Batbugs (Cimex pipistrelli group, Heteroptera: Cimicidae) are morphologically, but not genetically differentiated among bat hosts
Abstract
Members of the family Cimicidae (Heteroptera) are obligate haematophagous ectoparasites. The Cimex pipistrelli species group parasitizes on bats, the likely ancestral hosts of the whole family. Based on morphology, it was suggested that three species of the group were present in the West-Palaearctic region, although their validity remained a matter of discussion. Surprisingly, the status of these species has not been studied from the point of view of host specificity. We examined the diversification of the species group using morphological data, including the putative diagnostic characters, and sequences of one mitochondrial (cytochrome oxidase subunit I, COI) and four nuclear loci (internal transcribed spacer 2, 18S and 28s ribosomal genes and elongation factor 1 subunit α). This was carried out on a sample of 225 individuals from 69 bat roosts and 44 mist-netted bats, altogether representing 12 bat species from 13 European countries and Lebanon. We revealed 27 mitochondrial haplotypes representing two distinct haplogroups and one outlying haplotype. The extent of morphological variability of specimens representing both haplogroups covers the range of characters reported for all three recognized species; therefore, the haplogroups clearly do not correspond to any described species. Also, the very limited variability found in the nuclear sequences of the cimicid bugs examined suggests that separate species do not exist in the region. We found considerable morphological differentiation among samples from different bat species, although individuals representing particular mitochondrial haplogroups often live sympatrically and on the same host species. It seems that batbugs are morphologically adapted to a particular bat host despite the low genetic structuring among individuals parasitizing different species of bats.
Introduction
The examination of the functional, ecological and evolutionary patterns in a particular host–parasite system is necessary for the understanding of the diversity of a group of parasites (Poulin 2007). Generally, the degree of host specificity, the ability for adaptive phenotypic plasticity and the mode of parasite transmission are the underlying factors in the diversification of parasite populations (Greischar and Koskella 2007; Poulin et al. 2009; Reckardt and Kerth 2009) resulting in speciation or the emergence of distinct host races (Dres and Mallet 2002). Strong selective pressures on ectoparasites often lead to convergently evolved phenotypes among species (Johnson et al. 2012) or even within a single species (McCoy et al. 2005). Reconstruction of phylogenetic relationships and species determination in parasites based solely on morphology can then be misleading and therefore the implementation of molecular methods is often essential in the identification of species (Raeymaekers et al. 2008) or genera (Johnson et al. 2002) as well as the assessment of their host specificity. Such studies frequently lead to taxonomic reinterpretation including the discovery of cryptic species (Light and Hafner 2007; Bruyndonckx et al. 2009; Roy et al. 2009; Westram et al. 2011), even in a parasite previously believed to be generalist (Whiteman et al. 2006).
The family Cimicidae (Heteroptera) is a group distributed worldwide containing about 110 described species classified into 24 genera in six subfamilies (Henry 2009). It constitutes a group of obligate haematophagous ectoparasites with a unique parasitic strategy. About two-thirds of the recognized species are associated with bats, which are the likely ancestral hosts of the family (Horváth 1913; Usinger 1966). However, the only phylogenetic hypothesis (Usinger 1966) is built exclusively on morphology and exhibits inconsistencies when compared with molecular data (Balvín and Vilímová 2009; Balvín 2010). The genus Cimex Linnaeus, 1758 from the subfamily Cimicidae is the best-known taxon of the family due to its holarctic distribution and inclusion of two of the most important human-associated species. The genus comprises 21 described species (Ryckman et al. 1981). Except for one, all are primarily associated with bats including those found on humans (Usinger 1966). Cimex pipistrelli Jenyns, 1839 group is associated exclusively with bats and from the other taxa, it is delimited by narrow lateral lobes of the pronotum and cleft and naked paragenital sinus. The group consists of 10 described Palaearctic species (Usinger 1966; Ueshima 1968; Bhat 1974a,b). Three of these (C. pipistrelli, C. dissimilis Horváth 1910 and C. stadleri Horváth 1935) and one form of unclear taxonomic status (C. pipistrelli form singeri China, 1938) have been described from the Western Palaearctic region. Different opinions have been voiced on the validity of these taxa. Some authors considered the described taxa as subspecific forms (Stichel 1938, 1959; Wendt 1941; Lansbury 1961), whereas some accepted three distinct species (Hedicke 1935; Usinger 1966; Wagner 1967). Some authors considered C. stadleri synonymous to C. dissimilis (Stichel 1959; Péricart 1972, 1996), while yet others accepted only a monotypic species C. pipistrelli (Stichel 1938; Wendt 1941; Povolný 1957; Lansbury 1961; Kerzhner 1989). The discussion was based on delimiting the species by a few variable metric morphological characters. Overall, only Wendt (1941), Kerzhner (1989) and partially Usinger (1966) supported their opinions with a substantial amount of data. Wendt (1941) reported that he found such a large variability in the proposed discriminating morphological characters that progeny of even a single female could be assigned to all three described species. However, based on laboratory crossing experiments, Usinger (1966) suggested the existence of a reproductive barrier between the populations of batbugs of the Cimex pipistrelli group from the British Isles and those of Czechoslovakia, supporting the existence of at least two separate species in Europe. The need for the application of molecular techniques to solve the problem is evident. It is also surprising that the adaptation to a different host was not taken into account as a driving force for differentiation within the Cimex pipistrelli group [cf. for instance to studies on the morphological variation related to host identity in Cimex lectularius (Balvín et al. 2012a; Johnson 1939; Usinger 1966)]. For each of the species of the Cimex pipistrelli group, different host ranges were reported (e.g. Usinger 1966; Péricart 1972), but host specificity and its relation to morphological differentiation or species identity was not discussed.
