The supplementation of nutrient additives in broad bean-based diet improved the growth of “crisped” grass carp, Ctenopharyngodon idellus
Funding information National Natural Science Foundation of China, Grant/Award Number: 31772825
Abstract
To investigate the effects of supplemental nutrient additives in broad bean-based diets on growth, flesh quality, and collagen gene expression of grass carp, five diets were prepared, including complete formula diet (control), soaked broad bean (SBB), and three broad bean-based diets containing 80% broad bean without (BBD1) or with the supplementation of methionine (BBD2), and methionine+vitamins+minerals (BBD3), and were fed to grass carp (171.9 ± 1.1 g), Ctenopharyngodon idellus, for 84 days. The results showed that broad bean-based diets significantly increased weight gain (WG) and reduced the feed conversion ratio (FCR) of grass carp when compared to the SBB (p < .05). The BBD3 group reached levels similar to the WG and FCR of the control group (p > .05). Grass carp fed the BBD3 diet had lower steaming loss of flesh, higher muscle fiber density, and higher collagen content in muscle and skin than the control (p < .05). The relative expressions of COL1A1 and COL1A2 mRNA in muscle and skin were significantly higher in the BBD3 and SBB groups than in other groups (p < .05). In conclusion, the combination of methionine, vitamins, and minerals in broad bean-based diets promoted the growth of “crisped” grass carp and improved flesh quality and collagen gene expression when compared to the control formula diet.
1 INTRODUCTION
Grass carp (Ctenopharyngodon idellus) is an important cultured freshwater fish in China, and its annual output reached 5.346 million tons in 2017 (Fisheries Administration Bureau of the Ministry of Agriculture and Rural Affairs, 2018). However, the low market value of the bulk products cannot provide an effective livelihood for grass carp farmers; thus, it is necessary to promote grass carp as a high-value featured product. Decades ago, farmers in Guangdong Province, China, accidentally found that the flesh characteristics of grass carp were greatly changed by feeding the fish broad bean (Vicia faba L.), which resulted in what was referred to as “crisped” grass carp (C. idellus C.et V). Crisped grass carp is very popular in Guangdong and Hongkong, China, for its special flesh quality, and its annual production is estimated to be about 50,000 tons. It was reported that the flesh hardness, chewiness, elasticity, and adhesiveness were significantly increased by dietary broad bean when compared with that of common grass carp (Lin, Zeng, & Zhu, 2009; Lin, Zeng, Zhu, & Song, 2012; Zhu, Ruan, Li, Meng, & Zeng, 2013). In addition, the length and diameter of muscle fibers in crisp grass carp became shorter, and the density became higher (Lin et al., 2009).
Collagen, the major component of connective tissues, has been demonstrated to be closely related to muscle structure, flexibility, and texture (Gordon & Hahn, 2010; Johnston et al., 2000). The high positive correlation between muscle hardness and collagen has been established (Gaston, Armida, Begona, Pedro, & Jose, 2003). It was found that the collagen content of muscle in crisp grass carp was significantly higher than that of common grass carp (An, Xu, Li, & Xiong, 2015; Liu et al., 2011; Zheng et al., 2016). The collagen superfamily of proteins contains at least 19 proteins formally defined as collagens (Prockop & Kivirikko, 1995), and the main form of intramuscular connective tissue of carp, lizardfish, Japanese eel, sturgeon, and spotted shark is type I collagen (Sato, Yoshinaka, Itoh, & Sato, 1989; Yata, Yoshida, Fujisawa, Mizuta, & Yoshinaka, 2001). Type I collagen has three peptide chains, two α1 chains and one α2 chain, that are encoded by COL1A1 and COL1A2 genes, respectively.
To obtain crisped flesh, it is necessary to feed grass carp with soaked broad bean (SBB) for 90–120 days. Obviously, it is hard work to soak broad bean and feed the fish manually every day, yet the fish usually show a lower growth than the common grass carp. Thus, a broad bean-based formula diet was developed, and grass carp fed the diet showed better growth performance than those fed SBB (Liang, 2017; Mao et al., 2014; Zheng et al., 2016). However, no study was conducted to investigate the effects of dietary nutrient additives on the crisped flesh of grass carp, including amino acids, vitamins, and minerals. Considering the fact that broad bean is the only feed source for crisped grass carp in practice, it is not clear if dietary nutrient additives would positively or negatively affect the flesh quality as their inclusion promotes the growth of grass carp.
Therefore, this study was conducted to investigate the effects of broad bean-based diets without or with the supplementation of amino acids, vitamins, and minerals on growth performance, flesh quality, and collagen gene expression of grass carp.
