Morphological vs. molecular delineation of taxa across montane regions in Europe: the case study of Gammarus balcanicus Schäferna, † (Crustacea: Amphipoda)
Abstract
Mountainous areas are characterized by substantial biodiversity and endemicity due to their complex geological history and habitat fragmentation. Hence, it can be assumed that particularly high species richness can be found in organisms with limited dispersal capabilities that inhabit mountain streams. A number of scientific papers focus on molecular phylogeography or traditional taxonomy of species or species groups inhabiting such habitats. However, there is a lack of studies that integrate morphological and molecular data to identify and delineate cryptic species. For practical reasons, uncovering cryptic diversity is crucial in taxa used in biomonitoring. Distinct species, hard to separate based on morphology only, may have different tolerance ranges towards a variety of factors. Thus, our goal is to combine the two approaches to reveal potential patterns of diversification within a species widely distributed across European mountains: the amphipod crustacean Gammarus balcanicus. The data were obtained from 13 populations spread across the range of the species. Individuals were initially ascribed to G. balcanicus based on conventional fauna key morpho-anatomical diagnostic features and were further analysed for 23 additional features to explore any putative diversification. Morphometric data were analysed with use of the multiple correspondence analysis and anova. Molecular distances were calculated for 551-bp-long COI sequences. Test for isolation by distance was performed for both morphological and molecular data. The morphometric studies showed that some of the analysed features differed significantly between populations, although there was only a weak correlation between the morphological divergence and the between-population geographical distances. Moreover, high morphological diversity was present within sites. A set of 42 COI haplotypes was identified among the 135 individuals sequenced. No haplotype was shared among populations. The molecular p-distances within the nine localities presenting more than one haplotype were either almost null (ca. <0.003 for 7 localities) or relatively low (ca. 0.01–0.02 for 2 localities). In opposite, the molecular p-distances between localities were mostly at a high level (94% of pairwise comparisons being >0.14), similar as between other well-defined species of the genus Gammarus. Surprisingly, G. balcanicus appears to be polyphyletic based on topology of the neighbour-joining tree. The level of genetic distance between localities was not correlated with their geographical proximity. Globally, combining spatial patterns of morphological versus molecular divergence indicates a high level of cryptic diversity within a species conventionally defined based upon fauna key morphological features. In this context, the name G. balcanicus should be applied only to the population from locus typicus, while the other populations represent a number of putative distinct species. We may expect that such phenomenon would apply also to other animal taxa with conserved morphology, which are widespread over different mountain ranges in Europe.
Introduction
Mountainous areas are characterized by high species richness due to the dynamic patterns of speciation they hosted, associated with ecological diversification, high local extinction risk and limited dispersal between fragmented habitats (Nagy and Grabherr 2009). In temperate areas, for example in Europe, these biodiversity hot spots are also known as important glacial refugia in which fauna and flora survived and diversified along Pleistocene glacial cycles (Hewitt 2000; Schmitt 2007; Bhagwat and Willis 2008; Provan and Bennett 2008; Holderegger and Thiel-Egenter 2009).
Thus, phylogeography of mountain organisms is currently under intensive studies. Yet, most of them focus on terrestrial species (e.g. Schönswetter et al. 2003, 2004; Hewitt 2004; Schmitt and Hewitt 2004; Schönswetter and Tribsch 2005; Magri et al. 2006; Varga and Schmitt 2008; Homburg et al. 2013). Already Bănărescu and Boscaiu (1978) as well as Malicky (1983) indicated that freshwater animals cannot be grouped with the terrestrial fauna from zoogeographical and distributional aspects. According to Bănărescu and Boscaiu (1978), the distribution of rheophilous taxa without terrestrial dispersal stages coincides with the catchment areas of rivers limited by watersheds, while the distribution of rheophilous insects coincides with mountain ranges. Different is also the case of subterranean species, which may be defined by paleodrainages, crossing the borders of recent river drainages (Sket 2002). Many aquatic taxa are little studied, and more extensive data sets are available only for vertebrates such as fishes (e.g. Bănărescu 1990; Bernatchez and Wilson 1998; Doadrio et al. 2002; Schreiber 2002; Pasko and Maslak 2003; Sonstebo et al. 2007) or amphibians (e.g. Szymura et al. 2000; Babik et al. 2003; Garcia-Paris et al. 2003; Martinez-Solano et al. 2006; Hofman et al. 2007; Fijarczyk et al. 2011). Among invertebrates, most studies concerned caddisflies (Pauls et al. 2006, 2009; Balint et al. 2008), snails (Falniowski et al. 2012) and beetles (Ciamporova-Zatovicova and Ciampor 2011). Generally, less attention was paid to species inhabiting mountain streams and rivers (Pauls et al. 2006, 2009; Balint et al. 2008; Falniowski et al. 2012), which remain mostly unexplored. Aquatic crustaceans have been the object of little attention, focusing predominantly on mountain lakes (Meyran and Taberlet 1998; Vainio and Väinölä 2003; Hamrova et al. 2012). In addition, most studies were either dealing with molecular phylogeography of particular species or species groups or were based on traditional taxonomic approach. Strikingly, there is deficiency of studies employing both molecular and morphological data to uncover and delineate cryptic taxa in case of mountain aquatic species or species groups. This is surprising, as we can expect that the above-mentioned properties of mountainous areas generate substantial cryptic diversity that could be easily detected, for example, with the DNA barcoding approach. Balint et al. (2008) demonstrated that allopatric populations of montane aquatic invertebrate species may diverge in their morphometric features. However, it would be interesting to find out whether such diversification is congruent with divergence observed on the molecular level. On one side, such studies would be very useful in addressing the problem of phenotypic plasticity in montane species. On the other side, they would stimulate quest for new species-diagnostic morphological features to differentiate between molecularly distinct lineages traditionally regarded as conspecific based on other morphological features.
Good model organisms for studying diversification in mountain aquatic environments are amphipod crustaceans, being among the most abundant taxa in mountain streams. They have a wide geographical distribution, narrow range of environmental tolerance and limited dispersal capabilities (Väinölä et al. 2008).
