 (ZFMK-TIS-2898_Diptera) | MF446527 | MF446475 | MF446430 |
| Eupeodes (Metasyrphus) rojasi Marnef, 1999 | Venezuela: Lara, Lomas de Cubiro, 1710 m., 9°47′N 59°33′W, 31.iii.2015. Leg.: E. Arcaya, D. Medina, A. Arcaya, K. Arcaya, S. Matute. Det.: X. Mengual. | ZFMK_D275 | MF446541 | MF446489 | MF446445 |
| Eupeodes (Metasyrphus) rojasi Marnef, 1999 | Chile: Limari prov., Fray Jorge Natl. Park, 30.ix–4.x.1997. Leg.: M. E. Irwin & D.K. Yeates. Det.: G. Ståhls. | MZH_S197 | MF446560 | MF446507 | – |
| Fagisyrphus cinctus (Fallén, 1817) | Czech Republic: Bohemia, PLA Kokorinsko, Vojtechov, 14.v.2005. Leg.: L. Mazánek. Det.: L. Mazánek. | MZH_S558 | KM270869 | KM270837 | KM270789 |
| Graptomyza longirostris Wiedemann, 1820 | Singapore: Dairy Farm N.P., 02.v.2012. Leg.: V. Gowda. Det.: X. Mengual. | ZFMK_D007 | KM270878 | KM270847 | KM270816 |
| Ischiodon aegyptium (Wiedemann, 1830) | South Africa: Kwazulu-Natal, Royal Natal N.P., trail to The Crack, walking back in the evening, gorge forest and adjacent grassland. 1600 m., 09.xii.2012, 28°41′04.3″S 28°56′14.7″E. Leg.: S. Rojo. Det.: X. Mengual. | ZFMK_D077 | MF446529 | MF446477 | MF446434 |
| Ischiodon aegyptium (Wiedemann, 1830) | Algeria: Mostaganem Province, Mazagran, 35°54′N 0°43′E, 04.iii.2014, feeding on Myzus perscicae in pepper plants. Det.: S. Rojo. | ZFMK_D190 | MF446534 | MF446482 | MF446438 |
| Ischiodon scutellaris (Fabricius, 1805) | French Polynesia: Bora Bora, Vairupe, 13.xi.2012, Malaise trap. Leg.: T. Ramage. Det.: X. Mengual. | ZFMK_D267 | MF446540 | MF446488 | MF446444 |
| Ischiodon scutellaris (Fabricius, 1805) | China: Hong Kong, Park, 7.x.2001. Leg.: D. Iliff. Det.: G. Ståhls. | MZH_S157 | AY603768 | EF127429 | KM270790 |
| Lapposyrphus lapponicus (Zetterstedt, 1838) | Germany: Nordrhein-Westfalen, Euskirchen, Schleiden, Kermeter, 475 m., 50°36′05″N 06°26′55.7″E, 30.v.2011. Leg. J. Esser. Det. A. Ssymank. | ZFMK_D210 (ZFMK-TIS-2876_Diptera) | MF446538 | MF446486 | MF446442 |
| Lapposyrphus lapponicus (Zetterstedt, 1838) | U.S.A.: New Mexico, San Doval Co., 4 km NW La Cueva, FR144, 35°54.25′ N 106°40.10′ W, 9–17.vii.2002, malaise trap. Leg.: M. Hauser. | MZH_S358 | MF446564 | MF446512 | – |
| Lapposyrphus lapponicus (Zetterstedt, 1838) | Czech Republic: 13.v.2000. Leg.: L. Mazánek. Det.: L. Mazánek. | MZH_S65 | DQ158897 | DQ158897 | KM270791 |
| Leucozona (Ischyrosyrphus) glaucia (Linnaeus, 1758) | Spain: Pyrenees, Aran Valley, nr Arties, 1500 m., 1.viii.2003. Leg. G. Ståhls. Det.: G. Ståhls. | MZH_XP5 | EF127292 | EF127372 | KM270793 |
| Leucozona (Leucozona) inopinata Doczkal, 2000 | Germany: Nordrhein-Westfalen. TK: 54034, 50°30′51.1″N, 06°17′09.9″E; Fuhrtsbachtal, nahe Antoniusbrücke, Narzissen-Bärwurzwiese, Meum athamaticum in Vollblüte. NP Eifel, 537 m., 09.vi.2012. Leg. A. Ssymank. Det. A. Ssymank. | ZFMK_D004 | MF446524 | MF446472 | MF446427 |
| Leucozona (Leucozona) lucorum (Linnaeus, 1758) | Italy: South Tirol, Val Venosta, vii.2001. Leg.: G. Ståhls. Det.: G. Ståhls. | MZH_S139 | EF127346 | EF501965 | EU431548 |
| Megasyrphus erraticus (Linnaeus, 1758) | Finland: Ab: Karislojo, Karkalinniemi, v.2004. Leg.: G. Ståhls. Det.: G. Ståhls. | MZH_Y183 | EF127357 | EF127438 | EU431545 |
| Megasyrphus laxus (Hull, 1925) | Canada: AB, Jasper NP, Valley o–t Five Lakes, 117°98′E 52°48′N, 27.viii.2004. Leg. W. van Steenis. Det. W. van Steenis. | MZH_XP27 | EF127302 | EF127381 | KM270794 |
| Melangyna (Austrosyrphus) collata (Walker, 1852) | Australia: Victoria, Tarra Bulga NP, near Tarra Bulga Visitor Centre, AMG 55,462–5746, 26.i.2006. Leg.: W. van Steenis. Det.: W. van Steenis. | MZH_XP124 | KM270871 | KM270839 | KM270795 |
| Melangyna (Austrosyrphus) viridiceps (Macquart, 1847) | Australia: Victoria, Mt. Buffalo NP, The Horn top, AMG 55,479–5929, 1723 m., 29.i.2006. W. van Steenis. Det.: W. van Steenis. | MZH_XP123 | MF446550 | MF446497 | MF446454 |
| Melangyna (Melangyna) lasiophthalma (Zetterstedt, 1843) | Finland: N: Mäntsälän Mustametsä, 10.v.2003. Leg.: G. Ståhls. Det.: G. Ståhls. | MZH_Y5 | EF127361 | EF501966 | KM270796 |
| Melangyna (Melangyna) subfasciata (Curran, 1925) | Canada: Kluane, Whitehorse Airport, 135°05′E 60°45′N, 4.viii.2004. Leg.: W. van Steenis. Det.: W. van Steenis. | MZH_XP28 | EF127303 | EF127382 | KM270797 |
| Melanostoma scalare (Fabricius, 1794) | Finland: Ok: Kuhmo, Lentuankoski, 15.viii.2006. Leg.: G. Ståhls. Det.: G. Ståhls. | MZH_Y441 | EU431500 | EU431468 | EU431549 |
| Meligramma guttata (Fallén, 1817) | Finland: Ab: Mietoinen, Perkko, 6733:222, 21.vii.2004. Leg.: A. Haarto. Det.: G. Ståhls. | MZH_Y478 | EF501960 | EF501968 | KM270800 |
| Meligramma triangulifera (Zetterstedt, 1843) | Czech Republic: Jizerské Mountains, Rybí loucky-peat-bog, sq. 5158, 850 m, (malaise trap with alcohol), 5–20.viii.2003. Leg.: Preisler. Det.: G. Ståhls. | MZH_S560 | EF127316 | EF501967 | KM270799 |
| Meliscaeva auricollis (Meigen, 1822) | Greece: Lesbos island, iv.2001. Leg.: S. Rojo & C. Pérez. Det.: L. Mazánek. | MZH_S123 | EF127341 | EF127423 | EU241844 |
