Morphological evidence that the molecularly determined Ciona intestinalis type A and type B are different species: Ciona robusta and Ciona intestinalis
Abstract
Ciona intestinalis is considered a widespread and easily recognizable tunicate, the sister group of vertebrates. In recent years, molecular studies suggested that C. intestinalis includes at least two cryptic species, named ‘type A’ and ‘type B’, morphologically indistinguishable. It is dramatic to certify that two different species may be hidden under the name of a species widely used as a model species in biological researches. This raised the problem of identifying diagnostic morphological characters capable of distinguishing these types. We compared the morphology of specimens belonging to the two types and found that only type A specimens possess tunic tubercular prominences, allowing unambiguous discrimination. Remarkably, these structures were already described as distinctive of the Japanese species Ciona robusta, Hoshino and Tokioka, 1967; later synonymized under C. intestinalis (sensu Millar, 1953). In this study, we have confirmed that C. intestinalis type A corresponds to C. robusta. Based on the geographic distribution of C. intestinalis type B, and considering that the original C. intestinalis species was described from North European waters, we determined that C. intestinalis type B corresponds to C. intestinalis as described by Millar in 1953 and possibly to Linnaeus' Ascidia intestinalis L., 1767 for which we have deposited a neotype (from Roscoff, France) and for which we retain the name Ciona intestinalis (Linnaeus, 1767).
Introduction
In the last decades, Ciona intestinalis, a putatively cosmopolitan representative of the tunicates – likely, the sister group of vertebrates – has become a model chordate in various fields of biology, from comparative genomics to evo-devo and developmental biology (Satoh 2003). The nuclear genome draft of an individual sampled in California was published in 2002. This promoted the study of this organism and helped to clarify the evolutionary origin of chordate novelties (Dehal et al. 2002).
C. intestinalis is a shallow water species often present in harbours or semi-enclosed basins in extensive communities. The species has been recorded in all oceans and from high to low latitudes. The original, very short description made by Linnaeus – as Ascidia intestinalis Linnaeus 1767 – was extended by Roule (1884) and subsequently by Millar (1953). Despite the large number of articles devoted to specimens collected in different localities, populations with morphological characters substantially deviating from Millar's description (1953) have not been described. In general, the wide geographic distribution of C. intestinalis might be explained by its adaptability to environmental variation as well as by the effect of natural spread of the larvae and of human-related transportation of adults (e.g. on vessel keels) (Darbyson et al. 2009; Davidson et al. 2010; Kanary et al. 2011). Indeed, experiments showed that populations from different Scandinavian sites have different ranges of salinity tolerance for the development of fertilized eggs and larvae (Dybern 1967). A similar experimental study on a population from the Lagoon of Venice, testing various combinations of salinity and temperatures on different stages of the biological cycle, also suggested that populations from different localities might be somewhat genetically separated among them (Marin et al. 1987). More recently, another work on Scandinavian C. intestinalis populations found that differences in salinity tolerance at larval metamorphosis were primarily plastic and driven by parental experience (Renborg et al. 2014).
Recent molecular studies of specimens sampled in several localities around the world have shown that different, cryptic, species might be grouped under the name C. intestinalis, leading to the hypothesis that C. intestinalis constitutes a complex of species (Suzuki et al. 2005; Caputi et al. 2007; Iannelli et al. 2007; Nydam and Harrison 2007, 2011; Zhan et al. 2010; Sato et al. 2012). In particular, these studies have identified two forms, named ‘type A’ and ‘type B’, as highly genetically divergent and with disjoint global distributions (Suzuki et al. 2005; Caputi et al. 2007; Nydam and Harrison 2007; Zhan et al. 2010). Type A specimens have been found primarily in the Mediterranean Sea, the Pacific Ocean (Australia, Japan, New Zealand, South Korea, and West coast of North America), and the Atlantic coasts of South Africa (Caputi et al. 2007; Zhan et al. 2010). Type B individuals have been found on the coasts of both the NE and NW North Atlantic Ocean, as well as in the Bohai and Yellow Seas (China) (Zhan et al. 2010). Remarkably, type A and type B coexist in the English Channel and in some localities of the French Atlantic coasts (i.e. in Plymouth, UK, and in Brest, France) which, accordingly, represent sympatric areas (Sato et al. 2012).
