When snails inform about geology: Pliocene emergence of islands of Vanuatu indicated by a radiation of truncatelloidean freshwater gastropods (Caenogastropoda: Tateidae)
Abstract
The South Pacific archipelago Vanuatu has a very complex geological history including three major phases of volcanism creating island belts and phases of repeated submergence and re-emergence. An important issue for the evolution of the biota of Vanuatu ambiguously discussed in the geological literature is the question whether the entire archipelago has been submerged until the early Pleistocene or if at least parts of the island of Espiritu Santo have remained subaerial throughout the Pliocene. We used a time-calibrated phylogenetic analysis of freshwater gastropods of the family Tateidae based on COI, 16S rRNA and ITS2 to infer the colonization history of Vanuatu. Our analyses suggested that Espiritu Santo was colonized c. 3 Mya. Espiritu Santo was probably the place of origin for the subsequent colonization of the island of Erromango (2 Mya) and the Pleistocene radiation across the remaining archipelago. We describe 10 new species largely based on morphological and anatomical data. The genetic data in particular of the species from the young islands are taxonomically incongruent probably due to incomplete lineage sorting typical for young radiations. In contrast, the paraphyly of Fluviopupa espiritusantoana appearing in three distant clades indicates either the existence of cryptic species or the long survival of the stem species of almost the entire radiation.
Introduction
Small freshwater gastropods of the family Tateidae may represent one of the oldest radiations across South Pacific islands (Haase et al. 2010a; compare Gillespie et al. 2008; Neall and Trewick 2008; Keppel et al. 2009). They occur in New Zealand (Haase 2008), New Caledonia (Haase and Bouchet 1998); Vanuatu (Haase et al. 2010a), Fiji (Haase and Bouchet 2006) and the Austral Islands, the southernmost archipelago of French Polynesia (Haase et al. 2005). These islands and archipelagos have at least partly been above the Ocean surface since at least the late Miocene (Kroenke 1996; Bonneville et al. 2002). Only for Vanuatu, the geological record is ambiguous (see below). Tateids have not been found on any of the many younger, interspersed islands. The only younger islands that do have a tateid record are Norfolk Island whose single species probably got extinct in historical times (Ponder 1981) and Lord Howe Island (Ponder 1982). Marine or brackish water representatives occur only in coastal waters of Australia (Ponder and Clark 1988) and New Zealand (Haase 2008). The freshwaters of the latter were invaded probably three times independently (Haase 2005). These large land masses in the west and south-west were thus probably the source areas for the colonization of the tropical Pacific islands.
In the course of a project aiming at the reconstruction of the tateid ‘conquest’ of the South Pacific, six islands of Vanuatu were visited in 2011, viz. Aneityum, Erromango, Efate, Malekula, Pentecost and Gaua. This expedition complemented earlier collecting efforts on Espiritu Santo (short: Santo) and the Torres Islands in 2006 and 2007 (Bouchet et al. 2011), which led to the description of 10 new species (Haase et al. 2010a). The only other islands Tateidae – recently separated from Hydrobiidae and raised to family status within Truncatelloidea and no longer Risooidea (Criscione and Ponder 2013; Wilke et al. 2013) – were reported from until then are Efate and Gaua. The samples from Efate and Santo were attributed to Fluviopupa brevior (Ancey 1905) (Ancey 1905; Solem 1959; Starmühlner 1976) and the one from the crater lake on Gaua was classified as Potamopyrgus sp. (Baker 1929).
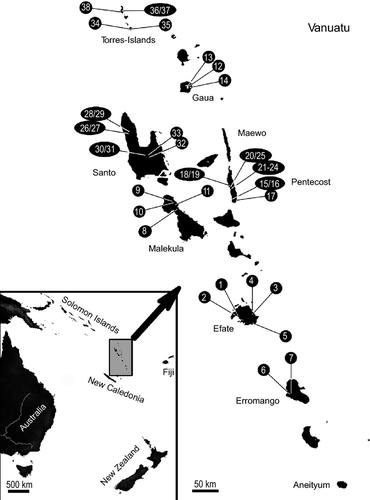
The Archipelago of Vanuatu (Fig. 1) consisting of more than 80 islands spread over more than 900 km is situated about 2000 km east of Australia and 400 km north-east of the main island of New Caledonia in the Pacific Ocean. Following Simeoni (2009; see also references cited therein), the Archipelago can be divided into three parts being the result of different volcanic episodes: The western belt comprising the largest Islands Malekula and Santo as well as the Torres Islands is the presumably oldest part with active volcanism from the Oligocene to the middle Miocene. Volcanism leading to the formation of the eastern belt consisting of Pentecost and Maewo dates from the end of the Miocene to the early Pliocene. The Central Chain comprising all islands between Aneityum and the Banks Islands is the presumably youngest part with continuous volcanism from the early Pleistocene to today. In addition to the complex history of formation, the archipelago has experienced stages of submergence and re-emergence. However, the knowledge about the final emergence of the different islands is partly ambiguous. The Torres Islands are entirely covered by Quaternary coral formations indicating a very young subaerial history (Taylor et al. 1985). Large parts of Malekula are covered by Pliocene (southern part) to Pleistocene (northern part) coral limestone. A window of Miocene rocks in the north has been interpreted as paleoisland, which, however, probably also submerged and re-emerged only in the Pleistocene (Taylor 1992). Total submergence since the early Pliocene is assumed for Santo. But at least parts have probably remained above sea level since uplift in the earliest Pleistocene (Taylor 1992; Robin 1993). Yet, Santo has also wide areas covered by Quaternary coral reef limestone. This indicates that, like on Malekula, there are younger and older parts regarding the time since emergence (Robin 1993). The Eastern belt islands are practically entirely covered by Pliocene to Pleistocene coral reef limestone suggesting a late Quaternary uplift (Taylor 1992). Most islands of the central chain including Aneityum, Efate and Gaua owe their emergence to Pleistocene volcanic activity (Robin 1993; Greene et al. 1994). In contrast, the western part of Erromango seems to be continuously emergent since 2.6–2.3 Myr except for the Mount Rantop peninsula (Neef and Hendy 1988). The earliest volcanism of Erromango dates already to the late Miocene, though. The island seems to have surfaced and submerged repeatedly since then. However, interpretations if volcanism was submarine or subaerial differ between authors (cf. Robin 1993; Greene et al. 1994). The uncertainty concerning the emergence of the islands of Vanuatu is illustrated by Figure 12 of Akimoto (1994) where the sea surface is tangent to the peaks of the islands throughout Plio- and Pleistocene. Clear islands exist only in the panels representing Holocene and present age. Neglecting the ambiguities, Hamilton et al. (2010) assumed ‘the last period of emergence only in the last 2 myr’. (p. 150) in their recent biogeographical analysis of ‘a nascent oceanic archipelago’.
The aims of our paper are threefold: (1) we describe the new species found during our expedition in 2011 based on morphology, anatomy and molecular phylogenetic analyses, (2) based on the phylogenetic analyses, we inferred the colonization history of the archipelago, and (3) the age of the radiation, the latter with possible implications for the reconstruction of the geological history of Vanuatu. Due to their low potential of active dispersal, snails often preserve old distribution patterns (e.g. Cook et al. 1990; Haase et al. 2007; Holland and Cowie 2009). These patterns may be informative also for the reconstruction of geological histories (e.g. Upton and Murphy 1997; Pfenninger et al. 2010). To understand the origin and evolution of flora and fauna of oceanic islands, it is important to know when islands emerged. Regarding in particular the older islands of Vanuatu with their complex history of sea-level fluctuations, the above estimates had to be presented in subjunctive. Therefore, we implemented a molecular clock approach to test the geological scenario outlined above and eventually refine hypotheses regarding the emergence of islands.
Material and Methods
Material
Most specimens examined in this study were collected in May and June 2011. They were preserved in 70% ethanol in the field and transferred to propylene glycol for shipment. Upon arrival in our laboratory, snails were returned to 96% ethanol. Sampling stations are indicated in Fig. 1 and described in detail in the supplementary material. Material from Santo and the Torres Islands was provided by the Museum National d′Histoire Naturelle Paris (no exact collection numbers available), and species from New Zealand included in the outgroup of phylogenetic analyses came from the Museum of New Zealand Te Papa Tongarewa in Wellington. As outgroup taxa, we selected two tateid species from New Zealand [Opacuincola delira Haase 2008 (MNZ M.174126), Potamopyrgus estuarinus Winterbourn 1971 (MNZ M.174052/1)], as well as one each from New Caledonia (Hemistomia winstonefi Haase and Bouchet 1998), Australia (Tatea huonensis Tension Woods 1876) and Brazil (Potamolithus ribeirensis Pilsbry 1911) based on the latest phylogeny of rissooidean/truncatelloidean gastropods (Wilke et al. 2013). Sequence data of the three latter outgroup taxa were taken from GenBank (Appendix 2).
Museum acronyms: MNHN, Museum National d′Histoire Naturelle Paris; MNZ, Museum of New Zealand Te Papa Tongarewa; ZMB, Museum für Naturkunde Berlin.
DNA isolation and sequencing
DNA was isolated from two snails of each sample (we crushed entire snails after taking photographs without leaving remnants qualifying as voucher) using QIAGEN's DNeasy Blood and Tissue Kit (QIAGEN GmbH, Hilden, Germany). The primers used to amplify a 715-bp-long fragment of the nuclear (nc) 16S rRNA gene (16S) were 16Sar (Palumbi et al. 1991) and 16Sr (5′-tcttctgccacctttatt-3′; this study). The primers L1460 and H1298 (modified at position 12; G → A) by Folmer et al. (1994) amplified a 658-bp-long fragment of the mt cytochrome oxidase subunit I gene (COI) gene and the primer ITSfn (5′ gacacattgaacatcgaca 3′; this study) and ITSr4 (Oliverio and Mariottini 2001) a ca. 435-bp-long fragment of the nuclear (nc) internal transcribed spacer 2 region (ITS2). Polymerase chain reactions were performed in 12.5 μl containing 1.1 μl 10X BH4 buffer (BIOLINE GmbH; Luckenwalde, Germany), 4.4 mM MgCl, 0.28 pM of each primer, 0.2 mM dNTP, 0.5 μl BSA (1%), 0.25 U DNA-Polymerase (BIOLINE), 5–50 ng DNA and water.
The PCR conditions were 5 min at 95°C for initial denaturation, followed by 40 cycles with a denaturation step at 95°C for 60 s (45 s for ITS2), an annealing step as outlined in the following, and an extension step at 72°C for 60 s (75 s for ITS2), as well as a final extension step at 72°C for 10 min. Annealing was performed at 46°C for 90 s for COI, 53°C for 45 s for ITS2 and as a touchdown with a temperature drop of one degree in each of the first 10 cycles starting at 60°C followed by 30 cycles at 51°C for 60 s for 16S. Products were purified enzymatically using exonuclease and shrimp alkaline phosphatase (ExoSAP-IT Affymetrix, Santa Clara, CA, USA) and sequenced using ABI's Big Dye Terminator Ready Reaction Mix v3.1 (Carlsbad, CA, USA) and the PCR primers on an ABI 3130xl Genetic Analyzer. All Sequences are available from GenBank with accession numbers listed in Appendix 2.
For a better resolution of deeper nodes, we attempted to sequence also the nc genes ATP synthetase α and the Elongation factor 1α, however, failed to establish primers working in at least the majority of specimens. The lack of markers amplifying across a wider range of gastropod groups is commonly deplored (Dayrat et al. 2011).
Phylogenetic analyses
Sequences were edited using BioEdit (Hall 1999). Due to the lack of indels, the protein coding mt COI gene could be aligned by eye. The mt 16S and the nc ITS2 sequences were initially aligned using Clustal W (Thompson et al. 1994). This alignment was then refined in RNAsalsa (Stocsits et al. 2009) using secondary structure information of Cacozeliana lacertina Gould 1861 [16S, http://www.rna.icmb.utexas.edu/SIM/4D/Mollusk/ (Cannone et al. 2002), AF101007] and Haliotis discus Reeve 1846 [ITS2, http://its2.bioapps.biozentrum.uni-wuerzburg.de (Koetschan et al. 2010), GI 28627842] and finally manually edited in BioEdit (the final Alignment is available from TreeBASE http://purl.org/phylo/treebase/phylows/study/TB2:S14586).
Partition Finder (Lanfear et al. 2012) was used to decide whether stem and loop structures in 16S and ITS2 and codon positions in COI should be treated as partitions with individual substitution models and to find the best fitting models according to the corrected Akaike information criterion. A test for substitution saturation was performed in DAMBE (Xia and Xie 2001) treating gaps as unknown states. Phylogenetic analyses were performed in a maximum likelihood framework. Initial attempts of implementing Bayesian analyses using MrBayes (Ronquist and Huelsenbeck 2003) had to be aborted, because the Markov chains did not reach stationarity (see below). For tree reconstruction, we used Garli (Zwickl 2006) with 500 search-replicates for the concatenated data set and 100 replicates for separated nc and mt data, respectively. To assess the robustness of the topology based on the concatenated data, a bootstrap analysis with 500 replicates was performed. The bootstrap consensus tree was composed using Phyutiliy (Smith and Dunn 2008). Bootstrapping was repeated after exclusion of three taxa with leaf stability (calculated also with Phyutility) index <0.6. Trees were rooted with Potamolithus ribeirensis which is the least closely related to the genus Fluviopupa among the selected outgroup taxa (Wilke et al. 2013).
