Niche properties constrain occupancy but not abundance patterns of native and alien woody species across Hawaiian forests
Funding information
DC received funding from the Agencia Nacional de Investigación y Desarrollo (Chile; FONDECYT Regular No 1201347). HK acknowledges funding from the Deutsche Forschungsgemeinschaft (DFG, grant FOR2716 DynaCom)
This article is a part of the Special Feature Macroecology of Vegetation, edited by Meelis Pärtel, Francesco Maria Sabatini, Naia Morueta-Holme, Holger Kreft and Jürgen Dengler.
Abstract
Questions
Islands harbour a disproportionate amount of global plant diversity, yet their unique native assemblages are particularly vulnerable to biological invasions. It is therefore critical to identify the macroecological constraints that mediate spatial distributions of alien species on islands. Here, we examined abundance–occupancy relationships of native and alien woody plant species, and the role of niche properties and functional traits related to dispersal and competition in shaping occupancy and abundance patterns.
Location
Hawaiian Islands.
Methods
We calculated relative abundance and occupancy for 64 woody species (42 natives, 22 naturalized aliens), and estimated each species' niche breadth and position. We fitted phylogenetic hierarchical Bayesian models to evaluate abundance–occupancy relationships and the impacts of niche properties and functional traits on occupancy and abundance of native and alien species.
Results
Our analyses revealed that locally more abundant native species were also more widespread, but that the abundance of alien species was unrelated to their occupancy. Yet, we found evidence that alien species with longer residence times on the Hawaiian Islands were more widespread. While widespread native and alien woody species both had broad niches, widespread alien woody species exhibited a tendency to occur in more marginal niche positions than widespread native woody species. Niche properties did not affect abundances of either native or alien woody species. Traits associated with dispersal capacity and competitive ability had minimal impacts on either occupancy or abundance of native and alien woody species.
Conclusions
We found that niche properties shape the occupancy but not the abundance of native and alien woody species across Hawaiian forests. Our results suggest that, because of substantial invasion debts, the impacts of alien woody invasions in native forests have yet to fully manifest.
1 INTRODUCTION
Biological invasions threaten the unique and highly endemic floras of oceanic islands (Sax & Gaines, 2008) and the ecosystem processes that this diversity underpins (Vilà et al., 2011). Transport and trade networks spread alien species globally (Capinha et al., 2015; Seebens et al., 2015), driving the continued increase of alien species (Seebens et al., 2017; Moser et al., 2018) and the homogenization of species composition (Helmus et al., 2014) and biotic interactions on islands (Fricke & Svenning, 2020). While spatial patterns of alien species diversity at macroecological scales are increasingly well understood (e.g., van Kleunen et al., 2015; Dawson et al., 2017; Moser et al., 2018; Guo et al., 2021), the cross-scale drivers of the occupancy and abundance of alien species are unclear, yet are essential to understand for efforts to manage biological invasions within archipelagos and islands (Blackburn et al., 2011).
Abundance–occupancy relationships (AOR) are one of the most prominent cross-scale macroecological patterns and reveal how species’ occupancy across landscapes may influence species’ dominance within communities (Brown, 1984). Similar ecological processes are thought to underpin both occupancy and abundance patterns, resulting in generally positive AORs across taxa (Blackburn et al., 2006). Species with broad niches are more likely to occur in more locations and to dominate communities more strongly than species with narrow niches (niche breadth hypothesis; Gaston et al., 1997). Similarly, species that occur in common, non-marginal environments, i.e. habitat generalists, are predicted to be more common across a region and are more likely to dominate local communities than species that occur in less common environments, i.e. marginal environments (niche position hypothesis; Gaston et al., 1997). Both hypotheses of niche properties have received empirical support to varying degrees for native species (e.g., Slatyer et al., 2013; Díaz et al., 2020; Marino et al., 2020; Sporbert et al., 2020), but may not be applicable in high-diversity ecosystems dominated by neutral species dynamics (Hubbell, 2005; Gravel et al., 2006). In such ecosystems, AORs are possibly mediated by other ecological processes, such as dispersal (Arellano & Macía, 2014). Moreover, the degree to which human activity or niche properties may influence AORs of alien species is also uncertain, although the strong impacts of environmental filtering on the establishment of alien species at larger spatial grains suggest that AORs of alien species are likely shaped by niche properties (Ma et al., 2016; Park et al., 2020).
