The leaf turtle population of Phnom Kulen National Park (northwestern Cambodia) has genetic and morphological signatures of hybridization
Abstract
Cambodia is known to harbour three distinct species of Southeast Asian leaf turtles (Cyclemys spp.), which are heavily traded and common in seizures of wildlife. Confiscated leaf turtles are often released to natural habitats. Thus, an exact knowledge of the distribution of the individual species is of great importance to avoid the introduction of non-native turtles, posing the risk of competition and hybridization. In this study, we examine a recently discovered leaf turtle population from Phnom Kulen National Park using external morphology, 17 unlinked microsatellite loci and the mitochondrial cytochrome b gene. Leaf turtles from the Phnom Kulen National Park morphologically resemble C. oldhamii, but harbour mitochondrial haplotypes of C. atripons. With respect to microsatellite loci, the turtles are distinct from C. atripons. Unfortunately no material of C. oldhamii was available from Cambodia. We propose that the Phnom Kulen population represents either a natural hybrid swarm of C. atripons and C. oldhamii or a distinct undescribed species with introgressed mitochondria of C. atripons. This underlines that genetic differentiation of wild leaf turtle populations in Cambodia is complex and suggests that this differentiation pattern becomes increasingly threatened by translocations of confiscated individuals. For drawing a definite conclusion about the taxonomic status of the Phnom Kulen population, denser sampling of other Cambodian leaf turtle populations would be required, in particular of C. oldhamii.
Introduction
Until recently, the Southeast Asian leaf turtle genus Cyclemys (Testudines: Geoemydidae) was thought to contain only one or two species, Cyclemys dentata (Gray, 1831) and C. tcheponensis (Bourret, 1939). However, starting in the mid-1990s, several studies resurrected and described additional leaf turtle species (Fritz et al. 1996, 1997, 2008; Iverson and McCord 1997; Stuart and Fritz 2008), so that currently seven distinct species are recognized (van Dijk et al. 2014). Cambodia is thought to harbour three species: C. atripons Iverson and McCord, 1997; C. oldhamii Gray, 1863 and C. pulchristriata Fritz et al., 1997; although the exact distribution ranges are not well understood (van Dijk et al. 2014). Yellow-bellied leaf turtles from southwestern Cambodia represent C. atripons, while yellow-bellied leaf turtles from the southeast of the country belong to C. pulchristriata, a species found mainly in Vietnam. Dark-bellied leaf turtles from the inland regions of Cambodia are assigned to C. oldhamii (Iverson and McCord 1997; Fritz et al. 2008; Stuart and Fritz 2008; van Dijk et al. 2014).
Leaf turtles are among the more frequently encountered turtle species in Cambodia, as indicated both by wildlife surveys and confiscations from the illegal wildlife trade (Som et al. 2006; Som and Kheng 2007; TRAFFIC 2014). They can be found in various freshwater habitats, including rivers and streams, swamps, grassland ponds and flooded rice paddies. Adult leaf turtles also utilize terrestrial habitats nearby freshwater (Durkin 2012). Leaf turtles are heavily traded and common in seizures of smuggled wildlife en route from Cambodia to food and traditional medicine markets in Vietnam, indicating that wild populations continue to be depleted. In 2013, all Cyclemys species were placed onto CITES Appendix II (CITES 2013). In Cambodia, confiscated turtles are often released (pers. observ.). To avoid introduction of non-native turtles with the risks of competition and hybridization, the exact knowledge of the local distribution of the different leaf turtle species is of great importance.
Durkin et al. (2010) reported dark-bellied leaf turtles from Phnom Kulen National Park in northwestern Cambodia which they tentatively identified as C. aff. atripons. However, the turtles' morphology conflicted with this determination, and based on coloration and pattern, Kim (2011) and Durkin (2012) assigned the Phnom Kulen population to C. oldhamii. Phnom Kulen National Park is located in between the known distribution ranges of C. atripons and C. oldhamii and is geographically isolated in terms of Cyclemys records. Thus, the taxonomic identity of the Phnom Kulen population remains unclear. In this study, we compare Cyclemys samples from Phnom Kulen National Park with previously published mitochondrial DNA (mtDNA) sequence data of all currently recognized Cyclemys species. In addition, we compare the Phnom Kulen turtles with representatives of C. atripons by genotyping them at 17 unlinked microsatellite loci and compare their coloration and pattern with C. atripons and C. oldhamii.