The present study reports the genetic population structure and morphological differentiation of the population of the Cimex pipistrelli group in the West-Palaearctic region and among different host species of bats. The main aims are to resolve taxonomic controversies concerning members of the West-Palaearctic Cimex pipistrelli group, and the examination of genetic and morphological differentiation within this group of batbugs and its corresponding relationship to host specificity.
Materials and Methods
Material
A total of 225 batbug individuals were collected from a combination of 69 bat roosts and 44 mist-netted bats from 14 countries (see Table S1). The total number of host bat species recorded during collections was 14. However, the number includes two members of the genus Rhinolophus recorded in the mixed colonies. These when roosting alone do not host batbugs. The batbugs were collected either by the first author when accompanying bat specialists during fieldwork at roosts or by the bat specialists themselves. The sample was supplemented by two collections of cimicids from Vespertilio superans bats and four from martins (Delichon dasypus) from Japan (Table S1, no. 350; 351: assigned as C. japonicus Usinger 1966; no. 895–898: Cimex sp.). The samples were preserved in 96% ethanol and deposited in the collection of O. Balvín at the Faculty of Science, Charles University in Prague. The determination of the C. pipistrelli group followed Usinger (1966).
DNA extraction, PCR and sequencing
The tissue for DNA extraction was obtained from half of the thorax and legs. Extraction was performed using DNeasy® Blood & Tissue kit (QIAGEN, Duesseldorf, Germany). We analysed the cytochrome oxidase subunit I (hereinafter COI) of 206 individuals from 66 bat roosts and from 41 mist-netted bats from the West-Palaearctic region as well as additional 11 individuals from two bat roosts and three colonies of martins from Japan. Sequences of COI were usually obtained from two individuals from each bat roost. We examined more specimens from a few of the localities. A total of 18 individuals from the roost of Myotis daubentonii in Ruda, Veselí nad Lužnicí (collections no. 1, 17, 35, 36, 75, 112, 225, Table S1) were examined to test their differentiation from that of the batbugs sampled from individuals of Nyctalus noctula mist-netted from the nearby surroundings. Individuals from the colony of M. daubentonii were known to use the same tree holes as N. noctula in this area. Also, we examined as many individuals as possible from the collections from Great Britain, where there is the type locality of C. pipistrelli.
Amplification of the 658 bp long gene fragment of COI was performed using modified DNA barcoding primers LepF (5′-ATT CAA CCA ATC ATA AAG ATA TNG G-3′) and LepR (5′-TAW ACT TCW GGR TGT CCR AAR AAT CA-3′) designed for Lepidoptera (e.g. Hajibabaei et al. 2006). The annealing temperature in the PCR was 48°C.
Tto evaluate the status of the two major haplogroups revealed by COI, we examined the variation within and between these groups using nuclear markers as well. Each haplogroup was represented by eight individuals from different localities.
We used the internal transcribed spacer 2 (ITS2) and elongation factor 1 subunit α (EF1α), which are often shown polymorphic within species (e.g. Damgaard et al. 2000; Kim and Lee 2008). We also included less polymorphic regions of the 18S and 28S ribosomal genes, which are usually used for phylogeny of higher taxa, but may occasionally show informative on species level as well (Deng et al. 2012). The 945 bp long fragment of ITS2 was amplified using primers CAS5p8sFc and CAS28sB1d (Kim and Lee 2008), the whole 18S gene of total length of 1886 bp using primers 18S-1, 18S-2, 18S-3 and 18S-4 (Tian et al. 2008), 650 bp of 28S using primers 28S-DD and 28S–FF (Tian et al. 2008) and 581 bp of EF1α using primers Shirley and Prowler (Damgaard et al. 2000). The annealing temperatures in PCR were 62°C for ITS2, 48°C for both 18S fragments and 28S and 58°C for EF1α.
The PCR products were purified using QIAquick® PCR purification kit (QIAGEN). The sequencing was carried out in both directions using a BigDye® Terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA, USA) and ABI PRISM® 3100-Avant Genetic Analyzer (Applied Biosystems) or using a commercial sequencing service (Macrogen Inc., Seoul, South Korea).