A key assumption of AORs is that species’ populations are in equilibrium with their environment and thus have filled their fundamental niches (Brown, 1995). Yet many species' populations are likely not in equilibrium (Wolkovich et al., 2014), and are either expanding or contracting in response to climate or land-use change (Lenoir et al., 2008; Fadrique et al., 2018; Newbold et al., 2018; Feeley et al., 2020) or are facilitated by human introductions. The amount of time for a local population to reach equilibrium is largely unknown for natural ecosystems. Evidence from experiments with model systems and microcosms suggests that this may occur over ecological time scales, but likely takes considerably longer in natural populations (Ricklefs, 2004). Biological invasions on islands represent a natural experiment that can overcome this limitation, as the year of introduction (or residence time) of naturalized alien species is at times well documented (e.g. Seebens et al., 2015) and can be leveraged to explore its effects on occupancy and abundance patterns. More generally, occupancy patterns of alien species on islands have implications for native species diversity (Powell et al., 2011), as alien species rapidly alter community structure across island forests (Craven et al., 2019; Vizentin-Bugoni et al., 2021). For example, AORs can be used to determine commonness and rarity patterns of alien species at multiple spatial scales (Rabinowitz, 1981); mismatches between occupancy and abundance patterns could indicate which alien species have the potential to become invasive (Catford et al., 2016).
A trait-based approach to AORs, by providing a direct, mechanistic link between plant ecological strategies (Reich, 2014; Díaz et al., 2016) and abundance and occupancy patterns (e.g., Buckley & Freckleton, 2010; Heino & Tolonen, 2018; Díaz et al., 2020; Marino et al., 2020; Fried et al., 2021), has the potential to identify the underlying ecological processes. For example, wood density is strongly associated with interspecific variation in competitive ability (Lasky et al., 2014; Kunstler et al., 2016) and the trade-off between growth and survival in tropical forests (Rüger et al., 2018). Similarly, variation in plant height and seed mass determines dispersal capacity (Muller-Landau et al., 2008; Thomson et al., 2011) and reproductive effort (Rüger et al., 2018). Together, there is strong evidence that particular combinations of trait values, e.g. tall stature, light seeds, and dense wood, could facilitate the spread of a species across a region and enhance its ability to compete within a local community (but see Díaz et al., 2020). While traits may have similar impacts on abundance and occupancy, they also may reveal different underlying ecological processes, contrary to expectations that abundance and occupancy are shaped by similar ecological processes (Brown, 1984). For example, a positive plant height–occupancy relationship could indicate that greater dispersal capacity (Thomson et al., 2011) enables species to occupy more locations, while a positive plant height–abundance relationship could suggest that dominant species compete more efficiently for light than less abundant ones (King et al., 2006; Onoda et al., 2014). Moreover, trait–abundance or trait–occupancy relationships could differ between native and alien species, possibly reflecting differences in dispersal vectors, e.g. native birds vs feral pigs, tolerance to anthropogenic disturbances (Huenneke & Vitousek, 1990; Denslow, 2003; Fricke & Svenning, 2020), adaptive strategies (Guo et al., 2018), or symbiotic relationships with fungi (Delavaux et al., 2019; Moyano et al., 2020).