Materials and Methods
Sampling and laboratory procedures
Samples from 62 leaf turtles were used for analysis (Appendix). Twelve of these were Cyclemys atripons, most of which had been previously studied (Fritz et al. 2008). These turtles conform morphologically to the original species description (Iverson and McCord 1997). The remaining 50 individuals were sampled between 2010 and 2012 at Kbal Spean in western Phnom Kulen National Park, northwestern Cambodia. The turtles were captured using baited funnel traps set in the Kbal Spean River (Durkin et al. 2010; Kim 2011; Durkin 2012). Samples were either tail tips or marginal scute clippings stored in 95% ethanol. DNA was extracted using the innuPREP DNA Mini Kit (Analytik Jena AG, Jena, Germany) or the NucleoSpin Tissue Kit (Macherey-Nagel, Düren, Germany).
As a mitochondrial marker, the cytochrome b gene (cyt b) was sequenced for 45 freshly collected samples (Appendix) using the PCR primers CytbG (Spinks et al. 2004) and mt-E-Rev2 (Fritz et al. 2006) or mt-c-For2 and mt-f-na (Fritz et al. 2006). PCR was performed in a final volume of 20 μl using 1 unit DFS-Taq polymerase (Bioron GmbH, Ludwigshafen, Germany) with the buffer recommended by the supplier (2 μl PCR buffer 10× incl. MgCl2) and a final concentration of 0.2 mM of each dNTP (Thermo Fisher Scientific Biosciences GmbH, St. Leon-Rot, Germany), 0.5 μM of each primer (biomers.net GmbH, Ulm, Germany) and approximately 10–40 ng of total DNA. For PCR, an initial 5-min-long denaturation step at 95°C was followed by 35–40 cycles with denaturation at 95°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 1 min and a final elongation step of 10 min at 72°C. PCR products were purified using the ExoSAP-IT enzymatic clean-up (Affymetrix USB, Cleveland, OH, USA; modified protocol: 0.2 μl ExoSAP-IT per sample 30 min at 37°C, 15 min at 80°C) and sequenced using the forward primer mt-c-For2 and the reverse primer mt-E-Rev2 on an ABI 3730 Genetic Analyzer (Life Technologies, Carlsbad, CA, USA) using the BigDye Terminator v3.1 Cycle Sequencing Kit (Life Technologies). Cycle sequencing reactions were purified by gel filtration using the Performa DTR V3 96-Well Short Plate Kit (EdgeBio, Gaithersburg, MD, USA) and 400 μl of a 5% Sephadex solution (GE Healthcare, München, Germany). DNA sequences were aligned using bioedit 7.0.5.3 (Hall 1999) and trimmed to a length of 1166 bp. This fragment comprised the complete cyt b gene (1140 bp) plus adjacent DNA coding for tRNA-Thr (26 bp).
In addition, 17 unlinked microsatellite loci (Table S1) were analysed for each sample. Two to four loci each were combined in multiplex PCRs; one locus (GP81) was processed alone (Table S1). The final volume of each PCR was 10 μl, containing 0.5 unit Taq polymerase (Bioron, Ludwigshafen, Germany) with the incomplete buffer recommended by the supplier and a final concentration of 1.5 mM MgCl2 (Bioron), 0.2 mM of each dNTP (Fermentas, St. Leon-Rot, Germany), 2.5 μg bovine serum albumin (Fermentas), approximately 10–20 ng of total DNA, 0.25 μM forward and reverse primers. Forward primers were fluorescent-labelled (Table S1). PCR cycling conditions were: After 5-min denaturation at 94°C, 35 cycles were run with denaturation at the same temperature for 45 s, annealing as in Table S1 for 1 min and elongation at 72°C for 1 min, followed by a 10-min-long final elongation step. PCR products were diluted with water in a ratio of 1:100. Fragment lengths were determined using an ABI 3730 Genetic Analyzer, the GeneScan-600 LIZ Size Standard and the software genemapper (all: Life Technologies).
