Impact of higher temperatures on yolk sac absorption and early development in hybrid catfish between Clarias gariepinus and C. macrocephalus
Abstract
Climate change has driven global temperature increases, resulting in severe heat waves, altered precipitation patterns, and extreme weather events. In April 2024, a massive die-off of hybrid catfish larvae and fry occurred in Thai aquaculture farms, coinciding with elevated temperatures of air (37–41°C) and freshwater (30–32°C). This study aimed to investigate the correlation between elevated temperatures and abnormal embryonic development in hybrid catfish. Controlled mating of male North African catfish (Clarias gariepinus) and female bighead catfish (Clarias macrocephalus) was conducted in May and June 2024. We examined the effects of water temperature changes in hybrid catfish, focusing on fertilization, hatching, yolk sac absorption, and mouth morphology, while keeping the conditions of water quality, feeding, and disease management constant. The fertilization rate was 67.7% at 29°C, whereas it significantly decreased to 59.0% at 32°C. The hatching rate decreased from 43.4% at 29°C to 26.6% at 32°C. Survival rates plummeted, with no larvae surviving beyond 72 h post-hatching (hph) at 32°C. Yolk sac absorption was notably accelerated at 32°C, being completely absorbed by 48 hph. Mouth morphometry revealed that a gap in the mouth started to develop 12 hph, and the opening size of the mouth increased out to 72 h. These findings indicate that higher temperatures (32°C) lead to faster yolk absorption, causing embryos to deplete yolk reserves rapidly, potentially before full development and independent feeding. This results in a smaller body size and lower survival rates. Present study provides crucial insights for enhancing breeding practices and creating management protocols for hybrid catfish hatcheries during the period season of high temperature in the context of climate change.
1 INTRODUCTION
Rising temperatures from climate change are negatively affecting aquaculture, particularly fish hatching and development, through extreme weather events such as heat waves and droughts that damage production (Maulu et al., 2021). The planet has warmed by 1.1°C since the preindustrial era, and scientists warn that it could reach 4°C by 2100 (Jain et al., 2020). Higher temperatures are expected to shift climate zones, alter plant and animal distributions, increase mortality, reduce productivity, and damage infrastructure. These changes will affect phenology, behavior and life cycles, increasing pests and diseases, and diminishing agricultural, livestock and aquacultural yields, as well as essential ecosystem services (Islam et al., 2022; Makrinos & Bowden, 2016). In Thailand, changes in temperature and rainfall patterns are urgent issues that need to be addressed immediately. According to the Thai Meteorological Department (TMD), the average temperature has increased since 1951 (Sooksuwan, 2009). In 2021, the annual average temperature was 27.5°C, which was 0.4°C higher than that for the past 30-year period (Limsakul, 2020; Senapeng et al., 2022). The transition from El Niño to La Niña by 2024 is expected to cause higher temperatures, heat waves, delayed seasonal rains, and droughts (Hagen & Azevedo, 2024), heavily affecting non-irrigated areas, agriculture, related industries, and consumption. Extreme heat driven by the abnormally high surface temperature of the equatorial Pacific Sea is expected to peak at 42.0–44.5°C this year, which affects growth, reproduction, and yield of aquaculture animals (Ruby & Ahilan, 2018; TMD, 2024a; Vogel et al., 2019).
The massive loss of large freshwater fish in aquaculture sectors in Thailand has garnered attention because of its potential impacts on food shortages and increased prices of fish products. Temperature critically affects the development, survival, and physiological functions of aquatic organisms, particularly fish (Ruby & Ahilan, 2018). Temperature, a measure of the average kinetic energy linked to particle movement, is crucial for thermodynamic and chemical reactions, being the main abiotic factor affecting fish viability (Fry, 1958; Fry & Hart, 1948). Ectothermic organisms such as fish are strongly affected by increased temperature at the molecular level, such as in reaction rates and chemical bond stability, and at the macro level, such as in behaviors, including locomotion (Baldisserotto & Val, 2002; Little et al., 2020; Schulte, 2011). Hatching rates and embryonic development of fish are significantly affected by temperature, although there are large differences between species (Pereira et al., 2016; Radael et al., 2016; Thépot & Jerry, 2015). Temperature also affects growth performance, enzymatic activity, and metabolic rate in fish (Kamler et al., 1994; Qiang et al., 2019; Romanova et al., 2019). Therefore, controlling environmental temperature in aquaculture is crucial for maintaining species-specific breeding conditions and preventing abnormalities and low hatching rates (Arenzon et al., 2002; Tucker Junior et al., 2002).