Here, we use interspecific AOR to examine spatial distributions of native and alien woody species across the Hawaiian archipelago, and the ecological drivers underlying them. We anticipate that native and alien woody species will exhibit contrasting AORs, because alien woody species are likely not at equilibrium as many have naturalized recently and are still expanding geographically. We also expect that invasion history, measured as residence time, will influence the strength of the AOR of alien woody species. We therefore test whether alien woody species that have been present for a longer period of time on the Hawaiian archipelago occupy more locations and are more abundant than those introduced more recently. To understand the underlying drivers of AORs, and potential differences in AORs between native and alien woody species, we assess the impact of drivers on occupancy and abundance patterns that capture species' niche requirements and how species disperse across space and compete. We expect that species with broad niches or that occur in non-marginal environments, and with high competitive ability (e.g. high wood density, high maximum height) and high dispersal capacity (e.g. light seeds, high maximum height) will occupy more locations and be more abundant than species with narrow niche requirements, low competitive ability, or a limited dispersal capacity. We also expect that dispersal capacity may be less important in determining the occupancy and abundance patterns of alien species if dispersed by humans. Lastly, we use null models to evaluate if AORs emerge due to sampling artefacts, related to the undersampling of rare species, or underlying ecological processes (Gaston et al., 1997).
2 METHODS
2.1 Species selection
Our analyses were based on a forest plot database with 527 plots including species identity and stem size (in diameter at breast height [1.3 m], DBH) from across the Hawaiian archipelago (Craven et al., 2018). We selected non-monocot woody species that occurred in at least three plots across the archipelago and included only native and naturalized alien species, i.e. species that have established self-reproducing populations outside of their native range (Richardson et al., 2000), resulting in a total of 42 native and 22 alien woody species (Appendix S1). We standardized species names with The Plant List v. 1.1 using the R package Taxonstand (Cayuela et al. 2017).
2.2 Species occurrence and abundance
We downloaded occurrence data with spatial coordinates from iDigBio (www.idigbio.org), BIEN (https://bien.nceas.ucsb.edu/bien/), and GBIF (www.gbif.org/) using the R packages spocc and BIEN (Chamberlain, 2020; Maitner, 2020), which we combined with occurrences from the forest plot database. Given known biases of plant occurrence data (Meyer et al., 2016), we cleaned geographic coordinates by removing duplicates, occurrences in the ocean or within 100 m of biodiversity institutions, and those with rasterized coordinates using the clean_coordinates function in the R package CoordinateCleaner (Zizka et al., 2019). We then intersected the 7,587 species’ occurrences with a 32-km2 hexagonal grid (Figure 1 and Appendix S2), converting species’ occurrences to presences or absences per grid cell. If a species occurred within a grid cell, we assumed that it occupied the area of the entire grid cell. We estimated species’ occupancy as the proportion of the area occupied by a species in relation to the total area occupied by all species for each island.
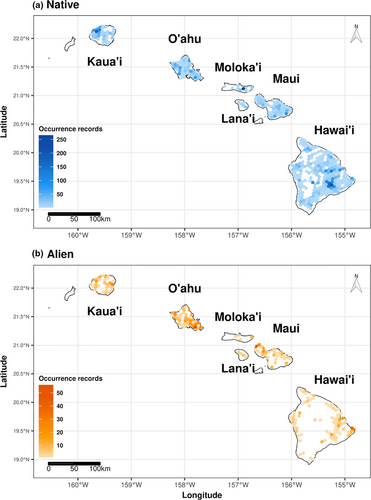
We estimated mean and median relative abundance for each species within each island using individuals with stems >5 cm in diameter at a height of 1.3 m from the plot data set (Craven et al., 2018). Because our results were similar for both measures of relative abundance, we present results using only the mean relative abundance. In total, the data set contains 194 species × island observations representing 64 species (42 native, 22 alien) across the six largest islands of the Hawaiian archipelago, many of which occur on a limited subset of these islands.
2.3 Residence time
We obtained the year of first record of alien woody species on the Hawaiian Islands from Seebens et al. (2017; Appendix S1). We note that two species in our data set (Aleurites moluccanus and Morinda citrifolia) were introduced by Polynesians prior to the 19th century, which occurred in approximately 1000 CE. We then calculated residence time for each species by subtracting the year of first record from 2020.
2.4 Phylogeny
We matched all species to a large seed plant phylogeny (Smith & Brown, 2018), using the ALLOTB phylogeny with 353,185 terminal taxa. We conservatively bound species to the backbone using dating information from congeners with the congeneric.merge function in the pezpackage (Pearse et al., 2015) allowing genera of missing species to collapse into polytomies.