Alignments and population genetics analyses
The sequences were aligned using MAFFT with default settings (Katoh et al. 2009). We constructed a median-joining network (for the algorithm and rationale for using this type of network, see Bandelt et al. 1999; Huson et al. 2010) in Network 4.516 (fluxus-engineering.com) using default parameters to visualize the data. Basic population genetic polymorphism analyses (nucleotide and haplotype diversities) were performed using the program Arlequin 3.01 (Excoffier et al. 2005). As the morphological analysis showed a large variation coupled with an association to a particular host species, we examined the components of genetic variability at hierarchical levels in the West-Palaearctic sample using the analysis of the molecular variance (amova) in Arlequin 3.01. The components of diversity in the hierarchical model were comprised of within localities, among localities/within populations on particular host bat species and among populations on particular host species.
Morphological analysis
The specimens were photographed in a standardized manner in a Petri dish with ethanol and flattened by a smaller dish using a stereoscopic microscope (Olympus SZX9) and a digital camera (Olympus C-5060, Olympus, Tokyo, Japan) operated by Photo Micro 2.0. The measurements were taken using MeasureIT (Olympus).
For the examination of morphological variation, we primarily focused on recording characters previously suggested for the determination of the relevant taxa (Table 1). For a deeper insight into the morphological variation, we measured the dimensions of other body parts as well (see Fig. 1 for definitions of most of the measured characters). If not stated otherwise, the largest possible dimension of a body part or the longest hair was measured. In total, 61 characters were measured (abbreviation in brackets): Body: total body length (to). Head: clypeus width (cw), eye diameter (ed), eye width (ew), head width (hw), intraocular space dorsally (is) and ventrally (iv), length (al1-4) and width (aw1-4) of antennal segments, length (rl1-3) and width (rw1-3) of rostral segments, length of hairs between the eye and antenna (se), length of hairs on the clypeus (sc). Pronotum: pronotum width (pw), width between the frontal tips (wl), pronotum length (pl), length measured medially (pm), depth of the frontal concavity (pc), length of hairs (sp), length of 10 hairs closest to the frontal tips (sl; the length of these hairs was taken as a separate character because we supposed that due to their position, they were probably used for comparison with eye diameter by Horváth (1910) and authors following him (e.g. Hedicke 1935)). Scutellum: scutellum width (sw), number of hairs (sn). Hemelytra: hemelytra length (hl), width (wh), length of hairs (sh), ratio of length of hairs to their mutual distance on the inner half of the disc of hemelytra (ih). Abdomen: length of hairs on the posterior-lateral angle of the 2nd–8th tergite (sa2-8), length of hairs on the 9th tergite (sa9), average length of hairs of the anterior (st3, 5 and 7) and length of hairs of the posterior (ts3, 5 and 7) row in the medial third of the 3rd, 5th and 7th tergum. Legs: length (fl1-3) and width (fw1-3) of femora, length (tl1-3) and width (tw1-3) of tibiae. The pubescence of pygophore as a status character was evaluated by eye. The measurements were taken from 195 adult individuals collected from 63 bat roosts and from 42 mist-netted bats in West-Palaearctic region by a single person (OB).
| Review of published characters and their states and values | Values of metric characters in our sample | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Descriptive characters | C. pipistrelli | C. dissimilis | C. stadleri | C. singeri | Author | Expression of characters; for abbreviations see Methods | Haplogroup 1; N = 81 | Haplogroup 2; N = 95 | ||||||
| Pubescence on tergum | hairy | hairless | 0 | 0 | Horváth (1910) | |||||||||
| Pubescence on tergum | long | short | short | long | Stichel (1926, 1937, 1938) | |||||||||
| Shape of hair tips on pronotal edges | tapering | truncate | 0 | 0 | Southwood and Leston (1959) | |||||||||
| Pubescence on pygophorus | all over | tip only | tip only | 0 | Hedicke (1935) | |||||||||
| Metric characters | C. pipistrelli | C. dissimilis | C. stadleri | C. singeri | Author | Mean | Min | Max | St.Dev. | Mean | Min | Max | St.Dev. | |