One of the major fishery stocks of aquaculture in Thailand is the clariid catfish (Clariidae family), accounting for approximately 5.88% of the fish market. Of these, 95% are sterile hybrid catfish, which were firstly developed in 1987 by crossbreeding male North African catfish (Clarias gariepinus) and female bighead catfish (Clarias macrocephalus), which exponentially improved the productivity and meat quality of catfish (Lisachov et al., 2023, 2024; Nukwan, Lawanyawut, et al., 1990; Nukwan, Tangtrongpiros, et al., 1990; Ponjarat et al., 2019). The hybrid exhibits rapid growth, high adaptability to low-oxygen environments, wide distribution, tolerance to low water quality, and increased disease resistance (Lisachov et al., 2023). However, in April 2024, a massive die-off of hybrid catfish larvae and fry occurred in several aquaculture farms, when the temperature elevated to 37 to 41°C in the air and 30 to 32°C in freshwater in Thailand (TMD, 2024b). The effects of temperature on hatching rates and early larval development have been studied in various fish species. Studies on fish species other than catfish have shown that suboptimal temperatures cause delayed hatching, embryonic growth defect, and reduced survival rates (Arula et al., 2015; Kamler, 2002; Motta et al., 2023; Otterlei et al., 1999; Viader-Guerrero et al., 2021; Wen et al., 2013); however, the impacts of high temperature are less understood in hybrid catfish. To investigate the correlation between temperature and developmental defects of embryos in hybrid catfish, male North African catfish and female bighead catfish were mated and bred under different temperature conditions (29°C and 32°C), while keeping the conditions of water quality, feeding, and disease management constant, during the rainy season in May and June 2024. Hatching rates and mouth development and survival rates of larvae were examined. These findings provide valuable insights that can guide future enhancement and optimization of breeding practices and management protocols for hybrid catfish in fish hatcheries.
2 MATERIALS AND METHODS
2.1 Procurement of broodstocks and mating
Forty broodstocks of fish, comprising male North African catfish with a mean weight of 1.5 kg and female bighead catfish with a mean weight of 0.35 kg, all of which reached reproductive age (2 years on average), were procured from Yang Talat, Kalasin, Thailand (16°39′29.2″ N, 103°29′09.5″ E). All animal care and experimental procedures were approved by the Animal Experiment Committee of Kasetsart University, Thailand (approval no. ACKU65-SCI-003, ACKU66-SCI-006, and ACKU66-SCI-0014), in accordance with the Regulations on Animal Experiments at Kasetsart University and the ARRIVE guidelines (https://arriveguidelines.org).
Broodstocks were maintained at a density of five individuals per square meter. The ponds were equipped with inlet and outlet pipes to ensure water exchanges and maintain water quality (Komugisha & Rajts, 2021). The water quality standard was set with dissolved oxygen level above 4 mg/L, temperatures of 28 to 31°C, pH of 6.5 to 7.5, and ammonia (NH3) levels below 0.5 mg/L. The catfish were acclimatized for 2 weeks in the concrete tank facility of the farm and were fed Catfish Grower Feed 831, containing 30% protein (Betagro Public Company Limited, Nakhon Ratchasima, Thailand).
Ten male North African catfish and 30 female bighead catfish were randomly selected for this study. The broodstock was anesthetized with tricaine methanesulfonate (MS-222) at a concentration of 40 mg/L of water for 3 to 5 min to facilitate hormone injection (Park, 2019). The catfish were injected with a mixture of CinnaFact containing buserelin (CinnaGen Co., Tehran, Iran), a synthetic gonadotropin-releasing hormone (GnRH) analog that inhibits the release of luteinizing hormone (LH), follicle-stimulating hormone (FSH), and Dany, containing domperidone (Manx Healthcare, Warwick, United Kingdom), a dopamine antagonist that is used to treat gastrointestinal motility disorders and prevent nausea. This mixture was administered using a 23-gauge, 1-inch (0.60 × 25 mm2) hypodermic needle in muscle tissue in front of the dorsal fin at a dosage of 1 mL/kg body weight (Neiffer & Stamper, 2009). The catfish were maintained in four separate tanks according to their sex and species.