2.5 Functional trait data
We selected three functional traits to explain variation in dispersal and competition among native and alien woody species:, maximum plant height (m), seed mass (mg), and wood density (g/cm3; Chave et al., 2009; Muller-Landau, 2010; Thomson et al., 2011). We obtained data for wood density from Chave et al. (2009) and Zanne et al. (2009) using the getWoodDensity function in the R package BIOMASS (Rejou-Mechain et al., 2017) and from the BAAD, BIEN, TRY, and USFS databases (Falster et al., 2015; Burrill et al., 2018; Kattge et al., 2020; Maitner, 2020). For seed mass, we obtained seed mass from accessions stored in the National Tropical Botanical Garden's Seed Bank and Laboratory, Shiels (2011), and the BIEN and TRY databases (Kattge et al., 2020; Maitner, 2020). Trait values were averaged by species across data sources. When species-level data were not available for seed mass, and because it is strongly conserved phylogenetically (Igea et al., 2017), we used genus-level means. For native species, genus-level values were calculated using only native species that occur in Hawaii. We obtained maximum height from the online Flora of the Hawaiian Islands (Wagner et al., 2005).
Because data were not available for all species in our data set (wood density: 20.3% unavailable; seed mass: 18.8% unavailable; maximum height: 0% unavailable), we used phylogenetic trait imputation to fill gaps in our data set. Trait imputation can provide reliable information for up to 60% of missing data, and adding phylogenetic information to trait imputation has been shown to strongly reduce error of estimation (Penone et al., 2014). However, estimation error may be higher when closely related species have missing data. We used a random forest imputation algorithm in combination with phylogenetic information with the missForest function in the R package missForest (Stekhoven & Buehlmann, 2012). For each trait, we assessed imputation error for a range of phylogenetic eigenvectors (1–30) and used the imputed trait values for the number of phylogenetic eigenvectors that minimized imputation error. We log-transformed maximum height and seed mass prior to imputation and back-transformed imputed values and estimation errors. Our gap-filled trait data had similar distributions and median values as the observed trait data (Appendix S3). Imputation errors, calculated as the percent normalized root mean squared error, were 4.3% for wood density and 43.0% for seed mass. We retained imputed trait data in our subsequent analyses, as removing species with missing values from the analysis likely creates more bias than using imputed data (Penone et al., 2014), yet also present results excluding species with imputed trait data.
2.6 Niche properties
To quantify the environmental niche of native and alien woody species, we included both climatic and biogeographic factors in our analysis. We therefore extracted mean annual temperature (MAT), mean annual precipitation (MAP), vapour pressure deficit (VPD), and potential evapotranspiration (PET; calculated using the Penman–Monteith equation) for each species’ occurrence from interpolated climate data for the Hawaiian Islands at a resolution of 250 m (Giambelluca et al., 2013; Giambelluca et al., 2014). We then calculated aridity, a widely used measure to assess the impacts of water availability on plant communities (e.g., Berdugo et al., 2020), as 1-MAP/PET (Zomer et al., 2008). We also extracted elevation range, terrain rugosity, and soil substrate age at a resolution of 1 km (Sherrod et al., 2007; Amatulli et al., 2018). We took the average of each variable within each 32-km2 grid cell in which each species occurred and standardized all data using a z-transformation, and imputed missing values (MAT, MAP, aridity, PET, and VPD: 0.3% unavailable; soil substrate age: 8.9% unavailable) with a principal components analysis model using the imputePCAfunction in the R package missMDA (Josse & Husson, 2016). We performed principal components analysis on the gap-filled environmental data using the PCA function in the R package FactoMineR (Lê et al., 2008) and retained the first three principal component axes, which explained 85.3% of variation in environmental variables (Appendix S4), for estimating n-dimensional hypervolumes.