Gammarus balcanicus is a freshwater amphipod inhabiting streams in various, often isolated, mountain ranges. It is widely distributed in Europe, from Southern Alps through the Carpathians and Balkan Peninsula to the Crimean Peninsula (Karaman and Pinkster 1987). Its alpha-taxonomy has been the topic of debates (Schäferna 1922; Karaman 1929, 1935; Martynov 1931, 1935; Dobreanu and Manolache 1942; Cărăuşu et al. 1955) that, in the past, led to description of many local species and subspecies. However, Karaman and Pinkster (1987) could not define any diagnostic features that would differentiate these forms and synonymized them with G. balcanicus. This led us to the assumption of a putative substantial diversification pattern. Such pattern would occur between mountain ranges but might also occur between localities within a given range. Thus, our aim is to study the morphological and molecular variation within this species (as initially delineated based on conventional fauna diagnostic features) from a set of localities representing the actual distribution of the morpho-species to reveal putative pattern of its diversification.
To achieve this goal, we will answer the following questions:
- What is the pattern of morphological variability of G. balcanicus populations from various European mountain ranges?
- What is the level of molecular divergence within and between different populations of G. balcanicus as well as between G. balcanicus and other morphologically well-defined species from the genus Gammarus?
- Is morphological and molecular variation exhibiting the same patterns?
- If divergent molecular lineages are recognized within G. balcanicus, could they be associated with a given set of morphological features that could serve to distinguish the species during further taxonomic revisions?
Material and methods
Sample collection and identification
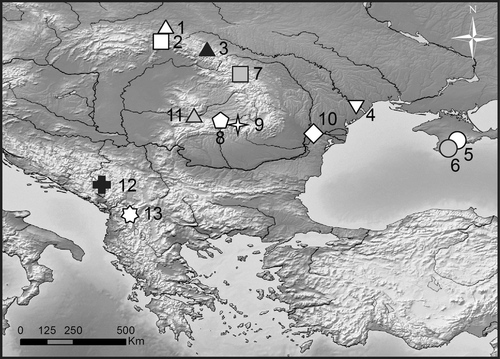
Samples were collected from 13 locations from different regions inhabited by Gammarus balcanicus in Europe, including the Crimean Peninsula, Carpathian Arch and Balkan Peninsula (Table 1, Fig. 1). Most of the samples were collected during several scientific expeditions organized by the Department of Invertebrate Zoology and Hydrobiology from 2005 to 2010. The samples from Crimea were collected within the bilateral (Slovenia-Ukraine) research project of Ljubljana and Kharkiv Universities in 2006. The animals were gathered from a variety of stream and spring habitats with use of a benthic hand-net, sorted at a site and preserved in 96% ethanol. In the laboratory, the collected individuals were identified under stereomicroscope Nikon SMZ-800 according to the available literature (Karaman and Pinkster 1977a,b, 1987; Jażdżewski and Konopacka 1989).
| Code | Species | Locality | Mountain/region | River basin | Altitude (m asl) | Country | Coordinates | Collection date | N1 | N2 | Accession number |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Gammarus balcanicus | Close to Dwerniczek | Eastern Carpathians | Vistula | 576 | Poland | 49.212° N, 22.739° E | 26.06.2008 | 20 | 12 | KF053224, KF053225 |
| 2 | G. balcanicus | North of Muczne | Eastern Carpathians | Vistula | 697 | Poland | 49.149° N, 22.715° E | 26.06.2008 | 20 | 9 | KF053226, KF0532267 |
| 3 | G. balcanicus | Between Yaroslyak and Vorokhta | Eastern Carpathians | Danube | 983 | Ukraine | 48.190° N, 24.558° E | 22.07.2010 | 20 | 12 | KF053228, KF053229, KF053230, KF053231 |
| 4 | G. balcanicus | Near Ovidiopol | Black Sea Lowlands | Dniestr | 24 | Ukraine | 46.257° N, 30.419° E | 22.08.2009 | 20 | 10 | KF053232 |
| 5 | G. balcanicus | South East from Simferopol | Crimea | Salhir | 505 | Ukraine | 44.846° N, 34.213° E | 26.08.2006 | 12 | 12 | KF053233 |
| 6 | G. balcanicus | Near Kizil-Koba (Red Cave) | Crimea | Salhir | 503 | Ukraine | 44.867° N, 34.343° E | 17.08.2006 | 10 | 10 | KF053234, KF053235, KF053236, KF053237, KF053238 |
| 7 | G. balcanicus | Near Catrinari | Eastern Carpathians | Danube | 1004 | Romania | 47.191° N, 25.499° E | 25.08.2005 | 20 | 12 | KF053239, KF053240, KF053241, KF053242, KF053243, KF053244 |
| 8 | G. balcanicus | 20 km south of Cârţişoara | Southern Carpathians | Danube | 933 | Romania | 45.667° N, 24.593° E | 30.08.2005 | 20 | 11 | KF053245, KF053246, KF053247, KF053248, KF053249, KF053250, KF053251 |
| 9 | G. balcanicus | Zărneşti | Southern Carpathians | Danube | 834 | Romania | 45.542° N, 25.296° E | 29.08.2005 | 20 | 9 | KF053252, KF053253, KF053254, KF053255, KF053256 |
| 10 | G. balcanicus | Close to Luncavica | Dobrogea Plateau | Danube | 93 | Romania | 45.228° N, 28.311° E | 02.09.2005 | 20 | 8 | KF053257, KF053258 |
| 11 | G. balcanicus | Hunedoara | Transylvanian Plateau | Danube | 255 | Romania | 45.751° N, 22.888° E | 07.09.2005 | 20 | 8 | KF053259, KF053260, KF053261, KF053262, KF053263 |