The reproductive isolation of type A and type B has been investigated in the context of the biological species concept (Mayr 1963), using experiments of in vitro fertilization between individuals coming from different localities (Suzuki et al. 2005; Caputi et al. 2007; Sato et al. 2012, 2014). However, available data are currently insufficient to clarify this issue and the problem of the existence of distinct species under this species concept. Indeed, fertilization experiments have sometimes produced partially contradictory results. This was probably due to the usage of different experimental conditions (i.e. developmental temperature-salinity) and of specimens coming from different localities/populations (see also the discussion in Sato et al. 2014). The most comprehensive study, performed on sympatric type A and type B populations of the English Channel, indicates that hybrids can mature and produce viable gametes. Yet, naturally introgressed animals are present only at low percentage, probably as consequence of several pre- and postzygotic mechanisms of reproductive isolation (Sato et al. 2014).
In spite of the wealth of molecular data, only two studies have compared the morphology of Ciona intestinalis type A versus type B (Caputi et al. 2007; Sato et al. 2012). Caputi et al. (2007) found that, with some exceptions, the two C. intestinalis types can be told apart by the sperm duct pigmentation, as type A individuals have orange genital papillae and an uncoloured duct, while type B shows pigmentation only in the duct. Subsequently, Sato et al. (2012) described three external inherited morphological characters as useful markers to distinguish types A and B ‘in the field’ and within the region of sympatry. The identified characters, all observable with the unaided eye and without the help of any laboratory facilities, are as follows: (1) body colour, (2) presence/absence of tubercular prominences on the siphons and (3) yellow/orange pigments at the distal end of siphons. However, none of these three characters was found exclusively in a particular C. intestinalis type, and the existence of a number of exceptions makes them unreliable indicators of the specific type. For example, in the sympatric area of Plymouth, 92% of the specimens with yellow or orange pigmentation at the distal end of siphons were type B and only 78% of all specimens without pigmentation at the distal end of siphons were type A (Sato et al. 2012). In conclusion, although outwardly useful in the field, all the identified morphological differences between type A and type B appear devoid of taxonomic value (Caputi et al. 2007; Sato et al. 2012). That is a critical situation for scientists, because C. intestinalis is used as a model organism in biological research (Satoh and Jeffery 1995; Corbo et al. 2001; Satoh 2003; Satoh et al. 2003).
The genus Ciona includes deep water and shallow water species. The two arctic taxa C. longissima Hartmeyer 1899 (a poorly described species) and C. gelatinosa Bonnevie 1896 (redescribed by Sanamyan and Sanamyan 2007) range from subtidal zone down to bathyal–abyssal depths; both species are characterized by the presence of a posterior abdomen into which the lateral muscle bands extend. Other deep water species are as follows: C. antarctica Hartmeyer 1911 (redescribed by Monniot and Monniot 1983); the abyssal C. mollis Ritter 1907 (redescribed by Monniot 1998); C. imperfecta Monniot and Monniot 1977 (whose belonging to the genus Ciona is doubtful); and C. pomponiae Monniot and Monniot, 1989 (redescribed by Sanamyan and Sanamyan 2007).
The six shallow water species are as follows:
- C. intestinalis (Linnaeus 1767) sensu Millar 1953 (not including C. intestinalis sensu Hoshino and Tokioka 1967 and Pisano et al. 1972, both of which correspond to C. savignyi);
- C. edwardsi (Roule 1886) redescribed by Copello et al. (1981);
- C. savignyi Herdman 1882 (C. intestinalis sensu Hoshino and Tokioka 1967 and Pisano et al. 1972);
- C. roulii Lahille 1890 of which only the original description is available, eventually transcribed by Harant and Vernières (1933) in the Faune de France volume on ascidians, who unjustifiably changed the original name into roulei which is an incorrect subsequent spelling (ICZN 1999, art 33.3), and therefore, it must be corrected (art 32.5);
- C. robusta Hoshino and Tokioka 1967; described also by Pisano et al. (1972), later regarded as a junior synonym of C. intestinalis (Hoshino and Nishikawa 1985); and
- C. sheikoi Sanamyan 1998 (a shallow subtidal species collected at −80 m from north-west Pacific Ocean).