Regarding the low resolution in parts of the resulting tree, the analysis of the concatenated data set was repeated using constraint files to test different hypotheses of island colonization. The hypotheses compared were (1) unconstrained tree; (2) every island was colonized only once, hence samples from each island formed reciprocally monophyletic groups; (3) samples from Santo and Malekula, the presumably oldest Islands, are sister group to the samples of the remaining islands of which each was colonized only once; and (4) samples from Santo were paraphyletic with respect to the samples from the other islands, of which each was colonized only once. The resulting trees were tested against each other using the approximately unbiased test implemented in Consel (Shimodaira and Hasegawa 2001).
The phylogeny was dated based on the substitution rate estimated for COI in tateid gastropods from New Zealand (3.26 ± 0.14% MY−1), the closest relatives for which rates have been estimated (Haase et al. 2007). A likelihood ratio test for the molecular clock was performed in DAMBE (Xia and Xie 2001). Divergence times were estimated in two approaches, first, based on average COI-distances between clades calculated in Paup*4.10b (Swofford 2003) and corrected after the HKY + G substitution model, the best fitting model according to the corrected Akaike information criterion as shown by jModeltest (Posada 2008). The second approach considering all three gene fragments was a Bayesian analysis implemented in BEAST 1.7.5 (Drummond et al. 2012) assuming a birth–death model and a strict clock based on the COI substitution rate to calculate divergence times. As these analyses had no outgroup taxa, COI was no longer partitioned due to the very restricted variability of first and second codon positions. Topologically instable taxa were omitted. Using the whole taxon set resulted again in problems of parameter optimization most likely caused by the many short branches in clade I (Fig. 2). Therefore, clade I was reduced to nine randomly chosen taxa. Finally, eight independent analyses each run for 40 Mio generations logging every 10,000th were combined to obtain effective sample sizes of >200 for all parameters setting a burn in of 10%.
Morphology
Five shell dimensions, shell height and width, aperture height and width, and width of the body whorl, were measured (parallel and perpendicular to the coiling axis, respectively) from digital photographs taken under a dissecting microscope using Zeiss Axio Vision 4.8 of up to 20 adult individuals of each sample. Whorls were counted to the nearest eighth of a whorl. Statistical comparisons including the multivariate techniques CVA, manova and Hotelling's T2-tests and univariate Student's t-tests were performed using past 2.0 (Hammer et al. 2001).
Snails were dissected and anatomies drawn after dissolving shells in 1N HCL. A 5% sodium hypochlorite solution was used to clean shells, radulae and opercula for scanning electron microscopy. Cephalopodia were dried using hexamethydisilazane (Nation 1983). The objects were coated in gold and investigated in a Zeiss EVO LS10 Scanning Microscope.
Species descriptions
The phylogenetic analysis based on molecular data revealed both morphologically and genetically cryptic species (see below), that is, species, which are morphologically very similar and can reliably only be distinguished genetically, and other species, which are genetically indistinguishable but differ in morphology or anatomy. In the following, we describe new species only if the evidence was unambiguous. Populations, for whose variability it was impossible to decide whether it was intra- or interspecific, were regarded as single species. For the taxonomic treatment of morphological and anatomical characters, we refer to the ‘pragmatic species concept’ of Haase and Bouchet (2006).
As a service to the reader, we use the new names already in the phylogenetic analyses although they are formally introduced only in the second part of the article.
Abbreviations
In the figures, the islands of Vanuatu are indicated by two letters: Am, Ambae; An, Aneityum; Ef, Efate; Er, Erromango; Ga, Gaua; Ma, Malekula; Me, Maewo; Pe, Pentecost; Sa, Espiritu Santo; Ta, Tanna; To, Torres Islands.
Results
Phylogenetic analysis
Partition Finder suggested the following partitioning scheme with respective substitution models: HKY + I + Γ for combined stems and loops of 16S; K81 + Γ for ITS2 loop structures and F81 + Γ for the stems; and F81 + I, HKY and GTR+Γ for first to third codon positions of COI. The test for substitution saturation reported only negligible saturation for all three genes and all three codon positions of COI. Sequence divergence based on pairwise distances in the ingroup reached up to 5.1% in 16S, 10.2% in COI and 5.4% in ITS2.
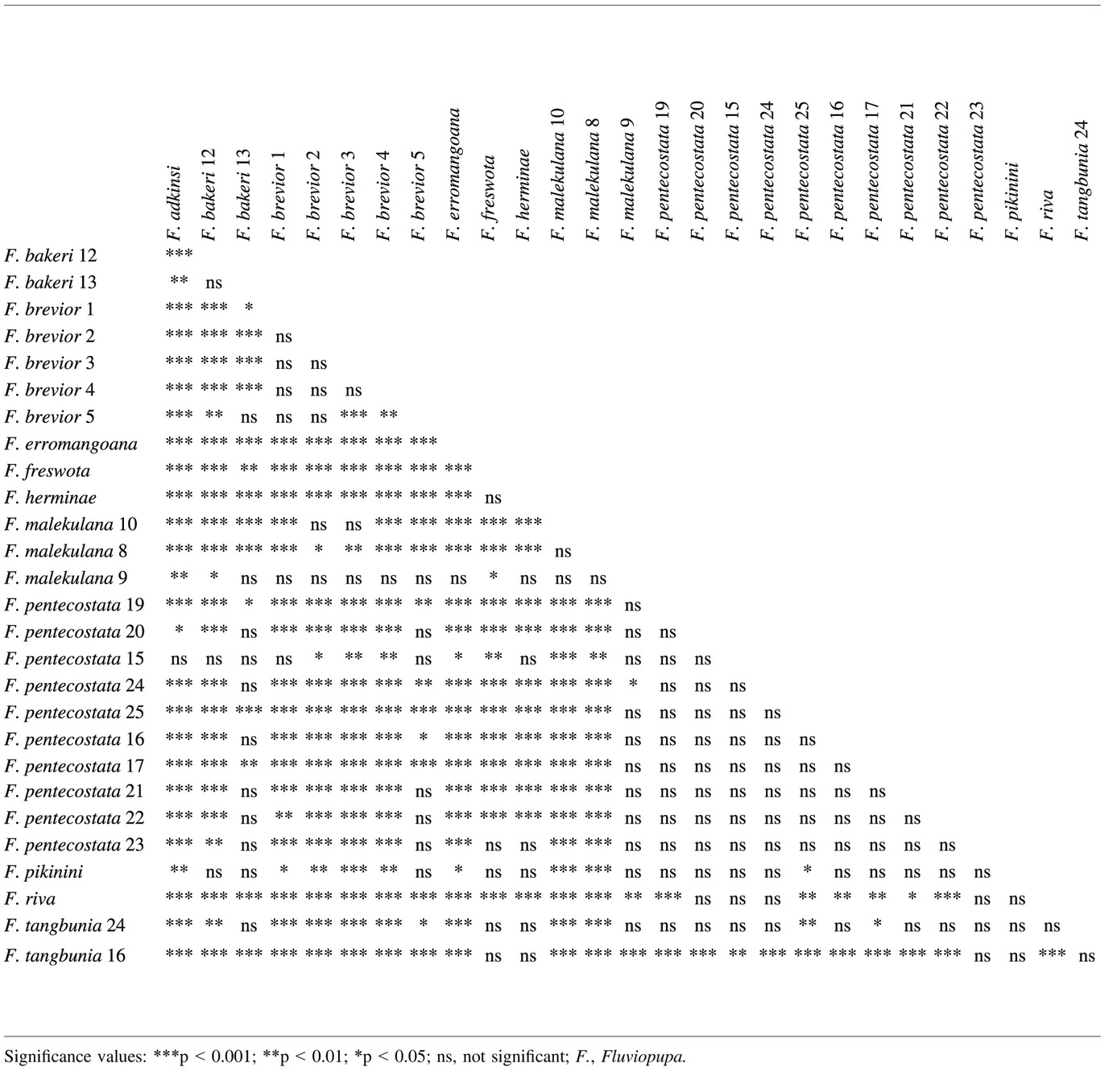
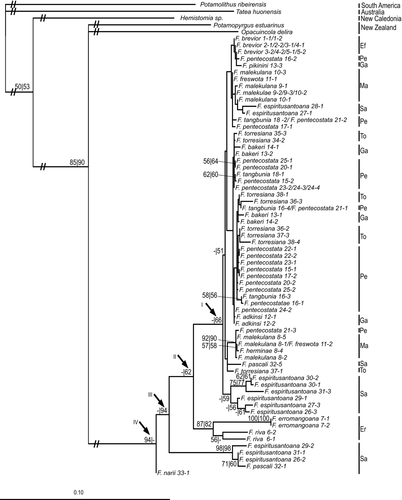
In the phylogenetic analysis (Fig. 2), the snails from Vanuatu formed a well-supported monophylum. Interestingly, the closest relatives to the Vanuatu clade were the species from New Zealand. This relationship was also well supported. The sister group relationship of Hemistomia winstonefi from New Caledonia with the other island taxa received a bootstrap support of only 67. The Australian Tatea huonensis was drawn to Potamolithus ribeirensis, the root. Most of the specimens collected in Vanuatu were genetically very similar. Animals from Efate, Malekula, Pentecost, Gaua and the Torres Islands and some from Santo formed the large, hardly resolved clade I with the moderate support of 66. There was also only little geographical structure in this clade characterized by short branch lengths. Fluviopupa brevior and F. adkinsi nov. sp. were the only species with more than one representative that were recovered each in a single subclade and only few subclades were composed of a single species. Only one subclade comprising more than two specimens received bootstrap support > 50. This subclade contained three specimens of F. malekulana nov. sp., the single representative of F. herminae nov. sp. and one of F. freswota nov. sp., all from Malekula, and one snail ascribed to F. pentecostata nov. sp. Clade II, the sister clade of clade I, was well defined and formed by the two reciprocally monophyletic species from Erromango. The next deeper clade, clade III, contained three specimens again of F. espiritusantoana and a single one of F. pascali, both occurring on Santo and both also represented in the youngest clade I. The oldest lineage in the Vanuatu clade was formed by F. narii Haase, Fontaine & Gargominy 2010 from Santo. However, together with F. pikinini nov. sp. (13-3) and F. espiritusantoana (28-1), this specimen of F. narii was topologically unstable with a leaf stability index of 0.53 and removed in a second bootstrap analysis. Thus, there is not much confidence in the branch connecting nodes III and IV. Bootstrap support of nodes I-III was only achieved after removal of these three unstable individuals. The topologies of the analyses separately based on either ITS2 or the mt fragments were practically identical to the concatenated analysis with only slight differences in the positions within clade I and F. riva nov. sp. being paraphyletic in the mtDNA tree.
The comparison of different hypotheses of island colonization revealed that none of the alternative hypotheses was at least as likely as the unconstrained tree (AU-test, p < 0.01).
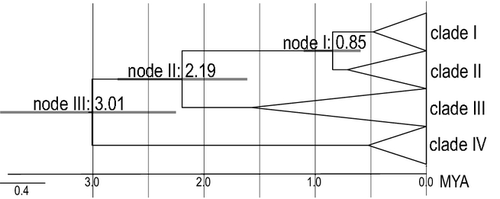
The molecular clock was not rejected (likelihood ratio test, p > 0.79) for all three genes. Assuming a sequence divergence of 3.26 ± 0.14% MYR−1, the following ages for the three nodes relevant for the global picture of the geological evolution of Vanuatu (Figs 2 and 3) were estimated based on the average COI-distances between clades: node I: 0.68 ± 0.03 Mya, node II: 2.62 ± 0.11 Mya and node III: 4.81 ± 0.20 Mya. The deepest but uncertain (see above) split concerning F. narii could not be dated because sequencing COI failed in this individual. The phylogenetic tree resulting from the BEAST analysis (Fig. 3) was topologically basically identical to the ML tree; only the relationships within clade I were differently reconstructed. All basal nodes including nodes I-III were supported by posterior probabilities of 1. The following ages were estimated: node I: 0.84 ± 0.26 Mya, node II: 2.20 ± 0.57 Mya and node III, 3.01 ± 0.77 Mya.
Systematic descriptions
Fluviopupa Pilsbry, 1911
Type species: Fluviopupa pupoidea Pilsbry, 1911 (original designation, by monotypy).
Synonymy: Fluviopupa Pilsbry, 1911.
The following description is based on the latest comprehensive diagnosis of the genus by Haase et al. (2010a).
Shell measurements and whorls are given in Appendix 1 and therefore not repeated in species descriptions. The following features are invariant across all species and therefore not mentioned again in species descriptions:
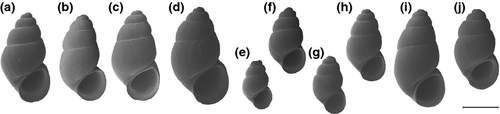
Shell (Figs 4 and 5): conical; protoconch well differentiated from teleoconch, surface with wrinkles gradually becoming finer towards the teleoconch; teleoconch smooth apart from growth lines; umbilicus narrow; aperture simple.

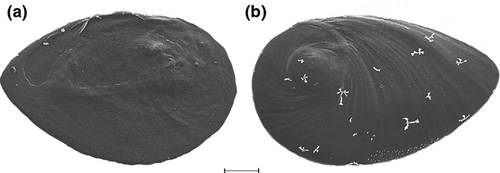
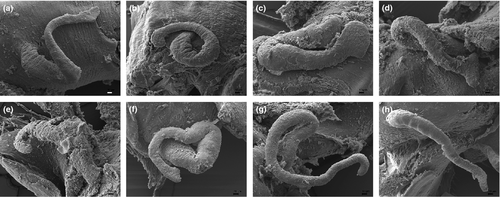
Operculum: Corneous, yellow, elongate-ellipsoidal, paucispiral; nucleus submarginal, with variable extent of white smear and small, rudimentary peg (Fig. 6).