2 MATERIALS AND METHODS
2.1 Experimental diets and design
Five diets were prepared, including complete formula diet (control), SBB, and three broad bean-based formula diets. Broad bean-based diets contained 80% broad bean without (BBD1) or with the supplementation of methionine (BBD2) and methionine+vitamins+minerals (BBD3). As the content of crude protein in dried broad bean was about 28%, all formula diets were designed with a crude protein content of 28% (Table 1). In the preliminary trial, the maximum inclusion level of broad bean powder in the formula diet to form a stable pellet was found to be about 80%, and more inclusion would lead to the failure in pelleting. Besides, methionine was the most deficient amino acid (the first limiting amino acid) in the broad bean and BBD1 diet (Table 2), so methionine was supplemented in BBD2 and BBD3 to reach levels similar to the control diet.
| Ingredienta | Control | BBD1 | BBD2 | BBD3 | SBB |
|---|---|---|---|---|---|
| Soybean meal | 10.00 | 10.00 | 10.00 | 10.00 | |
| Cottonseed meal | 17.00 | ||||
| Rapeseed meal | 22.00 | ||||
| Rice bran | 14.55 | 10.00 | 9.60 | 7.35 | |
| Wheat middling | 34.00 | ||||
| Choline chloride | 0.50 | 0.50 | |||
| Vitamin premixb | 0.25 | 0.25 | |||
| Mineral premixc | 0.30 | 0.30 | |||
| Monocalcium phosphate | 1.20 | 1.20 | |||
| Microcapsuled lysine | 0.20 | ||||
| Microcapsuled methionine | 0.40 | 0.40 | |||
| Broad bean | 80.00 | 80.00 | 80.00 | 100.00 | |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Proximate composition | |||||
| CP | 27.45 | 27.87 | 27.69 | 27.42 | 27.93 |
| EE | 2.52 | 2.07 | 2.38 | 2.24 | 1.72 |
| Ash | 4.92 | 4.59 | 4.56 | 4.36 | 3.88 |
| Moisture | 7.52 | 7.06 | 7.86 | 8.44 | 7.28 |
- Abbreviations: BBD, broad bean-based diet; CP, crude protein; EE, ether extract; SBB, soaked broad bean.
- a The protein contents of ingredients are as follows: soybean meal (44.2%), cottonseed meal (50.0%), rapeseed meal (37.7%), rice bran (14.3%), and wheat middling (16.9%).
- b Vitamin premix (mg or IU/kg diet): vitamin A 10,000 IU, vitamin D3 3,000 IU, vitamin E 150 IU, vitamin K3 12.17 mg, vitamin B1 20 mg, vitamin B2 20 mg, vitamin B3 100 mg, vitamin B6 22 mg, vitamin B12 0.15 mg, vitamin C 1,000 mg, biotin 0.6 mg, folic acid 8 mg, and inositol 500 mg.
- c Mineral premix (mg/kg diet): I 1.5 mg, Co 0.6 mg, Cu 3 mg, Fe 63 mg, Zn 89 mg, Mn 11.45 mg, Se 0.24 mg, and Mg 180 mg.
| Amino acid | Control | BBD1 | BBD2 | BBD3 | SBB |
|---|---|---|---|---|---|
| EAA | |||||
| Thr | 0.907 | 0.689 | 0.692 | 0.698 | 0.670 |
| Val | 0.840 | 0.735 | 0.715 | 0.777 | 0.662 |
| Met | 0.470 | 0.119 | 0.431 | 0.410 | 0.040 |
| Ile | 0.538 | 0.436 | 0.432 | 0.443 | 0.443 |
| Leu | 1.861 | 1.770 | 1.759 | 1.733 | 1.834 |
| Phe | 1.304 | 1.134 | 1.172 | 1.148 | 1.097 |
| His | 1.237 | 1.165 | 1.227 | 1.197 | 1.184 |
| Lys | 1.266 | 1.441 | 1.385 | 1.401 | 1.438 |
| Arg | 2.050 | 2.197 | 2.194 | 2.260 | 2.312 |
| NEAA | |||||
| Asp | 2.932 | 3.246 | 3.211 | 3.208 | 3.330 |
| Ser | 1.628 | 1.527 | 1.659 | 1.553 | 1.646 |
| Glu | 5.270 | 3.980 | 4.112 | 4.031 | 4.050 |
| Gly | 1.344 | 1.042 | 1.030 | 1.082 | 1.077 |
| Ala | 1.191 | 1.106 | 1.087 | 1.065 | 1.020 |
| Cys | 0.273 | 0.166 | 0.172 | 0.171 | 0.112 |
| Tyr | 0.663 | 0.617 | 0.581 | 0.679 | 0.639 |
| Pro | 1.023 | 0.800 | 0.803 | 0.871 | 0.715 |
| TAA | 24.797 | 22.170 | 22.662 | 22.727 | 22.269 |
- Abbreviations: BBD, broad bean-based diet; EAA, essential amino acids; NEAA, nonessential amino acids; SBB, soaked broad bean; TAA, total amino acids.
Broad bean was produced in Hubei Province, China, and contained 27.93% crude protein, 7.28% moisture, and 1.72% crude lipid (Table 1). In the SBB group, broad bean was immersed in pure water for 24 hr to make the bean soft, and it then cut into small blocks to feed the fish directly. In the four groups of formula diets, ingredients were ground and sifted out using a 40-μm sieve and then weighed and mixed with water to form a homogeneous mixture. The mixture was pelleted through a 2.5-mm die with a plane-die granulator (HKJ-218, Hua-ming Mechanical Factory, Wuxi, Jiangshu province, China). The pelleting temperature was 80–85°C. The pellets were air-dried immediately and then stored in a cool dry space. The formulation and proximate composition of the experimental diets are shown in Table 1, and the amino acid composition is shown in Table 2.