Hoshino and Nishikawa (1985) suggested a classification of the Ciona species based essentially on the presence/absence of the endostylar appendage and on the arrangement of the pharyngo-epicardiac openings, two characters often ignored by previous authors. In our opinion, this classification had probably overestimated these two characters to the detriment of other morphological considerations. Based on this new classification, these authors then synonymized C. robusta and C. edwardsi under C. intestinalis, undervaluing the careful description previously given for both these species (Hoshino and Tokioka 1967; Copello et al. 1981). As a proof of this overestimation, later experiments of hybridization performed by Lambert et al. (1990) showed that C. intestinalis, C. roulii and C. edwardsi are distinct species.
Here, we perform detailed morphological comparisons between C. intestinalis type A and type B specimens from different localities, with the aim of identifying significant morphological differences that unambiguously distinguish the two types.
Material and methods
Specimen collection and analyses
The source localities and the sampling dates of the analysed 51 specimens are reported in Table 1. For morphological analyses, the specimens were anaesthetized in seawater saturated with menthol, fixed in 10% seawater formalin and then dissected. Organs were stained with Mayer's haemalum and dehydrated with ethanol at crescent concentrations. Morphological observations were made on dissected whole animals, on stained organs, and also on specimens before dissection according to the ‘in field’ morphological characterization developed by Sato et al. (2012). Specimens were analysed with a dissecting stereomicroscope using reflected light as well as transmitted light. Ciona intestinalis type A and type B specimens were identified based on the analysis of the mitochondrial genome, according to the two PCR-based screening tests described in the Supplementary Materials of Iannelli et al. (2007). These tests take into account the translocation of the trnC and the presence/absence of a 85-bp-long non-coding region to discriminate type A from type B individuals. Specimens coming from the sympatric zone (Plymouth) were also analysed according to the genotyping test and the ‘in field’ morphological characters described in Sato et al. (2012). In particular, the used genotyping test is based on restriction enzyme digestion at one mitochondrial (cox1) and three nuclear loci (vAChTP, CiCesA and patched) according to Sato et al. (2012) and Nydam and Harrison (2010).
| Locality | Date | C. robusta (formerly ‘type A’) | C. intestinalis (formerly ‘type B’) | Details |
|---|---|---|---|---|
| Plymouth, UK | Autumn 2011 | 3 | 3 | Panels to the Queen Anne's Battery Marina (DD: 50.364, −4.131) |
| Lagoon of Venice, IT | Autumn 2013 | 20 | 0 | Piers to the south-west of Chioggia centre (DD: 45.2138, 12.2732; 45.2135, 12.2738) |
| Lagoon of Venice, IT | Autumn 2013 | 10 | 0 | Floats to the West of Sottomarina (DD: 45.2180, 12.2898) |
| Roscoff, FR | Autumn 2013 | 0 | 8 | Marina of Roscoff (DD: 48.7274, −3.9872) |
| Roscoff, FR | Summer 2014 | 0 | 7 | Marina of Roscoff (DD: 48.7274, −3.9872) |
Histology
Specimens of Ciona intestinalis type A from Plymouth and Venice, and type B from Plymouth and Roscoff were anaesthetized with menthol, fixed in 4% paraformaldehyde in phosphate-buffered saline (pH 7.4). A total of ten specimens were then dehydrated with ethanol at crescent concentrations, embedded in paraplast, dissected, and serial sections 7 μm thick were cut and counterstained with haematoxylin–eosin.
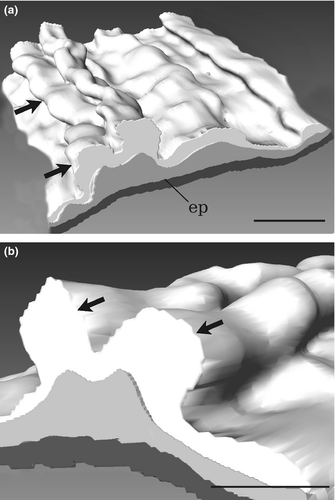
For 3D reconstructions, 84 histological sections 7 μm thick belonging to an anterior part of one of the ten sectioned C. intestinalis type A specimens were recorded with a digital camera (Leica DFC 480) mounted on a Leica DMR compound microscope. Images were aligned using Adobe Photoshop CS on a Windows 7 computer. Based on the resulting stack of images, 3D models of the anatomy of the tunic corresponding to the area near the oral siphon were created in amira 5.3.3 software (Mercury Computer Systems, Berlin, Germany).