| Ratio of lengths of 2nd and 3rd antennal segment | ≤1 | ≫1 | 0 | 0 | Horváth (1910) | al2/al3 | 1.10 | 0.97 | 1.28 | 0.06 | 1.10 | 0.90 | 1.24 | 0.06 |
| >1 | >1 | <1 | <1 | Stichel (1926, 1937, 1938, 1959) | ||||||||||
| – | ♀1.12; ♂1.24 | ♀1.08; ♂0.96 | 0 | Horváth (1935) | ||||||||||
| ≪1 | 1.2 | ≈1 | 0 | Hedicke (1935) | ||||||||||
| 1.125 (18/16) | 1.125 (18/16) | 0.94 (16/17) | 0.94 (16/17) | Lansbury (1961) | ||||||||||
| >1 | >1 | 1 | 1 | Usinger (1966) | ||||||||||
| Ratio of length of hairs on pronotum to eye diameter | >1 | <1 | 0 | 0 | Horváth (1910) | sl/ed | 0.67 | 0.41 | 1.07 | 0.15 | 0.61 | 0.37 | 0.96 | 0.10 |
| ≫1 | <1 | <1 | 0 | Hedicke (1935) | sp/ed | 0.71 | 0.47 | 1.12 | 0.15 | 0.68 | 0.46 | 1.11 | 0.11 | |
| Ratio of length of hairs on pronotum to width of 1st antennal segment | >1; >130 μm | ≤1; <130 μm | ≤1; <130 μm | 0 | Usinger (1966) | sl/aw1 | 0.92 | 0.61 | 1.41 | 0.19 | 0.86 | 0.58 | 1.31 | 0.12 |
| sp/aw1 | 0.98 | 0.68 | 1.57 | 0.20 | 0.96 | 0.72 | 1.52 | 0.13 | ||||||
| 1.2–1.4 | 0.8–1 | 0 | 0 | Péricart (1972) | sl | 139 | 97 | 198 | 23.85 | 133 | 88 | 175 | 17.30 | |
| sp | 149 | 105 | 219 | 23.89 | 148 | 118 | 191 | 16.78 | ||||||
| Ratio of lengths of hairs on tergum medially and laterally | =1 | ≪1 | ≪1 | ≪1 | Stichel (1959) | ts3/sa3 | 0.40 | 0.00 | 1.61 | 0.40 | 0.43 | 0.00 | 1.29 | 0.41 |
| ts5/sa5 | 0.41 | 0.00 | 1.35 | 0.37 | 0.47 | 0.00 | 1.20 | 0.38 | ||||||
| ts7/sa7 | 0.57 | 0.00 | 1.83 | 0.38 | 0.55 | 0.00 | 1.11 | 0.34 | ||||||
| Ratio of length of hairs on tergum medially to width of 1st antennal segment | ≥1 | ≤0.8 | 3/11–4/11 | ≤0.8 | Lansbury (1961) | ts3/aw1 | 0.30 | 0.00 | 1.11 | 0.31 | 0.30 | 0.00 | 0.88 | 0.29 |
| ts5/aw1 | 0.33 | 0.00 | 1.14 | 0.30 | 0.36 | 0.00 | 0.99 | 0.30 | ||||||
| ts7/aw1 | 0.51 | 0.00 | 1.25 | 0.33 | 0.50 | 0.00 | 1.13 | 0.32 | ||||||
| Length of hairs on tergum medially | – | – | 30 μm | 40–80 μm | Stichel (1959) | ts3 | 44 | 0 | 141 | 44.52 | 47 | 0 | 130 | 44.50 |
| ts5 | 49 | 0 | 138 | 43.80 | 55 | 0 | 150 | 45.68 | ||||||
| ts7 | 76 | 0 | 190 | 48.59 | 77 | 0 | 170 | 48.04 | ||||||
| Ratio of length of hairs on hemelytrae medially to their mutual distance | – | >1; >0.1 mm | <1; <0.1 mm | 0 | Usinger (1966) | ih | 0.93 | 0.00 | 2.00 | 0.51 | 0.95 | 0.00 | 2.00 | 0.53 |
- 0: taxon not mentioned. – : state of character not given. For values for the total sample of West-Palaearctic region and C. japonicus and their values of these diagnostic characters and diagnostic characters delimiting other Palaearctic species of C. pipistrelli group see online supplementary Table 2.
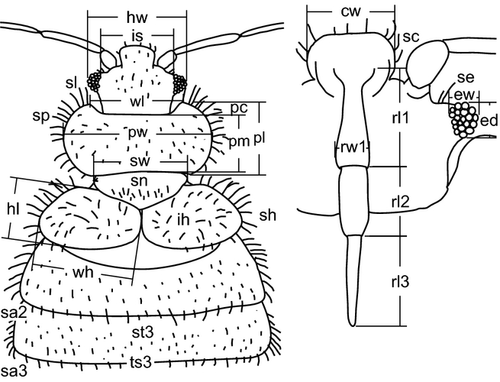
We divided the specimens into groups according to the haplogroups revealed by the analysis of COI sequences and the host species. Specimens from Pipistrellus spp. were grouped together as the closely related and similar species P. pipistrellus and P. pygmaeus were not distinguished in some collections.
Relationships among morphological variables measured among West-Palaearctic batbugs were first explored by principal component analysis (PCA). Since the variables expressing hair lengths poorly correlated with the variables expressing dimensions of body regions, two other PCAs were performed using these two subsets of variables to examine the morphological variation in more detail. In all cases, we analysed only the principal components covering significant portions of variation according to the broken-stick model (Jackson 1993). Specimens of Cimex japonicus were added to an additional PCA to test the position of this species relatively to the morphospace occupied by the West-Palaearctic C. pipistrelli group.
We performed full-factorial analyses of variance (anovas) of factor scores for particular principal component (PC) using haplogroups and host association as categorical predictors. Only the samples from M. myotis, N. noctula and Pipistrellus spp. were included as they contained a considerable amount of specimens from both haplogroups. A sample from M. daubentonii was added to one-way anovas testing differences in factor scores among different hosts. The pairwise differences between groups of specimens from different hosts and haplogroups were tested using the unequal N HSD (honestly significant difference) post hoc test.