External features: tentacles without conspicuous pattern of ciliation.
Mantle cavity: Ctenidium well developed, abutting directly on pericardium; osphradium ovate-elongate; hypobranchial gland not recognized in dissections; kidney not protruding into pallial roof.
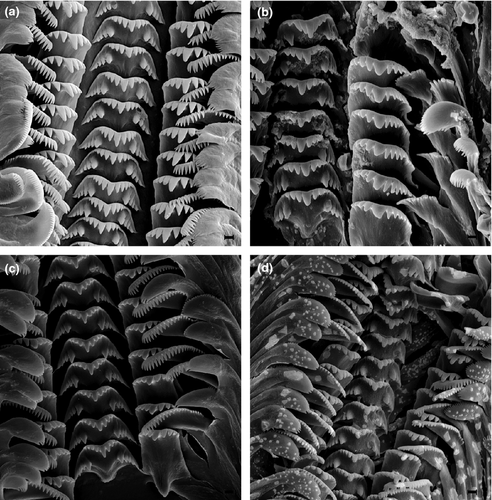
Digestive system: Radula (Fig. 7) taenioglossate; stomach with fan-shaped caecum (Fig. 8).
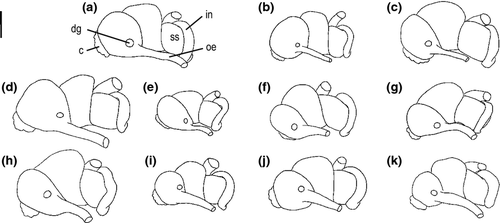
Female genitalia (Fig. 9): Oviparous; ovary sac-shaped, only occasionally extending to stomach; renal oviduct coiling first 180° clockwise and than 270° counter-clockwise, the proximal loop often bent towards albumen gland; one distal receptaculum seminis globular with moderately wide, short duct, lying against left side of middle part of bursa copulatrix; bursa copulatrix behind albumen gland, pyriform to elongate; pallial oviduct with ovate cross-section; genital opening terminal or subterminal.
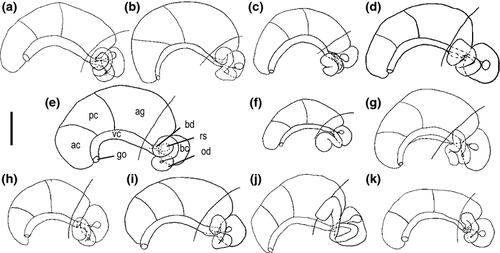
Male genitalia: Testis lobate, usually covering proximal chamber of stomach; vas deferens initially coiling as vesicular seminalis; vas deferens entering prostate in posterior third; penis simple, usually tapering more or less continuously from broad base, occasionally bulging out to form a flange on right side (Fig. 10).
Fluviopupa brevior (Ancey 1905)
Synonymy: Potamopyrgus brevior Ancey 1905, Nautilus 19: p. 46.
Type material: Holotype lost; neotype here designated; neotype: ZMB Moll 117880, Paratypes: ZMB moll 117891 (n = 50).
Type locality: Efate Island, Mangaliliu village, station 1 (17°38′19.5′′S, 168°12′23.7′′E).
Additional material: Efate Island; road to Benjor Beachclub, station2 (17°41′00.6′′S, 168°14′53.6′′E; MNZ C477745 n > 50, MNHN n = 15); Lamin, station 3 (17°39′10.0′′S, 168°31′16.5′′E; MNZ C477746 n > 50, MNHN n > 50); East coast, station 4 (17°38′43.7′′S, 168°30′48.0′′E; MNZ C477748 n > 50, MNHN n = 15) and White Sands, station 5 (17°48′31.7′′S, 168°24′01.4′′E; MNZ C477747 n = 50, MNHN n = 15).
Diagnosis: Due to its variable shell size and shape, F. brevior is not easy to distinguish from several species occurring on the other islands of Vanuatu. Among species with a terminal penial lappet and similar shell shape and size, F. pentecostata nov. sp. is significantly more slender (Hotelling's T2-test: p < 0.01; t-tests: p < 0.01 for sw and sw/sh at type localities), F. espiritusantoana Haase, Fontaine & Gargominy 2010 has less denticles on the outer teeth and lacks the U-shaped loop in the male's rectum, and F. pascali Haase, Fontaine & Gargominy 2010, anatomically similar to F. espiritusantoana, is much broader (Haase et al. 2010a).
Description
Shell (Figs 4a and 5a): Grayish-white to light brown; conical; about 1.75–1.8 times higher than wide; protoconch 0.875–1.125 whorls; aperture little higher than wide.
External features: Variable black epidermal pigmentation, with some animals being nearly unpigmented, mantle rim and head with very little or no pigment.
Mantle cavity: Ctenidium with 18–22 filaments; osphradium short, behind middle of ctenidium, reaching third to half of its length.
Digestive system: Rectum close to pallial oviduct in females but making U-shaped loop at anterior end of prostate in males; radula R: 3-5 1 3-5/3-4 3-4, L: 3-4 1 3-4, M1: 20-23, M2: 29-31.
Female genitalia (Fig. 9a, b): Ovary starting 0.75–1.5 whorls below apex, comprising up to 1.5 whorls; receptaculum seminis pyriform, lying against anterior third to middle of bursa copulatrix, bursal duct short, entering slightly below anterior edge of bursa; length of albumen gland two-third of capsule gland, about 1/3 of albumen gland extending into pallial roof, anterior section of capsule gland translucent milky-white, posterior one opaque white, albumen gland translucent; renal oviduct without any special features, proximal loop bent towards albumen gland.
Male genitalia: Testis lobate starting 0.75–1.5 whorls below apex, comprising 0.75–1.5 whorls, partly overlapping posterior chamber of stomach; seminal vesicle leaving testis 0.25–0.5 (in samples 2 and 5 up to 0.75) whorls proximal to anterior end; penis (Fig. 10a) with terminal lappet.
Remarks: The samples from stations 3 and 5 showed a sexual dimorphism with the females being significantly larger in sh, sw, ah and bww at station 3 and in sw, ah, aw and bww at station 5 (t-tests, p < 0.05).
Fluviopupa brevior was the only species we found on Efate. Shell size and shape varied between samples (Fig. 11) and partly between sexes. Genetically, the samples were practically identical and formed a monophylum (Fig. 2).
The species has first been described by Ancey (1905) as Potamopyrgus brevior based on a single specimen. This holotype has apparently been lost which is why we designated a neotype for the species now described more comprehensively.
Fluviopupa riva nov. sp
Type material: holotype ZMB Moll 117881; paratypes ZMB Moll 117892 (n = 50), MNZ C477766 (n = 20), MNHN (n = 10).
Type locality: Erromango Island, south-east of the village Dillons Bay, station 6 (18°49′24.3′′S, 169°01′36.2′′E).
Etymology: The noun riva means river in Bislama, the lingua franca of Vanuatu, and is reminiscent of our 10 fordings of the Williams River before we discovered the type locality.
Diagnosis: F. riva nov. sp. is significantly different from its sister species F. erromangoana nov. sp. described below (Hotelling's T2-test: p < 0.01). It is smaller in all five dimensions and wider considering the relation of shell height and width (t-tests: p < 0.01). The species also differ in the denticle number of the marginal teeth. F. melissae Haase, Fontaine & Gargominy 2010 has about the same size and shape but a more ovate aperture and is almost entirely unpigmented (Haase et al. 2010a,b).
Description
Shell (Figs 4b and 5b): Light brown-grey; conical, about 2 mm high and 1.85 times higher than wide; protoconch comprising one whorl; aperture nearly as wide as high.
External features: black epidermal pigmentation, head and mantle rim with less or no pigmentation.
Mantle cavity: Ctenidium with 16–18 filaments; osphradium short, behind middle of ctenidium, reaching half of its length.
Digestive system: Rectum close to pallial oviduct in females but making U-shaped loop at anterior end of prostate in males; radula R: 4 1 4/3 3, L: 4-6 1 4-5, M1: 24-25, M2: 30-32 (Fig. 7a).
Female genitalia (Fig. 9c): Ovary starting 1.25–1.5 whorls below apex, comprising up to one whorl; receptaculum seminis pyriform, lying against anterior third of bursa copulatrix, bursal duct short, entering slightly below anterior edge of bursa; length of albumen gland two-third of capsule gland, about two-third of albumen gland extending into pallial roof, anterior section of capsule gland translucent milky-white, posterior one opaque white, albumen gland translucent milky-white; renal oviduct without any special features.
Male genitalia: Testis lobate starting one whorl below apex, comprising 1.25–1.5 whorls, overlapping posterior chamber of stomach; seminal vesicle leaving testis 0.25–0.5 whorls proximal to anterior end; penis (Fig. 10b) with terminal lappet.
Remarks: In the phylogenetic analysis (Fig. 2), specimens of F. riva nov. sp. formed a well-defined monophyletic group.
Fluviopupa erromangoana nov. sp
Type material: holotype ZMB Moll 117882; paratypes ZMB Moll 117883 (n = 50), MNZ C477767 (n = 25), MNHN (n = 10).
Type locality: Erromango Island, property of Harry and Emili along road between Dillons Bay and Airfield, station 7 (18°46′56.0′′S, 169°01′29.3′′E).
Etymology: This new species is named after the Island of Erromango where it occurs.
Diagnosis: for distinction from F. riva nov. sp. see above.
Description
Shell (Figs 4c and 5c): Light brown-grey; conical, more than 2 mm in height and about 1.8 times higher than wide; protoconch comprising 7/8 whorls; aperture nearly as wide as high.
External features: Black epidermal pigmentation, mantle rim, pallial roof over genital glands, snout and tentacles with less or nor pigmentation.
Mantle cavity: Ctenidium with 17–20 filaments; osphradium short, behind middle of ctenidium, reaching third of its length.
Digestive system: Rectum close to pallial oviduct in females but making U-shaped loop at anterior end of prostate in males; radula R: 4 1 4/4 4, L: 3 1 4, M1: 21-22, M2: 26-27.
Female genitalia (Fig. 9d): Ovary starting 1–1.5 whorls below apex, comprising up to 1.25 whorls; receptaculum seminis pyriform, lying against central third of bursa copulatrix, bursal duct short, entering slightly below anterior edge of bursa; length of albumen gland two-third of capsule gland, about three-fourth of albumen gland extending into pallial roof, anterior section of capsule gland translucent milky-white, posterior one opaque white, albumen gland translucent opaque white; renal oviduct without any special features, proximal loop bent towards albumen gland.
Male genitalia: Testis lobate starting one to 1.25 whorls below apex, comprising one to 1.5 whorls, overlapping posterior chamber of stomach; seminal vesicle leaving testis 0.5 whorls proximal to anterior end; penis (Fig. 10c) with terminal lappet.
Remarks: The species showed a sexual dimorphism (Hotelling's T2-test: p < 0.01) with females being significantly larger than males in all dimensions (t-tests: p < 0.01). In the phylogenetic analysis, specimens of F. erromangoana nov. sp. formed the well-supported sister clade to the other species occurring on Erromango, F. riva nov. sp. (Fig. 2).
Fluviopupa malekulana nov. sp
Type material: holotype ZMB Moll117883; paratypes ZMB Moll 117894 (n > 50), MNZ C477769 (n = 25), MNHN (n = 15).
Type locality: Malekula Island, Teboune, station 10 (15°59′18.2′′S, 167°19′37.6′′E).
Additional material: Malekula Island; Toros, station 9 (16°01′27.6′′S, 167°21′22.8′′E; MNZ C477749 n = 14); Lakatoro, station 8 (16°06′40.8′′S, 167°25′13.7′′E; MNZ C477750 n > 50, MNHN n = 20).
Etymology: Malekulana refers to the island of Malekula, where this species occurs.
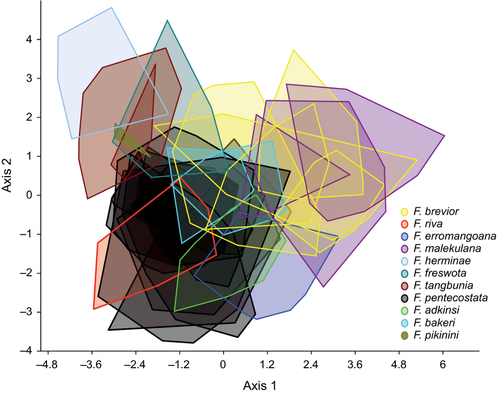
Diagnosis: F. malekulana nov. sp. is significantly larger (Hotelling's T2-tests: p < 0.01) in all shell dimensions (t-tests: p < 0.01) than F. freswota nov. sp. and F. herminae nov. sp., both also occurring on Malekula and described below (see also CVA Fig. 11). Moreover, it has a unique hammer-shaped terminal penial lappet. Genetically, F. malekulana nov. sp. could not be differentiated from the other two species (Fig. 2). Compared with the equally sized F. brevior, F. malekulana is in general less pigmented and has a more pronounced terminal penis lappet.
Description
Shell (Figs 4d and 5d): Light brown; conical, height and width quite variable, 1.7–1.75 times higher than wide; protoconch comprising 0.875 to one whorl; aperture always higher than wide.
External features: Intensity of epidermal pigmentation varies, many individuals nearly unpigmented, tentacles, snout and mantle roof over genital glands always less pigmented, mantle rim always unpigmented.
Mantle cavity: Ctenidium with 18–23 filaments; osphradium behind middle of ctenidium reaching one-third of its length.