To synchronize ovulation, the bighead catfish were injected with the hormone mixture 2 h earlier than the North African catfish because of their latency periods of 10 h for the bighead catfish and 8 h for the North African catfish. North African catfish were sacrificed, and their testes were collected. The gonadosomatic index (GSI) was calculated using the formula: (gonad weight/total fish weight × 100) (Yoneda et al., 2013). Testicles were extracted from male North African catfish by making an incision in the abdomen, and then semen was collected. Semen was mixed with normal saline solution (0.9% NaCl) (A.N.B. Laboratories Co., Ltd., Bangkok, Thailand) at a ratio of 1 mL semen to 10 mL NaCl (Sarder et al., 2020; Uiuiu et al., 2017). Eggs were collected from female bighead catfish by gently squeezing the abdomen toward the cloaca. Fecundity was estimated using the gravimetric fecundity method: (eggs weight × number of eggs in the sample) ÷ weight of eggs (Eyo et al., 2013). The average GSI of North African catfish was 1.72 ± 0.75%. The fecundity per female bighead catfish was 15,264 ± 1637 eggs. Eggs and sperm were mixed at a ratio of 100 g eggs to 100 mL of sperm solution and stirred with a chicken feather for 1 min (Cook et al., 2023; Ochokwu et al., 2015). Subsequently, the sperm solution was discarded, and clay water was added to 500 mL and stirred for 1 min to remove the stickiness of the eggs, allowing them to hatch more easily in hatching jars (Siddique et al., 2016). The residual clay water was then discarded, and the eggs were rinsed with clean water for 1 min. This process was repeated two to three times. After fertilization, the eggs were incubated in jar-shaped containers with a flowing water system at a density of 45,000 eggs per container for 1 day. The eggs were incubated at temperatures of 29°C and 32°C, with dissolved oxygen level ranging from 6.5 to 7 mg/L, pH of 7 to 7.5, and NH3 level less than 0.1 mg/L.
After 24 h of incubation in plastic hatching jars filled with a volume of 2500 mL of fresh water and a flow rate of 3–4 L/min, 135,000 eggs were spread on floating trays made of polyvinyl chloride (PVC) pipe and hapa net. The tray was placed at the bottom in concrete tanks (180 × 250 cm, 20 cm depth) with aeration. The floating trays were used to facilitate the separation of egg shells and larvae. Egg shells were retained on the hapa net, while the larvae fell to the bottom of the pond. After 24 h, the floating trays were removed from the concrete tanks. Water quality was consistently monitored three times a day at 6:00 a.m., 2:00 p.m., and 9:00 p.m. The water used in the hatchery was processed through a recirculating aquaculture system (RAS) provided by the Betagro hatchery farm. The quality of water was maintained through a series of physical treatments such as separating sediment, chemical treatments involving ozonation, UV sterilization for disinfection, and biological treatments using a biofilter that supports the growth of nitrifying bacteria to convert ammonia into nitrate. Temperature was measured using a mercury-in-glass thermometer, whereas dissolved oxygen, ammonia, pH, and NH3 levels were measured using an AquaCare test kit (Maidenhead Aquatics, Maidenhead, United Kingdom) following the protocols. On the third day post-hatching, the larvae were fed live water flea (Moina sp.) and pelleted feed containing 30% protein. The pelleted feed was mixed with a small amount of water, crushed or ground, and fed to the larvae. The larvae were fed 25% of their body weight per day and reared for 7 days.