| 12 | G. balcanicus | Near Kolašin (locus typicus) | Dinarides | Skadar Lake | 1069 | Montenegro | 42.836° N, 19.569° E | 24.09.2006 | 20 | 10 | JX899354 |
| 13 | G. balcanicus | Close to Burrel | Albanian central mounatin range | Mat | 224 | Albania | 41.584° N, 20.031° E | 16.09.2006 | 20 | 12 | KF053264 |
| BOS | Gammarus bosniacus | Vrelo Bosne | Dinarides | Danube | 497 | Bosnia and Herzegovina | 43.820° N, 18.270° E | 27.08.2006 | – | 1 | JX899355 |
| PLJ | Gammarus pljakici | Close to Andrijevica | Dinarides | Danube | 828 | Montenegro | 42.729° N, 19.801° E | 30.08.2006 | – | 1 | KF053265 |
| SAL | Gammarus salemaai | Lake Ohrid, Sv. Stefan | Helenides | Ohrid Lake | 694 | Macedonia | 41.080° N, 20.799° E | – | – | 1 | JX899266 |
| SKE | Gammarus cf. sketi | Sv. Naum springs | Helenides | Ohrid Lake | 697 | Macedonia | 40.914° N, 20.742° E | – | – | 1 | JX899272 |
| MAC | Gammarus macedonicus | Lake Ohrid, Sv. Zaum | Helenides | Ohrid Lake | 761 | Macedonia | 40.949° N, 20.774° E | – | – | 1 | JX899221 |
| PUL | Gammarus pulex | Öland | – | – | – | Sweden | – | – | – | 1 | JF965943 |
| FOS | Gammarus cf. fossarum | Bled | Alps | – | – | Slovenia | – | – | – | 1 | JF965879 |
| LAC | Gammarus lacustris | Liubliaz | Polesia | Pripyat | 141 | Ukraine | 51.848° N, 25.472° E | 09.08.2009 | – | 1 | JX899356 |
| VAR | Gammarus varsoviensis | Korostianka | Polesia | Pripyat | 147 | Ukraine | 51.770° N, 25.354° E | 09.08.2009 | – | 1 | JX899357 |
| LEO | Gammarus leopoliensis | Between Borsa and Prislop Pass | Eastern Carpathians | Danube | 837 | Romania | 47.626°N, 24.768° E | 25.08.2005 | – | 1 | KF053266 |
| ROE | Gammarus roeseli | Preševo | Preševo Valley | Danube | Serbia | – | – | – | 1 | JF965948 | |
| STO | Gammarus stojicevici | Near Pirot | Balkan Mts. | Danube | 414 | Serbia | 43.217° N, 22.537° E | 28.08.2007 | – | 3 | KF053221, KF053222, KF053223 |
Morphological analysis
In amphipods, sexual dimorphism as well as ontogenetic and seasonal morphological variability is a well-known phenomenon (Goedmakers 1972, 1980; Pinkster 1983, 1988). Thus, most of the identification keys for this group are based only on morphology of adult males. Therefore, to minimize associated (sexually and developmentally based) variation, only adult males collected during the summer (June to September) were used for the analysis – altogether, 242 individuals (Table 1). Based on the taxonomic revision by Karaman and Pinkster (1987) and literature cited therein (e.g. Schäferna 1922; Jażdżewski 1975; Karaman 1977), twenty-three features known to vary within G. balcanicus were taken into account (A to W, Table 2). The raw data set was deposited in the Dryad repository (http://doi.org/10.5061/dryad.tg8jg). To test which of the selected morphological features were significantly differing among the studied populations, the Kruskal–Wallis nonparametric anova with multiple post hoc comparisons were performed in Statistica 10 (StatSoft 2011). The features found to be differentiated between populations (Table 2) were subsequently used as data matrix for the multiple correspondence analysis (MCA) conducted in SPSS 20 (IBM Corp. 2011) to explore whether there is a geographical pattern in the observed variability. The MCA was performed on three dimensions (3D), of which two dimensions (2D) were presented on the plot and all three dimensions were used for the subsequent statistical analyses. To test whether there are statistically supported differences between populations, the Kruskal–Wallis nonparametric alternative for anova followed by pairwise comparisons using Dunn's (1964) procedure with a Bonferroni correction for multiple comparisons was performed on the object scores obtained from the 3D MCA. For every population, the distance of each individual from group centroid was calculated with use of a modified formula for the distance of points in three-dimensional space (see Balint et al. 2008 for details). The same formula was used to calculate the distances among centroids of each group. To compare whether morphological variance (i.e. distances of individuals from the group centroids) was significantly different among studied sites, we performed anova with post hoc Tukey test for independent samples in Statistica 10 (StatSoft Inc. 2011). Prior to the analysis, to meet the anova assumptions, the data were log-transformed. To test whether the distances among group centroids are correlated with the geographical distances among the sampled points, the Mantel test of isolation by distance was performed with Genepop 4.2 (Rousset 2008). The level of this correlation was assessed in SPSS 20 with Spearman's rank correlation coefficient, and its statistical significance was tested with 1000 bootstrap replicates.