2.2 Experimental fish and feeding management
The grass carp were obtained from Qingpu Aquaculture Farm (Shanghai, China). After transportation to the laboratory, grass carp were fed with the basal diet for 7 days to acclimate to the environment. A total of 225 grass carp with an initial body weight of 171.9 ± 1.1 g were randomly allocated to 15 cages (1.0 × 3.0 × 1.2 m) with a water depth of 1.0 m and 15 fish per cage. The cages were located in five indoor cement pools (5.0 × 3.0 × 1.2 m), and each pool contained three cages. The feeding trial was conducted at the Aquaculture Station of Shanghai Ocean University (Shanghai, China) for 84 days.
During the feeding period, fish were fed manually thrice a day (7:30 a.m., 12:00 p.m., 5:00 p.m.), with a daily feeding rate of about 3% of body weight. The feed intake was adjusted according to water temperature and feeding behavior to ensure no feed residue was left in the cage within 15 min of feeding. The waste in pools was cleared by siphoning every 5 days, and about one-third of cultured water was exchanged with pond water after filtration and dark sedimentation. The whole system used natural light without direct sunshine on the pools. Dissolved oxygen, temperature, pH, ammonia nitrogen, and nitrite of water were ≥5 mg/L, 25–30°C, 7.5–8.0, ≤0.2 mg/L, and ≤0.1 mg/L, respectively.
2.3 Sample collection
At the end of the feeding trial, grass carp were deprived of diets for 24 hr and then counted and weighed to calculate weight gain (WG), feed conversion ratio (FCR), and survival. Three fish per cage were randomly selected for individual measurement of body weight and body length and then dissected to weigh the visceral, liver, and intestinal fat to calculate the condition factor (CF), hepatosomatic index (HSI), viscerosomatic index (VSI), and intraperitoneal fat (IPF).
The skin and ventral muscle below the lateral line were sampled from the left side of the body and stored at −80°C for analysis of proximate composition, amino acid, and collagen contents. The ventral flesh of the other side was sampled to determine the immediate water-holding capacity, and the flesh sample (0.5 × 0.5 × 2.0 cm) for histological preparation was also collected from the same side and stored in Bouin's Fluid (saturated picric acid solution: methanol: acetic acid = 15:5:1).
Another three fish were randomly selected from each cage, and their skin, muscle, and liver were sampled and quickly frozen with liquid nitrogen and then stored at −80°C to detect collagen gene expression.
2.4 Measurement indicators and methods
2.4.1 Growth performance and physical indices
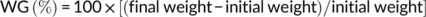
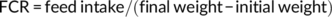
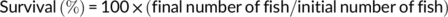
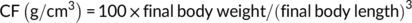
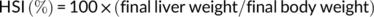
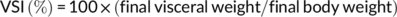

2.4.2 Proximate composition of muscle and diets
The moisture content was estimated by drying the samples to a constant weight at 105°C in a drying oven. The crude protein content was estimated using the Kjeldahl system method (2300 Auto-analyzer; FOSS, Sweden). The ether extract content was determined after ether extraction using Soxtherm (Sox 416 Macro, Gerhardt, Germany). The ash content was determined by combusting samples in a muffle furnace at 550°C for 6 hr.
The amino acid analysis of diets and flesh was performed using an automatic amino acid analyzer (S-433D, Sykam, Germany) after hydrolysis with 6 M HCl (adding 1 g/L phenol) for 24 hr at 110°C. For methionine (Met) analysis, the samples were hydrolyzed in 2 mL performic acid for 15 min at 55°C.
2.4.3 Determination of collagen content
The collagen content was calculated by multiplying the hydroxyproline (Hyp) content (% of sample) by eight as in the method provided by the Association of Official Analytical Chemists (2000). Total Hyp content in tissues was measured by kits (A030-2, Jian-cheng Bioengineering Institute, China). The ultraviolet spectrophotometer (UV759, Shanghai Precision Scientific Instrument, China) was used for measuring Hyp content at 560 nm wavelength.
2.4.4 Water-holding capacity of flesh
Steaming loss: A block of flesh (about 3 g) (W1) was placed on a gauze and steamed at 100°C for 5 min, and then, absorbing paper was used to remove surface water of the flesh, which was then weighed (W2).
Centrifuge loss: A block of flesh (about 3 g) (W1) was placed in a centrifuge tube and centrifuged at 5,000 r/min for 10 min, and then, absorbing paper was used to remove surface water of the flesh and weighed (W2).
Thawing loss: A block of flesh (about 3 g) (W1) was frozen at −20°C for 24 hr and then thawed at room temperature. After removing surface water with the absorbing paper, the flesh was weighed (W2).