2.2 Observation of embryonic development
To examine fertilization rate, average egg diameter, and embryo development, egg samples were collected using 3/16-inch airline tubing. The eggs were then placed in a Petri dish and observed under an Olympus CX23LEDRFS1 microscope (Olympus, Tokyo, Japan) equipped with a Toupcam XP1080HB camera and using ToupLite software (ToupTek Photonics Co., Ltd., Hangzhou, China) for capturing images at 4× magnification. Three hundred eggs were sampled, which provided a sufficiently large sample size for an accurate and reliable estimation of the overall fertilization rate (Cardona et al., 2021; Paufve et al., 2020; Siddique et al., 2016). The fertilization rate was measured to assess the sperm capability of fertilization and calculated at the division stage using the following formula: (N − b) ÷ N × 100, where N represents the total number of eggs spawned, and b is the number of unfertilized eggs (opaque white) as obtained from the relation: y ÷ x × N, where x is the total number of eggs and y is the number of unfertilized eggs. The number of fertilized eggs, g, is determined as g = N–b (Olufeagba et al., 2016).
To determine the average egg diameter and observed embryonic development, 10 eggs were randomly collected from each incubator, placed on a petri dish and monitored continuously. The developmental stages of North African catfish have been previously documented by Olaniyi and Omitogun (2014) and Olufeagba et al. (2016). The hatching rate (%) was calculated using the formula: number of hatched eggs/total number of fertilized eggs × 100 (Viader-Guerrero et al., 2021). Time required for hatching was recorded from fertilization until 50% of the fertilized eggs hatched. The hatching rate was monitored every hour, and observation times were recorded once hatching began (Kamler, 2002). The survival rate (%) was calculated using the following formula: number of fish survived/number of fish released × 100 (Hassan et al., 2021). Ten larvae were examined daily to evaluate the yolk sac volume, the absorption of the yolk sac, and the observed embryonic development using an Olympus CX23LEDRFS1 microscope (Olympus, Tokyo, Japan) equipped with an additional Toupcam XP1080HB camera (ToupTek Photonics, Zhejiang, China). To evaluate the volume consumption of the yolk sac, the yolk sac volume (mm3) was calculated using the formula: 0.1667 π L A2 where L is the length of yolk sac (mm) and A is the height of yolk sac (mm) (Blaxter & Hempel, 1966). The differences in the statistics of yolk sac absorption, fertilization rate, hatching rate, and survival rate were examined using Welch's two-sample t-test implemented in the statistical software R version 3.4.3 and the “stats” package (R Core Team, 2023). The estimated values were expressed as mean ± standard deviation, and a significant level of 5% (p < 0.05) was defined as significant.
2.3 Observation of mouth development
Mouth development was observed for 12 hph embryos by monitoring the initial opening, frequency of mouth movements at 24, 48 and 72 hph, and morphological changes based on Reyes-Mero et al. (2022). Observations were made using an Olympus CX23LEDRFS1 microscope (Olympus, Tokyo, Japan) equipped with a Toupcam XP1080HB camera (ToupTek Photonics). Maximum mouth opening (MMO) was estimated using the methodology described in, following Shirota (1970), which considers a right angle. In this method, MMO was calculated as the maximum mouth length (MUL) × . This calculation assumed that the mouth opened at a right angle (90°), forming an isosceles right triangle with one side representing the mouth opening length (MUL). The hypotenuse of this triangle, obtained by multiplying the side length by , provided a precise estimation of MMO. The importance of MMO lies in its role in understanding the feeding ability and growth potential in fish larvae (Reyes-Mero et al., 2022). Statistical differences in yolk sac absorption, fertilization rate, hatching rate, and survival rate were examined using Welch's two-sample t-test implemented in the statistical software R version 3.4.3 and the “stats” package (R Core Team, 2023). The estimated values were expressed as mean ± standard deviation, and a significant level of 5% (p < 0.05) was defined as significant.