| Merodon equestris (Fabricius, 1805) | Finland: N, Askola, 12.i.2007. Leg. G. Ståhls. Det.: Ståhls. | MZH_Y690 | EU431486 | EU431455 | EU431523 |
| Myolepta dubia (Fabricius, 1850) | Germany: Nordrhein-Westfalen, NP Eifel, FO: 7757 R 2532921, H 5613552. Odenbachtal-Felskuppen, 320 m., 24.vi–08.vii.2010, Malaise. Leg.: J. Esser. Det. A. Ssymank. | ZFMK_D012 | KM270877 | KM270846 | KM270815 |
| Neocnemodon vitripennis (Meigen, 1822) | Finland: N: Sibbo, Hindsby, 26.v.2004. Leg.: G. Ståhls. Det.: G. Ståhls. | MZH_Y211 | EU431503 | KM270845 | EU431559 |
| Notosyrphus goldbachi (Fluke, 1950) | Argentina, Tucuman, San Javier, Hotel Sol, Ruta 340 – Km 23, 1250 m., 26°47′52″ S 65°21′31″ W, 01.xi.2008. Leg.: X. Mengual. Det.: X. Mengual. | MZH_Y916 | MF446556 | MF446503 | MF446459 |
| Ocyptamus funebris Macquart, 1834 | Costa Rica: San José, Heredia, INBioparque, 15–21.i.2005, malaise trap. Det.: F.C. Thompson. | MZH_S487 | EF127364 | EF127443 | EU409242 |
| Paragus (Afroparagus) caligneus Ssymank & Mengual, 2014 | Gabon: Ogooué-Ivindo, Ivindo NP, Makokou (518 m), 16–19.ix.2012. Am Ivindo nahe Station (Malaisetrap 4). Leg.: R. Peters. Det. X. Mengual. | ZFMK_D067 | KJ158454 | KJ158455 | MF446432 |
| Paragus (Pandasyopthalmus) haemorrhous Meigen, 1822 | Spain: Alicante, 2000. Leg.: A. Vujić. Det.: A. Vujić. | MZH_S48 | AY174470 | AY476866 | EU409259 |
| Paragus (Paragus) bicolor (Fabricius, 1794) | Tajikistan: Tigrovaya Balka Nature Reserve, 21.iv.2014. Leg.: A. Barkalov. Det.: A. Barkalov. | ZFMK_D168 | MF446533 | MF446481 | MF446437 |
| Paragus (Paragus) pecchiolii Rondani, 1857 | Montenegro: Durmitor, 26.vi.2000. Leg.: A. Vujić. Det.: A. Vujić. | MZH_S71 | AY476844 | AY476864 | KM270803 |
| Paragus (Serratoparagus) crenulatus Thomson, 1869 | Malaysia: Sabah, Danum Valley, viii.1999. Det.: A. Vujić. | MZH_S62 | AY476862 | AY476880 | KM270802 |
| Parasyrphus malinellus (Collin, 1952) | Germany: Nordrhein-Westfalen, Monschau, Döppeskaul, 50°30′14.4″N 06°17′50.6″E, 560–580 m., 16.vi.2009. Leg.: J. Esser. Det.: A. Ssymank. | ZFMK_D207 (ZFMK-TIS-2914_Diptera) | MF446535 | MF446483 | MF446439 |
| Parasyrphus lineolus (Zetterstedt, 184) | Italy: South Tirol, Val Venosta, vii.2001. Leg.: G. Ståhls. Det.: L. Mazánek. | MZH_S137 | EF127342 | EF127424 | KM270804 |
| Pipiza quadrimaculata (Panzer, 1804) | Finland: Ka, Joutseno, Riikanmaa. KKJ-Y, 05.vii.2007. Leg.: M.P. van Zuijen & W. & J. van Steenis. Det.: G. Ståhls. | MZH_XP218 | EU431506 | EU431474 | EU431562 |
| Platycheirus albimanus (Fabricius, 1781) | Sweden: 2000. Leg.: J. van Steenis. Det.: J. van Steenis. | MZH_E38 | EF127351 | EF127432 | KM270805 |
| Pseudodoros nigricollis Becker, 1903 | Cyprus: Pafos, Polis Chrysochou, 14.xi.2015. Leg.: Ch. Makris; wing sheltered spot in reed bed of Phragmites australis close to coast line. Det.: A. van Eck. | ZFMK_D280 | MF446543 | MF446491 | MF446447 |
| Pseudodoros nigricollis Becker, 1903 | Egypt: 3 km W of Siwa town, Siwa oasis, Fatnas spring and conservancy, Reed swamp, −42 m., 29°11′34.8″ N 25°28′48″ E, 13.v.2009. Leg.: K.-D.B. Dijkstra. Det.: G. Ståhls. | MZH_Y912 | MF446565 | MF446513 | MF446465 |
| Pseudodoros nigricollis Becker, 1903 | Tanzania: Kigoma region, 35–38 km W of Uvinza, Lower Malagarasi Basin, Igamba and Kasagwe, 05.18°S 30.06°E, 7.viii.2009. Leg.: K.-D.B. Dijkstra. Det.: G. Ståhls. | MZH_Y1108 | MF446557 | MF446504 | – |
| Rohdendorfia alpina Sack, 1938 | Italy: Stelvio Pass. Leg.: G. Ståhls. Det.: G. Ståhls. | MZH_G344 | EF127338 | EF127420 | EU431552 |
| Salpingogaster nigra Schiner, 1868 | Colombia: Dpto Meta, PNN Sumapaz, Cabaña Las Mirlas, 710 m., 3°48′N 73°52′W, 29.v–19.vi.2004. Leg.: H. Vargas. Det.: X. Mengual. | MZH_XP77 | EU241748 | EU241796 | EU241853 |
| Scaeva (Scaeva) albomaculata (Macquart, 1842) | Greece: Lesvos island, iv.2001. Leg.: S. Rojo & C. Pérez. Det.: S. Rojo. | MZH_S105 | AY603765 | MF446510 | – |
| Scaeva (Scaeva) albomaculata (Macquart, 1842) | Spain: Alicante, 1999. Leg.: S. Rojo. Det.: X. Mengual. | MZH_S57 | EF127329 | EF127410 | EU431553 |
| Scaeva (Scaeva) caucasica Kuznetzov, 1985 | China: Gansu prov., Bayin, vii.2001. Leg.: J. Bancroft. | MZH_S190 | MF446561 | MF446508 | – |
| Scaeva (Scaeva) pyrastri (Linnaeus, 1758) | Germany: Nordrhein-Westfalen, Watchberg–Bonn, Oberbachem, Werthovener Weg,. FO: 7891, TK: 530842, 138 m, 27.vii.2012. Leg. A. Ssymank. Det. A. Ssymank. | ZFMK_D009 | KM270874 | KM270842 | KM270809 |
| Scaeva (Semiscaeva) dignota (Rondani, 1857) | Greece: Lesvos island, iv.2001. Leg.: S. Rojo & C. Pérez. Det.: S. Rojo. | MZH_S100 | MF446562 | MF446509 | – |
| Scaeva (Semiscaeva) komabensis (Matsumura, 1918) | Japan: Nagano Prefecture, Ueda, Sugadaira Kogen, near SMRC, grassland, 1330 m., 05–19.vii.2014, 36°31′18″N 138°21′03″E, Malaise trap. Leg.: A. Blanke. Det. X. Mengual. | ZFMK_D279 | MF446542 | MF446490 | MF446446 |
| Scaeva (Semiscaeva) komabensis (Matsumura, 1918) | South Korea: Gaeweon-do, Hongcheon-gun Nae-myeon, 6.vi.2000. Leg.: D.S. Choi. Det.: X. Mengual. | MZH_Y100 | EF127354 | EF127435 | MF446460 |