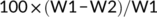
2.4.5 Muscle fiber density and diameter
Muscle tissues were gradually dehydrated, paraffin-embedded, and sectioned (7 μm; RM2245, Leica, Germany) and then stained with hematoxylin–eosin to evaluate muscle morphology. Muscle tissue images were captured by an optic microscope equipped with a DS-Ri1 camera (80i, Nikon, Japan). Spot Advance software (Spot Imaging Inc., Sterling Heights, MI) and ImagePro Plus software (Meyer Instruments, Houston, TX) were used to identify muscle fiber characteristics, including fiber density and diameter.
2.4.6 Expression of collagen genes
Real-time quantitative polymerase chain reaction (PCR) primers of COL1A1 and COL1A2 were designed according to reports by Liu et al. (2012) and Yu et al. (2014). Primers of the grass carp reference gene were designed based on the 18srRNA (Table 3). The real-time quantitative PCR analysis was performed using the SYBR® Premix Ex Taq (Perfect Real Time) kit (TaKaRa, Japan) following official instructions. The total reaction volume was 20 μL, which included 10 μL of SYBR® Premix ExTaq™ (Tli RNaseH Plus, TaKaRa, Japan), 0.5 μL of the upstream primer (10 μmol/L), 0.5 μL of the downstream primer (10 μmol/L), 0.5 μL of ROX Reference DyeII (50X), 2 μL of the cDNA template, and 6.5 μL of ddH2O. The real-time quantitative PCR was conducted using CFX96 Touch real-time PCR (Bio-Rad), and its conditions were as follows: denaturing at 95°C for 30 s and 40 cycles of denaturing at 95°C for 5 s, annealing at 60°C for 30 s, and extension at 72°C for 1 min. The melting curve was created after the extension (60–95°C, 30 s, the plate was read every 0.5°C).
| Primer | GenBank accession no. | Sequence (5′-3′) | Application |
|---|---|---|---|
| 18s-F | EU047719.1 | GGAATGAGCGTATCCTAAACCC | qRT-PCR |
| 18s-R | CTCCCGAGATCCAACTACAAGC | ||
| COL1A1-F | HM363526.1 | ACGCACACAAACAATCTCAAGT | |
| COL1A1-R | GCATGGGGCAAGACAGTCA | ||
| COL1A2-F | HM587241.1 | ACTCCGATAGAGCCCAGCTT | |
| COL1A2-R | ACATTGGTGGCGCAGATCA |
- Abbreviation: qRT-PCR, quantificational real-time polymerase chain reaction.
The samples of the control group were used for calibration, and the relative expressions of COL1A1 and COL1A2 mRNA in muscle, skin, and liver were calculated using the 2−△△Ct method.
2.5 Statistical analysis
The data were presented as (mean ± SD), and statistical analysis was performed using SPSS 22.0 statistical software (Chicago, IL). All data were subjected to one-way analysis of variance. If significant differences were detected (p < .05), Duncan's multiple range test was applied.
3 RESULTS
3.1 Growth performance
During the feeding period, no mortality was recorded. Grass carp fed the control and SBB diets showed the highest and lowest WG among groups, respectively. Compared with the SBB group, BBD1, BBD2, and BBD3 diets significantly increased WG and decreased FCR (p < .05). Especially in the BBD3 group, the WG and FCR reached similar levels to the control group (p > .05). HSI, VSI, and IPF of BBD1, BBD2, and SBB groups were significantly lower (p < .05) than those of the BBD3 and the control group. CF showed no significant difference among all treatments (p > .05) (Table 4).
| Indicator | Control | BBD1 | BBD2 | BBD3 | SBB |
|---|---|---|---|---|---|
| Initial body weight (g) | 172.56 ± 0.42 | 171.69 ± 0.73 | 172.61 ± 0.21 | 172.21 ± 0.43 | 172.13 ± 0.39 |
| Final body weight (g) | 337.52 ± 5.15a | 252.44 ± 5.64c | 268.97 ± 3.00b | 334.22 ± 3.45a | 220.28 ± 3.18d |
| WG (%) | 95.59 ± 3.11a | 47.03 ± 3.14c | 55.82 ± 1.80b | 94.08 ± 1.91a | 27.97 ± 1.69d |
| FCR | 2.64 ± 0.11d | 3.82 ± 0.16b | 3.61 ± 0.14c | 2.66 ± 0.19d | 6.54 ± 0.12a |
| Survival (%) | 100 | 100 | 100 | 100 | 100 |
| HSI (%) | 2.35 ± 0.18a | 1.72 ± 0.12c | 1.93 ± 0.16b | 2.22 ± 0.08a | 1.62 ± 0.15c |
| VSI (%) | 6.98 ± 0.32a | 6.26 ± 0.28b | 6.54 ± 0.36b | 6.82 ± 0.31a | 5.98 ± 0.21c |
| IPF (%) | 1.53 ± 0.15a | 0.68 ± 0.06b | 0.71 ± 0.07b | 1.52 ± 0.15a | 0.64 ± 0.07b |
| CF (g/cm3) | 1.79 ± 0.10 | 1.76 ± 0.12 | 1.83 ± 0.13 | 1.78 ± 0.08 | 1.80 ± 0.10 |
- Note: Values in the same row with different letters are significantly different (p < .05).