Since the test of relation of morphology and host association could be biased due to unequal sampling from different bat species and also unequal distribution of the bat species throughout the sampled area, we tested the geographic variation in morphology by the partial Mantel test of matrix association (Smouse et al. 1986). We computed the mean factor scores on PC1 and PC2 for each locality. Batbugs from mist-netted bats were regarded as separate localities. The pairwise distances among PC1 and PC between the localities were used as the dependent matrix. We disregarded geographically outlying specimens by including the samples only from Czech and Slovak Republics and Hungary. The Mantel tests were run on 10 000 randomization in the program ibd 1.52 (Bohonak 2002).
We tested the differences between the haplogroups in individual characters previously used for the taxonomy of the West-Palaearctic C. pipistrelli group within (1) all specimens from the haplogroups and (2) groups of specimens from M. myotis and N. noctula of which a considerable number of individuals were counted. We used analysis of covariance (ancova) to test the ratio of lengths of the 2nd (dependent variable) and 3rd (covariate) antennal segment. The hair lengths did not correlate with the dimensions of body regions in PCA. Moreover, as the hairs were sometimes missing their lengths rarely showed normal distribution. Therefore, they were tested for the differences between the haplogroups using the two-group nonparametric Mann–Whitney U test (MWU). Using the same test, we examined the differences in the relative characters comprising hairs used for the distinction of species of C. pipistrelli group in the literature (Table 1). The ratio of the hair length on the pronotum to eye diameter or width of 1st antennal segment was represented as fractions sp/ed, sl/ed, sp/aw1, sl/aw1, ratio of the hair length on the tergum medially and laterally as ts3/st3, ts5/st5, ts7/st7 and ratio of the hair length on the tergum medially to width of 1st antennal segment as ts3/aw1, ts5/aw1, ts7/aw1.
We also performed a discriminant function analysis (DFA) using the specimens from M. myotis, M. daubentonii, N. noctula and Pipistrellus spp. In DFA, we used characters not correlated with body size and body dimensions statistically controlled for body size. For this purpose, we created a set of 80 characters using hair length (which do not correlate with body size) and residuals from simple linear regressions of pairs of body dimensions. Head width, a precisely measurable character which showed the highest correlations (0.92–0.93) with the principal component 1 (PC1) reflecting a largely generalized body size and low correlations with the principal component 2 (PC2) in all PCAs, was chosen as a predictor for all other dimensions used as dependent. Secondly, we used the widths of antennal and rostral segments, femora, tibiae and hemelytrae as dependent and their lengths as a predictors. Thirdly, we used lengths of pronotum (pl, pm, pc), width of pronotum between the frontal tips and scutellum width as dependent and total pronotum width as predictor. Following on from this, we performed two PCAs using the residuals and hair lengths and chose 18 characters according to their mutual correlations and the results of ancovas and Kruskal–Wallis tests of differences between specimens from different hosts. This elimination of redundant variables allowed us to keep important information on the differences in body shape while at the same time minimizing the number of variables under the smallest sample size in a group of specimens from a single host (19). The selected 18 variables were included to DFA, where we made a further selection of variables useful for specimen classification using backwards stepwise selection.
The data were analysed using Statistica 8.0 software (StatSoft Inc., 2007).
Results
Molecular analysis
We found 42 polymorphic sites and revealed 28 haplotypes among COI sequences in West-Palaearctic populations of the C. pipistrelli species group (GenBank accession numbers from KC503512 to KC503539, for details see Table S4). The haplotype diversity was 0.7750 ± 0.0284, the nucleotide diversity 0.0147 ± 0.0075. In each of the samples from bats (four individuals from two bat roosts) and martins (seven individuals from four colonies) from Japan, only one haplotype was revealed (GenBank accession numbers KC503540, KC503541). The number of polymorphic sites in the sample of C. pipistrelli group including the collections from Japan was 56, the haplotype diversity was 0.7961 ± 0.0262 and the nucleotide diversity 0.01604 ± 0.0081. It is necessary to note that the individuals from martins from Japan morphologically represent a species of the genus Oeciacus although they are much closer to C. pipistrelli group than to the described Oeciacus species according to mtDNA (Balvín 2010; Balvín et al., 2012c). They are included to show how such a large phenotypic diversification of a genetically close group can exist.
The estimated haplotype network shows five distinct and quite equally distant groups of haplotypes (Fig. 2). Three consist of only one haplotype; two of them represent the collections from Japan and the third represents one of the collections from central Russia (h26; collection no. 201; see the Table S1). The other two haplogroups are similarly abundant in central Europe. The distribution of the haplogroup 1 was, in addition, stretched as far as Ukraine and many peripheral areas of West-Palaearctic region, namely Great Britain, Spain, Bulgaria, Greece, Lebanon and central Russia. The geographic distribution of haplotypes in the most densely sampled areas of the Czech and Slovak Republics and Hungary (Fig. 3) often suggests a locally restricted genetic diversity.