Data analysis
mtDNA
Phylogenetic relationships of previously published mtDNA sequences (Fritz et al. 2008) combined with the newly generated data from this study (Appendix) were inferred by Maximum Likelihood (ML) analyses using RAxML 7.2.6 (Stamatakis 2006), the implemented evolutionary GTR + G model, and a GenBank sequence of Heosemys spinosa (AY434578) as outgroup. Five independent ML searches were performed with different starting conditions and the fast bootstrap algorithm to explore the robustness of the results by comparing the best trees. Subsequently, 1000 non-parametric thorough bootstrap replicates were calculated and the values plotted against the best tree.
Using the aligned sequence data, the mutational relationships of haplotypes were also displayed as a parsimony network with TCS 1.21 (Clement et al. 2000).
Microsatellites
For the Phnom Kulen turtles and the Cyclemys atripons studied, pairwise linkage disequilibrium between loci and Hardy–Weinberg equilibrium were tested using arlequin 3.5.2.1 (Excoffier and Lischer 2010). Furthermore, the 17 microsatellite loci were analysed using an unsupervised Bayesian clustering approach as implemented in structure 2.3.4 (Pritchard et al. 2000; Hubisz et al. 2009). In doing so, the admixture model and correlated allele frequencies were used. structure searches in the data set for partitions which are, as far as possible, in Hardy–Weinberg equilibrium and linkage equilibrium. Unsupervised analyses were chosen because this approach clusters samples strictly according to their genetic information, but without any presumptions about population structuring (e.g. geographical distances, sampling sites). Because microchecker 2.2.3 (van Oosterhout et al. 2004) suggested the presence of null alleles for three loci (Mauca01, Maucas14, Test56) in the Phnom Kulen population, the data set was corrected for null alleles according to Falush et al. (2007). All calculations were run for K = 1–10, and the most likely number of clusters (K) was determined using the ∆K method (Evanno et al. 2005) with structure harvester (Earl and vonHoldt 2012). Calculations were repeated 10 times for each K using a MCMC chain of 750 000 generations for each run, including a burn-in of 250 000 generations. Population structuring and individual admixture were then visualized using distruct 1.1 (Rosenberg 2004).
Diversity within the Phnom Kulen population and comparison with Cyclemys atripons
For the Phnom Kulen population and Cyclemys atripons, diversity and divergence parameters were estimated using microsatellite data. For doing so, number and size of microsatellite alleles were compared by a frequency table produced in convert 1.31 (Glaubitz 2004). For inferring locus-specific observed (HO) and expected heterozygosities (HE) and for performing a locus-by-locus analysis of molecular variance (AMOVA; 10 000 permutations), arlequin 3.5.2.1 was used. Locus-specific excess or deficiency of heterozygotes as expressed by the inbreeding coefficient FIS (Weir and Cockerham 1984) was calculated with fstat 2.9.3.2 (Goudet 1995). The same software was also used for computing values for locus-specific allelic richness and testing statistical significance of FIS for each locus and across all loci using randomizations and Bonferroni correction (Rice 1989).
Coloration and pattern
From each turtle from Phnom Kulen National Park, morphometric characters were taken to the nearest millimetre using callipers. The animals were weighed to the nearest gram using spring scales or electronic scales and uniquely marked using either a microchip, a marginal scute notch or both. Each turtle was photographed in dorsal and ventral aspect. In addition, photographs of the temporal region were taken, where possible. Recapture of marked turtles over several years allowed observations of the progression of plastral patterning, particularly in younger individuals that show more growth and change from year to year.