- Abbreviations: BBD, broad bean-based diet; CF, condition factor; FCR, feed conversion ratio; HSI, hepatosomatic index; IPF, intraperitoneal fat; SBB, soaked broad bean; VSI, viscerosomatic index; WG, weight gain.
3.2 Muscle composition
As shown in Table 5, there were no significant differences in muscle crude protein and ash content among groups (p > .05). The BBD1, BBD2, and SBB groups showed significantly lower moisture and EE contents than the control group (p < .05).
| Composition | Control | BBD1 | BBD2 | BBD3 | SBB |
|---|---|---|---|---|---|
| Moisture | 78.48 ± 0.50a | 77.47 ± 0.63b | 77.27 ± 0.86b | 77.87 ± 0.68ab | 77.37 ± 0.83b |
| CP | 18.30 ± 1.38 | 18.99 ± 0.62 | 18.97 ± 1.23 | 18.95 ± 0.78 | 18.87 ± 0.74 |
| EE | 1.71 ± 0.11a | 1.44 ± 0.15b | 1.46 ± 0.09b | 1.65 ± 0.07a | 1.46 ± 0.12b |
| Ash | 1.12 ± 0.04 | 1.17 ± 0.03 | 1.14 ± 0.07 | 1.20 ± 0.06 | 1.15 ± 0.03 |
- Note: Values in the same row with different letters are significantly different (p < .05).
- Abbreviations: BBD, broad bean-based diet; CP, crude protein; EE, ether extract; SBB, soaked broad bean.
3.3 Flesh water-holding capacity
Compared with the control group, steaming loss of the other four groups significantly decreased (p < .05), while SBB group showed the lowest value (p < .05). The SBB group also showed significantly lower centrifuge loss than the control group (p < .05). There was no significant difference in thawing loss among all treatments (p > .05) (Table 6).
| Indicator | Control | BBD1 | BBD2 | BBD3 | SBB |
|---|---|---|---|---|---|
| Steaming loss | 22.91 ± 1.15a | 21.79 ± 1.17b | 21.42 ± 0.78b | 21.85 ± 0.97b | 19.82 ± 0.98c |
| Centrifuge loss | 7.78 ± 0.36a | 7.47 ± 0.63ab | 7.39 ± 0.53ab | 7.42 ± 0.51ab | 7.10 ± 0.30b |
| Thawing loss | 4.56 ± 0.37 | 4.69 ± 0.46 | 4.77 ± 0.41 | 4.79 ± 0.31 | 4.81 ± 0.20 |
- Note: Values in the same row with different letters are significantly different (p < .05).
- Abbreviations: BBD, broad bean-based diet; SBB, soaked broad bean.
3.4 Muscle amino acid composition
As shown in Table 7, the contents of total amino acids (TAA), total essential amino acids (TEAA), and total nonessential amino acids (TNEAA) showed no significant differences among groups (p > .05). Compared with the control group, the Val, Met, Ile contents of the BBD1 group and the Val, Met contents of the SBB group significantly decreased (p < .05).
| Amino acid | Control | BBD1 | BBD2 | BBD3 | SBB |
|---|---|---|---|---|---|
| EAA | |||||
| Thr | 3.20 ± 0.17 | 3.13 ± 0.28 | 3.33 ± 0.25 | 3.10 ± 0.08 | 3.15 ± 0.31 |
| Val | 2.68 ± 0.16a | 2.41 ± 0.11b | 2.60 ± 0.14ab | 2.59 ± 0.07ab | 2.42 ± 0.13b |
| Met | 1.72 ± 0.13a | 1.50 ± 0.07b | 1.67 ± 0.02a | 1.57 ± 0.13ab | 1.45 ± 0.09b |
| Ile | 2.04 ± 0.16a | 1.60 ± 0.09b | 1.93 ± 0.17a | 1.95 ± 0.04a | 1.90 ± 0.06a |
| Leu | 6.72 ± 0.30 | 6.56 ± 0.33 | 6.67 ± 0.59 | 6.56 ± 0.19 | 6.70 ± 0.41 |
| Phe | 3.34 ± 0.11ab | 3.14 ± 0.12b | 3.51 ± 0.27a | 3.31 ± 0.06ab | 3.32 ± 0.22ab |
| His | 4.20 ± 0.04 | 4.25 ± 0.29 | 4.25 ± 0.21 | 4.10 ± 0.20 | 4.36 ± 0.12 |
| Lys | 7.30 ± 0.29 | 6.95 ± 0.45 | 7.47 ± 0.53 | 7.00 ± 0.19 | 7.50 ± 0.45 |