| Code | Examined features | H | p | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First antenna: | ||||||||||||||||
| A | No. of setae on peduncle segment 4 | 117.05 | 0.000 | 0–1 | 0–1 | 0–2 | 1–2 | 1–2 | 1–2 | 0–1 | 0–3 | 0–2 | 0–2 | 0–1 | 0–2 | 0–2 |
| B | No. of setae on peduncle segment 5 | 162.32 | 0.000 | 0 | 0 | 0–1 | 1 | 1 | 1 | 0–1 | 0–1 | 0–1 | 0–1 | 0 | 0–1 | 0 |
| C | Length of setae on peduncle segment 4 and 5 | NA | NA | S | S | S | S | S | S | S | S | S | S | S | S | S |
| D | Length of setae on flagellum | 11.10 | 0.520 | L-S | S | S | S | S | S | S | S | S | S | S | S | S |
| Second antenna: | ||||||||||||||||
| E | Presence of calceoli on flagellum | 105.42 | 0.000 | Y/N | Y/N | Y/N | N | N | N | Y/N | Y/N | Y/N | Y/N | Y/N | Y/N | N |
| F | No. of setae on peduncle segment 4 | 180.37 | 0.000 | 2–4 | 3–5 | 2–3 | 2–3 | 2–4 | 2–3 | 3–5 | 4–5 | 1–3 | 2–3 | 2–4 | 1–2 | 2–5 |
| G | No. of setae on peduncle segment 5 | 141.88 | 0.000 | 2–5 | 2–5 | 2–5 | 2–4 | 3–4 | 3–4 | 4–6 | 3–5 | 1–4 | 2–4 | 2–4 | 2–3 | 3–5 |
| H | Length of setae on peduncle segments | 174.20 | 0.000 | M-L | S-L | S-L | S | S | S | S-L | M-L | S-M | S | S-M | S-L | S-L |
| I | Length of setae on flagellum | 144.44 | 0.000 | S-L | S-L | S-L | S | S | S | S-L | S-L | S-M | S-M | S-L | M-L | M-L |
| Third and fourth pereiopod. Posterior margin: | ||||||||||||||||
| J | Length of setae on segments 3 to 6 | 133.14 | 0.000 | S-L | S-L | S-M | S | S | S | S-M | S-M | S-M | S-M | S-M | M-L | S-M |
| Third uropod: | ||||||||||||||||
| K | Endopodit/Exopodit length ratio | 210.49 | 0.000 | 0.5–0.6 | 0.6–0.75 | 0.4–0.6 | 0.8 | 0.8 | 0.8 | 0.5–0.6 | 0.33–05 | 0.8 | 0.67–0.8 | 0.6–1 | 0.5–0.6 | 0.6 |
| Third uropod. Exopodit outer margin: | ||||||||||||||||
| L | Presence of free setae | 85.44 | 0.000 | Y/N | N | Y/N | Y/N | N | N | Y/N | Y/N | Y/N | Y/N | Y/N | N | Y/N |
| M | Type of setae (simple or plumose) | 18.08 | 0.113 | Simple | Simple | Simple | Simple | Simple | Simple | Simple | Simple | Simple | Simple | Simple | Simple | Simple |
| N | No. of spine groups | 91.32 | 0.000 | 2–4 | 3 | 2–4 | 2–4 | 3–4 | 3–4 | 2–4 | 2–4 | 2–4 | 3–6 | 3–6 | 2–3 | 2–5 |
| Third uropod. Exopodit inner margin: | ||||||||||||||||
| O | Presence of free setae | 169.34 | 0.000 | Y | Y | Y | Y/N | Y | Y | Y | Y | Y | Y/N | Y | Y/N | Y |
| P | Type of setae (simple or plumose) | 156.81 | 0.000 | Both | Both | Both | Both | Both | Both | Both | Both | Both | Both | Both | Simple | Both |
| Q | No. of spine groups | 76.22 | 0.000 | 0 | 0–1 | 0–1 | 0–1 | 0–1 | 0 | 0–2 | 0–1 | 0–3 | 0–3 | 0–1 | 0–4 | 0–1 |
| Third uropod. Endopodit outer margin: | ||||||||||||||||
| R | Presence of free setae | 58.53 | 0.000 | Y | Y | Y | Y | Y/N | Y/N | Y | Y/N | Y | Y | Y | Y/N | Y |
| S | Type of setae (simple or plumose) | 149.32 | 0.000 | Both | Both | Both | Both | Both | Both | Both | Both | Both | Both | Both | Simple | Both |
| T | No. of spine groups | 120.20 | 0.000 | 0–5 | 0–3 | 0–4 | 2–4 | 1–3 | 2–3 | 0–4 | 0–3 | 3–8 | 2–9 | 2–8 | 0–3 | 2–6 |
| Third uropod. Endopodit inner margin: | ||||||||||||||||
| U | Presence of free setae | 163.87 | 0.000 | Y | Y | Y | Y | Y | Y | Y/N | Y | Y | Y/N | Y/N | Y/N | Y |
| V | Type of setae (simple or plumose) | 153.74 | 0.000 | Both | Both | Both | Both | Both | Both | Both | Both | Both | Both | Both | Simple | Both |
| W | No. of spine groups | 79.70 | 0.000 | 0–1 | 0–1 | 0–2 | 0 | 0 | 0 | 0–1 | 0 | 0–2 | 0–2 | 0–3 | 0–1 | 0–2 |
- H, values of Kruskal–Wallis nonparametric anova (df = 12, N = 242) and p: probabilities; NA, not applicable; Y, yes; N, no; S, short; M, medium and L, Long.
Molecular methods
Eight to twelve individuals of G. balcanicus from each sampled location were used to define the level of genetic divergence within and between populations.
To compare the level of intraspecific divergence within G. balcanicus to the distance between G. balcanicus and other European freshwater Gammarus species, the following set of morphologically well-defined species was selected (Karaman and Pinkster 1977a,b, 1987): G. bosniacus Schäferna, 1922; G. pljakici G. Karaman, 1964, G. salemaai G. Karaman, 1985 G. cf. sketi G. Karaman, 1989, G. macedonicus G. Karaman, 1976, G. pulex Linnaeus, 1758, G. cf. fossarum Koch, 1836 G. lacustris G.O. Sars, 1864, G. varsoviensis Jazdzewski, 1975, G. leopoliensis Jażdżewski and Konopacka, 1989; G. roeselii Gervais, 1835 and G. stojicevici S. Karaman, 1929;. Within this set, sequences of G. pljakici, G. leopoliensis and G. stojicevici were produced within this study, while all the others were acquired from GenBank (see Table 1 for details).
From each individual, about 3 mm3 of the muscle tissue was carved out with a sharp-edged tweezers and incubated over night at 55°C in a 1.5-ml tube containing 200 μl of Queen's lysis buffer with 5 μl of proteinase K (20 mg ml−1) (Seutin et al. 1991). Subsequently, the DNA was extracted based on a standard phenol/chloroform method described in Hillis et al. (1996). Air-dried DNA pellets were resuspended in 100 μl of TE buffer, pH 8.00, stored at 4°C until amplification and finally long-term stored at −20°C.