3 RESULTS
3.1 Overview of hybrid catfish embryo development
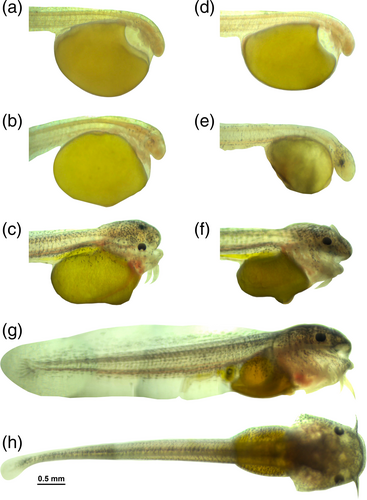
After fertilization, the pronuclei of the sperm and egg fused, and the egg yolk slightly shrank away from the membrane. The cortical cytoplasm moved toward the animal pole, forming the blastodisc. This zygote stage continued for about 20 min until the first cell cleavage began, where the one-cell eggs could be observed from the dorsal view (Figure 1a). The average diameter of fertilized eggs was 1.47 ± 0.04 mm. Embryos exhibited meroblastic cleavage, with cell divisions occurring only in the blastodisc region. This division was vertically oriented from the animal pole to the vegetal pole. At the first cleavage, two blastomeres were visible (Figure 1b). The cell continues to divide, forming four cells, eight cells, and 16 cells (Figure 1c–e). As divisions continued, the cell size decreased, forming multiple layers by the fifth division (Figure 1f). The morula stage featured a multicellular blastodisc at the animal pole (Figure 1g,h), progressing to the blastula stage where the blastocoel was formed (Figure 1i). During the gastrula stage, the blastoderm covered the yolk in epiboly, forming a germ ring and later the embryonic shield (Figure 1j–l). The blastoderm continued to grow, covering the yolk and forming the anterior and posterior buds by the end of epiboly. Somite formation began as paired blocks of cells, progressing in a cephalocaudal direction and contributing to the vertebral column and other tissues (Figure 1m,n). Tail flexion in the embryo is observed shortly before hatching (Figure 1o,p). This movement involves muscle contractions in the tail region, producing repetitive or jerking motions that help weaken the egg membrane from within, thereby allowing the embryo to emerge during hatching (Figure 1q). Key anatomical structures such as the aortic arch, eyes, fins, blood vessels, pericardial cavity, and brain parts developed during the primordial stage, with pigmentation appearing in the retina and dorsal skin.

Embryonic development accelerated at 32°C, resulting in shorter hatching times. Hatching began at 22–24 hpf (Table 1). Upon hatching, larvae lacked the ability to swim and sank to the bottom of the tank. By 24 h post-hatching, their swimming ability improved, but they mostly gathered in the bottom corner of the tank (Figure 1r). Hybrid catfish larvae bred at 29°C developed fully formed mouths with distinguishable jaws by 24 hph. In contrast, larvae bred at 32°C did not have fully open mouths by 24 hph, despite greater yolk sac utilization (71.4%), leading to nutritional deficiencies.
| Stage | Period (hours post-fertilization) | Description |
|---|---|---|
| Fertilization | 0 | Shrinkage of the yolk away from the perivitelline membrane. |
| First cleavage | 0–2 | The cytoplasm is concentrated at the anterior region, forming the animal pole or reddish blastodisc, where cell divisions occur. Cell division produced a larger number of smaller-sized cells. |
| Morula | 2–3.5 | Repeated cell divisions resulted in the formation of a multicellular blastodisc, where the cells became very small and densely packed at the animal pole. |
| Blastula | 3.5–5.5 | Small cells formed compact and spherical structures. Two layers of cells, the epiblast and hypoblast, were formed. |
| Gastrula | 5.5–8.5 | The gastrulation stage was indicated by the percentage of epiboly and the formation of the three germ layers: ectoderm, mesoderm, and endoderm. |
| Segmentation of somites | 8.5–22 | Somites were formed. The anterior–posterior axis became distinguishable. The cephalic portion became broader, and the embryonic rudiment became distinct with somites. The number of somites reached 22 to 25, and the yolk was well-formed. |
| Hatching | 22–24 | The wriggling movements increased as the chorion still covered the embryos, which was attempting to break through the chorion. |
3.2 Fertilization rate, hatching rate, and survival rate of hybrid catfish
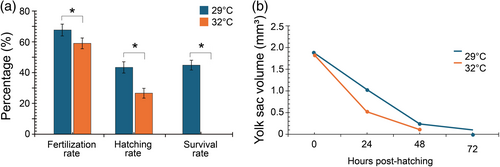
In egg incubation at 29°C, the average fertilization rate was 67.7 ± 3.9%. By contrast, the average fertilization rate decreased to 59.0 ± 3.5% at 32°C. Welch's two-sample t-test revealed a significant difference in fertilization rate between the two temperatures (p < 0.05) (Figure 2a). The average hatching rate was 43.4 ± 3.7% at 29°C, whereas it decreased to 26.6 ± 3.2% at 32°C. A significant difference was also seen in the hatching rate between the two temperatures (p < 0.05) by Welch's two-sample t-test (Figure 2a). At 29°C, the average survival rate was 44.9 ± 3.2% at 29°C; however, no larvae survived until the third day (72 hph) (Figure 2a).