Results
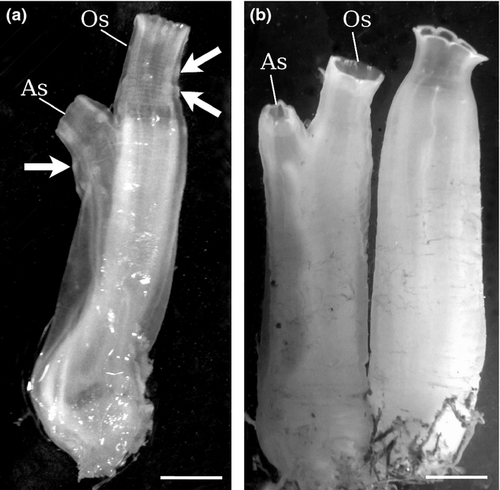
We first analysed six individuals from Plymouth (the region of sympatry): three classified as Ciona intestinalis type A and three as type B. Then, we compared 8 ‘type B’ from Roscoff and 30 ‘type A’ from the Venetian lagoon. Both specimen sets ranged from 2 to 12 cm in height (Fig. 1). All analysed individuals were molecularly classified as described in Material and Methods, and the type identification was found in accordance with their source locality. In each animal, we carefully inspected the tunic and some internal characters described by Millar (1953) as characteristics of Ciona intestinalis. These characters are all detailed below, but only the tunic allowed us to clearly distinguish type A from type B individuals.
Tunic
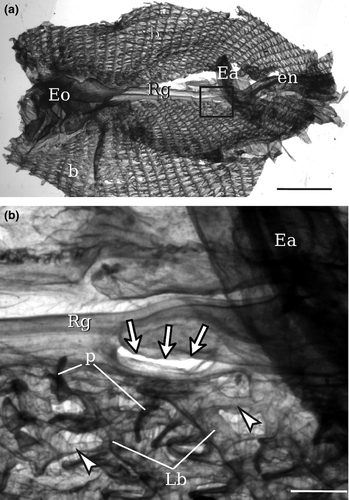
In all type A specimens, the tunic presented tubercular prominences, papilla shaped or elongate, distributed along the whole body and prevalently arranged around the siphons, in more or less longitudinal rows (Fig. 2). These tubercular prominences are present in all type A specimens, also in young and small individuals, although in these cases, they are invisible to the unaided eye and their identification requires the usage of a dissecting stereomicroscope equipped with transmitted light. These structures are present also in the thin layer of tunic lining the internal surface of the oral siphon (Fig. 2c–e), where they can be lobed; in Ciona robusta, they were named by Hoshino and Tokioka (1967) ‘endocarps’, a term commonly used to describe internal features of the parietal body wall in the atrium of stolidobranch ascidians; see, for example Kott 1985, p 11.
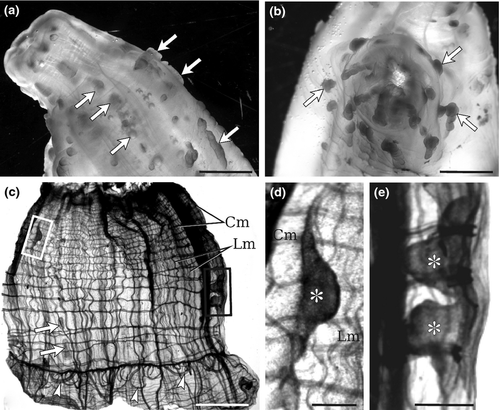
Histological analyses confirmed the existence of the tubercular prominences along the whole body and showed that these structures are not due to pathologic conditions. No signs of cell-mediated events, such as encapsulation or tissue injury, were observed. The oral siphons of type A individuals (three from Plymouth and three from Venice) were transversely cut and analysed by light microscopy (Fig. 3). In general, the tunic around the oral siphon was thin except in tubercular prominences, which were outgrowths of both the firm outer layer and the gelatinous inner layer of the tunic (Fig. 3a,b; Fig. 4). The tunic was crossed by numerous dense fibres, prevalently oriented parallel to the epidermis. The cuticle limiting the tunic was dense and irregular in profile (Fig. 3c,d). Both in tubercular prominences and in surrounding tunic regions, tunic cells were particularly dense in the vicinity of the cuticle.