A ca. 600–650-bp-long fragment of cytochrome oxidase subunit I (COI) of mitochondrial DNA was chosen for amplification. Two sets of primers amplifying the same region of the gene were used: LCO1490/HCO2198 (Folmer et al. 1994) or UCOIF/UCOIR (Costa et al. 2009). Polymerase chain reaction was performed in a total volume of 20 μl containing 10 μl of DreamTaq Master Mix (2x) Polymerase (Fermentas), 1.6 μl of each primer (concentration 5 μM) and 2 μl of DNA template. PCR was performed in a MaxyGen Gradient Thermocycler, under the following conditions: initial denaturation at 94°C for 3 min, 35 cycles of denaturation at 94°C for 20s, annealing at 50°C for 45s and elongation at 65°C for 60s, followed by a final extension for 2 min at 65°C. For samples with poor or no amplification under the above program, we used the reaction conditions described by Hou et al. (2007). A 2 μl aliquot of the PCR products was visualized in MidoriGreen-stained (Nippon Genetics) 1.0% agarose gels to check PCR product quality and length. Sequencing of the PCR product was performed through BigDye technology by Macrogen Inc., Korea. Altogether, 135 sequences were obtained. First, all sequences were positively verified as Gammarus DNA using GenBank BLASTn searches (Altschul et al. 1990). Then, they were assembled and trimmed in Geneious 6.0.5 (Biomatters 2013) to 551 bp. Alignment was performed with ClustalW (Larkin et al. 2007). Acquired sequences were deposited in GenBank with accession numbers given in Table 1.
Estimates of genetic diversity
All the produced COI sequences were assigned to haplotypes using program DnaSP 5 (Librado and Rozas 2009). A phenetic tree was constructed using the haplotype data in MEGA 5.05 (Tamura et al. 2011) using the neighbour-joining method (Saitou and Nei 1987) based on the p-distance (Nei and Kumar 2000) with bootstrap test performed on 10 000 replicates (Felsenstein 1985). The genetic distances and standard errors (SE) among sequences were estimated in MEGA 5.05 using uncorrected p-distances and Kimura two-parameter (K2P) model (Kimura 1980). Similarly, as in the morphological analyses described above, we defined groups based on their geographical origin (sampled locations). Following the approach of Costa et al. (2007), we calculated the average frequency distribution of COI distances within and between analysed localities with G. balcanicus as well as of COI distances between other morphologically defined species of Gammarus. To test whether the molecular distances between localities are correlated with the geographical distances among the sampled locations, the Mantel test of isolation by distance was performed in Genepop 4.2. The level of correlation was assessed in SPSS 20 with Spearman's rank correlation coefficient, and its statistical significance was tested with 1000 bootstrap replicates. The same testing procedure was applied to find out whether the levels of molecular and ‘morphological’ distances between populations are correlated.
Results
Morphological analyses
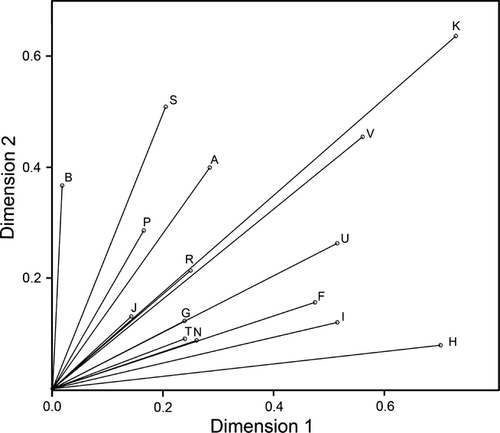
Among the 23 features used for the morphological analysis, 20 were differentiating populations at the statistically significant level (Table 2). The first two dimensions of the MCA ordinance analysis performed on the above-mentioned 20 morphological traits explained only 47.32% of the total observed variance within the data set. Thus, we reduced the data set based on the criterion of the discrimination measure (DM) values associated to particular features (Table S1). Only the 15 features with mean DM values above 0.1 were included in the final MCA (Fig. 3), with 61.45% of the total variance explained by the first two dimensions, and 81.13% explained by the first three dimensions. The results of this analysis are presented on the 2D graph (Fig. 2). The observed ordination pattern was affected mostly by the following features with the associated mean DM values above 0.20 (Fig. 3, Table S1), (symbols given in brackets correspond to Table 2): number of setae on peduncle of antenna 1 (A, B), number and length of setae on peduncle of antenna 2 (F, G, H), length of setae on flagellum of antenna 2 (I), the length ratio of endopodit to exopodit of uropod 3 (K), type of setae on exopodit outer margin of uropod 3 (P), presence and type of setae on endopodit of uropod 3 (S, U, V). Other analysed features contributed to a lesser extent to separation of individuals, with discrimination measures from 0.13 to 0.17 (Fig. 3, Table S1). The 2D MCA ordinance graph does not provide clear distinction of individuals associated with different geographical localities, although many statistically significant differences may be observed between particular populations (Table S2). In 31 cases, the dimension 1 significantly differentiates the populations (Table S2); the features with highest DM scores (DM > 0.47) for this dimension are as follows: number and length of setae on peduncle of antenna 2 (F, H), length of setae on second antenna flagellum (I), the length ratio of endopodit to exopodit of uropod 3 (K), presence and type of setae on endopodit of uropod 3 (U, V). The dimension 2 significantly differentiates populations in 38 cases. The features with highest DM (DM > 0.36) for this dimension include the following: number of setae on peduncle of antenna 1 (A, B), the length ratio of endopodit to exopodit of uropod 3 (K), type of setae on endopodit outer margin of uropod 3 (S) and type of setae on endopodit inner margin of uropod 3 (V). The dimension 3 significantly differentiates populations in the same number of cases as does the dimension 2. The features with a highest DV values (DM > 0.48) for the dimension 3 are number of setae on 4th and 5th peduncle segments of antenna 2 (F, G).