3.3 Yolk sac absorption and month morphometry
At 29°C, as the stage progressed, the average yolk sac volume decreased from 1.873 ± 0.054 mm3 at 0 hph to 1.03 ± 0.10 mm3 at 24 hph, 0.24 ± 0.06 mm3 at 48 hph, and 0.10 ± 0.01 mm3 at 72 hph, with volume absorption percentages of 45.2%, 87.1%, and 94.5%, respectively. At 32°C, the average yolk sac volume also decreased from 1.83 ± 0.13 mm3 at 0 hph to 0.52 ± 0.05 mm3 at 24 hph, 0.11 ± 0.03 mm3 at 48 hph, and was undetectable at 72 hph. The volume absorption percentages were 71.36% at 24 h and 94.05% at 48 h (Figure 3a–h). The yolk sac was almost completely absorbed by 48 hph at 32°C, compared to 72 hph at 29°C (Figure 2b). The yolk sac volume was not significantly different between the two temperatures at 0 h (p > 0.05); however, higher temperatures significantly accelerated yolk sac absorption in larvae, particularly at 24, 48, and 72 hph (p < 0.05). At 0 hph, no mouths were formed in the larvae. The initial formation of the mouth gap was observed at 12 hph, and at 24 hph, the mouth was fully formed with distinguishable upper and lower jaws, and the mandible became larger than the maxilla (Figure 3a−f). At 29°C, MMO was 447.22 ± 7.27 μm at 24 hph, and it increased to 474.88 ± 8.19 μm at 48 hph and 546.80 ± 11.87 μm at 72 hph. MMO was 446.12 ± 9.09 μm at 24 hph at 32°C, increasing to 474.08 ± 5.47 μm at 48 hph and 545.32 ± 9.41 μm at 72 hph (Table 2). Welch's two-sample t-test revealed no significant differences in MMO between 29 and 32°C at all stages (24 hph, p = 0.901; 48 hph, p = 0.915; 72 hph, p = 0.898).
| Age (hph) | Jaw length in larvae bred at 29°C (μm) | Jaw length in larvae bred at 32°C (μm) | Maximum mouth opening (μm) | |||
|---|---|---|---|---|---|---|
| Upper | Lower | Upper | Lower | 29°C | 32°C | |
| 0 | Not yet formed | Not yet formed | Not yet formed | Not yet formed | Not yet formed | Not yet formed |
| 12 | Initial formation of a mouth gap | Initial formation of a mouth gap | Initial formation of a mouth gap | Initial formation of a mouth gap | Initial formation of a mouth gap | Initial formation of a mouth gap |
| 24 | 316.23 ± 5.14 | 331.81 ± 2.26 | 315.46 ± 6.43 | 330.16 ± 8.82 | 447.22 ± 7.27 | 446.12 ± 9.09 |
| 48 | 335.79 ± 5.79 | 372.96 ± 5.51 | 335.23 ± 3.38 | 371.11 ± 5.25 | 474.88 ± 8.19 | 474.08 ± 5.47 |
| 72 | 386.64 ± 8.39 | 414.86 ± 3.76 | 385.60 ± 6.65 | 415.47 ± 5.26 | 546.80 ± 11.87 | 545.32 ± 9.41 |
- Note: Values are represented as mean ± standard deviation.