| Scaeva (Semiscaeva) mecogramma (Bigot, 1860) | France: Rhone-Alpes, RNN Gorges de L'Ardec, TM02, Dune de Guad, 27.v–08.vi.2015. Leg.: N. Bazin. Det.: M.C. Speight. | ZFMK_D281 | MF446544 | MF446492 | MF446448 |
| Scaeva (Semiscaeva) mecogramma (Bigot, 1860) | Spain: Alicante, 1999. Leg.: S. Rojo. Det.: S. Rojo. | MZH_S16 | MF446547 | MF446495 | – |
| Scaeva (Semiscaeva) selenitica (Meigen, 1822) | Germany: Nordrhein-Westfalen. TK: 5304, R 2531820, H 5607402. MF Hellberg (Kermeter, südexponierter Waldrand, Grenze Buchenwald, totholzreicher Douglasien-Kahlschlag). NP Eifel, 475 m., 16–30.v.2011. Leg. J. Esser, Det. A. Ssymank. | ZFMK_D015 | MF446528 | MF446476 | MF446431 |
| Scaeva (Semiscaeva) selenitica (Meigen, 1822) | Czech Republic: distr. Ostrava, Polanecký les, 3.iv.2000. Leg.: T. Kuras. Det.: L. Mazánek. | MZH_S69 | AY603764 | EF127404 | KM270808 |
| Simosyrphus grandicornis (Macquart, 1842) | Australia: Brisbane, 5.x.2002. Leg.: H. Hippa. Det.: G. Ståhls. | MZH_S288 | AY603770 | MF446511 | – |
| Simosyrphus grandicornis (Macquart, 1842) | Australia: Victoria, Mt. Buffalo NP, Dicksons Falls, AMG 55 481–5929, 1440 m., 29.i.2006. Leg.: W. van Steenis. Det.: W. van Steenis. | MZH_XP125 | KM270875 | KM270843 | KM270810 |
| Sphaerophoria scripta (Linnaeus, 1758) | Spain: Alicante, Aspe. Partida Tolomó, 07.ii.2006. Leg.: P. Hurtado. Det.: X. Mengual. | MZH_XP142 | EU241752 | EU241800 | EU241860 |
| Syrphus shorae Fluke, 1950 | Venezuela: Edo. Aragua. P.N. Henri Pittier, Portachuelo, 1152 m., 10°20.828′N 067°41.309′W, 26.i.2007. Leg.: X. Mengual. Det.: X. Mengual. | MZH_XP158 | EU409136 | EU409191 | EU409252 |
| Syrphus vitripennis Meigen, 1822 | Greece: Lesbos island, iv.2001. Leg.: S. Rojo & C. Perez. Det.: S. Rojo. | MZH_S53 | AY212797 | AY261728 | EU431554 |
| Xanthogramma citrofasciatum (De Geer, 1776) | Germany: Nordrhein-Westfalen. TK: 5304, 50°37′53″N, 06°26′37″E; Heimbach-Hasenfeld, Parkplatz Büdenbach, trockene Böschung mit Euphorbia cyparissias. NP Eifel, 303 m., 19.v.2012. Leg. A. Ssymank. Det. A. Ssymank. | ZFMK_D002 | MF446523 | MF446471 | MF446426 |
| Xanthogramma laetum (Fabricius, 1794) | Germany: Nordrhein-Westfalen. TK: 5304, R 2531820, H 5607402. MF Hellberg (Kermeter, südexponierter Waldrand, Grenze Buchenwald, totholzreicher Douglasien-Kahlschlag). NP Eifel, 475 m., 16–30.v.2011. Leg. J. Esser. Det. A. Ssymank. | ZFMK_D011 | MF446526 | MF446474 | MF446429 |
| Xanthogramma flavipes (Loew, 1863) | USA: NE, Cass Co., Louisville, Platte River SP. 19.v.2005. Leg. W. van Steenis. Det. W. van Steenis. | MZH_XP31 | EF127306 | EF127385 | KM270814 |
| Xanthogramma pedissequum (Harris, 1776) | Greece: Lesbos island, iv.2001. Leg.: S. Rojo & C. Perez. Det.: S. Rojo. | MZH_S120 | EF127339 | EF127421 | EU431557 |
2.3 Laboratory protocols
One to three legs, the entire abdomen or the entire specimen, either dry pinned or ethanol preserved, were used for DNA extraction. Extractions were carried out using the NucleoSpin Tissue DNA Extraction kit (Machery-Nagel, Düren, Germany) following the manufacturer's instructions; samples were resuspended in 50–100 μl ultra-pure water. Entire specimens or remnants of specimens were preserved and labeled as DNA voucher specimens for the purpose of morphological studies and deposited at the Zoological Museum of the Finnish Museum of Natural History [MZH], at the Colección Entomológica de la Universidad de Alicante [CEUA], and at the Zoological Museum Alexander Koenig [ZFMK], as listed in Table 1.
DNA primers and PCR amplification protocols for mitochondrial COI, and nuclear 28S and 18S rRNA genes were the same as described in Mengual, Ståhls, and Rojo (2008b), Mengual et al. (2012). Amplified DNA was electrophoresed on 1.5% agarose gels for visual inspection of amplified products. PCR products were enzymatically treated with ExoSap-IT (USB, Cleveland, OH, USA) and then sequenced (using the PCR primers) in both directions. The sequences were edited for base-calling errors and assembled using Geneious R7 (version 7.1.3, Biomatters Ltd.). All new sequences were submitted to GenBank (see Table 1 for accession numbers).
2.4 Sequence alignment
The alignment of the protein-coding COI gene was done manually, and it was not necessary to include gaps in this alignment. The COI data matrix contained a total of 1,371 nucleotide characters. The alignment of 18S and 28S rRNA genes was done using the secondary structure of these genes, as explained by Kjer (1995) and implemented by Gillespie, Cannone, Gutell, and Cognato (2004), Gillespie, Johnston, Cannone, and Gutell (2006), Mengual (2015a) and Mengual et al. (2012, 2015). As a result, 650 bps were included in the analysis for the D2–D3 regions of 28S, and 605 bp for 18S, both numbers including gaps. A nexus file with the final alignment is provided as online Supporting Information (File S3), and two additional text files are also given in the Supporting Information with the original structural alignment for 28S and 18S before trimming (Files S4 and S5, respectively).