Digestive system: Rectum close to pallial oviduct in females, making U-shaped loop at anterior end of prostate in males; radula R: 4-1-4/4 4, L: 3-6 1 3-4, M1: 19-20, M2: 26-27 (Fig. 7b).
Female genitalia (Fig. 9e): Ovary starting 0.75–1.25 whorls below apex, comprising up to one whorl; receptaculum seminis globular to pyriform, lying against anterior third of bursa copulatrix, bursal duct short, entering slightly apical to ventral edge of bursa; length of albumen gland two-third of capsule gland, about two-third of albumen gland extending into pallial roof, anterior section of capsule gland translucent white, posterior one opaque yellowish white, albumen gland translucent milky-white; renal oviduct without any special features, proximal loop bent towards albumen gland.
Male genitalia: Testis lobate starting 1–1.25 whorls below apex, comprising 0.75–1.5 whorls, partially covering posterior chamber of stomach; seminal vesicle leaving testis 0.5–0.75 whorls proximal to anterior end; penis (Fig. 10d) with terminal lappet.
Remarks: At station 10, the species showed a sexual dimorphism (Hotelling's T2-test: p < 0.05) with females being significantly larger in sh, aw and bww than males (t-test: p < 0.05). For some shells collected at station 9, not all parameters could be measured because of calcareous incrustations. Therefore, the smaller appearance of these shells could not be confirmed statistically. Anatomically, there was no difference to the other two samples which is why they were deemed conspecific. In the phylogenetic analysis (Fig. 2), station 8 seemed to be well separated from stations 9 and 10. However, F. freswota nov. sp. known from a single site occurred in both clades, indicating recent common ancestry. The short branch lengths of clade I (Fig. 2) obviously have no taxonomic relevance (see Phylogenetic Analysis and 4).
Fluviopupa herminae nov. sp
Type material: holotype ZMB Moll 117884; paratype ZMB Moll 117895 (n = 1).
Type locality: Malekula Island, Lakatoro, station 8 (16°06′40.8′′S, 167°25′13.7′′E).
Etymology: This species is named after the first author's niece Hermine-Maria who was born in August 2011 at about the time we discovered this species.
Diagnosis: F. herminae nov. sp. is significantly smaller (Hotelling's T2-test: p < 0.01) in all shell dimensions than F. malekulana nov. sp. and F. freswota nov. sp. (t-tests: p < 0.01) which also occur on Malekula. In comparison with the other species this one is entirely unpigmented except for the eyes and the proximal loop of the renal oviduct is not bent towards the albumen gland.
Description
Shell (Figs 4e and 5e): White to light brown; conical; less than 1.7 mm high and about 1.7 times higher than wide; protoconch comprising 0.875 whorls; aperture higher than wide.
External features: No epidermal pigmentation, eyes black.
Mantle cavity: Ctenidium with 17–18 filaments; osphradium short, behind middle of ctenidium, reaching one-third to one half of its length.
Digestive System: Rectum close to pallial oviduct in females, making U-shaped loop at anterior end of prostate in males; caecum small (Fig. 8e).
Female genitalia (Fig. 9f): Ovary starting 1–1.5 whorls below apex, comprising up to 0.75 whorls; receptaculum seminis pyriform, lying against anterior third of bursa copulatrix, bursal duct short, entering slightly apical to ventral edge of bursa; length of albumen gland two-third of capsule gland, about two-third of albumen gland extending into pallial roof, anterior section of capsule gland translucent milky-white, posterior one opaque white, albumen gland translucent white; renal oviduct without any special features.
Fluviopupa freswota nov. sp
Type material: holotype ZMB Moll 117885; paratypes ZMB Moll 117896 (n = 20).
Type locality: Malekula Island, Betel, station 11 (16°02′21.5′′S, 167°22′03.3′′E).
Etymology: Freswota, used as noun in apposition, means fresh water or river in Bislama, the lingua franca of Vanuatu.
Diagnosis: This species is significantly smaller than F. malekulana nov. sp. and significantly larger than F. herminae nov. sp. (see above). The U-shaped loop of the rectum is lying under the anterior half and not under the end of the prostate in contrast to the other species.
Description
Shell (Figs 4f and 5f): Whitish gray to light brown; conical, quite variable in size but always less than 2 mm height, about 1.7 times higher than wide; protoconch comprising 0.875–1 whorl; aperture higher than wide.
External features: Black epidermal pigmentation varying in its intensity with some animals being unpigmented except for the head; pallial roof over genital glands, mantle rim and snout with very little or no pigmentation.
Mantle cavity: Ctenidium with 17–20 filaments; osphradium short, behind middle of ctenidium, reaching one-third of its length.
Digestive system: Rectum close to pallial oviduct in females, making U-shaped loop at anterior half of prostate in males; radula R: 4-5 1 4-5/4 4, L: 4 1 4, M1: 21-22, M2: 27-29; caecum very small (Fig. 8f).
Female genitalia (Fig. 9g): Ovary starting 1–1.5 whorls below apex, comprising up to 1 whorl; receptaculum seminis globular to pyriform, lying against anterior third of bursa copulatrix, bursal duct short, entering slightly apical to ventral edge of bursa; length of albumen gland three-fourth of capsule gland, about three-fourth of albumen gland extending into pallial roof, anterior section of capsule gland yellowish milky-white, posterior one opaque white, albumen gland translucent milky-white; renal oviduct without any special features, proximal loop bent towards albumen gland.
Male genitalia: Testis lobate starting 1–1.25 whorls below apex, comprising 0.75–1.25 whorls, covering posterior chamber of stomach; seminal vesicle leaving testis 0.375 whorls proximal to anterior end; distal end of penis (Fig. 10e) blunt.
Remarks: In this species, females seemed to be larger than males, but statistical tests were not significant, which may have been caused by the unbalanced sample structure.
Fluviopupa tangbunia nov. sp
Type material: holotype ZMB Moll 117886; paratypes ZMB Moll 117897 (n = 50).
Type locality: Pentecost Island, Litli, station 16 (15°56′18.7′′S, 168°12′23.3′′E).
Additional material: Pentecost; Waterfall Village, station 18 (15°47′08.5′′S, 168°09′56.4′′E; MNZ C477751 n = 4); Vetlul, station 20 (15°49′05.2′′S, 168°10′22.0′′E; MNZ C477752 n = 1); Vanlvibang, station 24 (15°50′58.3′′S, 168°11′20.8′′E; MNZ C477753 n = 6).
Etymology: This species is named after the Tangbunia bank based on Pentecost, which is dealing in items of customary wealth such as mats, shells or pig tusks rather than monetary currencies.
Diagnosis: This species is smaller and proportionally wider than F. pentecostata nov. sp. described below (p < 0.01 for Hotelling's T2-test and t-tests of shell parameters and sh/sw ratio at type localities; see also CVA Fig. 11). Moreover, it is in general less pigmented and has unpigmented tentacles, and the posterior section of the capsule gland is always without a tinge of yellow. Genetically, both species are indistinguishable (Fig. 2).
Description
Shell (Figs 4g and 5g): Very light brown transparent to brown; conical, less than 1.8 mm high, about 1.75 times higher than wide; protoconch comprising 1 whorl; aperture higher than wide.
External features: Variable black epidermal pigmentation, most animals only little pigmented; mantle rim, snout and tentacles unpigmented, pallial roof over genital glands less pigmented.
Mantle cavity: Ctenidium with 17–19 filaments; osphradium in middle of ctenidium, reaching one-third of its length.
Digestive System: Rectum close to pallial oviduct in females, making U-shaped loop at anterior end of prostate in males; radula R: 4-5 1 4-5/3-4 3-4, L: 4-5 1 4-5, M1: 21-23, M2: 25-26.
Female genitalia (Fig. 9h): Ovary starting 1–1.25 whorls below apex, comprising up to 1 whorl; receptaculum seminis globular to pyriform, lying against anterior third of bursa copulatrix, bursal duct short, entering slightly apical to ventral edge of bursa; length of albumen gland three-fourth of capsule gland, about two-third of albumen gland extending into pallial roof, anterior section of capsule gland milky-white, posterior one opaque white, albumen gland translucent milky-white; renal oviduct without any special features, proximal loop bent towards albumen gland.
Male genitalia: Testis lobate starting 0.75–1 whorl below apex, comprising 1–1.25 whorls, covering posterior chamber of stomach; seminal vesicle leaving testis 0.25 whorls proximal to anterior end; terminal end of penis with terminal lappet.
Fluviopupa pentecostata sp. nov
Type material: Holotype ZMB Moll117887; paratypes ZMB Moll 117898 (n > 50), MNZ C477765 (n > 50), MNHN (n = 15).
Type locality: Pentecost Island,Ranwas, station 17 (15°58′03.6′′S, 168°15′53.3′′E).
Additional material: Pentecost Island; Salap, station 15 (15°56′55.6′′S, 168°11′48.0′′E; MNZ C477763 n = 8); Litli, station 16 (15°56′18.7′′S, 168°12′23.3′′E; MNZ C477754 n = 18); Vanibunger, station 25 (15°49′09.3′′S, 168°10′40.3′′E; MNZ C477755 n > 50, MNHN n = 15); Ravesedle, station 21 (15°51′01.3′′S, 168°11′05.4′′E; MNZ C477756 n > 50, MNHN n = 15) and 22 (15°51′02.2′'S, 168°11′06.4′′E; MNZ C477757 n = 39, MNHN n = 15); Vasovee, station 23 (15°51′01.7′′S, 168°11′09.9′′E; C47758 n = 10); Vanlvibang, station 24 (15°50′58.3′′S, 168°11′20.8′′E; MNZ C477759 n = 19); Waterfall Village, station 19 (15°47′07.6′′S, 168°09′53.8′′E; MNZ C477760 n = 25).; Vetlul, station 20 (15°49′05.2′′S, 168°10′22.0′′E; MNZ C477761 n = 12).
Etymology: This species is named after the island of its occurrence Pentecost.
Diagnosis: For distinction from F. tangbunia nov. sp., see above.
Description
Shell (Figs 4h and 5h): Light to dark brown; conical, quite variable in size about 1.85–1.9 times higher than wide; protoconch comprising 0.875–1.125 whorls; aperture higher than wide.
External features: Variable black epidermal pigmentation, which covers the whole visible part of the visceral mass; mantle rim, snout and pallial roof over genital glands with less or no pigmentation.
Mantle cavity: Ctenidium with 17–21 filaments; osphradium short behind middle of ctenidium reaching one-third to half of its length.
Digestive System: Rectum close to pallial oviduct in females, making U-shaped loop at anterior end of prostate in males; radula R: 4-5 1 4-5/3- 4 3-4, L: 4-5 1 4-5, M1: 20-24, M2: 29-35 (Fig. 7c).
Female genitalia (Fig. 9i): Ovary starting 0.75–1.5 whorls below apex, comprising up to 1.5 whorls; receptaculum seminis globular to pyriform, lying against anterior third of bursa copulatrix, bursal duct short, entering slightly apical to ventral edge of bursa; length of albumen gland two-third of capsule gland, about two-third of albumen gland extending into pallial roof, anterior section of capsule gland milky-white, posterior one opaque white to yellowish, albumen gland translucent milky-white; renal oviduct without any special features, proximal loop bent towards albumen gland.
Male genitalia: Testis lobate starting 0.75–1.25 whorls below apex, comprising 0.75–1.75 whorls, covering posterior chamber of stomach; seminal vesicle leaving testis 0.375–0.5 whorls proximal to anterior end; penis (Fig. 10f) with terminal lappet.
Remarks: In the type locality and samples taken from station 19 and 25, this species showed a sexual dimorphism (Hotelling's T2-test p < 0.05). Males were significantly smaller than females in all shell dimensions (t-tests: p < 0.05). At stations 16 and 20, the five shell dimensions sh, sw, ah, aw and bww indicated a sexual dimorphism (t-tests: p < 0.05), but the Hotelling's T2-tests were not significant, which may have been caused by the unbalanced sample structure with 12 females and 3 males at station 16 and the very small sample size (n = 8) at station 20. For the sample taken at station 22, only sh, aw and bww showed a sexual dimorphism (t-tests: p < 0.05). At stations 21 and 24, no sexual dimorphism was found. For samples taken from stations 15 and 23, statistical tests could not be performed due to lack of material.
Fluviopupa adkinsi nov. sp
Type material: Holotype ZMB Moll 117888; paratypes ZMB Moll 117899 (n > 50), MNZ C477768 (n = 25), MNHN (n = 15).
Type locality: Gaua Island, track to Lake Letas starting at airfield, station12 (14°14′45.5′′S, 167°33′55.3′′E).
Etymology: This species is dedicated to John Adkins, who, in drenching rain, guided us to Lake Letas, the crater lake of Gaua.
Diagnosis: This species is distinguished from F. bakeri nov. sp. co-occurring on the island of Gaua and described below by its larger and more slender shell (p < 0.01 for Hotelling's T2-test and t-tests of all five shell dimensions and the sh/sw ratio). Genetically, the species from Gaua are not differentiated (Fig. 2). Regarding species of similar size, F. pentecostata nov. sp. and F. brevior nov. sp. have a terminal penis lappet and F. epiritusantoana has a higher number of denticles on the outer marginal tooth.
Description
Shell (Figs 4i and 5i): Brown; conical, more than 2.1 mm high and about 1.9 times higher than wide; protoconch comprising 0.875 to one whorl; aperture higher than wide.
External features: Epidermis black except for snout, tentacles and mantle rim.
Mantle cavity: Ctenidium with 19–22 filaments; osphradium short, behind middle of ctenidium, reaching one-third of its length.