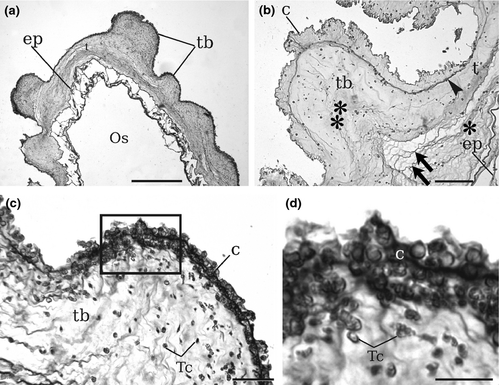
In all Ciona intestinalis type B specimens, the tunic was quite soft, its colour was pale, translucent and greyish (Millar's colour type 3; Millar 1953), and it was completely transparent in young individuals. The tunic around the siphons of large individuals could sometimes be lightly wrinkled. Remarkably, the tubercular prominences were absent in all type B specimens, both in small and in large individuals.
In conclusion, we found that the presence of tubercular prominences in the tunic is a character exclusive to Ciona intestinalis type A specimens and can unambiguously discriminate type A from type B samples. Remarkably, these tubercular prominences are a characteristic of Ciona robusta Hoshino and Tokioka 1967; the species identified in Japanese waters, and later synonymized to C. intestinalis (Hoshino and Nishikawa 1985) (see 1). Indeed, Hoshino and Nishikawa (1985) observed that C. robusta does not differ in any other characteristic from C. intestinalis (L.; not Hoshino and Tokioka 1967) except for the aspect of the tunic. Thus, we conclude that C. intestinalis type A corresponds to C. robusta.
Siphons
No differences in length and arrangement of the siphons were detected between Ciona intestinalis types A and B specimens, both corresponding to Millar's description (1953).
Body muscles
In both types, there were six longitudinal bands on each side: four extending up to the oral siphon and two to the atrial one. This is essentially the situation described by Millar (1953), although the latter description was highly schematic and did not consider intraspecific variability, which is present in the arrangement of muscles.
Tentacles and ciliated funnel
Tentacles were simple, of different lengths, and variable in numbers in relation to the size of individuals (Fig. 2c). In both types A and B, the aperture of the ciliated funnel (=dorsal tubercle) was horseshoe shaped, opened upward, with the ends curled inwards as described by Millar (1953).
Structure of the branchial wall
Both A- and B-type individuals presented the same structure figured by Brunetti (1979, Pl. IV fig. A; Pl V fig. C) and Copello et al. (1981, fig. 4A); that is the branchial wall is horizontally pleated as in an accordion (Fig. 5). This important morphological character distinguishes the typical Ciona intestinalis from Ciona edwardsi (Copello et al. 1981) and was already noted by Roule (1884, Pl 7 fig 66) but not by other authors, probably because it is clearly visible only in well-anaesthetized animals.
Anus and genital openings
No difference was detected between types A and B. In both types, the anus was situated about halfway along the branchial sac. Its edge was irregularly lobed (in the examined individuals, there were 13–15 lobes). The gonoducts ended forward of the anus almost at the base of the atrial siphon; their ends were orange pigmented in both type A and type B specimens.
Endostylar appendix and pharyngo-epicardiac openings
These two structures were present in both types. The pharyngo-epicardiac openings were situated near the endostylar appendix (Fig. 5). Their size and number (one or two) were variable, as previously observed by Millar (1953).
Stomach
Again, no difference was detected between the two types. In general, the stomach had a very delicate wall, with a large number of longitudinal folds in its internal surface.
Discussion
Our morphological analyses show that Ciona intestinalis type A and type B individuals are indistinguishable according to the characters proposed by Millar (1953) as characteristics of C. intestinalis and also according to the pigmentation of the distal ends of the siphons (Sato et al. 2012). Indeed, a survey of the siphon pigmentation on molecularly identified C. intestinalis type A and type B was recently performed by Sato et al. (2012), on a large number of individuals collected at Plymouth, where they occur in sympatry. However, this study concluded that this character does not provide an absolute distinction between the types. Thus, the siphon pigmentation is able to discriminate type A from type B in the sympatric area in the majority of specimens (Sato et al. 2012), but there are some exceptions. In general, the ascidian taxonomic literature reports a very large number of polychromatic species in which the colorations change according to the environmental parameters (e.g. Mukai 1974). This fact led taxonomists to doubt the taxonomic value of animals' coloration (‘…there is often so much variation in colour in different individuals of the same species that in some genera little reliance can be placed on colour as a diagnostic character’. Van Name 1945, p. 19). In ascidians, the colorations may be due to tunic cells (Endean 1961), epithelial cells (Ishii et al. 1993), tunic spicules and symbionts (Monniot et al. 1991), but the best known cause of colour is the blood cell containing pigments (Millar 1953; Wright 1981; Hirose et al. 1998). Moreover, different proportions of the same chromocytes can be the cause of ascidian different colour. Finally, fixative liquids usually change or completely extract colours; therefore, Sato's suggested method of identification based on siphon colours is not verifiable on fixed specimens.