2.5 Phylogenetic analyses
For the combined dataset, maximum-likelihood (ML) analysis and Bayesian inference were performed to infer the phylogenetic relationships of the members of the Scaeva-Eupeodes clade. For both approaches, the dataset was divided into five partitions: first codon position of COI, second codon position of COI, and third codon position of COI, 28S gene, and 18S gene. We determined the best choice of model for each partition using jModelTest 2.1.1 (Darriba, Taboada, Doallo, & Posada, 2012) under the Akaike Information Criterion (AIC), as recommended by Posada and Buckley (2004). The model chosen for positions 1 and 2 of COI was GTR + I + G, and TrN + I + G for position 3. The model GTR + I + G was selected for 28S, and the preferred model for 18S gene was TVM + I + G. Data were analyzed under the recommended models using Garli v.2.01 (Zwickl, 2006, 2011). Forty-eight independent runs were conducted using scorethreshforterm = 0.05 and significanttopochange = 0.0001 settings and the automated stopping criterion, terminating the search when the ln score remained constant for 50,000 consecutive generations. The tree with the highest likelihood was retained and is presented here (Figure 1). Bootstrap support values (BS) were estimated from 1,000 replicates using the same independent models in Garli.
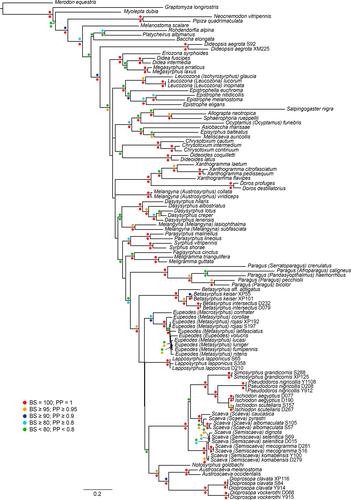
Phylogenetic estimation using the Markov Chain Monte Carlo algorithm as implemented in MrBayes 3.2.6 (Huelsenbeck & Ronquist, 2001; Ronquist & Huelsenbeck, 2003) was performed using a parallelized version of the software (XSEDE in CIPRES Science Gateway). Data were divided into the above five partitions, and a separate GTR + I + G model for each partition was specified in the analysis where each partition has its own set of parameters. Priors were applied with default values. Six runs, with four chains each (one “cold” chain and three heated chains; temp = 0.2), were performed simultaneously for 20,000,000 generations which were sufficient to bring the convergence (average standard deviation) to a value <0.03 (Ronquist, Huelsenbeck, & van Mark, 2005), sampling trees every 2,500 generations. The program Tracer 1.5 (Drummond & Rambaut, 2007; Rambaut & Drummond, 2007) was used to check convergence and acceptable mixing. The initial 2,000 trees (25%) were discarded as burn-in, and Bayesian posterior probabilities (PP) were calculated using a 50% majority-rule consensus tree inferred from the data.
Analytical runs were performed on CIPRES Science Gateway (Miller, Pfeiffer, & Schwartz, 2010). All trees were drawn with the aid of FigTree v.1.3.1 (Rambaut, 2009).
3 RESULTS
3.1 Phylogenetic analyses
The ML tree with the best likelihood score (−31,292.223746) is presented in Figure 1 (a .tre file, File S6, is provided as online Supporting Information). Although the taxonomic sampling was focused on the diversity within the Eupeodes-Scaeva clade and most Syrphini genera were included in our analyses, we would like to comment on the topology for the other taxa. The two members of Pipizinae were resolved as sister group of Syrphinae, and members of the tribe Bacchini were not recovered in a single clade, as in previous analyses (Mengual et al., 2015; Young et al., 2016). Most of the relationships among genera of Syrphinae are in agreement with previous analysis (Mengual, 2015a) with well-supported clades such as (Eriozona Schiner, 1860 + (Didea Macquart, 1838 + Megasyrphus Dušek & Láska, 1967)), (Leucozona Schiner, 1860 + (Epistrophella Dušek & Láska, 1967 + Epistrophe), or the clade comprising the genera Salpingogaster Schiner, 1868, Allograpta Osten Sacken, 1875, Sphaerophoria Lepeletier & Serville, 1828, Ocyptamus Macquart, 1834, Asiobaccha Violovitsh, 1976, Episyrphus Matsumura & Adachi, 1917, and Meliscaeva Frey, 1946.
Inside Syrphini, Dideopsis was resolved as the sister group of the remaining Syrphini + Paragini. Regarding the taxa tested in molecular phylogenetic analysis for the first time, Doros was placed as sister group of Xanthogramma Schiner, 1860, while Notosyrphus was recovered in a clade as sister group of Dioprosopa Hull, 1949 and Austroscaeva gen. nov., all with high support values (Figure 1). The genus Pseudodoros was resolved as sister group of Ischiodon with very high support (BS = 100).
The genus Paragus Latreille, 1804 was resolved as the sister group of the Scaeva-Eupeodes clade, wherein five clades received high support values: the genera Betasyrphus Matsumura, 1917, Eupeodes, and Lapposyrphus, the clade (Notosyrphus + (Austroscaeva + Dioprosopa)) and the clade comprising the two subgenera of Scaeva, Simosyrphus, Pseudodoros, and Ischiodon. Within the last clade, three groupings received very high support (BS = 100) and posterior probabilities with a value of 1, that is, Scaeva (Semiscaeva), Scaeva (Scaeva), and (Simosyrphus + (Pseudodoros + Ischiodon)).
The topology of the majority-rule consensus tree resulting from Bayesian inference compares favorably with the most likely tree, with some small differences (Fig. S2). The most remarkable difference between both approaches is the placement of Betasyrphus as the sister group of Eupeodes, being Lapposyrphus sister group of these two genera with a posterior probability of 0.70. Both topologies also disagree in the relationship between the two subgenera of Scaeva. Our ML analysis placed the subgenus Scaeva (Scaeva) as the sister group of Simosyrphus (Pseudodoros + Ischiodon) with very low support (BS = 51) and the subgenus Scaeva (Semiscaeva) as the sister group of this set of taxa, while in the Bayesian inference, Scaeva (Scaeva) and Scaeva (Semiscaeva) were resolved as sister groups with low probability (PP = 0.63) (Figure 1; Figs S1 and S2). These results imply that the two subgenera are valid clades (both subgenera have BS = 100 and PP = 1), but our molecular data were not sufficient to infer their relationship with confidence and high support values.
3.2 Systematics
Our results based on molecular characters showed no doubt about the relationship of the Neotropical taxa of Scaeva and the other Scaeva species. The placement of S. melanostoma and S. occidentalis in a different clade from Palaearctic Scaeva species, with Notosyrphus and Dioprosopa, provided robust support to the species group established by Dušek and Láska (1985), which together with the morphological differences mentioned by Dušek and Láska (1985) and Kassebeer (1999) prompted us to erect a new genus, Austroscaeva gen. nov., for this Neotropical species group.