Results
Mitochondrial relationships
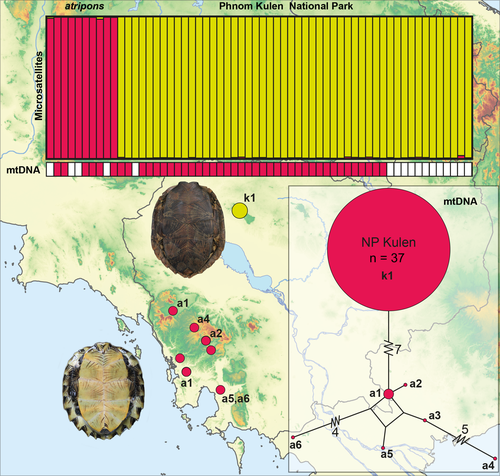
All individuals of the Phnom Kulen population yielded the same mitochondrial haplotype (k1; Fig. 1; Appendix) which differed in parsimony network analysis by seven mutational steps from the haplotypes of Cyclemys atripons. However, among the haplotypes of C. atripons, up to 12 mutations were observed (Fig. 1). Accordingly, in ML analyses, the haplotype of the Phnom Kulen population clustered with maximum support with the sequences of C. atripons and within this clade was sister to the remaining haplotypes (Fig. S1).
Population genetic analyses using microsatellite data
The ∆K method of Evanno et al. (2005) revealed K = 2 as the optimal number of structure clusters (Fig. S2), with one cluster corresponding to leaf turtles from the Phnom Kulen population and the other to Cyclemys atripons, without any indication of admixture (Fig. 1). Across the two clusters, the numbers of alleles per locus ranged from 1 to 22; from a total of 165 alleles, 114 private ones were found. Genetic diversity indices of the two clusters were similar, despite the difference in the sample size. In the Phnom Kulen population, pronounced linkage disequilibrium (6%) was evident (Tables 1 and 2). The Phnom Kulen population and C. atripons differed by an FST value of 0.21; thus, 21% of the observed global molecular variance occurred among and 79% within clusters.
| Locus | n A | AR | H O | H E | F IS | HWE | Null alleles | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PKNP | atripons | PKNP | atripons | PKNP | atripons | PKNP | atripons | PKNP | atripons | PKNP | atripons | PKNP | atripons | |
| Maucas01 | 6 | 7 | 4.000 | 5.539 | 0.34694 | 0.30000 | 0.49548 | 0.82632 | 0.302* | 0.649* | No | No | Yes | Yes |
| Maucas06 | 4 | 9 | 7.918 | 3.679 | 0.73469 | 0.80000 | 0.67873 | 0.60526 | −0.083 | −0.346 | Yes | Yes | No | No |
| Maucas12 | 2 | 4 | 2.940 | 2.000 | 0.50000 | 0.50000 | 0.40343 | 0.39474 | −0.242 | −0.286 | Yes | Yes | No | No |
| Maucas14 | 9 | 12 | 8.000 | 7.414 | 0.58000 | 0.80000 | 0.80081 | 0.87895 | 0.278* | 0.094 | No | Yes | Yes | No |
| Maucas17 | 5 | 5 | 2.000 | 4.618 | 0.22000 | 0.90000 | 0.28505 | 0.77368 | 0.230 | −0.174 | Yes | Yes | No | No |
| Maucas18 | 6 | 11 | 8.937 | 5.865 | 0.90000 | 0.90000 | 0.82768 | 0.86316 | −0.088 | −0.045 | Yes | Yes | No | No |
| Test56 | 5 | 6 | 4.000 | 4.660 | 0.22000 | 0.11111 | 0.55697 | 0.62092 | 0.607* | 0.830* | No | No | Yes | Yes |
| Maucas20 | 5 | 5 | 2.000 | 4.603 | 0.58000 | 0.70000 | 0.47091 | 0.75263 | −0.235 | 0.074 | Yes | Yes | No | No |
| Emys2 | 10 | 16 | 10.000 | 8.100 | 0.98000 | 0.90000 | 0.88646 | 0.89474 | −0.107 | −0.006 | Yes | Yes | No | No |
| MR3 | 5 | 13 | 8.937 | 5.000 | 0.80000 | 0.28571 | 0.73859 | 0.82418 | −0.084 | 0.671* | Yes | No | No | Yes |
| GP19 | 1 | 4 | 3.937 | 1.000 | 0.42000 | n/a | 0.44949 | n/a | 0.066 | n/a | No | Yes | No | No |
| GmuD16 | 5 | 9 | 7.000 | 4.333 | 0.79167 | 0.44444 | 0.79386 | 0.66667 | 0.003 | 0.347 | Yes | Yes | No | No |
| GmuB08 | 5 | 6 | 5.000 | 4.511 | 0.80000 | 0.33333 | 0.72707 | 0.61438 | −0.101 | 0.473* | Yes | No | No | Yes |
| Test10 | 11 | 22 | 17.835 | 9.391 | 0.93878 | 1 | 0.91269 | 0.93464 | −0.029 | −0.075 | Yes | Yes | No | No |
| TWL221 | 3 | 4 | 2.997 | 2.778 | 0.20000 | 0.55556 | 0.18505 | 0.54248 | −0.082 | −0.026 | Yes | Yes | No | No |
| TWI61 | 6 | 20 | 16.814 | 5.072 | 0.94000 | 0.55556 | 0.91273 | 0.56209 | −0.030 | 0.012 | Yes | Yes | No | No |