Ciona intestinalis types A and B can best be discriminated morphologically by the presence/absence of tubercular prominences in the tunic. This is the first time that the presence of these tubercular prominences is described as a character exclusive to type A and therefore as a character able to unequivocally discriminate type A from type B individuals. In their article, Sato et al. (2012) also described the tubercular prominences as ‘small raised regions on the surface of the tunic of the siphons’ present mainly in C. intestinalis type A. However, they reported that 80% of the specimens with tubercular prominences were type A, and 81% of specimens without tubercular prominences were type B. Therefore, Sato et al. (2012) concluded that this character is just a ‘rough indicator’ of the type identity. The different result of our study is ascribable to the methodology employed for the detection of tubercular prominences. Sato et al. (2012) worked without magnification (Sato A., personal communication), as they aimed to find a method of ‘field identification’ of the two types defined by molecular data. On the contrary, we analysed our specimens in detail at the dissecting stereomicroscope, using reflected light as well as transmitted light: the most numerous and large tubercular prominences are visible with the reflected light system, while transmitted light is necessary to identify tubercular prominences of smaller size present in younger individuals. Unlike Sato et al. (2012), we also found that tubercular prominences are dispersed in the tunic along the whole body, although they are particularly abundant around the siphons.
Histological analyses of the tunic (in both type A and type B), and specifically of the tubercular prominences, also demonstrated that these structures are not due to pathologic conditions. No signs of cell-mediated events, such as encapsulation or tissue injury, typical of inflammatory responses already described in Ciona species, were recognized (Parrinello 1981; Parrinello and Patricolo 1984; Parrinello et al. 1984, 2007; Cammarata et al. 2008).
In this study, we also showed that the presence/absence of tubercular prominences is a distinctive character not only of the type A/type B specimens sampled in the region of sympatry but also of specimens from Roscoff (only type B) and Venice (only type A). Thus, this character does not seem to be related to the environmental conditions of the region of sympatry.
The absence of any mention of the presence of tubercular prominences in type A and, in general, in C. intestinalis specimens, in previous molecular, taxonomic, faunistic and ecological studies (except for Sato et al. 2012), is surprising. This is probably due to the widespread belief that C. intestinalis is the only abundant shallow water species of the genus, easy to recognize without particular attention to the morphological details and without dissecting stereomicroscope analyses.
It is striking to note that the tubercular structures here described and found in each of the type A specimens correspond to those described and figured by Hoshino and Tokioka (1967) in the Japanese C. robusta. Probably, Ciona individuals with large tubercular prominences were collected also in other localities, but based on the authoritative taxonomy of Hoshino and Nishikawa (1985), which synonymized C. robusta with C. intestinalis, they were not considered further; therefore, specimens were described merely as C. intestinalis. For example, individuals of Ciona with large tubercular prominences corresponding to those described in C. robusta collected by one of us, in the lagoon of Venice previously, were considered abnormal or affected by parasites, as had been initially supposed by Pisano and Rengel (1972). Outside Japan, a single record of C. robusta in Mar del Plata (Argentina) was reported (Pisano et al. 1972). These authors reported that this species is able to reproduce also in strongly polluted water. Moreover, it presents self-fertility (Pisano and Rengel 1972). Caputi et al. (2007) and Nydam and Harrison (2010) have performed molecular analyses on Japanese individuals from Onagawa (Miyagi Prefecture), a locality only 50–60 km south of the type locality of C. robusta (Mone Inlet, Karakuwa cho, Miyagi Prefecture) (Hoshino and Tokioka 1967): all these individuals belong to type A. Therefore, although the syntypes and paratypes of C. robusta deposited by Hoshino and Tokioka (1967) at the Seto Marine Biological Laboratory are not suitable for molecular analyses (i.e. they were not fixed in ethanol) and have not been seen by us, we hypothesize that the original C. robusta specimens should be molecularly indistinguishable from type A.