Digestive system: rectum close to pallial oviduct in females but making U-shaped loop at anterior end of prostate in males; radula R: 4 1 4/4 4, L: 4 1 4, M1: 21-23, M2:28-30.
Female genitalia (Fig. 9j): Ovary starting 1.25 whorls below apex, comprising up to 1.25 whorls; receptaculum seminis pyriform to elongate, lying against central third of bursa copulatrix, bursal duct short, entering slightly apical to ventral edge of bursa; length of albumen gland three-fourth of capsule gland, about three-fourth of albumen gland extending into pallial roof, anterior section of capsule gland translucent milky-white, posterior one opaque white, albumen gland translucent white; renal oviduct without any special features, proximal loop bent towards albumen gland.
Male genitalia: Testis lobate starting 0.75–1.25 whorls below apex, comprising 1.25–1.5 whorls, reaching posterior chamber of stomach; seminal vesicle leaving testis 0.625–0.5 whorls proximal to anterior end; distal end of penis (Fig. 10g) blunt.
Remarks: This species showed a sexual dimorphism with females being significantly larger than males (Hotelling's T2-test: p < 0.01) in all five shell dimensions (t-tests: p < 0.01, for aw p < 0.05).
Fluviopupa bakeri nov. sp
Synonymy: Potamopyrgus sp. – Baker 1929, Man and Animals in the New Hebrides: p. 148.
Fluviopupa brevior – Solem 1959; Fieldiana Zoology 43: p. 195, pl. 6, Fig. 11; pl. 27, Figs 5 and 6.
Type material: Holotype ZMB Moll 117889; paratypes ZMB Moll 117900 (n > 50), MNZ C477764 (n = 25), MNHN (n = 15).
Type locality: Gaua Island, Lake Letas, station 13 (14°15′42.4′′S, 167°32′21.9′′E).
Additional material: Gaua Island, Medsamavud, station 14 (14°15′48.3′′S, 167°36′15.6′′E; MNZ C477762 n = 15).
Etymology: This species is named after the British biologist John R. Baker who was the first to describe the occurrence of a Potamopyrgus-like snail occurring in the crater lake on Gaua without naming or describing it in detail (Baker 1929).
Diagnosis: For differentiation from F. adkinsi nov. sp., see above.
Description
Shell (Figs 4j and 5j): Brown; conical, about 2 mm in height and about 1.75–1.8 times higher than wide; protoconch comprising 7/8 to one whorl; aperture slightly higher than wide.
External features: Variable black epidermal pigmentation, snout and mantle rim nearly unpigmented.
Mantle cavity: Ctenidium with 18–21 filaments; osphradium short, behind middle of ctenidium, reaching one-third of its length.
Digestive system: Rectum close to pallial oviduct in females but making U-shaped loop at anterior end of prostate in males; radula R: 4-5 1 4-5/4 4, L: 4 1 5, M1: 23-24, M2:28-30 (Fig. 7d).
Female genitalia (Fig. 9k): Ovary starting 1.25–1.5 whorls below apex, comprising up to 0.75 whorls; receptaculum seminis pyriform to elongate, lying against central third of bursa copulatrix, bursal duct short, entering slightly apical to ventral edge of bursa; length of albumen gland three-fourth of capsule gland, about three-fourth of albumen gland extending into pallial roof, anterior section of capsule gland translucent milky-white, posterior one opaque white, albumen gland translucent white; renal oviduct without any special features, proximal loop bent towards kidney.
Male genitalia: Testis lobate starting 0.75–1 whorl below apex, comprising 0.75–1.25 whorls, reaching posterior chamber of stomach; seminal vesicle leaving testis 0.25–0.375 whorls proximal to anterior end; distal end of penis (Fig. 10h) blunt.
Remarks: The sample from station 13 showed a sexual dimorphism in sh and bww (t-test: for sh p < 0.01, for bww p < 0.05), for station 14 tests could not be performed due to the lack of material.
Fluviopupa pikinini nov. sp
Type material: Holotype ZMB Moll 117890; paratypes ZMB Moll 117901 (n = 1).
Type locality: Gaua Island, Medsamavud, station 14 (14°15′48.3′′S, 167°36′15.6′′E).
Etymology: Pikinini, used as noun in apposition, is the Bislama word for child and refers to the relatively small size of this species.
Diagnosis: With a height of less than 1.7 mm, this species is significantly smaller than the other two species occurring on Gaua, F. bakeri nov. sp. and F. adkinsi nov. sp. (see Remarks).
Description
Shell (Fig. 4g): Colour varies from whitish gray to light brown; conical, about 1.6 mm high and 1.7 times higher than wide; protoconch comprising one whorl; aperture about as wide as high.
External features: Partially black epidermal pigmentation.
Mantle cavity: Ctenidium with 15 filaments (n = 1).
Remarks: Statistical comparisons with other species were not possible, because only four individuals were found. This is also the reason why the anatomical description remained incomplete. Genetically, this species is not distinct from the sympatric F. bakeri nov. sp.
Morphometrics
Despite the apparent similarity of the shells (Figs 4 and 5), the plot of the CVA based on five shell measurements of population samples illustrates yet considerable differentiation (Fig. 11). The manova was highly significant (p < 0.01). Hotelling's pairwise post hoc T2 comparisons are summarized in Table 1 and reported in the Diagnoses of the species descriptions above if taxonomically relevant. However, it is also obvious that shell shape alone cannot be used to identify all species. Anatomical and genetic data have to be taken into consideration as well.
Discussion
Our analyses revealed that tateids have reached Vanuatu at least as early as in the late Pliocene, indicating unambiguously that Santo as the first island where they could establish has at least partly remained above the sea level for more than 3 My. This even predates the assumptions of Taylor (1992) and Robin (1993) who did not rule out that Santo has re-emerged during the earliest Pleistocene. Our analyses thus significantly increases the time frame assumed by Hamilton et al. (2010) for the colonization of Vanuatu and the evolution of its flora and fauna in situ. The general relevance of these conclusions cannot be assessed, yet, because we are not aware of many other phylogenetic analysis concerning taxa from Vanuatu. In an analysis of the trichopteran genus Apsilochorema, the mean time of arrival on Vanuatu was estimated to 5.4 Mya within the highest posterior density interval of 12–2 Mya (Strandberg and Lohanson 2010). This time frame covers more than the ambiguities reported in the geological literature, which remained undiscussed in this paper, though, and would thus still support also Hamilton et al.'s (2010) assumption of a very young subaerial history. Our Bayesian estimate of the origin of the tateids from Vanuatu of 3.01 ± 0.77 Mya was much more accurate but still encompassed the early Pleistocene at the younger end of the density interval at 2.25 Mya. However, considering that islands, once emergent, still have to ‘evolve’ before they can support biota, we can safely assume that the subaerial history of Santo began considerably earlier already in the late Pliocene. The COI-distance-based estimate for node 3 of 4.81 ± 0.20 Mya even suggested a much earlier time of emergence and did not even overlap with the Bayesian estimate in contrast to the younger nodes. This may indeed be an overestimate balanced in the Bayesian analysis by the substitution rates of the other two gene fragments.
According to our reconstruction, Erromango was the next island to be settled after Santo in the early Pleistocene about 2 Mya, which fits into the geological scenario of Neef and Hendy (1988) and means that Erromango is much older than the other central chain islands. Erromango may well have served as stepping stone towards the southern-more and definitely much younger islands Tanna and Aneityum. Yet, there is no record of tateids from either island. We could not visit Tanna in 2011 and on Aneityum the northern part with presumably more suitable habitats (personal communication of locals) could not be surveyed due to bad weather conditions, while in the southern part, no truncatelloidean gastropods where found.
Eastern belt and central chain radiation with the inclusion of species from Malekula have to be of Pleistocene or even Holocene origin as indicated by the starburst-like clade I with short branch lengths lacking a clear geographical structure. Morphological and anatomical differentiations are limited as well. The origin of this radiation is certainly Santo. Scenarios suggesting single colonization events for Malekula, Efate, Pentecost, Gaua and the Torres Islands and somewhat relaxed assumptions were rejected. Only the samples from Efate seem to descend from a single colonizer. For the other islands, multiple introductions and possibly also multiple sources have to be inferred, although the representatives from Pentecost and Gaua (like those from Efate and Erromango) can be characterized by penial morphology suggesting reciprocal monophyly.
The fact that all species found on Malekula are part of the starburst of clade I suggests that Malekula has surfaced only in the rapid uplift phase in the late Quaternary, which has affected the entire region (Taylor 1992). However, we have surveyed only a small part of this large island and may have missed older lineages.
The most plausible vectors of the snails are probably birds (see Haase et al. 2010b for a recent discussion concerning truncatelloidean gastropods). Van Leeuwen et al. (2012a) and Wada et al. (2012) have demonstrated that small snails can even survive the passage through the gut of water fowl. Considering the relatively small distances between the islands, one can safely assume that birds have frequently crossed the open water and thereby occasionally transported snails and other invertebrates (Frisch et al. 2007; Green et al. 2008; Van Leeuwen et al. 2012b). In recent times, also humans have to be considered as transporters, for example, through the transplantation of plants cultivated in or at springs and streams. Yet, the young subaerial age of these islands and the short branches within the clade concerned also suggest that the picture of colonization events is probably blurred through incomplete lineage sorting.
Incomplete lineage sorting is probably also the main reason for the obvious taxonomic incongruence, which, however, is typical for young and rapid radiations (Funk and Omland 2003). Confusion and mislabelling of samples during laboratory work can be excluded. The taxonomic incongruence had already been realized after a preliminary analysis during data collection. Yet, adding specimens did not change the picture. In addition, clade I is not entirely chaotic. There are several subclades which are at least partly taxonomically and biogeographically coherent, and the topologies of the separate analyses only differ in their resolution. A systematic handling error concerning more than a few samples should have been more destructive.
A more sever taxonomical problem is caused by F. espiritusantoana and F. pascali from Santo, which are represented both in clades I and III. In clade I, F. espiritusantoana formed a larger sister subclade to the remaining clade members including another two specimens belonging to this species. Not only was F. espiritusantoana distributed across three clades, but also all but one individual samples were split. The same holds for F. pascali, which, however, has been known only from a single locality (Haase et al. 2010a). According to our dating, clades I and III were separated by c. 2.2 My. The problem could be reduced to a single species if we argued that F. pascali were an aberrant, morphologically well-defined form of F. espiritusantoana (see Haase et al. 2010a), which is, however, not convincing. Similarly, unconvincing is the assumption that the lineages of F. espiritusantoana represent cryptic species occurring sympatrically, although Haase et al. (2010a) did not rule out that the populations in the north and the centre of Santo represent two different species. Alternatively, F. espiritusantoana has to be considered the paraphyletic stem species of almost the entire radiation including the species from Erromango and clade I. The preservation of the unusually divergent lineages would probably be a consequence of repeated bottlenecks the species (and possibly also F. pascali) went through on a few islands remaining during repeated periods of partial submergence of Santo during the Pliocene. This would represent a case of ancient incomplete lineage sorting as described for radiations of cichlid fishes or crotaphydid lizards (Takahashi et al. 2001; McGuire et al. 2007). Provided F. espiritusantoana is indeed a single species, we would expect recombination between nc and mt lineages. We observed only a single case, where an individual switched position among the subclades of clade I between mt and ITS2-trees. Yet, our analysis included only two specimens per site. Therefore, disentangling the alternative hypotheses of cryptic species versus old stem species would require a far more comprehensive analysis and more extensive collecting of fresh material.
The phylogenetic position of F. narii was instable. However, according to the most common positions in the bootstrap replicates either as part of clade III or as sister species to all other samples from Vanuatu, it most likely descends from a very deep split.
Considering that fauna and flora of Vanuatu have predominantly western affinities (reviewed by Munzinger 2009; Siméoni 2009; Hamilton et al. 2010), it was surprising that the New Zealand taxa were closer related to the species from Vanuatu than H. winstonefi from New Caledonia and T. huonensis from Australia. However, considering that there are at least two more lineages of Tateidae occurring on New Caledonia that are unrelated to Hemistomia (Haase and Bouchet 1998) and that representatives from the majority of regions harbouring tateids were not included in our analyses, we refrain from a more comprehensive discussion of the potential origin of the species of the Vanuatu archipelago. This will be subject of a forthcoming analysis.
Acknowledgements
The authors like to thank a countless number of field guides and hosts, in particular Lissian Tabi from Pentecost, Donald Lovo from Erromango and Rodolphe Malero from Malekula. Touasi Tiwok from the Department of Environmental Protection and Conservation in Port Vila issued the research permit in accordance with the Convention on Biological Diversity. Philippe Bouchet and Benoît Fontaine from the Museum National d′Histoire Naturelle Paris are acknowledged for providing material from Santo and the Torres Islands and Bruce Marshall from the Museum of New Zealand Te Papa Tongarewa for local samples. At the University of Greifswald, we thank Rabea Schlüter, head of the Laboratory of Electron Microscopy, and our technician Christel Meibauer, who helped with DNA work. Comments of two reviewers helped to improve an earlier version of the manuscript. Financial support was received from the Deutsche Forschungsgemeinschaft (Grant HA4752/2-1).