| GP81 | 9 | 12 | 6.000 | 7.954 | 0.72340 | 0.88889 | 0.70991 | 0.87582 | −0.019 | −0.016 | Yes | Yes | No | No |
- PKNP, Phnom Kulen National Park population; atripons, Cyclemys atripons; nA, number of alleles; AR, allelic richness; HO, average observed heterozygosity; HE, average expected heterozygosity; FIS, inbreeding coefficient; HWE, Hardy–Weinberg equilibrium. FIS values indicated by an asterisk are statistically significant. Bold script highlights absence of HWE or presence of null alleles, respectively.
| n | n A | n Ā | n p | AR | H O | H E | F IS | LD | |
|---|---|---|---|---|---|---|---|---|---|
| PKNP | 50 | 97 | 5.71 | 68 | 6.960 | 0.63 | 0.64 | 0.02 | 6.00 |
| C. atripons | 10 | 165 | 9.71 | 46 | 5.089 | 0.59 | 0.68 | 0.13* | 0.72 |
- PKNP, Phnom Kulen National Park population; n, number of individuals; nA, number of alleles; nĀ, average number of alleles; nP, number of private alleles; AR, allelic richness; HO, average observed heterozygosity; HE, average expected heterozygosity; FIS, average inbreeding coefficient; LD, linkage disequilibria, per cent of pairwise comparisons between microsatellite loci. The FIS value with an asterisk is statistically significant.
Coloration and pattern
The leaf turtles from Phnom Kulen National Park had straight carapace lengths from 5.8 to 21.0 cm. All corresponded in coloration and pattern perfectly to the eastern coloration morph of Cyclemys oldhamii as described in Fritz et al. (2008). Phnom Kulen leaf turtles had a speckled pattern on the crown of the head and salmon striping on the neck on a dark background. The throat typically showed a mottled dark pattern on salmon background. The carapace of Phnom Kulen turtles was generally dark brownish. The plastron showed in the smallest individuals a spotted pattern on yellow background. With increasing size and age, this pattern was replaced by densely radiating dark streaks on each plastral scute. Aged individuals showed a gradient of patterns from dense dark brown radiations on a yellow background to a uniformly dark coloured plastron, as typical for C. oldhamii (Fig. 2).

Discussion
The taxonomy and species number of Southeast Asian leaf turtles was recently much in flux, with several species described as new to science or resurrected from synonymy (Fritz et al. 1996, 1997, 2008; Iverson and McCord 1997). The leaf turtles of Phnom Kulen National Park morphologically closely resemble Cyclemys oldhamii, a dark-bellied leaf turtle species which has been recorded at a distance of approximately 190 km from the national park in the Prey Long forest of central Cambodia (Som and Kheng 2007). Yet, in phylogenetic analyses the mitochondrial haplotypes of the Phnom Kulen turtles do not cluster with C. oldhamii (Fig. S1), but with C. atripons, another leaf turtle species that is morphologically clearly distinct and known to occur in the Cardamom Mountains of southwest Cambodia (Stuart and Platt 2004; Som et al. 2006). The nearest unambiguous record of C. atripons to Phnom Kulen National Park are two specimens collected approximately 175 km away in Phnom Samkos Wildlife Sanctuary in the Cardamom Mountains and deposited at the Centre for Biodiversity Conservation in Phnom Penh under collection numbers CBC 00576 and CBC 01515. However, a questionable extraterritorial record for C. atripons has recently been published from Krong Samraong District in northwestern Cambodia (Brakels et al. 2016), approximately 90 km from the park.