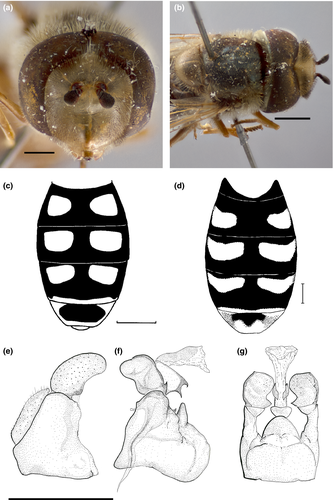
3.2.1 Austroscaeva Láska, Mazánek & Mengual gen. nov. Figures 2-4


Type species
Syrphus melanostoma Macquart, 1842.
Etymology
The name Austroscaeva is feminine and is a combination of Austro, meaning southern, and Scaeva, referring to the superficially similar genus Scaeva.
Included species
Austroscaeva melanostoma (Macquart, 1842) comb. nov., Austroscaeva occidentalis (Shannon, 1927) comb. nov., Austroscaeva patagoniensis (Kassebeer, 1999) comb. nov., and Austroscaeva penai (Marnef in Dušek & Láska, 1985) comb. nov.
Diagnosis
Very similar to the genus Scaeva (see diagnosis in Vockeroth, 1969: 70). Medium to large, moderately slender to robust species with very clear wings, pilose eyes, long pile on thorax and sometimes on abdomen as well, and oblique and often lunulate abdominal maculae.
Face produced forward; clypeus elongated. Front of male relatively broadened, strongly swollen (less in A. occidentalis). Dorsal 1/3–2/3 of eye of male with an extensive area of almost uniformly enlarged facets; eyes meeting anteriorly at an angle broader than 120°. Front of female only slightly swollen but broad. Face in both sexes moderately to strongly swollen. Mesonotum shining black, always pale pilose, without a clear lateral yellow vitta. Scutellum yellow, shiny medially or entirely pollinose, dull. Pleura black, densely pale pilose, subshining to moderately pollinose. Metasternum bare or pilose. Vein R4+5 nearly straight; basal section of vein R4+5 diverging from vein M, not running parallel as in Scaeva or Lapposyrphus; narrow and irregularly undulated wing membrane posterior to veins dm-cu and M1. Microtrichia greatly reduced, absent from at least basal 1/2 of wing. Abdomen narrowly to broadly oval, flattened, strongly margined from tergum 2 to tergum 5. Terga 2–4 each with a pair of slightly to strongly oblique, narrow to broad, whitish-yellow to bright yellow maculae.
Karyology
The karyology of Austroscaeva gen. nov. is different from that of Scaeva. Austroscaeva melanostoma and A. occidentalis have five pairs of chromosomes plus an heteromorphic pair XY, with X short telocentric and Y very short telocentric (Boyes, Boyes, van Brink, & Vockeroth, 1973). On the other hand, Scaeva albomaculata (Macquart, 1842), S. pyrastri, and S. selenitica have four pairs of chromosomes and a short heteromorphic pair XY (Boyes, van Bring, & Boyes, 1971; Boyes & van Brink, 1964).
Geographic distribution
Andean region (Argentina, Chile, Bolivia, Peru, Ecuador and Colombia) (see Kassebeer, 1999).
Natural history
Nothing is known about their larval biology or visited flowers. However, it is very likely that a U-shaped grasping organ (Rotheray, 1987) formed with the abdominal locomotory prominences of the larva is present also in these taxa. This structure is characteristic for the genera Scaeva, Eupeodes, Ischiodon (Rotheray & Gilbert, 1999), and Dioprosopa (Arcaya Sánchez, 2012).
Systematics
Austroscaeva gen. nov. comprises four species previously referred as the Neotropical Scaeva species group (Dušek & Láska, 1985; Kassebeer, 1999). Present molecular results place it as a sister group of Dioprosopa (BS = 90; PP = 1) in a clade with Notosyrphus (BS = 95; PP = 1) within the broader lineage Scaeva-Eupeodes. Láska et al. (2006) suggested that Austroscaeva gen. nov. might be sister to all Palaearctic Scaeva, Ischiodon, and Simosyrphus species according to the pattern of wing venation and other characters, a placement that agrees with our results in part.
Remarks
For more detailed information on species distribution and species descriptions, see Kassebeer (1999).
3.2.2 Austroscaeva patagoniensis (Kassebeer, 1999) comb. nov. Figures 4c and 5a–f
Description of the male
Robust, large species. Body length 13.5 mm; wing length 10 mm. Head (Figure 5d). Head width about 5 mm. Face very broad, rather produced in profile, entirely yellow, with black pile dorsally and laterally, pale pilose medially. Mouth edge mostly yellow. Gena yellow, pale pilose. Eye with pale pile. Area of enlarged facets distinct with marked border. Anterior angle of approximation of eyes wide, about 135°. Ocellar triangle equilateral. Pile on vertical triangle pale. Frontal triangle yellow, inflated, black pilose laterally and pale pilose in median line over antennae. Antenna brown; basoflagellomere dark, paler ventrally. Occiput densely whitish pollinose, with several rows of pale pile, rather broader than in, for example, S. pyrastri. Occiput dorsally with row of rather black cilia.
Thorax (Figure 5a,b). Black, postpronotum and anterior part of notopleuron pale, densely pale pilose, uniformly long. Pleuron black, yellow pilose and yellow microtrichose. Scutellum entirely yellow, shiny medially, yellow microtrichose on lateral margin; scutellar fringe with long yellow pile. Metasternum bare. Plumula pale. Halter pale. Wing: microtrichia reduced as in other species of genus; alula bare. Vein R4+5 almost straight. Wing membrane beyond apical cross-veins rather narrow and irregularly undulated. Legs: entirely yellow, except coxae and trochanters black, and apical tarsomeres a bit darker, entirely pale pilose.
Abdomen (Figure 5a,b). Robust, about 6.8 mm long and broad (3.8 mm). Terga 3 and 4 circa two times broader than long (tergum 3 about 2 times broader; tergum 4 about 1.8 times broader). Tergum 1 shiny black, with blue iridescence. Terga 2–4 black, mate with black pollinosity, with two square yellow maculae not reaching the lateral margin (maculae on tergum 4 might reach lateral margin on anterolateral corner of tergum), yellow pilose except some black pile on tergum 5 and genital segments. Maculae on terga 3 and 4 with anterior margin straight, but oblique. Tergum 4 with a yellow fascia on posterior margin. Tergum 5 mostly yellow with a medial dark macula. Sterna yellow, except a diffuse dark oval macula on sterna 2 and 3, yellow pilose. Male genitalia (Figure 5d–f): Rounded surstylus about as long as cerci. Hypandrium with a long, well-developed lingula (Figure 5e), longer than in male genitalia of A. penai (Figure 4f). Base of aedeagus (basiphallus) of the same shape as in other species of Scaeva with two teeth of similar size (Figure 5e). Distal part of aedeagus (distiphallus) cylindrical without basal bulge in lateral view. Superior lobe (postgonite) elongated with apical margin rounded. Superior lobe pointed dorsally, with anterodorsal corner triangular, and a remarkable tooth situated near distal margin of superior lobe (Figure 5e,f).