Appendix 1
Shell morphometry and sex ratio
| Species/locality/n/sex ratio | sh | sw | ah | aw | bww | Sh/sw | Ah/aw | Sw/bww | Sh/ah | Sw/aw | w | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
F. brevior Station 1 N = 20 8f/12m |
Holotype | 2.31 | 1.29 | 0.90 | 0.81 | 1.16 | 1.79 | 1.11 | 1.11 | 2.56 | 1.59 | 4.5 |
| Mean | 2.13 | 1.20 | 0.84 | 0.75 | 1.09 | 1.78 | 1.13 | 1.10 | 2.54 | 1.61 | 4.4 | |
| Median | 2.14 | 1.20 | 0.84 | 0.75 | 1.09 | 1.79 | 1.13 | 1.10 | 2.54 | 1.61 | 4.375 | |
| Min | 1.97 | 1.10 | 0.74 | 0.69 | 1.01 | 1.66 | 1.04 | 1.00 | 2.41 | 1.51 | 4.125 | |
| Max | 2.33 | 1.31 | 0.92 | 0.81 | 1.17 | 1.87 | 1.19 | 1.18 | 2.77 | 1.68 | 4.75 | |
| SD | 0.11 | 0.06 | 0.04 | 0.03 | 0.05 | 0.06 | 0.03 | 0.03 | 0.08 | 0.05 | 0.16 | |
| cv | 5.38 | 4.66 | 5.12 | 4.53 | 4.91 | 3.38 | 3.12 | 3.13 | 3.17 | 2.92 | 3.69 | |
|
F. brevior Station 2 N = 40 10f/10m |
Mean | 2.18 | 1.22 | 0.86 | 0.76 | 1.10 | 1.79 | 1.12 | 1.11 | 2.55 | 1.60 | 4.43 |
| Median | 2.21 | 1.23 | 0.87 | 0.76 | 1.10 | 1.78 | 1.11 | 1.11 | 2.56 | 1.60 | 4.5 | |
| Min | 1.69 | 0.98 | 0.66 | 0.60 | 0.86 | 1.65 | 1.06 | 1.06 | 2.44 | 1.51 | 4.125 | |
| Max | 2.56 | 1.43 | 0.99 | 0.88 | 1.27 | 1.89 | 1.20 | 1.20 | 2.68 | 1.73 | 4.75 | |
| SD | 0.22 | 0.11 | 0.09 | 0.07 | 0.11 | 0.05 | 0.04 | 0.03 | 0.06 | 0.06 | 0.18 | |
| cv | 10.17 | 9.01 | 10.04 | 9.34 | 9.66 | 2.90 | 3.60 | 3.16 | 2.33 | 3.51 | 4.11 | |
|
F. brevior Station 3 N = 20 9f/11m |
Mean | 2.33 | 1.31 | 0.93 | 0.81 | 1.17 | 1.78 | 1.15 | 1.13 | 2.51 | 1.62 | 4.5 |
| Median | 2.33 | 1.31 | 0.92 | 0.81 | 1.16 | 1.78 | 1.14 | 1.12 | 2.51 | 1.62 | 4.5 | |
| Min | 2.17 | 1.23 | 0.87 | 0.76 | 1.10 | 1.68 | 1.05 | 1.06 | 2.41 | 1.53 | 4.25 | |
| Max | 2.56 | 1.43 | 1.00 | 0.86 | 1.23 | 1.84 | 1.22 | 1.18 | 2.67 | 1.73 | 4.875 | |
| SD | 0.10 | 0.05 | 0.04 | 0.03 | 0.05 | 0.04 | 0.04 | 0.03 | 0.06 | 0.06 | 0.2 | |
| cv | 4.47 | 3.80 | 4.65 | 3.71 | 3.97 | 2.30 | 3.79 | 2.58 | 2.41 | 3.44 | 4.54 | |
|
F. brevior Station 4 N = 20 11f/9m |
Mean | 2.12 | 1.22 | 0.85 | 0.75 | 1.11 | 1.74 | 1.13 | 1.09 | 2.50 | 1.63 | 4.44 |
| Median | 2.10 | 1.21 | 0.85 | 0.74 | 1.11 | 1.74 | 1.13 | 1.09 | 2.50 | 1.63 | 4.375 | |
| Min | 1.82 | 1.11 | 0.75 | 0.66 | 1.00 | 1.64 | 1.04 | 1.00 | 2.33 | 1.54 | 4.125 | |
| Max | 2.40 | 1.33 | 0.96 | 0.83 | 1.30 | 1.85 | 1.21 | 1.15 | 2.66 | 1.70 | 5 | |
| SD | 0.15 | 0.06 | 0.05 | 0.05 | 0.07 | 0.06 | 0.04 | 0.03 | 0.08 | 0.04 | 0.2 | |
| cv | 7.02 | 5.11 | 6.31 | 6.15 | 6.57 | 3.45 | 3.39 | 2.98 | 3.19 | 2.46 | 4.46 | |
|
F. brevior Stattion 5 N = 25 7f/13m |
Mean | 2.06 | 1.15 | 0.80 | 0.71 | 1.02 | 1.79 | 1.13 | 1.12 | 2.57 | 1.63 | 4.44 |
| Median | 2.06 | 1.14 | 0.79 | 0.71 | 1.02 | 1.80 | 1.13 | 1.13 | 2.58 | 1.62 | 4.5 | |
| Min | 1.59 | 0.97 | 0.65 | 0.63 | 0.87 | 1.64 | 1.03 | 1.07 | 2.41 | 1.52 | 3.875 | |
| Max | 2.49 | 1.38 | 0.95 | 0.82 | 1.22 | 1.89 | 1.28 | 1.23 | 2.74 | 1.79 | 4.75 | |
| SD | 0.23 | 0.10 | 0.08 | 0.06 | 0.09 | 0.07 | 0.06 | 0.04 | 0.09 | 0.08 | 0.22 | |
| cv | 11.15 | 8.97 | 9.72 | 7.90 | 8.66 | 4.14 | 5.39 | 3.55 | 3.49 | 5.19 | 5.06 | |
|
F. riva Station 6 N = 20 11f/9m |
Holotype | 2.14 | 1.16 | 0.78 | 0.75 | 1.03 | 1.85 | 1.05 | 1.12 | 2.73 | 1.55 | 4.25 |
| Mean | 2.03 | 1.10 | 0.74 | 0.70 | 0.94 | 1.85 | 1.07 | 1.17 | 2.73 | 1.57 | 4.33 | |
| Median | 2.06 | 1.10 | 0.75 | 0.70 | 0.94 | 1.85 | 1.05 | 1.16 | 2.72 | 1.56 | 4.25 | |
| Min | 1.81 | 0.98 | 0.66 | 0.64 | 0.79 | 1.76 | 1.01 | 1.11 | 2.59 | 1.49 | 4 | |
| Max | 2.15 | 1.17 | 0.80 | 0.76 | 1.04 | 1.96 | 1.14 | 1.41 | 2.87 | 1.65 | 4.75 | |
| SD | 0.10 | 0.05 | 0.04 | 0.03 | 0.05 | 0.05 | 0.04 | 0.06 | 0.08 | 0.05 | 0.19 | |
| cv | 4.94 | 4.36 | 5.30 | 4.00 | 5.63 | 2.66 | 4.01 | 5.37 | 2.83 | 3.27 | 4.5 | |
|
F. erromangoana Station 7 N = 20 11f/9m |
Holotype | 2.24 | 1.26 | 0.81 | 0.81 | 1.14 | 1.78 | 1.00 | 1.10 | 2.79 | 1.56 | 4.375 |
| Mean | 2.26 | 1.26 | 0.83 | 0.81 | 1.12 | 1.80 | 1.03 | 1.12 | 2.72 | 1.56 | 4.25 | |
| Median | 2.28 | 1.27 | 0.84 | 0.82 | 1.12 | 1.80 | 1.03 | 1.12 | 2.68 | 1.55 | 4.25 | |
| Min | 2.02 | 1.15 | 0.72 | 0.74 | 1.00 | 1.71 | 0.97 | 1.08 | 2.62 | 1.46 | 3.875 | |
| Max | 2.44 | 1.33 | 0.89 | 0.89 | 1.19 | 1.86 | 1.12 | 1.18 | 2.89 | 1.73 | 4.625 | |
| SD | 0.12 | 0.06 | 0.05 | 0.04 | 0.06 | 0.05 | 0.04 | 0.03 | 0.08 | 0.05 | 0.21 | |
| cv | 5.35 | 4.50 | 5.52 | 5.62 | 5.04 | 2.62 | 3.47 | 2.37 | 2.94 | 3.52 | 5.07 | |
|
F. malekulana Station 10 N = 20 10f/10m |
Holotype | 2.02 | 1.18 | 0.84 | 0.76 | 1.01 | 1.70 | 1.10 | 1.18 | 2.40 | 1.55 | 4.125 |
| Mean | 2.21 | 1.30 | 0.91 | 0.81 | 1.13 | 1.71 | 1.12 | 1.15 | 2.43 | 1.60 | 4.33 | |
| Median | 2.27 | 1.32 | 0.92 | 0.81 | 1.13 | 1.70 | 1.12 | 1.14 | 2.45 | 1.59 | 4.375 | |
| Min | 1.82 | 1.11 | 0.77 | 0.71 | 0.98 | 1.62 | 1.02 | 1.10 | 2.32 | 1.51 | 3.875 | |
| Max | 2.53 | 1.41 | 1.08 | 0.91 | 1.23 | 1.82 | 1.20 | 1.26 | 2.60 | 1.72 | 4.625 | |
| SD | 0.19 | 0.09 | 0.08 | 0.06 | 0.07 | 0.06 | 0.05 | 0.04 | 0.08 | 0.05 | 0.21 | |
| cv | 8.47 | 6.68 | 8.35 | 6.94 | 6.09 | 3.34 | 4.25 | 3.52 | 3.40 | 3.40 | 4.88 | |
|
F. malekulana Station 8 N = 20 10f/10m |
Mean | 2.19 | 1.25 | 0.90 | 0.80 | 1.08 | 1.75 | 1.12 | 1.16 | 2.44 | 1.56 | 4.25 |
| Median | 2.18 | 1.24 | 0.90 | 0.80 | 1.08 | 1.74 | 1.12 | 1.14 | 2.44 | 1.56 | 4.25 | |
| Min | 1.87 | 1.11 | 0.78 | 0.70 | 0.97 | 1.63 | 1.04 | 1.10 | 2.24 | 1.47 | 3.875 | |
| Max | 2.63 | 1.38 | 1.02 | 0.89 | 1.23 | 1.92 | 1.19 | 1.32 | 2.58 | 1.65 | 4.625 | |
| SD | 0.19 | 0.08 | 0.07 | 0.05 | 0.08 | 0.07 | 0.03 | 0.05 | 0.08 | 0.04 | 0.19 | |
| cv | 8.86 | 6.89 | 7.85 | 6.41 | 7.08 | 4.19 | 3.04 | 4.45 | 3.15 | 2.91 | 4.51 | |
|
F. malekulana Station 9 N = 11 (for sh and w n = 5) 4f/2m |
Mean | 2.06 | 1.10 | 0.77 | 0.73 | 1.01 | 1.78 | 1.06 | 1.09 | 2.53 | 1.50 | 4.125 |
| Median | 2.09 | 1.06 | 0.79 | 0.74 | 0.99 | 1.74 | 1.04 | 1.09 | 2.55 | 1.50 | 4.125 | |
| Min | 1.79 | 1.03 | 0.67 | 0.66 | 0.92 | 1.73 | 0.99 | 1.04 | 2.42 | 1.43 | 4 | |
| Max | 2.18 | 1.26 | 0.89 | 0.84 | 1.14 | 1.86 | 1.16 | 1.14 | 2.63 | 1.58 | 4.25 | |
| SD | 0.16 | 0.08 | 0.07 | 0.06 | 0.07 | 0.07 | 0.06 | 0.03 | 0.09 | 0.05 | 0.13 | |
| cv | 8.08 | 7.55 | 8.94 | 8.36 | 7.48 | 4.02 | 5.50 | 2.85 | 3.75 | 3.58 | 3.07 | |
|
F. herminae Station 8 N = 8 3f/2m |
Holotype | 1.67 | 0.96 | 0.69 | 0.62 | 0.82 | 1.75 | 1.11 | 1.17 | 2.42 | 1.53 | 4 |
| Mean | 1.45 | 0.83 | 0.60 | 0.51 | 0.72 | 1.73 | 1.17 | 1.15 | 2.42 | 1.63 | 3.75 | |
| Median | 1.46 | 0.82 | 0.59 | 0.49 | 0.70 | 1.71 | 1.13 | 1.14 | 2.41 | 1.59 | 3.8 | |
| Min | 1.22 | 0.73 | 0.50 | 0.45 | 0.66 | 1.65 | 1.06 | 1.11 | 2.30 | 1.49 | 3.375 | |
| Max | 1.68 | 0.97 | 0.69 | 0.63 | 0.83 | 1.88 | 1.47 | 1.25 | 2.61 | 2.00 | 4 | |
| SD | 0.19 | 0.09 | 0.08 | 0.06 | 0.07 | 0.08 | 0.13 | 0.04 | 0.09 | 0.16 | 0.21 | |
| cv | 13.47 | 11.37 | 13.07 | 12.67 | 9.34 | 4.48 | 11.20 | 3.98 | 4.01 | 9.85 | 5.81 | |
|
F. freswota Station 11 N = 20 5f/15m |
Holotype | 1.59 | 0.96 | 0.65 | 0.57 | 0.86 | 1.66 | 1.14 | 1.11 | 2.46 | 1.69 | 3.875 |
| Mean | 1.66 | 0.98 | 0.67 | 0.60 | 0.87 | 1.70 | 1.11 | 1.13 | 2.49 | 1.62 | 3.97 | |
| Median | 1.65 | 0.97 | 0.66 | 0.59 | 0.87 | 1.69 | 1.09 | 1.13 | 2.50 | 1.60 | 3.94 | |
| Min | 1.37 | 0.87 | 0.60 | 0.55 | 0.78 | 1.57 | 1.03 | 1.07 | 2.28 | 1.53 | 3.625 | |
| Max | 2.00 | 1.16 | 0.77 | 0.69 | 1.00 | 1.85 | 1.27 | 1.28 | 2.68 | 1.81 | 4.5 | |
| SD | 0.18 | 0.08 | 0.06 | 0.04 | 0.05 | 0.07 | 0.06 | 0.05 | 0.10 | 0.08 | 0.23 | |