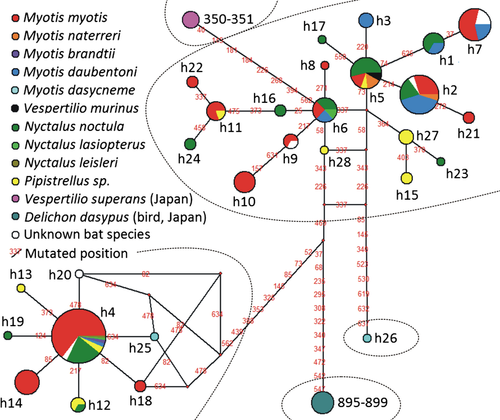

The haplogroup 1 is much more differentiated (the haplotype diversity 0.9012 ± 0.0148 and the nucleotide diversity 0.005377 ± 0.003057 among 95 individuals from 31 bat roosts and 20 mist-netted bats) than the haplogroup 2 (the haplotype diversity 0.2811 ± 0.0554 and the nucleotide diversity 0.000454 ± 0.000530 among 110 individuals from 35 bat roosts and 21 mist-netted bats). Two or more haplotypes were found in 12 of 56 samples from bat roosts represented by two or more individuals. In three of these bat roosts (no. 61, 73, 185; see Table S1), both haplogroups 1 and 2 were present.
Sequencing of the nuclear genes revealed null variation in the target loci, with a single exception of one variable site in ITS2 (GenBank accession numbers from KC503542 to KC503546, for details see Table S4). However, in both groups of specimens representing haplogroups revealed by COI both variants of ITS2 were present.
As visible in the network (Fig. 2), many haplotypes are shared among batbugs from different host species of bats. The most abundant haplotypes recorded from a single host in the network are either collections from two localities close to each other (h10) or due to the examination of more individuals from one location (h14). The results of amova (Table 2) also suggest a very low structuring of the populations according to host association.
| Variance component | Variance | % total | p | Φ-statistics |
|---|---|---|---|---|
| Among groups from different hosts | 0.342 | 6.82 | 0.086 ± 0.010 | ΦCT = 0.068 |
| Among localities | 3.708 | 73.98 | <0.001 | ΦSC = 0.794 |
| Within localities | 0.962 | 19.21 | <0.001 | ΦST = 0.808 |
Morphological analysis
The differences in the values of any diagnostic morphological character given in Table 1 between the haplogroups did not correspond to values reported for described species of the C. pipistrelli group. The character of pubescence of pygophore evaluated visually also did not show any consistency with the division of the specimens into the haplogroups.
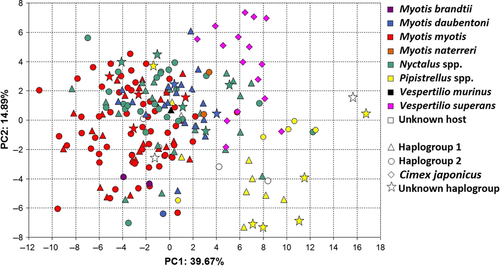
The result of PCAs using subsets of characters was congruent with the PCA using all characters. Therefore, only the results based on PCA using all measured characters are presented (Fig. 4). According to the broken-stick model, the first two principal components were significant. Most of the dimensions of body regions were strongly correlated with PC1 (Table S3), which thus can be interpreted as a representation of the overall body size. PC2 then largely represents the differences in hair length. The full-factorial anovas showed significant differences in factor scores of PC1 representing 40.46% of total variability among individuals associated with different bat species, while the haplogroups did not differ significantly (host species: F = 44.6, p < 0.001; haplogroup: F = 0.06, p = 0.811; host x haplogroup interaction: F = 1.53, p = 0.221). Both host-association and haplogroup identity contributed to significant differences among individuals in factor scores on PC2 representing 13.27% of variability (host: F = 10.13, p < 0.001; haplogroup: F = 4.12, p = 0.044; interaction of predictors: F = 1.54, p = 0.217). On PC1, the post hoc tests showed that all possible pairwise differences among groups from different bat species from each haplogroup were significant (all p < 0.038), except for both pairwise comparisons of specimens from M. myotis and N. noctula (p > 0.40). In PC2, the post hoc tests showed only a few significant pairwise differences among groups of specimens from different bats (N. noctula, haplogroup 1 from Pipistrellus sp., haplogroup 1: p = 0.04; N. noctula, haplogroup 2 from M. myotis, both haplogroups: p = 0.024 and from Pipistrellus sp. haplogroup 1: p = 0.004). No difference was shown between haplogroups within samples from single bat species (all p > 0.59). One-way anova using additional samples from M. daubentonii also showed the host species to affect the division of specimens both on PC1 (F = 31.9; p < 0.001) and PC2 (F = 7.8; p < 0.001). According to the post hoc tests, groups of batbugs from all particular host bat species mutually differed from each other in factor scores on PC1 (all P < 0.01), with the exception of batbugs from M. daubentonii and N. noctula creating a single homogenous group (p = 0.99). On the other hand, only the group from N. noctula significantly differed from the groups from M. myotis (p = 0.001) and Pipistrellus spp. (p < 0.001), and the group from M. daubentonii significantly differed from the group from the Pipistrellus spp. (p < 0.001) in PC2 scores.