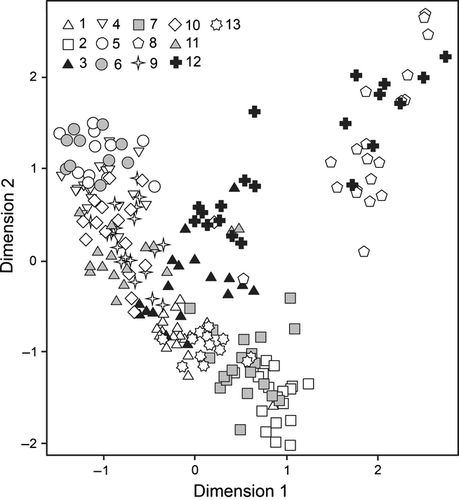
Variance within most localities is at a similar level. Three populations, one inhabiting Fagaras Mountains in Southern Carpathians (site 8), second from the Bjelasica Mountains in the Dinarid range on the Balkan Peninsula (site 12) and third from the Crimean Peninsula (site 6), are the exceptions (anova, F12,229 = 12.13, p < 0.001; unequal N HSD Tukey test, Table S3). Within the localities 8 and 12, the morphological variability is at the highest level, while it is the lowest in the site 6 (Table 3). Geographical distances between sites do not seem to be a very important factor structuring the observed variability (Mantel test, one-tailed p-value = 0.07), however, there is some weak yet statistically important positive correlation between pairwise group centroid distances (Table S2) and geographical distances among sampled localities (Spearman rank order correlation, R = 0.284, p = 0.006).While populations from such distant sites as those from Albanian Central Mountain Range (site 13) and Eastern Carpathians (site 1, 3, 7) show overlap in the range of morphological variability, no significant differences could be found for most of the analysed features (Fig. 2, Table 2, Table S2). In addition, some populations from mutually close localities within Eastern Carpathians (site 2, 3) or Southern Carpathians (site 8, 9) show less overlap in morphology and differ significantly in several features (Fig. 2, Table 2, Table S2).
| Code | H | D | SE | M |
|---|---|---|---|---|
| 1 | 2 | 0.0006 | 0.0004 | 0.207 |
| 2 | 2 | 0.0004 | 0.0004 | 0.225 |
| 3 | 4 | 0.0012 | 0.0006 | 0.345 |
| 4 | 1 | n/c | n/c | 0.227 |
| 5 | 1 | n/c | n/c | 0.232 |
| 6 | 5 | 0.0015 | 0.0007 | 0.103 |
| 7 | 6 | 0.0018 | 0.0007 | 0.248 |
| 8 | 7 | 0.0032 | 0.0011 | 0.448 |
| 9 | 5 | 0.0193 | 0.004 | 0.316 |
| 10 | 2 | 0.0009 | 0.0005 | 0.313 |
| 11 | 5 | 0.0096 | 0.0021 | 0.337 |
| 12 | 1 | n/c | n/c | 0.657 |
| 13 | 1 | n/c | n/c | 0.208 |
- H, number of haplotypes; D, mean molecular distances (p-distance); SE, standard errors for D; M, average morphological distance of individuals from group centroid; n/c, not calculated (only one haplotype present at a site).
Molecular analyses
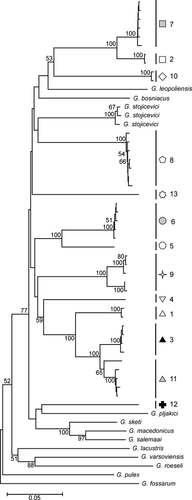
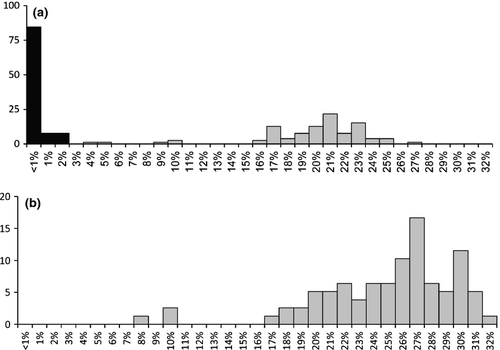
A total of 42 haplotypes have been identified of the 135 individuals sequenced. No haplotype was shared among populations. Nine of 13 locations showed more than one haplotype (Table 3). The p-distances between sequences within most sampled localities were <0.0032 (Table 3). The highest observed values still remained quite low, being 0.0193 and 0.0096, respectively, in Southern Carpathians and Transylvanian Plateau (sites 9 and 11). This pattern contrasts with the high overall mean molecular distance (p-distance: 0.153, SE: 0.009; K2P: 0.183, SE: 0.012) between localities (Table S4a and S4b). Most of these distances (94%) were high, ranging from 0.14 to 0.22 p-distance and from 0.16 to 0.27 K2P distance (Fig. 5a, Table S4a and S4b). They are in the same range as between-species distances calculated for several Gammarus species well delineated in morphological terms (Fig. 5b, Table S5a and S5b). However, there are also a few cases with between-locality distances below 0.11. Two cases concern the Carpathians (sites: 1, 3, 11; sites: 2, 7) and one Crimean Peninsula (sites: 5, 6). Interestingly, the distance between two other localities from the Southern Carpathians (sites: 8, 9) being in geographical proximity (57 km) is 0.18 p-distance and 0.21 K2P. Even between two sites remote by only ca. 7 km (sites: 1, 2) and 11 km (sites: 5, 6), the genetic p-distance is 0.19 and 0.10, respectively (Table S4a and S4b). The genetic distance between localities is not related to the geographical distance (Mantel test, one-tailed p-value = 0.54; Spearman rank order correlation, R = −0.026, p = 0.411). All the analysed populations of G. balcanicus and other species from G. balcanicus group (G. bosniacus, G. sketi, G. macedonicus, G. salemaai) cluster together within one clade on the neighbour-joining tree with 77% bootstrap support. The observed grouping pattern did not support monophyly of G. balcanicus versus other morphologically well-defined species. Interestingly, inside this clade, we can also find G. stojicevici belonging to the G. roeseli morphological group and G. leopoliensis belonging to the G. pulex group as defined by Karaman and Pinkster (1977a,b, 1987). The other species from G. pulex group (G. pulex, G. fossarum, G. lacustris, G. varsoviensis) and G. roeseli serve as an outgroup. Generally, inside the G. balcanicus clade, the branching order is poorly resolved. Only the localities from Crimean Peninsula (5 and 6) or Eastern and Southern Carpathians (1, 3 and 11) grouped with a 100% support (Fig. 4).