| cv | 10.88 | 8.67 | 8.60 | 6.94 | 6.41 | 4.16 | 5.20 | 4.46 | 4.07 | 4.87 | 5.93 | |
|
F. tangbunia Station 16 N = 27 10f/10m |
Holotype | 1.43 | 0.84 | 0.57 | 0.56 | 0.78 | 1.69 | 1.02 | 1.08 | 2.51 | 1.51 | 3.75 |
| Mean | 1.58 | 0.91 | 0.61 | 0.57 | 0.81 | 1.73 | 1.07 | 1.12 | 2.61 | 1.61 | 4.10 | |
| Median | 1.60 | 0.91 | 0.61 | 0.57 | 0.81 | 1.73 | 1.07 | 1.12 | 2.61 | 1.61 | 4.125 | |
| Min | 1.38 | 0.82 | 0.55 | 0.50 | 0.72 | 1.58 | 1.00 | 1.07 | 2.30 | 1.44 | 3.75 | |
| Max | 1.75 | 1.04 | 0.71 | 0.66 | 0.90 | 1.88 | 1.15 | 1.21 | 2.81 | 1.72 | 4.375 | |
| SD | 0.11 | 0.05 | 0.04 | 0.04 | 0.05 | 0.07 | 0.04 | 0.03 | 0.11 | 0.06 | 0.18 | |
| cv | 6.73 | 5.63 | 6.41 | 6.49 | 5.92 | 3.92 | 4.02 | 3.13 | 4.42 | 3.78 | 4.46 | |
|
F. tangbunia Station 18; N = 1 |
1.38 | 0.83 | 0.57 | 0.54 | 0.76 | 1.66 | 1.06 | 1.09 | 2.42 | 1.54 | 3.875 | |
|
F. tangbunia Station 20; N = 1 |
1.52 | 0.85 | 0.55 | 0.54 | 0.75 | 1.79 | 1.02 | 1.13 | 2.76 | 1.57 | 4.125 | |
|
F. tangbunia Station 24 N = 6 |
Mean | 1.62 | 0.94 | 0.62 | 0.60 | 0.83 | 1.72 | 1.04 | 1.13 | 2.59 | 1.56 | 3.98 |
| Median | 1.64 | 0.93 | 0.63 | 0.60 | 0.85 | 1.75 | 1.03 | 1.12 | 2.61 | 1.57 | 4.00 | |
| Min | 1.46 | 0.91 | 0.59 | 0.58 | 0.76 | 1.59 | 1.00 | 1.10 | 2.35 | 1.49 | 3.75 | |
| Max | 1.71 | 0.98 | 0.64 | 0.63 | 0.86 | 1.76 | 1.09 | 1.21 | 2.75 | 1.61 | 4.125 | |
| SD | 0.09 | 0.03 | 0.02 | 0.02 | 0.04 | 0.07 | 0.04 | 0.04 | 0.14 | 0.04 | 0.15 | |
| cv | 5.61 | 3.21 | 2.93 | 3.70 | 4.63 | 4.15 | 3.70 | 3.86 | 5.69 | 2.58 | 3.83 | |
| cv | 5.67 | 4.91 | 5.75 | 6.63 | 5.27 | 3.34 | 4.78 | 2.79 | 3.75 | 4.85 | 3.13 | |
|
F. pentecostata Station 17 N = 50 30f/20m |
Holotype | 2.26 | 1.17 | 0.81 | 0.77 | 1.08 | 1.94 | 1.05 | 1.08 | 2.81 | 1.52 | 4.5 |
| Mean | 2.12 | 1.12 | 0.78 | 0.72 | 1.02 | 1.89 | 1.08 | 1.10 | 2.72 | 1.55 | 4.5 | |
| Median | 2.13 | 1.12 | 0.77 | 0.72 | 1.02 | 1.89 | 1.08 | 1.11 | 2.74 | 1.54 | 4.5 | |
| Min | 1.72 | 0.94 | 0.65 | 0.61 | 0.85 | 1.79 | 0.99 | 1.03 | 2.59 | 1.39 | 4 | |
| Max | 2.52 | 1.28 | 0.89 | 0.85 | 1.18 | 2.01 | 1.15 | 1.19 | 2.88 | 1.69 | 5 | |
| SD | 0.18 | 0.09 | 0.06 | 0.05 | 0.08 | 0.06 | 0.04 | 0.04 | 0.07 | 0.07 | 0.20 | |
| cv | 8.71 | 7.76 | 7.82 | 6.63 | 7.90 | 2.95 | 3.73 | 3.52 | 2.53 | 4.21 | 4.57 | |
|
F. pentecostata Station 15 N = 6 -f/-m |
Mean | 2.20 | 1.17 | 0.81 | 0.72 | 1.04 | 1.89 | 1.12 | 1.12 | 2.74 | 1.62 | 4.5 |
| Median | 2.14 | 1.11 | 0.79 | 0.69 | 1.01 | 1.91 | 1.14 | 1.12 | 2.72 | 1.62 | 4.5 | |
| Min | 1.99 | 1.04 | 0.68 | 0.68 | 0.97 | 1.81 | 1.00 | 1.07 | 2.46 | 1.53 | 4.125 | |
| Max | 2.54 | 1.33 | 0.93 | 0.79 | 1.16 | 1.94 | 1.19 | 1.18 | 3.13 | 1.68 | 4.875 | |
| SD | 0.21 | 0.12 | 0.09 | 0.05 | 0.08 | 0.06 | 0.07 | 0.04 | 0.25 | 0.06 | 0.31 | |
| cv | 10.12 | 10.89 | 12.19 | 7.53 | 7.81 | 3.42 | 6.46 | 3.61 | 9.42 | 3.69 | 7.22 | |
|
F. pentecostata Station 16 N = 24 12f/3m |
Mean | 1.98 | 1.07 | 0.73 | 0.67 | 0.97 | 1.85 | 1.09 | 1.10 | 2.71 | 1.59 | 3.33 |
| Median | 2.00 | 1.07 | 0.73 | 0.66 | 0.96 | 1.85 | 1.09 | 1.09 | 2.70 | 1.59 | 4.375 | |
| Min | 1.73 | 0.97 | 0.66 | 0.58 | 0.86 | 1.70 | 1.03 | 1.06 | 2.54 | 1.50 | 4 | |
| Max | 2.24 | 1.19 | 0.83 | 0.78 | 1.10 | 1.94 | 1.22 | 1.16 | 2.89 | 1.74 | 4.725 | |
| SD | 0.13 | 0.06 | 0.04 | 0.05 | 0.06 | 0.06 | 0.04 | 0.03 | 0.09 | 0.05 | 0.17 | |
| cv | 6.77 | 5.71 | 6.20 | 7.00 | 6.15 | 3.26 | 3.64 | 2.67 | 3.55 | 3.40 | 4.01 | |
|
F. pentecostata Station 19 N = 20 11f/9m |
Mean | 1.98 | 1.05 | 0.74 | 0.68 | 0.96 | 1.89 | 1.09 | 1.09 | 2.68 | 1.55 | 4.40 |
| Median | 1.98 | 1.05 | 0.75 | 0.68 | 0.98 | 1.90 | 1.08 | 1.08 | 2.68 | 1.55 | 4.375 | |
| Min | 1.71 | 0.96 | 0.65 | 0.61 | 0.88 | 1.76 | 1.01 | 1.05 | 2.61 | 1.41 | 4.125 | |
| Max | 2.25 | 1.15 | 0.84 | 0.72 | 1.05 | 1.97 | 1.18 | 1.14 | 2.84 | 1.64 | 4.875 | |
| SD | 0.14 | 0.06 | 0.04 | 0.03 | 0.05 | 0.05 | 0.04 | 0.03 | 0.05 | 0.06 | 0.19 | |
| cv | 6.94 | 5.44 | 5.99 | 4.57 | 5.04 | 2.76 | 4.00 | 2.43 | 1.99 | 3.72 | 4.47 | |
|
F. pentecostata Station 20 N = 15 5f/3m |
Mean | 2.06 | 1.12 | 0.77 | 0.70 | 0.99 | 1.85 | 1.11 | 1.13 | 2.67 | 1.60 | 4.33 |
| Median | 2.02 | 1.10 | 0.78 | 0.69 | 0.99 | 1.86 | 1.10 | 1.13 | 2.68 | 1.60 | 4.375 | |
| Min | 1.82 | 1.00 | 0.69 | 0.64 | 0.90 | 1.78 | 1.03 | 1.05 | 2.55 | 1.46 | 4.125 | |
| Max | 2.33 | 1.29 | 0.86 | 0.77 | 1.08 | 1.92 | 1.17 | 1.20 | 2.73 | 1.72 | 4.625 | |
| SD | 0.14 | 0.08 | 0.05 | 0.04 | 0.05 | 0.05 | 0.04 | 0.04 | 0.05 | 0.06 | 0.13 | |
| cv | 6.76 | 7.51 | 6.68 | 5.37 | 5.21 | 2.68 | 3.60 | 4.02 | 1.86 | 3.88 | 3.02 | |
|
F. pentecostata Station 21 N = 20 7f/13m |
Mean | 2.11 | 1.14 | 0.79 | 0.73 | 1.03 | 1.85 | 1.08 | 1.11 | 2.68 | 1.57 | 4.5 |
| Median | 2.10 | 1.14 | 0.79 | 0.73 | 1.01 | 1.84 | 1.09 | 1.11 | 2.68 | 1.58 | 4.5 | |
| Min | 1.91 | 1.03 | 0.67 | 0.61 | 0.92 | 1.75 | 0.93 | 1.04 | 2.49 | 1.35 | 4.125 | |
| Max | 2.28 | 1.26 | 0.85 | 0.83 | 1.14 | 1.96 | 1.16 | 1.17 | 2.87 | 1.69 | 4.75 | |
| SD | 0.12 | 0.06 | 0.04 | 0.05 | 0.05 | 0.06 | 0.05 | 0.03 | 0.10 | 0.08 | 0.14 | |
| cv | 5.67 | 4.91 | 5.75 | 6.63 | 5.27 | 3.34 | 4.78 | 2.79 | 3.75 | 4.85 | 3.13 | |
|
F. pentecostata Station 22 N = 20 9f/11m |
Mean | 2.08 | 1.13 | 0.78 | 0.73 | 1.03 | 1.85 | 1.07 | 1.09 | 2.68 | 1.55 | 4.40 |
| Median | 2.09 | 1.14 | 0.77 | 0.72 | 1.02 | 1.84 | 1.07 | 1.10 | 2.69 | 1.56 | 4.375 | |
| Min | 1.93 | 1.05 | 0.72 | 0.69 | 0.98 | 1.72 | 1.00 | 1.00 | 2.56 | 1.41 | 4.125 | |
| Max | 2.23 | 1.19 | 0.83 | 0.80 | 1.11 | 1.95 | 1.13 | 1.15 | 2.82 | 1.68 | 4.625 | |
| SD | 0.08 | 0.04 | 0.03 | 0.03 | 0.04 | 0.07 | 0.04 | 0.04 | 0.08 | 0.07 | 0.11 | |
| cv | 3.89 | 3.19 | 3.63 | 3.94 | 4.06 | 3.69 | 3.52 | 3.39 | 3.09 | 4.30 | 2.62 | |
|
F. pentecostata Station 23 N = 7 1f/1m |
Mean | 1.81 | 0.99 | 0.67 | 0.64 | 0.89 | 1.84 | 1.04 | 1.12 | 2.70 | 1.54 | 4.25 |
| Median | 1.81 | 0.98 | 0.66 | 0.63 | 0.86 | 1.85 | 1.05 | 1.12 | 2.71 | 1.53 | 4.25 | |
| Min | 1.65 | 0.94 | 0.63 | 0.62 | 0.84 | 1.74 | 1.02 | 1.08 | 2.62 | 1.49 | 4.125 | |
| Max | 2.00 | 1.06 | 0.73 | 0.71 | 0.98 | 1.89 | 1.08 | 1.14 | 2.74 | 1.60 | 4.375 | |
| SD | 0.11 | 0.05 | 0.03 | 0.03 | 0.05 | 0.05 | 0.02 | 0.02 | 0.04 | 0.04 | 0.07 | |
| cv | 6.11 | 4.91 | 4.99 | 4.98 | 5.72 | 3.00 | 2.13 | 2.23 | 1.68 | 2.80 | 1.76 | |
|
F. pentecostata Station 24 N = 34 13f/14m |
Mean | 1.96 | 1.07 | 0.73 | 0.68 | 0.94 | 1.84 | 1.08 | 1.13 | 2.69 | 1.58 | 4.40 |
| Median | 1.97 | 1.07 | 0.73 | 0.68 | 0.95 | 1.84 | 1.08 | 1.13 | 2.68 | 1.59 | 4.375 | |
| Min | 1.75 | 0.96 | 0.64 | 0.59 | 0.80 | 1.71 | 0.99 | 1.07 | 2.49 | 1.48 | 4.125 | |
| Max | 2.21 | 1.21 | 0.81 | 0.77 | 1.05 | 1.99 | 1.16 | 1.20 | 2.86 | 1.69 | 4.625 | |
| SD | 0.14 | 0.07 | 0.05 | 0.05 | 0.06 | 0.06 | 0.04 | 0.04 | 0.08 | 0.05 | 0.15 | |
| cv | 7.04 | 6.76 | 6.76 | 6.73 | 6.88 | 3.09 | 3.80 | 3.17 | 3.12 | 3.21 | 3.49 | |
|
F. pentecostata Station 25 N = 20 12f/8m |
Mean | 2.18 | 1.14 | 0.80 | 0.74 | 1.03 | 1.92 | 1.09 | 1.10 | 2.72 | 1.54 | 4.5 |
| Median | 2.19 | 1.14 | 0.81 | 0.75 | 1.04 | 1.91 | 1.08 | 1.11 | 2.68 | 1.54 | 4.5 | |
| Min | 1.74 | 0.95 | 0.65 | 0.61 | 0.86 | 1.80 | 1.01 | 1.00 | 2.49 | 1.48 | 4 | |
| Max | 2.55 | 1.26 | 0.92 | 0.82 | 1.17 | 2.07 | 1.15 | 1.15 | 2.99 | 1.62 | 4.875 | |
| SD | 0.20 | 0.09 | 0.07 | 0.05 | 0.08 | 0.08 | 0.04 | 0.04 | 0.12 | 0.04 | 0.22 | |
| cv | 9.15 | 7.78 | 8.63 | 7.51 | 7.65 | 4.03 | 3.76 | 3.52 | 4.51 | 2.42 | 5.08 | |
|
F. adkinsi Station 12 N = 20 8f/12m |