The Mantel test did not show any correlation between the morphology and geography (r = −0.102; p = 0.97). Similar numbers were obtained when either of the matrices or both were log-transformed.
The ratio of lengths of the 2nd and 3rd antennal segments was not significantly different between the haplogroups in any of the tested groups of specimens. Among the hair related characters, using MWU, the characters sl (Z = −2.02; p = 0.043) and sl/ed (Z = −2.63; p = 0.008) in the sample from M. myotis and ts3 (Z = −2.05; p = 0.040) in the sample from N. noctula showed a significant difference between the haplogroups (for shorts see 2).
The DFA successfully discriminated between the groups of specimens from M. myotis, M. daubentonii, N. noctula and Pipistrellus spp. The characters included by backwards stepwise selection were the residuals of regression of al2, is, pw, wl, fl3 to hw, fw3 to fl3, wh to pw and the lengths of hairs sa3, sa8 and st3. The Wilks' Lambdas were 0.219, 0.529 and 0.777 for root 1, 2 and 3, respectively; p < 0.001 for all roots. The percentages of correctly classified specimens were 90.9%, 65.4%, 84.2% and 61.9% from the groups from M. myotis, N. noctula, Pipistrellus spp. and M. daubentonii, respectively and 79.4% in total.
Discussion
Our results show that populations of batbugs of the Cimex pipistrelli group are morphologically differentiated among host bat species (Fig. 4), but that this morphological diversity is not reflected by population structuring according to examined nuclear and mitochondrial DNA loci (Fig. 2, Table 2).
In the past, based on morphological characters, three species of the C. pipistrelli group were suggested to be present in the West-Palaearctic region (e.g. Horváth 1935; Usinger 1966; Wagner 1967). However, this view is not supported by our molecular analyses. Most notably, we revealed two distinct haplogroups of batbugs in the West-Palaearctic region (Fig. 2), neither of which morphologically corresponded to any of the described species. According to the proposeded key diagnostic characters (cf. Table 1), the specimens representing both haplogroups could be classified into all three described species C. pipistrelli, C. dissimilis and C. stadleri. Therefore, we preliminarily suggest treating C. dissimilis and C. stadleri as junior synonyms of C. pipistrelli (see also e.g. Kerzhner 1989; Lansbury 1961; Povolný 1957; Wendt 1941) until new evidence is available. At the current state, we cannot foreclose that further data, for example, from variable nuclear markers, will allow some of the newer names to be assigned to a taxon possibly defined in future.
Specimens of both haplogroups often parasitize the same host species and live in sympatry (Figs 2 and 3). Most morphological variability among batbugs can be attributed to different host association and we found only a slight morphological differentiation between individuals representing particular COI haplogroups. Moreover, although we checked the nuclear markers frequently used for the reconstructions of species phylogeny (e.g. Marcilla et al. 2002; Kim and Lee 2008), we found null variability in three of them (18S, 28S, EF1α), and the limited variability in ITS2 did not match the separation of the haplogroups. For these reasons, we suggest that the two COI haplogroups do not represent two independent batbug species and conclude that there is little evidence for the existence of separate species of batbugs from the C. pipistrelli group in the West-Palaearctic region.
Our results contrast with cross-mating experiments made by Usinger (1966), who reported a reproductive barrier between a ‘long-haired’ population representing C. pipistrelli s. str. from Great Britain and a ‘short-haired’ C. stadleri from Czechoslovakia. However, compared to recent standards, Usinger described the experiments rather vaguely and did not report the number of replications, original host species of experimental individuals or the nutritional conditions present during experiments, and so we consider that his results should not be taken as absolute.
We believe that the morphological differentiation among batbugs from different host bat species caused the former misunderstanding in the taxonomy of the West-Palaearctic C. pipistrelli group. A particularly strong example is the difference in physical appearance of batbugs from the Myotis myotis and Pipistrellus spp., the most frequently sampled hosts in continental Europe and British Isles, respectively (Fig. 5). The distribution range of M. myotis only extends to the edge of East England (Horáček 1985). The smaller and more hairy appearance of British batbugs can be explained by their common association with the Pipistrellus spp. Although host-associated morphological variation has been discussed in C. lectularius (Johnson 1939; Usinger 1966), such a hypothesis has never appeared in any discussions of the taxonomy of the C. pipistrelli group (e.g. Povolný 1957; Lansbury 1961; Usinger 1966; Péricart 1972).