Material examined
CHILE: Coquimbo region, 5 miles N of Laguna Dam, 2438 m. (8000 ft.), 06.xii.1950, R. & M. Bacher [1♂, CAS, ZFMK-DIP-00022587].
Differential diagnosis
This species differs from A. melanostoma and A. occidentalis by having the orange-yellow mouth edge (black in A. melanostoma and A. occidentalis), frontal triangle black pilose with pale pilose medially (entirely black pilose in A. melanostoma and A. occidentalis), and a broader abdomen, with terga 3 and 4 two or more times broader than long (in A. occidentalis and A. melanostoma the abdomen is narrower, with terga 3 and 4 about 1.6–1.7 times broader than long). See differential diagnosis under A. penai for the differences between A. patagoniensis and A. penai.
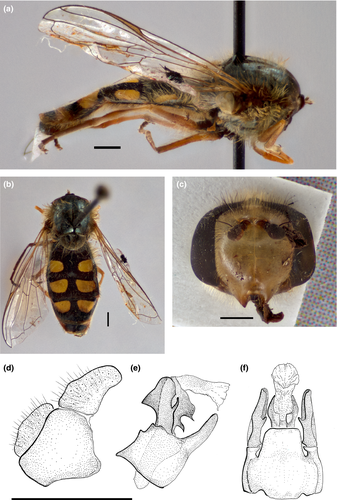
3.2.3 Austroscaeva penai (Marnef in Dušek & Láska, 1985) comb. nov. Figures 3b,c and 4a,b,d–g
Description of the male
Robust, large species. Body length 15 mm; wing length 10.5 mm. Head (Figure 4a,b). Head width about 4.5 mm. Face very broad occupying about 65% of head width, rather produced in profile, entirely yellow, with black pile dorsally and laterally, pale pilose ventrally. Mouth edge mostly yellow; clypeus very long (0.9 mm) and narrow (0.18 mm), broadened at base (0.25 mm). Gena yellow, pale pilose. Eye densely pilose, pile in dorsal part about 0.15 mm long. Area of enlarged facets distinct with marked border. Anterior angle of approximation of eyes wide, about 135°, posterior angle of approximation of eyes about 50°. Anterior ocellus about 0.75 mm distant from the apex of anterior angle of approximation of eyes. Pile on vertical triangle black on anterior 1/2, white on posterior 1/2. Occiput dorsally with row of rather black cilia. Frontal triangle yellow, inflated, with one brown macula at each antennal base, black pilose laterally and pale pilose in median line over antennae. Antenna brown; basoflagellomere dark, paler ventrally, 0.52 mm long and 0.5 mm wide; scape and pedicel 0.5 mm long together, arista 1 mm long. Occiput densely whitish pollinose, with several rows of pale pile, rather broader than in, for example, S. pyrastri.
Thorax (Figures 3b,c and 4b). Black, postpronotum and anterior part of notopleuron pale, densely pale pilose, uniformly long. Pleuron mostly black, except anterior anepisternum pale, posterior anepisternum pale on posterior 1/3, anepimeron yellow dorsally and posteriorly, katatergum yellow. Scutellum entirely yellow, black on anterolateral corners, pale pilose with some black pile on posterior margin; pile on disk 0.4–0.5 mm long, on posterior margin 0.75–0.85 mm long. Metasternum with several (about 15) long pale pile. Plumula pale. Halter pale. Wing: microtrichia reduced as in other species of genus; alula bare. Vein R4+5 almost straight. Wing membrane beyond apical cross-veins rather narrow and irregularly undulated. Legs: coxae and trochanters black, femora pale except dark very basally (1/6 or less), entirely pale pilose, except several black setae on distal ends of tarsomeres.
Abdomen (Figures 3b,c and 4d). Robust, about 6.7 mm long and very broad (4.7 mm), equivalent 70% of the length of abdomen. Terga 3 and 4 more than two times broader than long (tergum 3 about 2.4 times broader; tergum 4 about 2.3 times broader). Terga 2–4 black with two yellow maculae reaching the lateral margin on anterolateral corner of tergum, yellow pilose except posterolateral corners of terga 2–5. Maculae on terga 3 and 4 with anterior margin slightly concave. Tergum 4 with a yellow fascia on posterior margin. Tergum 5 mostly yellow with a medial dark fascia. Sterna yellow, except a diffuse dark oval macula on sterna 2 and 3, yellow pilose. Male genitalia (Figure 4e–g): Like genitalia of other Austroscaeva species, most similar to A. melanostoma but a little larger. Epandrium broader than high (width about 0.75 mm, height about 0.5 mm). Rounded surstylus about as long as cerci. Hypandrium about as high as broad (0.53 mm) with angular distal lateral lobes in ventral view and with a well-developed lingula (Figure 4f). Base of aedeagus (basiphallus) not enlarged, of the same shape as in other species of Scaeva with two teeth of similar size (Figure 4f). Distal part of aedeagus (distiphallus) cylindrical without basal bulge in lateral view. Superior lobe (postgonite) about 0.3 mm high and 0.2 mm broad, forming rounded lobe dorsally in apical part (Figure 4f,g). Basal tooth on superior lobe small, almost inconspicuous; apical tooth distinct and broadly pointed, situated near distal margin of superior lobe.
Material examined
ARGENTINA: Neuquén, Cabecera E Lago Huechulafquen, 16.xii.1997, on Phacelia sp., G.G. Roitman & N.H. Montaldo [1♂, ZFMK, ZFMK-DIP-00019582].
Differential diagnosis
This species differs from A. melanostoma and A. occidentalis by having the orange-yellow mouth edge (black in A. melanostoma and A. occidentalis), metasternum pilose (metasternum bare in A. melanostoma and A. occidentalis), frontal triangle black pilose with pale pilose medially (entirely black pilose in A. melanostoma and A. occidentalis), and a broader abdomen, with terga 3 and 4 more than two times broader than long (in A. occidentalis and A. melanostoma the abdomen is narrower, with terga 3 and 4 about 1.6–1.7 times broader than long). From the male of the close species A. patagoniesis, this species differs as stated in the identification key by having more abundant pile on metasternum, male genitalia (Figures 4e–g and 5d–f), and by the abdominal pattern. Females of. A. penai can easily be distinguished from females of A. patagoniensis by the frontal pilosity (pale pilose medially, black pilose laterally in A. penai, but uniformly black pilose in A. patagoniensis), mesonotal pilosity (uniformly long pile in A. penai, but long and very short pile in A. patagoniensis), and abdominal pattern (see Kassebeer, 1999).