Molecules vs. morphology
We observed partial congruence between patterns of morphological and molecular divergence in the studied localities of G. balcanicus as in some cases the level of molecular distance between localities reflected the degree of morphological similarity. That happened with the localities from the Carpathian Arch (sites: 1, 3; sites 2, 7) close in molecular terms (p-distances < 0.10, bootstrap 100%) which are also grouped together on the MCA ordination graph and did not show any significant differences in morphological features (Figs 2 and 4, Tables S2 and S4). Nonetheless, individuals from other genetically and geographically distant sites are overlapping with this group on the MCA graph. While morphological variability is at relatively high level within sites, the within-site genetic variability is low in terms of genetic distance even if up to seven haplotypes may be observed per site (Table 3). On the other side, populations from geographically distant localities (e.g. sites: 1, 3, 7, 13), clearly divergent in molecular terms, overlap in morphology. Generally, the correlation between the level of morphological and molecular divergence between localities is very weak (Mantel test, one-tailed p-value = 0.183; Spearman rank order correlation, R = 0.0159, p = 0.082).
Discussion
In his original description of G. balcanicus based on individuals from several montane and submontane locations representing both western and central parts of the Balkan Peninsula (Montenegro, Hercegovina, Bulgaria), Schäferna (1922) indicated that many morphological traits of this species were largely variable. He attributed this variation to the rather wide distribution of the species. Subsequently, similar forms were found in numerous montane or submontane localities in Europe ranging from South-Eastern Alps through the Balkan Peninsula, Carpathian Arch to Crimean Peninsula and described as separate species or subspecies (Schäferna 1922; Karaman 1929, 1935; Martynov 1931, 1935; Dobreanu and Manolache 1942; Cărăuşu et al. 1955). Later, Karaman (1977) and Karaman and Pinkster (1987) claimed lack of geographical pattern in the morphology of G. balcanicus in Europe, as well as instability of features defining its subspecies and some of the taxa close in morphology but erected to a species status. Therefore, they synonymized most of them with G. balcanicus. Our formal analysis of a large number of morphological characters revealed high level of morphological polymorphism yet without a clear geographical pattern. Most variability ranges of the analysed features did not differ between localities. In some cases, a substantial overlap in morphology could be observed, even between sites from geographically distant areas, such as western Balkan Peninsula and Southern Carpathians. Interestingly, that conforms to the opinion of Karaman (1977) and Karaman and Pinkster (1987) drawn only from simple observations without any statistical support. In contrary to the species we studied, the montane caddisfly Rhyacophlla aquitanica (McLachlan, 1879) showed clear diversification among montane ranges in Europe (Balint et al. 2008).
The relatively high morphological variability of G. balcanicus joined by high molecular diversity is not a surprising phenomenon as it was observed in many other taxa (e.g. Omland 1997; Kim et al. 2010; Seligmann 2010). However, in our case, the high morphological diversity within sites is contrasting with low molecular distances. Similar pattern was already observed in the cichlid fishes from the ancient lake Victoria (Seehausen 2006) as well as in gammarid crustaceans from the European ancient Lake Ohrid (Wysocka et al. 2013). Apparently, in case of the ancient lakes, the morphological diversification with incomplete sorting of genetic lineages may be related to rapid events of sympatric speciation caused by availability of free ecological niches (Elmer et al. 2010). Nevertheless, ancient lakes are ‘long-living’ and spatially complex systems (Delvaux et al. 1995; Albrecht and Wilke 2008). Many mountain rivers hold substantial endemic diversity (Balian et al. 2008; Tockner et al. 2009 and references therein) what can suggest that these structures are also ‘long living’ in spatiotemporal terms (Malicky 1983). Our study has shown that the levels of morphological variability between and within sites are similar. Strangely, akin limits of this polymorphism are kept even in populations from remote sites in different mountain ranges. Thus, we may suspect that high morphological polymorphism may be of some adaptive significance, and as such is maintained even in allopatry. Similar results were found in case of the terrestrial snail Cepaea nemoralis Linnaeus, 1758; however, the cause of this phenomenon has not been discussed (Ożgo 2005; Cameron et al. 2011). It is impossible to explain this mechanism in G. balcanicus, based on the results of our study, yet, we may suspect that it may be supported in some yet unknown way by a range of microhabitats available for the species in alpine streams (Nagy and Grabherr 2009).
In opposition to morphology that shows only a weak geographical pattern, the molecular divergence between sites is always very high, even in case of geographical proximity. Exclusive presence of private haplotypes in populations inhabiting different sites suggests that they are in complete allopatry (i.e. that no gene flow occurs). The high molecular distance between these allopatric populations indicates that their actual divergence is at least on a species level. In fact, the average frequency distribution of COI distances between the analysed localities of G. balcanicus falls within the range observed between other species of Gammarus well defined in morphological terms (Fig. 5b). This may support Hou et al.'s (2011) conclusion about the very old origin of the present gammarid diversity in freshwaters of Europe, with the oldest lineages possibly dating back to the Tethys regression in Tertiary. Besides, G. balcanicus appears to be polyphyletic as some of its populations group with such species as G. leopoliensis and G. stoicevici which are prominently different in their morphology and classified respectively to G. pulex and G. roeseli groups by Karaman and Pinkster (1977a,b). For example, the G. roeseli group was defined based on presence of sharp dorsal processes absent in most species of Gammarus. This points out the need for re-evaluation of morphological features used in the taxonomy of gammarids.
There were already many attempts to define limits between species based on DNA data, one of them being the DNA barcoding approach. Hebert et al. (2003) found out that in insects, molecular p-distance among most congeneric morpho-species was >0.03 for COI. For the same molecular marker, Costa et al. (2007) defined interspecific molecular distance among congeneric marine Gammarus species as ranging from ca. 0.06 to 0.31 K2P, while the intraspecific range was up to 0.03 K2P. Similarly, Hou et al. (2009) as well as Hou and Li (2010) observed at least 0.15 uncorrected pairwise divergence for a number of morphologically distinguishable freshwater species of Gammarus from China. Witt et al. (2006) proposed a different approach to the problem using a species screening threshold (SST) set at 10 times the average intrapopulation COI haplotype divergence.