| Arg | 4.11 ± 0.29ab | 3.71 ± 0.25b | 4.33 ± 0.26a | 4.44 ± 0.33a | 4.49 ± 0.25a |
| TEAA | 35.33 ± 1.54 | 33.77 ± 1.94 | 35.65 ± 2.94 | 34.62 ± 0.44 | 35.14 ± 2.05 |
| NEAA | |||||
| Asp | 9.55 ± 0.80 | 9.06 ± 0.47 | 8.93 ± 0.18 | 9.39 ± 0.31 | 9.62 ± 0.62 |
| Ser | 3.89 ± 0.08ab | 3.75 ± 0.08b | 4.00 ± 0.21a | 3.85 ± 0.13ab | 3.86 ± 0.08ab |
| Glu | 12.61 ± 0.72 | 12.79 ± 0.47 | 12.68 ± 0.88 | 12.22 ± 0.36 | 12.64 ± 0.81 |
| Gly | 3.62 ± 0.15 | 3.52 ± 0.08 | 3.93 ± 0.32 | 3.68 ± 0.10 | 3.54 ± 0.35 |
| Ala | 5.05 ± 0.17ab | 4.82 ± 0.07b | 5.21 ± 0.23a | 4.88 ± 0.18ab | 5.06 ± 0.16ab |
| Cys | 0.46 ± 0.03ab | 0.42 ± 0.03b | 0.47 ± 0.02a | 0.44 ± 0.02ab | 0.41 ± 0.04b |
| Tyr | 2.31 ± 0.10 | 2.23 ± 0.04 | 2.38 ± 0.14 | 2.32 ± 0.05 | 2.34 ± 0.18 |
| Pro | 1.68 ± 0.11 | 1.69 ± 0.05 | 1.81 ± 0.16 | 1.66 ± 0.03 | 1.71 ± 0.17 |
| TNEAA | 39.17 ± 1.88 | 38.28 ± 1.16 | 40.06 ± 2.21 | 38.43 ± 0.96 | 39.17 ± 2.37 |
| TAA | 74.50 ± 3.42 | 72.05 ± 2.95 | 75.71 ± 4.13 | 73.05 ± 1.39 | 74.32 ± 4.41 |
- Note: Values in the same row with different letters are significantly different (p < .05).
- Abbreviations: BBD, broad bean-based diet; EAA, essential amino acids; NEAA, nonessential amino acids; SBB, soaked broad bean; TAA, total amino acids; TEAA, total essential amino acids; TNEAA, total nonessential amino acids.
3.5 Muscle fiber density and diameter
As shown in Table 8, grass carp fed broad bean-based diets and SBB showed significantly higher muscle fiber density and lower muscle fiber diameter than those of the control group (p < .05). The SBB, BBD1, and BBD2 groups shared similar muscle fiber density and diameter indices, and the three groups showed higher fiber density and lower fiber diameter than the BBD3 group (p < .05).
| Group | Muscle fiber density (number/mm2) | Muscle fiber diameter (μm) |
|---|---|---|
| Control | 203.9 ± 12.2c | 79.1 ± 2.3a |
| BBD1 | 346.0 ± 11.3a | 60.9 ± 1.0c |
| BBD2 | 344.9 ± 20.6a | 60.8 ± 1.8c |
| BBD3 | 268.4 ± 13.8b | 68.9 ± 1.8b |
| SBB | 360.6 ± 21.7a | 59.5 ± 1.8c |
- Note: Values in the same column with different letters are significantly different (p < .05).
- Abbreviations: BBD, broad bean-based diet; SBB, soaked broad bean.
3.6 Collagen contents in muscle, skin, and liver
The collagen contents in muscle and skin of grass carp fed broad bean-based diets and SBB were significantly higher than those of fish fed the control diet (p < .05), while the BBD3 and SBB groups showed significantly higher collagen content in muscle than the BBD1 and BBD2 groups (p < .05). The BBD3 group had the highest collagen content in skin. There was no significant difference in liver collagen content among all treatments (p > .05) (Table 9).
| Group | Muscle | Skin | Liver |
|---|---|---|---|
| Control | 1.71 ± 0.09c | 16.68 ± 0.65c | 3.38 ± 0.24 |
| BBD1 | 1.87 ± 0.09b | 18.05 ± 0.34b | 3.64 ± 0.31 |
| BBD2 | 1.88 ± 0.07b | 18.06 ± 0.62b | 3.62 ± 0.27 |
| BBD3 | 2.04 ± 0.12a | 18.80 ± 0.69a | 3.53 ± 0.30 |
| SBB | 2.07 ± 0.09a | 18.08 ± 0.27b | 3.65 ± 0.34 |
- Note: Values in the same column with different letters are significantly different (p < .05).
- Abbreviations: BBD, broad bean-based diet; SBB, soaked broad bean.