Cyclemys atripons belongs to a phylogenetically well-supported clade comprised of three yellow-bellied species, C. atripons, C. dentata and C. pulchristriata. Cyclemys atripons is the sister taxon to C. dentata, a species distributed across the Great Sunda Islands and the Malay Peninsula (Fritz et al. 2008). The leaf turtles from Phnom Kulen National Park harbour mitochondrial haplotypes of C. atripons, but our analyses of microsatellite DNA provide evidence that they are genetically distinct from the latter species. Thus, we can dismiss the hypothesis that the Phnom Kulen turtles belong to the species C. atripons and that this species is polymorphic. Considering that the Phnom Kulen turtles are morphologically indistinguishable from C. oldhamii, it seems reasonable to hypothesize that the Phnom Kulen population represents a hybrid swarm of C. atripons and C. oldhamii. This hypothesis is also supported by the pronounced linkage disequilibrium found in the Phnom Kulen turtles and by the lack of Hardy–Weinberg equilibrium at several loci (Tables 1 and 2), as typical for hybridized populations. That also some loci of C. atripons are not in Hardy–Weinberg equilibrium is not astonishing because our sample of this species is very small and does not represent a natural population (unlike the Phnom Kulen turtles), but an assemblage of individuals from several distinct populations.
The illegal wildlife trade in Southeast Asia is extensive, and confiscations over the last two decades have included many hundreds of kilograms of live turtles and tortoises being smuggled from Cambodia to Vietnam and China for the food and traditional medicine markets (Compton 2000; own unpubl. observ.). Seizures of live animals are often released back into the wild without knowledge of their origin. For example, in 2008, 419 kg of live pythons and turtles of uncertain origin confiscated in Battambang, Cambodia, were released to the wild, including the Tonle Sap lake (TRAFFIC 2014). This practice has obvious implications for the genetic integrity of species and can threaten the persistence of morphologically or genetically distinct populations through gene pool mixing (Laikre et al. 2010). In certain conservation contexts, a low level of assisted immigration can produce fitness benefits for populations with outbreeding depression (‘genetic rescue’; Tallmon et al. 2004), but for species that do not require immediate conservation intervention, gene pool mixing can erode evolutionarily significant units (ESUs) and complicate baseline taxonomic work. While to our knowledge no bulk releases of leaf turtles have occurred in Phnom Kulen National Park, the study site Kbal Spean is a culturally significant site, with a history of ceremonial human presence dating back over 1000 years (Freeman et al. 1999). In Cambodia, the tradition of releasing captured or purchased turtles for religious reasons persists today (Ihlow et al. 2016). Whether releases of turtles occurred at Kbal Spean over the centuries due to human intervention is unknown. However, it is unlikely that the genetic situation found in the Phnom Kulen population is caused by the translocation of a few alien leaf turtles because the entire population seems affected. All studied leaf turtles were genetically similar in that they harboured the same mitochondrial haplotype and were assigned to the same cluster in structure analyses. In addition, both the Phnom Kulen population and C. atripons possess many private alleles (Table 2). This would not be expected if the Phnom Kulen population is derived from recent hybridization because in the latter case, a high number of shared alleles with C. atripons would be expected.
Unfortunately, no samples of Cambodian C. oldhamii were available for genetic examination, and our conclusion that the Phnom Kulen turtles are a hybrid swarm remains somewhat speculative. In particular, the inferred genetic impact of C. oldhamii is backed only by morphology. However, morphology alone is very convincing in this case and introgressed mitochondria have been previously described for another pair of yellow-bellied and dark-bellied Cyclemys species, C. dentata and C. enigmatica. The yellow-bellied C. dentata occurs throughout its distribution range sympatrically with a dark-bellied leaf turtle of which only few individuals have been characterized genetically (Fritz et al. 2008). All of these turtles yielded mitochondrial haplotypes of C. dentata, while nuclear genomic markers were distinct, which is why Fritz et al. (2008) described these dark-bellied turtles as the distinct species C. enigmatica. Thus, we also cannot exclude the possibility that the Phnom Kulen turtles represent an undescribed species with introgressed mitochondria from C. atripons. However, for the time being we refrain from taxonomic consequences. Further research, including extensive sampling of other leaf turtle populations from Cambodia, especially of C. oldhamii, is needed to clarify this situation.