It is important to mention some ambiguity associated with species definition through such DNA barcodes and problems according to the species concept used. For example, neither Hebert et al. (2003) nor Hou et al. 2009 nor Costa et al. (2007) did refer to any species concept and did not discuss this issue. Witt et al. (2006) were discussing on the level of the phylogenetic species concept. Various problems posed by implementation of DNA barcoding in taxonomy have been already widely discussed (e.g. Collins and Cruickshank 2013). The necessity of combining various sorts of data, including molecular divergence, ecological requirements, hybridization experiments and thorough morphological studies, for the proper definition of gammarid species was pointed already by Meyran et al. (1997) and further by Hou and Li (2010). Nevertheless, in practice, such multiproxy approach is difficult to use. Usually, only preserved specimens are available what limits the study only to their morphology and molecular diversity. Thus, such handy and simple approach as DNA barcoding is necessary for at least provisional definition of species, whose validity may be further tested by other scientists.
According to the criteria specified above, most of the studied populations attributed provisionally to G. balcanicus deserve a separate species status. Only the populations 3 and 11 from Eastern Carpathians and Transylvanian Plateau, respectively, as well as populations 2 and 7 from the Eastern Carpathians are divergent by 0.04 K2P and 0.05 p-distance and do not show statistically significant differences in morphology. Although, the values are greater than the 0.02 threshold set up by Hebert et al. (2003), they fall in between intraspecific and interspecific distance values given by Costa et al. (2007). Yet, taking into account the Witt et al. (2006) SST, these populations also differ enough to be called different species. Even taking into account the high interspecific divergence level observed by Hou and Li (2010), only a few population pairs diverged by <0.15. Two are the already mentioned populations 2 and 7. The interpopulation distance for three populations (1, 3, 11) from the Eastern Carpathians and Transylvanian Plateau is from 0.04 to 0.09 (p-distance), what is to be appreciated in the face of the lack of significant differences in morphology. The Crimean populations are mutually divergent by 0.11 (p-distance) and also do not differ significantly in morphological terms.
The molecular divergence between the studied populations of G. balcanicus is at the same level as between other European Gammarus species. Also, there are significant differences in case of some of the studied morphological features. Thus, we can assume that at least some of the numerous species and subspecies described years ago and later synonymized with G. balcanicus (see above) might in fact be distinct species, not even closely related in phylogenetic terms. We can conclude that the name G. balcanicus sensu Schäferna 1922 applies only to the population from type locality near Kolasin, Montenegro. In this paper, we neither aim to define the key diagnostic morphological features for identification of the analysed populations nor to perform formal taxonomic revision of the species. That is a goal for a separate study upon taxonomy of G. balcanicus – a difficult one taking into account that many of the original collections and type specimens were lost, and in many cases, the exact type localities are unknown. Yet, at present, we can conclude which features show the greatest variability among sites that could be used in further taxonomic studies upon G. balcanicus. These are as follows: number of setae on peduncle of antenna 1 (A, B), number and length of setae on peduncle and length of setae on flagellum of antenna 2 (F, G, H, I) and on third uropod: the length ratio of endopodit to exopodit (K), type of setae on endopodit outer margin (S), presence and type of setae on endopodit (U, V). Interestingly, all the analysed features localized on antennas were classified by Karaman and Pinkster (1987) as characters stable within species, while the armature of third uropod was recognized as showing intraspecific variability. Once again, it leads to the conclusions that the taxonomy of gammarids based on morphological characters should be definitely re-evaluated. In our opinion, a wider variety of phenotypic characters should be studied for the sake of delimitation of these, so far, cryptic species. Cuticle ultrastructure revealed by SEM is a particularly promising marker (Khalaji-Pirbalouty and Sari 2004, 2006). On the other side, implementation of further molecular markers such as 28S, 18S, EFα1 will help to illustrate the phylogenetic relationships among these putative taxa and their evolutionary history.
Proper identification of such cryptic species is crucial also for practical reasons. Various widely spread Gammarus species, including G. balcanicus, are commonly used in biomonitoring of waters and particularly for toxicological tests (e.g. Krupa and Guidolin 2003; Felten et al. 2008; Parvulescu and Hamchevici 2010; Maazouzi et al. 2011; Sroda and Cossu-Leguille 2011). Distinct species, even not separable based on morphology but distant in molecular terms, may have different tolerance ranges towards a variety of factors (e.g. heavy metals). For example, Scheepmaker (1990) reported that even very similar and closely related species of Gammarus differ in the minimum water temperature required for reproduction by 2–4°C. Recognition of the level of genetic diversity and its maintenance is also one of the key targets for nature conservation (WWF 2012). Taking into account the already mentioned (see 1) properties of montane areas, it may be expected that other aquatic species, widely distributed in European mountain ranges and poorly defined in morphological terms, will also reveal deep within-species molecular divergence and wide cryptic diversity. Generally, all this makes the recognition of cryptic diversity in aquatic taxa an important goal for future studies, notably in case of those distributed across various mountain ranges.
In conclusion, (1) there is a high morphological variability within populations of G. balcanicus, however, this variability shows no geographical pattern across European mountain ranges; (2) in contrary, the level of molecular divergence within sites is very low, while it is very high between different localities yet also not geographically structured; (3) analysis of molecular data suggests that G. balcanicus is a polyphyletic group of allopatric cryptic species; (4) a number of morphological features show substantial variability and help to separate some of these putative species in multiple correspondence analysis, however, so far we could not define any of them as being diagnostic.
Acknowledgements
This study was supported by the Polish Ministry of Science and Higher Education (projects no. N N303 579439 and 5818/B/P01/2010/39) and partially from the internal funds of the University of Lodz. We thank Małgorzata Kłonowska-Olejnik for providing some samples from the Ukrainian Carpathians. The sampling in Crimea was supported by the Research Agency of Slovenia with the Slovenian-Ukrainian bilateral agreement. The anonymous reviewers are greatly acknowledged for their constructive comments and suggestions.