3.7 The expression of COL1A1 and COL1A2 mRNA in tissues
As shown in Table 10, the expression of COL1A1 and COL1A2 mRNA in the muscle and skin of grass carp fed broad bean-based diets and SBB were significantly higher than those of the control (p < .05). The BBD3 and SBB groups showed significantly higher expression in muscle than the BBD1 and BBD2 groups (p < .05). However, there were no significant differences in the two gene expressions in liver among all treatments (p > .05).
| Group | Muscle | Skin | Liver | |||
|---|---|---|---|---|---|---|
| COL1A1 | COL1A2 | COL1A1 | COL1A2 | COL1A1 | COL1A2 | |
| Control | 1.00 ± 0.03c | 1.00 ± 0.10c | 1.00 ± 0.08b | 1.00 ± 0.03c | 1.00 ± 0.04 | 1.00 ± 0.09 |
| BBD1 | 1.52 ± 0.06b | 1.34 ± 0.12b | 1.32 ± 0.08a | 1.18 ± 0.08b | 0.95 ± 0.08 | 0.94 ± 0.08 |
| BBD2 | 1.62 ± 0.12b | 1.40 ± 0.11b | 1.24 ± 0.06a | 1.16 ± 0.07b | 0.93 ± 0.08 | 0.99 ± 0.10 |
| BBD3 | 2.75 ± 0.13a | 2.04 ± 0.18a | 1.33 ± 0.05a | 1.36 ± 0.11a | 0.97 ± 0.04 | 1.01 ± 0.08 |
| SBB | 2.60 ± 0.08a | 1.90 ± 0.11a | 1.29 ± 0.10a | 1.28 ± 0.09ab | 0.99 ± 0.04 | 1.06 ± 0.10 |
- Note: Values in the same column with different letters are significantly different (p < .05).
- Abbreviations: BBD, broad bean-based diet; SBB, soaked broad bean.
4 DISCUSSION
Previous studies have shown that feeding grass carp with broad bean reduces the growth performance, including decreased WG and increased FCR (Bie et al., 2012; Li et al., 2008; Li, Leng, Li, & Li, 2008; Liang, 2017; Zheng et al., 2016). In this experiment, the growth performance of grass carp fed SBB was significantly lower than that of the control fish. Results may be because of poor amino acid balance (Gan, Guo, Rong, & Xiong, 2010; Lin et al., 2012; Liu et al., 2011), high carbohydrate content, and low lipid content in broad bean (Wu, Lin, Bian, Chen, & Xia, 2015), which could not meet the nutritional needs of grass carp. In addition, broad bean contains some antinutritional factors, such as tannins, lectins, protease inhibitors, and phytic acid (Francis, Makkar, & Becker, 2001). These antinutritional factors reduced the activity of digestive enzymes (Li, Leng, Li, Liu, et al., 2008; Zheng et al., 2016) and thus negatively affected the digestion and absorption of nutrients. Moreover, broad bean was reported to induce intestinal inflammation in grass carp (Li et al., 2018). In the present trial, grass carp fed SBB were found to consume the diet slowly, and some fish even vomited after eating during the feeding period. After 24-hr soaking, the broad bean would be softened but tasted a little puckery, which might reduce the palatability.
Broad bean has a very low content of methionine (Table 2), which makes methionine the first limiting amino acid. The three broad bean-based diets in this study contained 80% broad bean powder, and the formula of BBD1 was very simple: only 80% broad bean powder and 20% soybean meal and rice bran. The latter two had a much higher methionine content than that of the broad bean diet. Therefore, BBD1 diet showed a higher methionine content than that of the broad bean diet (Table 2), but it was still insufficient to support normal growth of grass carp. For this reason, the WG of fish fed BBD1 diet was higher than that of fish fed SBB but lower than that of fish fed the complete formula diet. After the supplementation of methionine (BBD2) and methionine+vitamins+minerals (BBD3) in BBD1 diet, the nutrient profiles were balanced, and the feed palatability was improved; thus, the WG and feed utilization were further improved, and the BBD3 group reached a level similar to that of the control group. In Mao et al. (2014), grass carp fed the formula diet containing 80% broad bean did not show any difference in growth performance compared to the control fish. However, Zheng et al. (2016) found that the WG of grass carp fed the formula diet containing 75% broad bean was significantly lower than that of fish fed the normal diet. The different results might be related to the body size of grass carp used in the present study (172 g) and in the studies of Mao et al. (860 g) and Zheng et al. (2016) (59 g). In general, large-sized grass carp have a low nutrient requirement and a high tolerance for antinutritional factors.
Liang (2017) reported no significant difference in muscle moisture, crude protein, and ash content when grass carp were fed with SBB, broad bean-based diet, and complete formula diet, but the crude lipid content of the latter two groups was significantly reduced. In the study of Zheng et al. (2016), the muscle moisture content of grass carp fed SBB and the broad bean-based diet was significantly higher than that of control fish, whereas the crude protein content of the SBB group was significantly lower than that of the broad bean-based diet and the control group. In this study, muscle moisture and crude lipid contents of BBD1, BBD2, and SBB groups were significantly lower than those of the control group. The flesh quality showed a certain correlation with crude lipid content (Johnston et al., 2000). In general, the decrease of muscle crude lipid content increases the friction among muscle bundles and decreases the tenderness of muscle, thus increasing the chewiness of the flesh (Grigorakis & Alexis, 2005). The present study and the study of Zheng et al. (2016) also showed a reduced muscle crude fat in fish fed SBB and broad bean-based diets. However, in Mao et al. (2014), the broad bean-based diet increased the muscle crude fat content of grass carp. When feeding grass carp with broad bean for 100 days, the lipid content of muscle first decreased (0–20 day), then increased over 20–60 days, and decreased after that (An et al., 2015), which demonstrated that the flesh embrittlement needs a long time to reduce the lipid content. These might help to explain the different results of muscle lipids among the studies of Mao et al. (2014) (860 g, 20 weeks), Zheng et al. (2016) (59 g, 8 weeks), and the present study (172 g, 12 weeks).