4 DISCUSSION
The embryonic developmental stages from fertilization to hatching were examined in detail for the hybrids of male North African catfish and female bighead catfish. The one-cell stage at fertilization and first cleavage exhibited the typical developmental pattern of teleost embryos, which included pronuclei fusion, initial cytoplasmic movement, and formation of blastodisc. The cleavage stages exhibited meroblastic division in the blastodisc region as observed in other teleost species (Concha & Reig, 2022). The average diameter of fertilized eggs was 1.47 mm, ranging from 1.30 to 1.50 mm, similar to those of North African catfish (1.50 mm) and bighead catfish eggs (1.70 mm) (Behmene et al., 2022; Janssen et al., 1987; Morioka et al., 2013). From morula and blastula formation through the segmentation and pharyngeal stages, a developmental continuum culminated in larvae hatching at approximately 22–24 h post-fertilization. The larvae initially move passively and then improve their moving ability by 22 to 24 h post-fertilization as observed in various teleost species (Olaniyi & Omitogun, 2014). Embryonic development was accelerated at 32°C, resulting in shorter hatching time. Previous studies have shown that the optimal temperature for embryonic development of North African catfish ranges from 23 to 30°C (Nguenga et al., 2004). Temperature in excess of this range causes an increase of embryonic mortality and yolk absorption rates, suggesting that temperature critically influences of early survival of catfish embryos (Prokešová et al., 2015).
The fertilization rate, which is indicative of reproductive success and early embryonic viability, was significantly affected by temperature. At 29°C, the fertilization rate was significantly higher than at 32°C, suggesting that 32°C negatively affects fertilization. These findings are consistent with the results of Okunsebor et al. (2015), which showed the highest fertilization rate and hatching rate at 28 to 30°C for bagrid catfish (Heterobranchus bidorsalis), while a temperature of 32°C was correlated with the lowest hatching rate. Similarly, a higher temperature of 32°C also negatively affected the hatching rate (compared to 29°C) in the hybrid catfish in this study. This suggests that hybrid catfish are very sensitive and vulnerable to higher temperatures at an early stage of embryonic development. Additionally, the survival rate of hybrid catfish larvae was also significantly affected by temperature, with no individuals surviving until the third day post-hatching (72 h) at 32°C. This suggests that elevated temperatures have a detrimental effect on the post-hatching viability of fish larvae because survival rates at the early embryonic stage also substantially decrease in various fish species as water temperature rises (Hasan et al., 2023). Higher temperatures increase metabolic rates, leading to faster yolk utilization and a lack of energy storage that is required for proper development (Eme et al., 2015). Fertilization and hatching success rates are generally increased by moderate and stable temperatures. By contrast, extreme temperatures, whether too low or near tolerance limits, are associated with higher embryo mortality rates and developmental abnormalities (Nowosad et al., 2014).
Yolk sac absorption and mouth morphologies provide critical insights into hybrid catfish larval development, which is significantly influenced by temperature variations. The volume contraction of the yolk sac differed significantly between egg incubation at 29°C and 32°C. Rapid yolk sac absorption occurs 36 to 48 hph in striped catfish (Pangasianodon hypophthalmus), in which over 50% of total volume was decreased during this period (Subhan et al., 2018). However, higher temperatures significantly accelerated the yolk sac absorption by the larval stage in the hybrid catfish in this study. The yolk sac of the hybrid catfish was fully absorbed by 48 hph at 32°C. This suggests that higher temperatures (32°C) accelerated the transition from the absorption of yolk sac nutrients to spontaneous feeding and subsequent larval development (Melo et al., 2021).
Mouth morphometry showed that the hybrid catfish larvae bred at 29°C developed fully formed mouths with distinguishable jaws at 24 hph. The mouth slit is initially formed at 12 hph, and the supply of nutrients from the egg yolk is continuously required until the palate opens completely (Reyes-Mero et al., 2022). By contrast, in the larvae bred at 32°C, the mouth was not completely open by 24 hph, although the yolk sac utilization was greater than at 29°C, reaching 71.4%. This led to nutritional deficiencies in the larvae as a result of nearly complete yolk sac absorption. Despite being able to swim actively, the larvae faced difficulties in finding adequate food, which suggests a close link between rapid yolk sac absorption and premature mouth opening, leading to larval mortality because of nutritional deficiency (Reyes-Mero et al., 2022). No significant differences in MMO were observed between 29°C and 32°C at all stages measured. This collectively suggests that higher temperatures like 32°C lead to faster yolk absorption rates. This can cause embryos to deplete their yolk sac nutrients rapidly before they are fully developed and capable of independent feeding, resulting in smaller body sizes and lower survival rates. The MMO increased significantly, reaching 545–546 μm by 72 hph. This pattern aligns with previous findings, about the critical role of mouth development in prey capture during the first 3 days (Vu & Huynh, 2020). The development of a longer mandible than the maxilla allows the lower jaw to move more freely and to capture prey effectively (Kerdchuen & Legendre, 1994; Mukai et al., 2010).