3.2.4 Identification key to the species of Austroscaeva gen. nov. (translated and adapted from Kassebeer, 1999)
1. Scutellum entirely dull, grey pollinose (Figure 1a). Male: eye angle about 120° (Figure 1a)… … … …… ………occidentalis (Shannon)
– Scutellum densely grey pollinose, dull, except shiny mesobasally in dorsal view (Figures 1b and 2b). Male: eye angle broader than 120° (Figures 2c and 4b) ……………………………………… 2
2. Mouth edge and gena black (Figure 2d). Maculae on terga 2–4 with transverse striae of very fine pale pollinosity, in oblique or lateral view (Figures 2b and 3a). Metasternum bare. Smaller species (8.3 to 13.7 mm) ………………………melanostoma (Macquart)
– Mouth edge and gena predominantly orange-yellow (Figures 3c and 5c). Maculae on terga 2–4 without silvery gray pollinosity striae (Figures 3b,c and 5a). Metasternum usually pilose. Large species (12.1 to 15.3 mm) …………………………………………3
3. Maculae on terga 2–4 large, more less square with anterior margin almost straight and oblique, not reaching lateral margins (Figures 4c and 5b). Metasternum bare or only with a few long pile. Alula with a group of microtrichia basally. Female: frontal triangle uniformly black pilose (see Figures 2a,c,d); mesonotum with long and very short pile. Male: frons pale pilose medially, black pilose laterally (Figure 5c). Male genitalia as in Figures 5d–f……………………………………
………………………………………… patagoniensis (Kassebeer)
– Maculae on terga 2–4 narrower, elongated, with anterior margin slightly concave, reaching the lateral margins on the anterolateral corner (Figures 3b and 4d). Metasternum with numerous long pile. Alula bare. Male and female: frons or frontal triangle pale pilose medially, black pilose laterally (Figure 4a,b). Female: mesonotum with uniformly long pile (Figure 3c). Male genitalia as in Figure 4e–g…………………………………………………penai (Marnef)
4 DISCUSSION
The taxon sampling constricts our discussion on the tribal relationships within Syrphinae or on the placement of certain genera because this was not the major goal of our study. Nevertheless, present results agree with earlier phylogenetic molecular works on Syrphidae in general (Mengual, 2015a; Mengual et al., 2008a,b, 2015; Young et al., 2016) and indicate that the traditional systematic arrangement of Syrphinae (with current tribes Bacchini, Syrphini, Paragini, and Toxomerini) is in need of a revision. The tribe Bacchini was resolved in several clades and Paragini was placed within Syrphini, a result reported in the last phylogenetic analyses on Syrphidae (Mengual, 2015a; Mengual et al., 2008a, 2015; Young et al., 2016).
The phylogenetic relationships of the genus Paragus have always been controversial and unclear (Dušek & Láska, 1967; Shatalkin, 1975; Vujić, Ståhls, Rojo, Radenković, & Šimíc, 2008). Mengual et al. (2008a) resolved it as sister group of Allobaccha Curran, 1928, but this placement was probably an artifact as a result of a long-branch attraction effect (Mengual, 2015a). Mengual et al. (2015), using molecular and adult morphological characters, recovered Paragus as the sister group of the Scaeva-Eupeodes clade. The same result was obtained by Young et al. (2016) using anchored-hybrid enrichment data and in the present study. This position is in agreement with Rotheray and Gilbert (1999), who analyzed larval morphological characters. Based on the current evidence, Paragus is largely corroborated as the sister group of the Scaeva-Eupeodes clade.
Our results defined a Scaeva-Eupeodes clade with two major subclades. On one hand, the genera Eupeodes (including Macrosyrphus), Lapposyrphus, and Betasyrphus, but without clear relationships among them. On the other hand, the genera Simosyrphus, Pseudodoros, Ischiodon, Scaeva, Notosyrphus, Austroscaeva gen. nov., and Dioprosopa, with the last three genera forming a well-supported monophyletic group and the set of the other four genera resolved as a clade. Our data supported a new genus concept of Eupeodes, with only two subgenera, that is, Eupeodes s. str. and Macrosyrphus, which is the sister group of all the rest Eupeodes species. There was no evidence to support the subgenus Metasyrphus, as the single species of Eupeodes (Eupeodes) was resolved in the middle of the Eupeodes (Metasyrphus) species, and Eupeodes (Metasyrphus) corollae (Fabricius, 1794) was resolved as the sister group of the remaining species except E. (Macrosyrphus) confrater. The Eupeodes subdivision by Vockeroth (1969), with Metasyrphus and Eupeodes, is not supported. He mentioned three species groups within Metasyrphus: 1) the corollae group with only one species, E. corollae, that differs by the enlarged genitalia; 2) the confrater group, again with a single species and several undescribed ones, which refers to the subgenus Macrosyrphus; and 3) the luniger species groups, including all the other species of the genus. Vockeroth (1969) only kept in Eupeodes its type species, E. volucris. A later concept of Eupeodes by Vockeroth (1973, 1986) with Macrosyrphus and Metasyphus as synonyms is very close to our results. Regarding Macrosyrphus, its position was quite well supported (BS = 89) with a PP = 0.93, but its ranking as a synonym of Eupeodes, as a subgenus or as a valid genus, seems only a subjective opinion.
Lapposyrphus is a separate taxon and a valid genus always recovered as an independent lineage from Eupeodes in previous molecular studies (Mengual, 2015a; Mengual et al., 2008a). It is most likely related to Eupeodes and Betasyrphus, although our analysis did not resolve its relationships among them (Figures 1 and S2). Another interesting data source to separate these taxa is their karyology. As in Scaeva and Austroscaeva gen. nov., the number of chromosomes of Lapposyrphus and Eupeodes (including Metasyrphus) is different (Boyes et al., 1971). Lapposyrphus lapponicus has five pairs of chromosomes plus a small XY pair, while Eupeodes species have four pairs plus XY (Boyes et al., 1971). It must be noted that Syrphus aberrantis placed under Lapposyrphus by Vockeroth (1969) has only four pairs of chromosomes as all the studied species of Eupeodes. The placement of S. aberrantis needs further studies to corroborate its generic affinities.
Vockeroth (1969), however, suggested that Betasyrphus was probably related to those genera with a well-developed lingula and a margined abdomen (Syrphus, Epistrophe, Dasysyrphus Enderlein, 1938, Scaeva and Eupeodes, as Metasyrphus). Rotheray and Gilbert (1999) resolved Betasyrphus as sister to Episyrphus based on larval morphological characters, but Mazánek, Láska, Bičík, Dušek, and Novotný (1999) pointed out the similarity of Betasyrphus puparia to those of Scaeva and Eupeodes. More recently, Young et al. (2016) recovered Betasyrphus as sister group of Scaeva. Our results placed Betasyrphus either as sister group of Eupeodes (PP = 0.90; Figure S2) or as sister group of the rest of the Scaeva-Eupeodes clade without support (Figure 1).
There is a Neotropical radiation within the Scaeva-Eupeodes clade. All the Neotropical taxa, except the Neotropical species Eupeodes rojasi Marnef (1999), were resolved in a well-supported clade (BS = 95) with a PP = 1 as (Notosyrphus + (Dioprosopa + Austroscaeva). Vockeroth (1969) stated that Notosyrphus was certainly related to Dasysyrphus, although it shared the pilosity pattern of the katepisternum and the reduction in the wing microtrichia with Scaeva. Present results did not support a close relationship of Notosyrphus with Dasysyrphus, but with other Neotropical taxa of the Scaeva-Eupeodes clade.