We estimated niche breadth and niche position for each species across the entire archipelago as n-dimensional hypervolumes using the R package hypervolume (Blonder et al., 2018; Blonder & Harris, 2019). We estimated hypervolumes using Gaussian kernel density estimation, which provides a looser fit to the data than other algorithms and is recommended for fundamental niche modelling (Blonder et al., 2018). To ensure comparability of niche properties across species, we estimated bandwidths for each species using the cross-validation estimator and calculated the mean bandwidth for each dimension across species, which we then used to estimate hypervolumes for each species. To meet the criteria of log(number of species’ occurrences) > number of predictors (Blonder et al., 2018), we calculated hypervolumes for 58 species with at least eight occurrences using the first two axes of the principal components analysis. We then extracted the volume and centroid, here estimated from the first dimension of the hypervolume (Appendix S4), and used these measures to represent each species’ niche breadth and position respectively. To make our estimates of niche position comparable with related studies (e.g., Heino & Tolonen, 2018; Díaz et al., 2020) we took the absolute value of the hypervolume centroid, such that low values represent common environmental conditions, i.e. non-marginal niche positions, and high values represent rare environmental conditions, i.e. marginal niche positions.
2.7 Data analysis
To examine occupancy–abundance relationships and drivers of occupancy and abundance, we fitted three separate phylogenetic hierarchical Bayesian models. As we expected that closely related species are likely to be ecologically similar (Felsenstein, 1985; Freckleton et al., 2002), the failure to account for phylogenetic relationships may reduce estimation accuracy and increase type 1 error rates (Li & Ives, 2017). We therefore accounted for phylogenetic relationships among species by including a random effect term for species whose associated covariance matrix contains phylogenetic distances among species. We first fitted a ‘naive’ model with a beta distribution in which mean relative occupancy depends on mean relative abundance, native status, and their interaction. We included a crossed random-effects structure with random intercept terms for island and species, as not all species occur on all islands. The random intercept term for island accounts for differences among islands in environmental conditions. We included two random intercept terms for species, one (as mentioned above) that accounts for phylogenetic covariance and another that accounts for repeated measurements. In the second model, we examined variation in occupancy as a function of native status, mean relative abundance, niche position, niche breadth, wood density, maximum plant height, and seed mass, and their two-way interactions with native status. In the third model, we examined variation in mean relative abundance as a function of occupancy, native status, niche position, niche breadth, wood density, maximum plant height, and seed mass, and their two-way interactions with native status, which we fitted using a zero-one-inflated beta distribution. We fitted the second and third models to a subset of the data set that included 58 species and 145 species × island observations. Prior to fitting models we checked for multicollinearity among predictor variables; pairwise correlations among variables were all less than 0.7 (Appendix S5; Dormann et al., 2013).
To test the impacts of including imputed trait values in our full data set, we performed a sensitivity analysis in which we excluded species with imputed trait values. We then re-fitted phylogenetic hierarchical Bayesian models that examine the effects of niche properties and functional traits on relative abundance and occupancy to the subset of the data (n = 42 species, 131 species × island observations).
Lastly, we tested the impact of residence time on the occupancy and abundance of alien woody species that were introduced to the Hawaiian Islands after 1800. We first examined variation in occupancy as a function of residence time and mean relative abundance with a beta distribution and then examined variation in mean relative abundance as a function of residence time and occupancy with a zero-one-inflated beta distribution. Both models used similar random effect structures as used in the ‘naive’ model.
We fitted all models with weakly informative priors, four chains, and 1,500 burn-in samples per chain, after which 3,500 samples per chain (total post-warmup samples = 14,000) were used to calculate posterior distributions of model parameters. Priors were flat for population-level effects and were Student t priors with three degrees of freedom and a scale parameter of three for group-level effects. All variables were standardized using a z-transformation to facilitate comparisons among models, with the exception of the two models that test impacts of time of arrival. To ensure that there were no divergent transitions, we increased the ‘adapt_delta’ parameter to 0.99 in the brm function for all models. Model convergence was evaluated visually with trace plots and by estimating Rhat using the rhat function, where values considerably greater than one indicate that models have failed to converge (Appendix S6). Model fit was assessed visually by comparing observed data to simulated data from the posterior predictive distribution (Appendix S7). Additionally, we estimated a Bayesian R2 using the bayes_R2 function for each model, which is an estimate of the proportion of variation explained for new data (Appendix S6). Seed mass and maximum height were natural-log-transformed for all analyses. All models are fit using the R packages brms (Bürkner, 2017).