Gan et al. (2010) found that the main limiting amino acids in the flesh of crisped grass carp were tryptophan and methionine+cystine. TAA (Bie et al., 2012) and TEAA (Zhu, Li, Ruan, Meng, & Zeng, 2008) contents in the flesh of crisped grass carp were reported to be higher than those of common grass carp, but the study of Zheng et al. (2016) did not find differences in TAA between the two fishes, and the study of Liu et al. (2011) indicated that TAA, TEAA, and flavor amino acid content were decreased by feeding broad bean. In this experiment, the TAA, TEAA, and TNEAA content of grass carp showed no significant difference among groups.
Water-holding capacity is one of the main characteristics for measuring muscle quality (Brinker & Reiter, 2011), which affects the quantification and characterization of muscle and meat products (Kauffman, 1986). Lower water loss means more retention of water and soluble materials in flesh during storing and cooking. The studies of Mao et al. (2014) and Zheng et al. (2016), together with the present study, showed that grass carp fed SBB and broad bean-based diets had a lower steaming loss than fish fed a normal diet.
Crisped grass carp were reported to have a larger muscle fiber diameter (Li, Leng, Li, & Li, 2008; Lun, Leng, Li, & Li, 2008) and tighter muscle fiber arrangements (Li, Leng, Li, & Li, 2008) than did the common grass carp. The chewiness and hardness of flesh showed a positive correlation with muscle fiber density (Johnston et al., 2000). Fish muscle growth has two means of hyperplasia and hypertrophy (Egginton & Johnston, 1982). Hyperplasia was an increase in the number of muscle fibers, and hypertrophy was an increase in the diameter of muscle fibers. The hyperplasia usually continues until the fish grows to 40% of its maximum body length, after which muscle growth was dominated by hypertrophy (Johnston et al., 2003). In this experiment, the muscle fiber diameter of grass carp fed SBB, BBD1, and BBD2 was significantly smaller than that of fish fed the complete formula feed and BBD3. The final body weight of the former three groups was also significantly lower than those of the latter two groups, which may be one reason contributing to the difference in muscle histology, although the body weight differences were caused by the feed. In the future, grass carp of the same size should be used for the histological comparison to exactly reflect the effects of broad bean or a broad bean-based diet.
As the major component of connective tissues, collagen is closely related to muscle structure, flexibility, and texture, which largely determines the water retention and tenderness of muscle (Gordon & Hahn, 2010; Johnston et al., 2000). Collagen plays an important role in muscle fiber endurance, strength and quality, and movement of fish (Sato, Yoshinaka, Sato, & Shimizu, 1986). The increase of collagen content was found to result in high mechanical strength of muscle (Chang, Wang, Xu, & Zhou, 2011). Research by Liang (2017) and Zheng et al. (2016) showed that both SBB and broad bean-based diets significantly increased the muscle collagen content of grass carp, and the same results were also found in this study. It is worth noting that the combination of methionine, vitamins, and minerals (BBD3), rather than the sole supplementation of methionine, increased collagen content in muscle and skin. Further study may be needed on vitamins and minerals in the premix that promote the formation of collagen.
The main collagen in fish muscle and skin is type I collagen (Eckhoff, Aidos, Hemre, & Lie, 1998; Sato et al., 1989; Yata et al., 2001), which is encoded by two α1 chains (COL1A1) and one α2 chain (COL1A2). The COL1A1 and COLlA2 proteins of grass carp were found to be highly hydrophilic (Liu et al., 2012; Yu et al., 2014). A higher hydrophilic capability can promote the ability to combine with other substances and can thus enhance the function and the elasticity of tissues as well as the water-holding capacity, which is consistent with Lapière's (1990) findings of type I collagen. Studies have shown that the relative expression of type I collagen mRNA and the protein expression of COL1A1 and COL1A2 genes of crisped grass carp were significantly higher than those of common grass carp (Yu et al., 2014). The present study also indicated the increased expression of COL1A1 and COL1A2 mRNA in muscle and skin by feeding broad bean-based diets and SBB but not in the liver, which was consistent with the collagen content pattern in tissues. However, what active component(s) in broad bean stimulates the expression of the collagen genes of grass carp? It is a critical question related to the crisping mechanism of grass carp flesh by feeding broad bean, which needs more investigation in the future.
In conclusion, the present study showed that the combination of methionine, vitamins, and minerals in broad bean-based diets promoted the growth performance of the crisped grass carp and improved the flesh quality and the expression of collagen I genes in muscle and skin when compared to the control formula diet.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (31772825).