Ciona intestinalis type B is common on North Atlantic coasts (Suzuki et al. 2005; Caputi et al. 2007; Nydam and Harrison 2007), as well as in the Bohai and Yellow Seas (Zhan et al. 2010). Ciona intestinalis (L.) was first described – as Ascidia intestinalis – from the northern European Seas (Linnaeus' ‘Oceano europaeo’ Syst. Nat. 1791. Gmelin edition pag 3123), which may be assumed as its original type locality. Type B is common in the same ocean region of C. intestinalis (L.) type locality. As a consequence, because C. intestinalis (L.) is universally recognized to correspond to Millar's description (1953), we assume this to be the detailed description of the Linnaean species and assign type B to the valid Linnaean species. Moreover, as a deposited type of the C. intestinalis (L.) is lacking, and being faced with an important zoological problem, we have deposited a neotype from Roscoff (where the numerous samplings and molecular screening testify the presence of only type B) at the Natural History Museum in Venice and some topotypes at both the same museum and the Zoological Museum, University of Padova. Data on deposited specimens are reported in Table 2. As a further important step in the specimens deposit, prior to fixation in formalin, a small piece of each sample was fixed and stored in 95% ethanol for future possible molecular analyses. A similar operation should be performed even for Ciona robusta Hoshino and Tokioka 1967, even if syntypes and paratypes are still available for this species.
| Locality | Date | Size (cm high) | Depth (m) | Sex condition | Locationa | Catalogue No. |
|---|---|---|---|---|---|---|
| Roscoff, DD: 48.7274, −3.9872 | 21.08.2014 | 5 | 0–2 | Mature | VE |
MSNV-23282 neotype |
| Roscoff, DD: 48.7274, −3.9872 | 21.08.2014 | 5 | 0–2 | Mature | VE |
MSNV-23283 topotype |
| Roscoff, DD: 48.7274, −3.9872 | 21.08.2014 | 6 | 0–2 | Mature | PD |
TU26 topotype |
| Roscoff, DD: 48.7274, −3.9872 | 21.08.2014 | 2.5 | 0–2 | Mature | PD |
TU27 topotype |
- a PD: Zoological Museum, University of Padova (Italy); VE: Natural History Museum in Venice (Italy).
In conclusion, our morphological analyses provide a taxonomically valid character able to discriminate Ciona intestinalis type A from type B specimens and show that the two types are two distinct species, from both the genetic (Suzuki et al. 2005; Caputi et al. 2007; Iannelli et al. 2007; Nydam and Harrison 2007, 2010) and morphological perspectives. Our results are also confirmed by the identification of additional morphological differences at the larval stage (Pennati et al., 2015). Indeed, the larvae of the two types significantly differ in shape. In particular, late larvae of type B exhibit a longer pre-oral lobe, longer and relatively narrower total body length, and lower ocellus–tail distance than type A. Therefore, all together, our adult and larval morphological data support the conclusion that Ciona intestinalis type B is a different species from the molecularly defined type A, and we identify C. intestinalis type A as corresponding to C. robusta.
The results here illustrated permit to unambiguously distinguish the two formerly known Ciona intestinalis types A and B as the two species C. robusta and C. intestinalis, respectively. Therefore, we invite, encourage and advocate the use of the specific names C. robusta and C. intestinalis for types A and B, to clearly distinguish the individuals in future research and publications.
Considering the relevance of C. intestinalis in the study of chordate evolution and developmental biology, and the number of researchers working with this species all over the world, we expect that the impact on the scientific community of the easy morphological discrimination of type A and type B and finally the assigning of correct species names to both will be welcome.
Acknowledgements
The authors thank Atsuko Sato, John DD Bishop, Alessandro Minelli and Teruaki Nishikawa for helpful discussions. We also thank John DD Bishop (Marine Biological Association of the UK, Plymouth, UK) for kindly providing specimens from Plymouth. This work was supported by: MIUR PRIN Projects 2009 to LM and CG (http://www.istruzione.it, grant numbers 2009XF7TYT and 2009NWXMXX_003, respectively), University of Padova Senior postdoc 2012 Project to FG (http://www.unipd.it, grant number GRIC120LSZ); and by grant n. 2013-0752 from CARIPLO Foundation to RP. Authors have no conflict of interests.