Hull (1949) erected Dioprosopa as a new subgenus of Baccha Fabricius, 1805 for the species Syrphus clavatus Fabricius, 1794 based on the forward produced face. Thompson, Vockeroth, and Sedman (1976) synonymized Dioprosopa under Pseudodoros, although the distribution of Dioprosopa (New World) does not overlap with the geographic distribution of Pseudodoros (West Mediterranean, Iran, and Afrotropical region) (Dirickx, 1994; Gilasian, Vujić, Hauser, & Parchami-Araghi, 2017; Thompson, 2013; Van Eck & Makris, 2016). In his very detailed revisionary work, Kassebeer (2000) described a new species of Dioprosopa and explained and illustrated the differences between Dioprosopa and Pseudodoros. Mengual et al. (2008a) listed Dioprosopa as a subgenus of Pseudodoros and recovered Dioprosopa as sister group of (Ischiodon + Scaeva), but Mengual (2015) resolved Dioprosopa together with Lapposyrphus as sister group of the Scaeva-Eupeodes clade. Albeit some authors consider the separation of Dioprosopa and Pseudodoros an “excessive splitting” (Sinclair, Thompson, & Wyatt, 2016), the taxa are not closely related. Present results did confirm the validity of both as separate taxa with generic rank, and most importantly, the placement of Pseudodoros in our results helped to solve the problem between Ischiodon and Simosyrphus.
The genus Ischiodon was synonymized under Simosyrphus based on the similar larvae and puparia and the sister group relationship of these two taxa based on part of the COI gene (Láska et al., 2006). This close relationship of Ischiodon and Simosyrphus was also noted by Vockeroth (1969) and reported by Mengual (2015a). However, the addition of Pseudodoros in the molecular study revealed that both taxa are valid and corroborated the study of Kassebeer (2000), who considered Pseudodoros and Dioprosopa as separate genera. Thus, we resurrect the genus Ischiodon as a separate taxon from Simosyrphus with generic rank.
The three species groups of Scaeva as defined by Dušek and Láska (1985) were recovered in our analysis. Neotropical species were resolved as sister group of Dioprosopa, and a new genus, Austroscaeva gen. nov., has been erected for them. The other two species groups concur with the suggested Scaeva subgenera by Láska et al. (2013). Subgenera Scaeva s. str. and Semiscaeva were resolved with high support (BS = 100) and a PP = 1, but the relationship among these two subgenera and the clade (Simosyrphus + (Pseudodoros + Ischiodon)) was not. ML tree (Figure 1) did resolve Scaeva s. str. as the sister group of (Simosyrphus + (Pseudodoros + Ischiodon)) (BS = 51), in the same way as Mengual et al. (2008a) and Mengual (2015a), but the Bayesian inference placed Scaeva s. str. and Semiscaeva as sister group (PP = 0.63). The support is too low to make conclusions, and both placements seem plausible with the current evidence. Among the studied Scaeva species, the placement of S. mecogramma (Bigot, 1860) must be further discussed. Vockeroth (1969) did not study this species, and most of the previous identification keys to genera did not key properly out this taxon (Speight, 2016). This is a very peculiar Scaeva species with yellow fascia on abdominal terga 3 and 4, instead of the typical oblique and often lunulate abdominal maculae; so distinct from other Scaeva species that even Kuznetzov (1985) erected a subgenus only for it. Speight (2016) argued that S. mecogramma shared only one character with the other Scaeva species, the posterolateral continuity between the dorsal and ventral pile patches on the katepisternum, but this character also occurs in other genera, such as Dasysyrphus. In his argumentation, Speight (2016) doubted about the generic affinity of S. mecogramma and proposed a closer relationship of this species with Betasyrphus. Dušek and Láska (1985) and Láska et al. (2013) included S. mecogramma in the subgenus Semiscaeva (equivalent to the selenitica species group) and our results agreed with this placement.
During our study, we found two species names to refer to the same taxon, that is, Scaeva lunata (Wiedemann, 1830) and Scaeva opimia (Walker, 1852). There are two valid and different species originally described as Syrphus lunatus: Syrphus lunatus Fabricius, 1794 [currently Eumerus lunatus (Fabricius, 1794)], and Syrphus lunatus Wiedemann, 1830 [currently Scaeva opimia (Walker, 1852)]. Following the articles 57.2 and 60.2, a junior primary homonym is permanently invalid and has to be replaced by the oldest valid synonym if available, with its own authorship and date. In such case, Syrphus lunatus Wiedemann, 1830 is invalid, and its synonym Syrphus opimius Walker, 1852 must be used for this valid taxon as Scaeva opimia (Walker, 1852) (Dušek & Láska, 1985; Thompson, 2013).
As a novelty based on molecular characters, and outside the Scaeva-Eupeodes clade, our results recovered the genus Doros as sister group of Xanthogramma. Both genera resemble morphologically, and this relationship has been already suggested based on larval morphological characters (Rotheray & Gilbert, 1999) and on adult and genital characters (Dušek & Láska, 1967; Shatalkin, 1975). Olbiosyrphus Mik, 1897 was erected for Syrphus laetus Fabricius, 1794 (= Xanthogramma laetum) based on the presence of pile on eyes. Present results did not support the separation between Xanthogramma and Olbiosyrphus but agreed with Vockeroth (1969) who synonymized Olbiosyrphus under Xanthogramma.
5 CONCLUSION
The relationships of the Scaeva-Eupeodes clade of genera were studied, and the monophyly of all the genera was well supported, except for Scaeva and Eupeodes (Macrosyrphus) as part of Eupeodes. The two proposed subgenera of Scaeva by Láska et al. (2006), that is, Scaeva and Semiscaeva, were resolved as monophyletic groups with high support but not necessarily as sister groups. Scaeva subgenera were placed together with the clade (Simosyrphus + (Pseudodoros + Ischiodon)) in the same well-supported group, although the relationships between the Scaeva subgenera and this clade of genera were resolved with very low support.
Subgeneric division of Eupeodes should include only two taxa, Eupeodes s. str. and Macrosyrphus. Betasyrphus, Lapposyrphus, and Eupeodes were resolved as related, and our analysis resulted in two other very well-supported clades, that is, all Neotropical genera in one hand, and Scaeva, Simosyrphus, Pseudodoros, and Ischiodon on the other hand. Based on adult morphological characters and the present molecular results, a new genus was erected for the Neotropical species previously placed within Scaeva, namely Austroscaeva gen. nov. Despite the well-supported monophyly of each genus, the relationships among the genera belonging to the Scaeva-Eupeodes clade need further study as the support values for some nodes were very low. At the same time, we hope that immature stages of Austroscaeva gen. nov. become available to study their morphology and the biology of these Neotropical species, resulting in a better understanding of their phylogenetic relationships.
ACKNOWLEDGEMENTS
We thank collectors who sent us material for this molecular work, especially Nicolas Bazin and Martin Speight for providing material of Scaeva mecogramma, and Jean-Pierre Sarthou for making Doros species available for this study. Also, thanks to Elvira Rättel (MZH, Helsinki) and Claudia Etzbauer (ZFMK, Germany) for their support while obtaining molecular data. We are very grateful to Trevor O. Burt for the excellent drawings of male genitalia used in Figures 4 and 5. We thank Jeff Skevington and an anonymous reviewer for improving our manuscript with their comments and suggestions.