When the hybrid catfish are produced by mating of male (North African catfish and female bighead catfish) from April to May in Thailand, the influence of high temperature at the larval stage should be considered because temperature fluctuates widely by climate change in this period. However, this study had several limitations. Firstly, the sample size was relatively small, which might affect the generalizability of the results. Secondly, the study was conducted under controlled laboratory conditions, which may not perfectly reproduce the natural environment. Lastly, the long-term effects of temperature variations on the growth and development of hybrid catfish larvae were not assessed. Therefore, further comprehensive research is required to resolve these issues. Further research on the metabolic reaction and adaptive responses of hybrid catfish larvae to varying environmental conditions will provide deeper insights into their early life history strategies. This information will greatly contribute to the promotion of aquaculture practices aimed at optimizing larval survival and growth rate of catfish larvae and fry.
5 CONCLUSIONS
The critical role of temperature in early development of hybrid catfish embryos has been underestimated by highlighting the vulnerability of embryos and larvae to thermal stress. The responses to heat stress in embryonal development, including accelerated yolk sac absorption at higher temperatures, result in faster yolk utilization. This rapid absorption of the yolk sac can cause embryos to deplete yolk reserves rapidly before full development, leading to smaller body size and lower survival rate. This study has emphasized the pivotal role of temperature in proceeding with the process of early development of hybrid catfish embryos, which provides crucial insights for aquaculture. These insights are particularly important under (future) climate change scenarios, in which temperature fluctuations challenge a harmful impact on fish reproduction and sustainability. Future research should explore the metabolic and adaptive mechanisms of hybrid catfish larvae under varying environmental conditions to promote their health and growth rates and to optimize aquaculture practices.
ACKNOWLEDGMENTS
We would like to express our deepest gratitude to the Phu Sing Research and Training Center at Kalasin University and the Kalasin Fish Hatchery Farm (Betagro) for providing parental stock of North African catfish and bighead catfish, as well as the essential experimental environment and equipment. Additionally, we also thank the Faculty of Science, Kasetsart University (6501.0901.1/336).
FUNDING INFORMATION
This research was financially supported in part by the National Research Council of Thailand (NRCT) (N42A650233) awarded to KSh; National Research Council of Thailand: High-Potential Research Team Grant Program (N42A660605) awarded to WS, TP, EK, KSj, SH, NM, AC, PD, JP, and KSh. The Program Management Unit for Human Resources and Institutional Development and Innovation (PMU-B) has granted a proposal entitled “Fostering Excellence: Empowering Post-Doctoral Researchers in Agriculture and Food through Cutting-Edge Genome Linkage Analysis. Elevating Aquatic Animal Breeding Capabilities and Pioneering Selection of High-Value Species for Thriving Economies in the Aquatic Animal Industry” under the Program of National Postdoctoral and Postgraduate System approved by PMU-B Board Committees (Contract No. B13F670053), which was awarded to JR, WS, TP, EK, KSj, SH, NM, AC, PD, JP, and KSh.; a Postdoctoral Researcher award at Kasetsart University awarded to TP and KSh; Thailand Scholarship (Scholarship number: MHESI 0202.3/10651) awarded to THDN and KSh; a grant from Kasetsart University Research and Development Institute (FF(KU)25.64 and FF(S-KU)17.66) awarded to WS and KSh; and a support from the International SciKU Branding (ISB), Faculty of Science, Kasetsart University awarded to WS and KSh. This research was also supported by Phu Sing Research and Training Center at Kalasin University and Betagro Fish Breeding Farm. No funding source was involved in the study design, collection, analysis, and interpretation of the data, writing of the report, or decision to submit the article for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Detailed methodological protocols, raw data, and any supplementary materials are available to ensure the reproducibility of the results presented in this study.