At the current stage of knowledge, we are not able to identify the mechanism responsible for the morphological differentiation of batbugs among different hosts. One possibility is the genetic isolation of the populations of each host and the rapid adaptation to a particular host due to the strong selective pressure on loci controlling differences in morphology, although this genetic differentiation has not so far been reflected in the loci sampled by us. Populations of ectoparasites associated with particular hosts have been shown to maintain genetic integrity regardless of limited gene flow (e.g. Nadler et al. 1990; Alasaad et al. 2008). Also, some occurrences of host races (Dres and Mallet 2002) were identified in several ectoparasitic arthropods including cimicids (McCoy et al. 2001, 2003; Balvín et al. 2012a). In our case, the gene flow among the possible host races could be strongly indicated in the maternally inherited mitochondrial locus, which would concur with the observation that adult females are probably the main dispersal vehicle of batbugs (Heise 1988, Balvín et al., 2012b). Cimicids usually stay inactive in shelters; often only appearing on their host during feeding. However, it has been suggested that at times, they also seem to actively attach to their host in order to disperse (Heise 1988, Reinhardt and Jacobs 2006; Balvín et al., 2012b). The frequency of batbug transport differs greatly among host bat species; of individuals found attached to a host bat mostly come from the Nyctalus spp. It is therefore likely that N. noctula is an important agent for the dispersal of batbugs among colonies of other bat species, at least in central Europe (Balvín et al., 2012b).
The samples from mist-netted N. noctula and the roosts of Myotis daubentonii and M. brandtii from the area surrounding the field base of Charles University in Southern Bohemia (Fig. 3; most western box) can serve as anecdotic evidence of the frequent contacts of different bat species. Six haplotypes were found among 16 specimens from M. daubentonii, six specimens from N. noctula and two specimens from M. brandtii. Two of them were shared between the collections from N. noctula and M. daubentonii, and one between N. noctula and M. brandtii. A similar situation is reflected in batbugs collected from bat boxes inhabited by Pipistrellus sp. and mist-netted Nyctalus noctula from the Pálava region (Fig. 3; second most western box). The habitat of batbugs most often sampled by our researchers was colonies of Myotis myotis females, which are particularly faithful to their natal colonies (Castella et al. 2001) and any possible shifts most likely happen among neighbouring colonies (Horáček 1985). The geographic segregation of haplotypes (Fig. 3) suggests the exchange of batbugs between bat roosts within close regions, such as those characterized in the common overwintering areas of Bohemian, Moravian or Aggtelec-Slovakian Karsts.
However, the observed morphological differentiation of batbugs among particular hosts can be totally independent from the strength of gene flow and the degree of genetic differentiation. It can be also caused by phenotypic plasticity, the ability of the same genotype to produce different phenotypes according to the different environmental conditions present (West-Eberhard 1989). This phenomenon is rarely reported from ectoparasites, to our knowledge only from lice (Veracx and Raoult 2012). It can be tested by common garden or transplant experiments. A common garden experiment was carried out between Cimex lectularius and Cimex columbarius Jenyns, 1839 to decide whether these two species are indeed separate (Usinger 1966). This experiment suggested a limited phenotypic plasticity of bugs with regard to their host identity; however, only one diagnostic character (ratio of length of third antennal segment and head width) was observed, and the chosen hosts were artificially selected from outside the range of those commonly used.
According to COI sequences, both of the samples from Japan used in the present study are not more genetically distant from the West-Palaearctic batbugs than the two West-Palaearctic haplogroups are from each other (Fig. 2). The sample from martins (IC 895-7, see Table S1) morphologically represents a typical species of the genus Oeciacus. The other sample from Japan (IC 350-1, see Table S1) belongs to Cimex japonicus, which is one of seven species of the C. pipistrelli group described from the East-Palaearctic region (Usinger 1966; Ueshima 1968; Bhat 1974a,b). The knowledge on their morphology is either based on only a very small number of specimens, or possibly even just on the type material. In the light of our findings, the true diversity of the C. pipistrelli group in the East-Palaearctic region may be far from its description by current taxonomy as well.
In summary, we show that members of the West-Palaearctic Cimex pipistrelli group may represent an interesting model for the study of morphological diversification in ectoparasitic organisms. Future studies on these interesting organisms should consider such diverse factors as host association, differential geographic range of host species, frequency of host switches and the magnitude of (possibly adaptive) phenotypic plasticity to reveal the driving force responsible for the morphological differentiation in the face of limited genetic variation between populations occupying different bat host species.
Acknowledgements
Our deepest thanks has to be expressed to more than 60 bat specialists and colleagues who have taken part in the collection of batbug material for the study, regrettably we cannot name them all here. Special thanks is due to Anthony M. Hutson (IUCN/SSC Chiroptera Specialist Group, Winkfield, Great Britain), Klaus Reinhardt (University of Sheffield, Great Britain) and Phil Smith (Bournemouth, Great Britain) who helped to obtain material from Great Britain. We are thankful for the help with laboratory work and comments on the manuscript from Josef Bryja, Jan Zima (Academy of Sciences, Czech Republic), Petr Janšta, Pavel Munclinger and Jakub Straka (Charles University, Czech Republic). We would like to express our gratitude to Chris Johnson for the correction of English. The study was supported by the grant of Ministry of Education, Youth and Sports of the Czech Republic no. SVV-2013-267 201 and a grant from the Grant Agency of Charles University no. 122/2006 B/Bio.