Our study highlights that the taxonomy of leaf turtles is still incompletely known and that additional investigations will contribute to a significantly better understanding of their taxonomic and genetic diversity. Even though our results do not support the hypothesis that the observed genetic constitution of the leaf turtle population in Phnom Kulen National Park has been noticeably impacted by the introduction of leaf turtles from elsewhere, we provide evidence for a complicated genetic variation in wild populations that may become increasingly threatened by translocations of confiscated individuals.
Acknowledgements
We are grateful to H. E. Chay Smith (General Director of the General Department for Administration of Nature Conservation and Protection GDANCP, Ministry of Environment of the Royal Government of Cambodia) for kindly issuing the relevant permits. We thank Sy Ramony (Director of Department of National Park and Wildlife Sanctuary Director of Department of National Park and Wildlife Sanctuary of GDANCP, Ministry of Environment) and Hong Daravuth (Deputy Director of the Department of National Park and Wildlife Deputy Director of the Department of National Park and Wildlife Sanctuary of GDANCP and Director of Protected Areas in Siem Reap Province, Ministry of Environment) for their generous support. Field work in Phnom Kulen National Park was partially funded by Brian Malone (Department of Ecology, Environment and Evolution, La Trobe University) and the Angkor Centre for Conservation of Biodiversity (ACCB). David Emmett (Conservation International) provided samples of Cyclemys atripons. Thanks for lab work go to Anja Rauh (Senckenberg Dresden).
Appendix:
Studied leaf turtle samples, geographical sampling localities (WGS84), mitochondrial haplotypes (mtDNA, see Fig. 1) and cluster assignment in structure analyses. All leaf turtles from Phnom Kulen National Park yielded the same mitochondrial haplotype (ENA accession number LT595721); the newly sequenced sample of Cyclemys atripons (8588) had the same haplotype as a previously published turtle (AM931626). For accession numbers of other previously published mtDNA sequences, see Fritz et al. (2008).
| Sample | Locality | mtDNA | K = 2 | X | Y |
|---|---|---|---|---|---|
| 17 | Unknown | n/a | Red | n/a | n/a |
| 1382 | Cambodia: Koh Kong: Sre Ambel District: Sre Ambel | a6 | Red | 11.1222 | 103.7458 |
| 1383 | Cambodia: Koh Kong: Sre Ambel District: Sre Ambel | a5 | n/a | 11.1222 | 103.7458 |
| 4487 | Cambodia: Koh Kong: Tatai River, Central Cardamoms Protection Forest | a4 | Red | 11.8214058 | 103.53540227 |
| 4488 | Cambodia: Koh Kong: upper Tatai River, Central Cardamoms Protection Forest | n/a | Red | 11.8214058 | 103.53540227 |
| 4489 | Cambodia: Koh Kong: camp on Areng River, Central Cardamoms Protection Forest | n/a | Red | 11.6373879 | 103.5730672 |
| 4490 | Cambodia: Koh Kong: mid-reaches of the Tatai River, Central Cardamoms Protection Forest | a2 | Red | 11.6878695 | 103.6110616 |
| 4491 | Cambodia: Koh Kong: coastal forests, southern Cardamoms, near Trapeang Rung, estuary | a1 | Red | 11.3775173 | 103.2586019 |
| 4492 | Cambodia: Koh Kong: coastal forests, southern Cardamoms, near Trapeang Rung, estuary | a1 | Red | 11.3775173 | 103.2586019 |
| 4493 | Cambodia: Koh Kong: Tatai Krom village, flooded forest near river estuary, southern Cardamoms | n/a | Red | 11.5578674 | 103.1414840 |
| 8588 | Cambodia: Koh Kong: Phnom Samkos Wildlife Sanctuary, northern Cardamom Mountains | a1 | Red | 12.20419 | 103.07009 |
| 42 516 | Unknown | a3 | n/a | n/a | n/a |
| 6412 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | yellow | 13.68436609 | 104.0169064 |
| 6413 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.68175921 | 104.020699 |
| 6415 | Cambodia: Kbal Spean River, Phnom Kulen National Park | n/a | Yellow | 13.68660597 | 104.0186077 |
| 6416 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.6949395 | 104.0112127 |
| 8548 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.68675 | 104.01539 |