Holotype | 2.28 | 1.17 | 0.80 | 0.75 | 1.08 | 1.95 | 1.07 | 1.08 | 2.84 | 1.55 | 4.75 |
| Mean | 2.35 | 1.24 | 0.86 | 0.74 | 1.11 | 1.89 | 1.15 | 1.12 | 2.74 | 1.67 | 4.75 | |
| Median | 2.39 | 1.23 | 0.87 | 0.74 | 1.11 | 1.89 | 1.16 | 1.13 | 2.77 | 1.67 | 4.75 | |
| Min | 2.13 | 1.14 | 0.75 | 0.69 | 1.04 | 1.79 | 1.04 | 1.00 | 2.55 | 1.55 | 4.5 | |
| Max | 2.52 | 1.33 | 0.95 | 0.78 | 1.21 | 2.01 | 1.22 | 1.16 | 2.84 | 1.74 | 5.125 | |
| SD | 0.12 | 0.06 | 0.05 | 0.03 | 0.04 | 0.05 | 0.05 | 0.04 | 0.08 | 0.05 | 0.17 | |
| cv | 5.34 | 4.92 | 6.15 | 3.76 | 3.75 | 2.94 | 4.62 | 3.74 | 2.86 | 3.27 | 3.67 | |
|
F. bakeri Station 13 N = 20 10f/10m |
Holotype | 2.03 | 1.14 | 0.75 | 0.69 | 1.05 | 1.78 | 1.09 | 1.09 | 2.70 | 1.65 | 4.375 |
| Mean | 2.02 | 1.17 | 0.76 | 0.70 | 1.05 | 1.74 | 1.08 | 1.11 | 2.66 | 1.66 | 4.2 | |
| Median | 2.00 | 1.16 | 0.75 | 0.70 | 1.05 | 1.74 | 1.08 | 1.10 | 2.67 | 1.66 | 4.2 | |
| Min | 1.90 | 1.08 | 0.70 | 0.65 | 0.93 | 1.65 | 1.00 | 1.06 | 2.54 | 1.57 | 3.875 | |
| Max | 2.21 | 1.24 | 0.82 | 0.77 | 1.16 | 1.84 | 1.17 | 1.16 | 2.83 | 1.80 | 4.5 | |
| SD | 0.10 | 0.05 | 0.04 | 0.03 | 0.06 | 0.04 | 0.03 | 0.03 | 0.07 | 0.06 | 0.14 | |
| cv | 5.12 | 4.37 | 5.16 | 4.72 | 5.36 | 2.54 | 3.24 | 2.69 | 2.70 | 3.40 | 3.44 | |
|
F. bakeri Station 14 N = 14 6f/1m |
Mean | 1.98 | 1.11 | 0.74 | 0.68 | 1.01 | 1.79 | 1.09 | 1.10 | 2.69 | 1.64 | 4.2 |
| Median | 1.99 | 1.11 | 0.75 | 0.69 | 1.01 | 1.79 | 1.09 | 1.09 | 2.66 | 1.63 | 4.25 | |
| Min | 1.78 | 1.02 | 0.67 | 0.61 | 0.90 | 1.70 | 1.04 | 1.05 | 2.58 | 1.54 | 3.875 | |
| Max | 2.18 | 1.23 | 0.82 | 0.72 | 1.10 | 1.89 | 1.17 | 1.17 | 2.85 | 1.76 | 4.5 | |
| SD | 0.12 | 0.05 | 0.05 | 0.03 | 0.05 | 0.06 | 0.04 | 0.03 | 0.09 | 0.06 | 0.17 | |
| cv | 5.95 | 4.91 | 6.28 | 4.64 | 5.31 | 3.53 | 3.56 | 3.16 | 3.23 | 3.55 | 4.17 | |
|
F. pikinini Station 14 N = 4 -f/1m |
Holotype | 1.64 | 0.94 | 0.61 | 0.59 | 0.84 | 1.75 | 1.03 | 1.11 | 2.66 | 1.57 | 4 |
| Mean | 1.61 | 0.93 | 0.60 | 0.58 | 0.85 | 1.73 | 1.04 | 1.09 | 2.69 | 1.61 | 4 | |
| Median | 1.61 | 0.93 | 0.60 | 0.56 | 0.85 | 1.73 | 1.04 | 1.09 | 2.68 | 1.61 | 4 | |
| Min | 1.54 | 0.88 | 0.58 | 0.56 | 0.83 | 1.72 | 1.00 | 1.06 | 2.66 | 1.56 | 3.875 | |
| Max | 1.67 | 0.97 | 0.62 | 0.62 | 0.89 | 1.75 | 1.09 | 1.11 | 2.72 | 1.68 | 4.125 | |
| SD | 0.06 | 0.04 | 0.02 | 0.03 | 0.03 | 0.01 | 0.04 | 0.02 | 0.03 | 0.06 | 0.10 | |
| cv | 3.72 | 4.27 | 3.62 | 5.48 | 3.24 | 0.89 | 3.72 | 1.89 | 1.16 | 3.61 | 2.68 |
- Ah, aperture height; aw, aperture width; bww, body whorl width; cv, coefficient of variation adjusted for sample size; f, females; m, males; max, maximum; min, minimum; SD, standard deviation; sh, shell height; sw, shell width; w, whorls; F., Fluviopupa, all measurements are given in mm.
Appendix 2
List of individuals and sequences analysed as well as GenBank accession numbers
| Specimen | 16S | COI | ITS2 |
|---|---|---|---|
| F. adkinsi 12-1 | KC875032 | KC875104 | KC875177 |
| F. adkinsi 12-2 | KC875033 | KC875105 | KC875178 |
| F. bakeri 13-1 | KC875034 | KC875106 | KC875179 |
| F. bakeri 13-2 | KC875035 | KC875107 | KC875180 |
| F. bakeri 14-1 | KC875036 | KC875108 | KC875181 |
| F. bakeri 14-2 | KC875037 | KC875109 | KC875182 |
| F. brevior 1-1 | KC874996 | KC875076 | KC875147 |
| F. brevior 1-2 | KC874997 | KC875077 | KC875148 |
| F. brevior 2-1 | KC874998 | KC875078 | KC875149 |
| F. brevior 2-2 | KC874999 | KC875079 | KC875150 |
| F. brevior 3-1 | KC875000 | KC875080 | KC875151 |
| F. brevior 3-2 | KC875001 | KC875082 | KC875153 |
| F. brevior 4-1 | KC875002 | KC875081 | KC875152 |
| F. brevior 4-2 | KC875003 | KC875083 | KC875154 |
| F. brevior 5-1 | KC875005 | KC875085 | KC875156 |
| F. brevior 5-2 | KC875004 | KC875084 | KC875155 |
| F. erromangoana 7-1 | KC875008 | KC875088 | KC875159 |
| F. erromangoana 7-2 | KC875009 | KC875089 | – |
| F. espiritusantoana 26-2 | KC875010 | KC875090 | – |
| F. espiritusantoana 26-3 | KC875011 | KC875091 | KC875160 |
| F. espiritusantoana 27-1 | KC875013 | – | KC875161 |
| F. espiritusantoana 27-3 | KC875012 | KC875092 | – |
| F. espiritusantoana 28-1 | KC875014 | – | KC875162 |
| F. espiritusantoana 29-1 | KC875016 | KC875093 | – |
| F. espiritusantoana 29-2 | KC875015 | – | KC875163 |
| F. espiritusantoana 30-1 | KC875018 | KC875095 | KC875164 |
| F. espiritusantoana 30-2 | KC875019 | KC875096 | KC875165 |
| F. espiritusantoana 31-1 | KC875017 | KC875094 | – |
| F. espiritusantoana 31-3 | KC875020 | – | KC875166 |
| F. freswota 11-1 | KC875050 | KC875121 | KC875195 |
| F. freswota 11-2 | KC875040 | KC875111 | KC875185 |
| F. herminae 8-4 | KC875042 | KC875113 | KC875187 |
| F. malekulana 8-1 | KC875039 | KC875110 | KC875184 |
| F. malekulana 8-2 | KC875041 | KC875112 | KC875186 |
| F. malekulana 8-5 | KC875043 | KC875114 | KC875188 |
| F. malekulana 9-1 | KC875044 | KC875115 | KC875189 |
| F. malekulana 9-2 | KC875045 | KC875116 | KC875190 |
| F. malekulana 9-3 | KC875046 | KC875117 | KC875191 |
| F. malekulana 10-1 | KC875048 | KC875119 | KC875193 |
| F. malekulana 10-2 | KC875047 | KC875118 | KC875192 |
| F. malekulana 10-3 | KC875049 | KC875120 | KC875194 |
| F. narii 33-1 | KC875021 | – | KC875167 |
| F. pascali 32-1 | KC875022 | KC875097 | KC875168 |
| F. pascali 32-5 | KC875023 | KC879098 | KC875169 |
| F. pentecostata 15-1 | KC875057 | KC875128 | KC875202 |
| F. pentecostata 15-2 | KC875058 | KC875129 | KC875203 |
| F. pentecostata 16-1 | KC875059 | KC875130 | KC875204 |
| F. pentecostata 16-2 | KC875060 | KC875131 | KC875205 |
| F. pentecostata 17-1 | KC875063 | KC875135 | KC875209 |
| F. pentecostata 17-2 | KC875064 | KC875136 | KC875210 |
| F. pentecostata 20-1 | KC875068 | KC875140 | KC875214 |
| F. pentecostata 20-2 | KC875069 | KC875141 | KC875215 |
| F. pentecostata 21-1 | KC875062 | KC875134 | KC875208 |
| F. pentecostata 21-2 | KC875067 | KC875139 | KC875213 |
| F. pentecostata 21-3 | KC875070 | KC875142 | KC875216 |
| F. pentecostata 22-1 | KC875071 | KC875143 | KC875217 |
| F. pentecostata 22-2 | KC875072 | KC875144 | KC875218 |
| F. pentecostata 23-1 | KC875073 | KC875145 | KC875219 |
| F. pentecostata 23-2 | KC875054 | KC875125 | KC875199 |
| F. pentecostata 24-2 | KC875051 | KC875122 | KC875196 |
| F. pentecostata 24-3 | KC875052 | KC875123 | KC875197 |
| F. pentecostata 24-4 | KC875053 | KC875124 | KC875198 |
| F. pentecostata 25-1 | KC875055 | KC875126 | KC875200 |
| F. pentecostata 25-2 | KC875056 | KC875127 | KC875201 |
| F. pikinini 14-3 | KC875038 | – | KC875183 |
| F. riva 6-1 | KC875006 | KC875086 | KC875157 |
| F. riva 6-2 | KC875007 | KC875087 | KC875158 |
| F. tangbunia 16-3 | – | KC875132 | KC875206 |
| F. tangbunia 16-4 | KC875061 | KC875133 | KC875207 |
| F. tangbunia 18-1 | KC875065 | KC875137 | KC875211 |
| F. tangbunia 18-2 | KC875066 | KC875138 | KC875212 |
| F. torresiana 36-2 | KC875028 | – | KC875174 |
| F. torresiana 36-3 | KC875029 | KC875101 | KC875175 |
| F. torresiana 37-1 | KC875030 | KC875102 | – |
| F. torresiana 37-3 | KC875031 | KC875103 | KC875176 |
| F. torresiana 34-2 | KC875027 | KC875100 | KC875173 |
| F. torresiana 35-3 | KC875026 | – | KC875172 |
| F. torresiana 38-1 | KC875025 | KC875099 | KC875171 |
| F. torresiana 38-4 | KC875024 | – | KC875170 |
| Hemistomia sp. | JX970548 | JX970617 | – |
| Opacuincola delira | KC875075 | KC875146 | – |
| Potamolithus ribeirensis. | JX970549 | JX970618 | – |
| Potamopyrgus estuarinus | KC875074 | – | KC875220 |
| Tatea huonensis | JX970550 | JX970619 | – |