| 8549 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.68723 | 104.01516 |
| 8550 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.68723 | 104.01516 |
| 8551 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.68948 | 104.0147 |
| 8553 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69089 | 104.01386 |
| 8556 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.6924 | 104.01292 |
| 8557 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | yellow | 13.6924 | 104.01292 |
| 8559 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69524 | 104.01055 |
| 8560 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69524 | 104.01055 |
| 8561 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | yellow | 13.69547 | 104.01015 |
| 8562 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69547 | 104.01015 |
| 8563 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69751 | 104.00636 |
| 8564 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69808 | 104.00642 |
| 8565 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69774 | 104.00498 |
| 8566 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.68442 | 104.01686 |
| 8567 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69177 | 104.01341 |
| 8568 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69487 | 104.01079 |
| 8569 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69524 | 104.01055 |
| 8570 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69524 | 104.01055 |
| 8571 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69548 | 104.0096 |
| 8572 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69548 | 104.0096 |
| 8573 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69774 | 104.00498 |
| 8574 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.68992 | 104.01448 |
| 8575 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69177 | 104.01341 |
| 8576 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.68948 | 104.0147 |
| 8577 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69548 | 104.0096 |
| 8578 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69789 | 104.00679 |
| 8579 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69295 | 104.01207 |
| 8580 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69049 | 104.01389 |
| 8581 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69049 | 104.01389 |
| 8582 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69049 | 104.01389 |
| 8584 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69774 | 104.00498 |
| 8586 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69774 | 104.00498 |
| 8587 | Cambodia: Kbal Spean River, Phnom Kulen National Park | k1 | Yellow | 13.69089 | 104.01386 |
| 10 489 | Cambodia: Kbal Spean River, Phnom Kulen National Park | n/a | Yellow | 13.68914048 | 104.0148154 |
| 10 491 | Cambodia: Kbal Spean River, Phnom Kulen National Park | n/a | Yellow | 13.69497418 | 104.0108427 |
| 10 492 | Cambodia: Kbal Spean River, Phnom Kulen National Park | n/a | Yellow | 13.69734267 | 104.0062649 |
| 10 493 | Cambodia: Kbal Spean River, Phnom Kulen National Park | n/a | Yellow | 13.69362979 | 104.0115234 |
| 10 494 | Cambodia: Kbal Spean River, Phnom Kulen National Park | n/a | Yellow | 13.69362979 | 104.0115234 |
| 10 495 | Cambodia: Kbal Spean River, Phnom Kulen National Park | n/a | Yellow | 13.69542353 | 104.0101658 |
| 10 497 | Cambodia: Kbal Spean River, Phnom Kulen National Park | n/a | Yellow | 13.69107122 | 104.0137995 |
| 10 498 | Cambodia: Kbal Spean River, Phnom Kulen National Park | n/a | Yellow | 13.69154039 | 104.0135571 |
| 10 499 | Cambodia: Kbal Spean River, Phnom Kulen National Park | n/a | Yellow | 13.69734267 | 104.0062649 |
| 10 500 | Cambodia: Kbal Spean River, Phnom Kulen National Park | n/a | Yellow | 13.69542353 | 104.0101658 |
| 10 501 | Cambodia: Kbal Spean River, Phnom Kulen National Park | n/a | Yellow | 13.68950153 | 104.0146659 |
| 10 502 | Cambodia: Kbal Spean River, Phnom Kulen National Park | n/a | Yellow | 13.68715318 | 104.015249 |