2.8 Sampling artefacts
Positive AORs may reflect sampling artefacts, as occupancy of species with low abundances may be systematically underestimated (Gaston et al., 1997). We therefore developed a null model where (a) species are randomly distributed across the study region (to calculate occupancy) and (b) individuals are randomly distributed across plots (to calculate abundance) using the independentswap algorithm in the R package picante that maintains species’ occurrence frequency and species richness (Kembel et al., 2010). We calculated relative occupancy and mean relative abundance for each randomization and then fitted the ‘naive’ phylogenetic hierarchical Bayesian model to the data as described above. We repeated this procedure 1,000 times, and compared the model coefficients of the null abundance–occupancy models to those of the observed abundance–occupancy model. We quantified the differences between null and observed models as the proportion of null model coefficient estimates that were greater than or smaller than those of the observed abundance–occupancy model, and interpreted differences to be significant if more than 95% of null abundance–occupancy model coefficient estimates were greater or less than those of the observed abundance–occupancy model.
To further test the assumption that positive AORs emerge because species with low abundances are undersampled, we performed a sensitivity analysis in which we excluded species whose relative abundance is below the 25th quantile across all species. We then re-fitted the ‘naive’ phylogenetic hierarchical Bayesian model to the subset of the data (58 species, 145 species × island observations).
In addition to the R packages already mentioned, we used tidyverse (Wickham et al., 2019), including ggplot2 (Wickham, 2016), and phytools (Revell, 2012), sf (Pebesma, 2018) sjmisc (Lüdecke, 2018), dggridR (Barnes, 2020), sp ( Pebesma & Bivand, 2005; Bivand et al., 2013). All data manipulation, visualiation, and analyses were performed using R version 4.0 (R Core Team, 2020).
3 RESULTS
Despite their contrasting origins, native and alien woody species in this study exhibited considerable similarities in terms of functional traits but not niche properties. On average, native woody species had broader niches than alien woody species (Appendix S8). The mean niche position of alien woody species suggests that alien woody species tended to occur in areas with more marginal environmental conditions, i.e. higher MAT, aridity, PET, and VPD, than native woody species, which occurred in locations with non-marginal environmental conditions, i.e. higher MAP and lower MAT, aridity, PET, and VPD (Appendix S4). Mean values of seed mass, maximum plant height, and wood density were similar among native and alien woody species (i.e. 95% confidence intervals overlap; Appendix S9).
3.1 Abundance–occupancy relationships
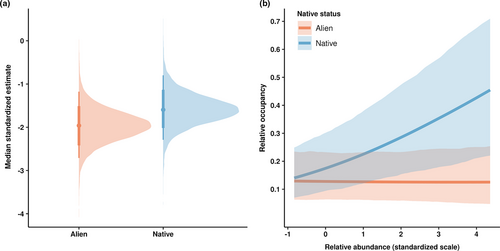
Across Hawaiian forests, native and alien woody species occupied a similar proportion of locations (Figure 2a). However, native and alien woody species showed contrasting abundance–occupancy relationships (βNative = 0.332, 95% credible interval: 0.106–0.563; βAlien = −0.015, 95% credible interval: −0.164–0.126; Figure 2b). For native woody species, occupancy increased sharply with increasing mean relative abundance, while alien woody species’ occupancy did not vary with mean relative abundance.
3.2 Sampling artefacts
Our null model analysis showed that estimates of the overall relationship between mean relative abundance and relative occupancy and the interactive effect of native status and mean relative abundance on relative occupancy did not differ significantly between the null and observed models (Appendix S10). In the case of the overall relationship between mean relative abundance and relative occupancy, only 82% of null model estimates were larger or smaller than those of the observed model. Similarly, only 78% of null model estimates of the interaction between native status and mean relative abundance of the null model were greater or less than that of the observed model. In contrast, our sensitivity analysis (Appendix S11) that excludes species with low relative abundance showed similar results when the ‘naive’ model is fitted to the full data set (Figure 2), i.e. that the AOR of native woody species differed from that of alien woody species.
3.3 Residence time
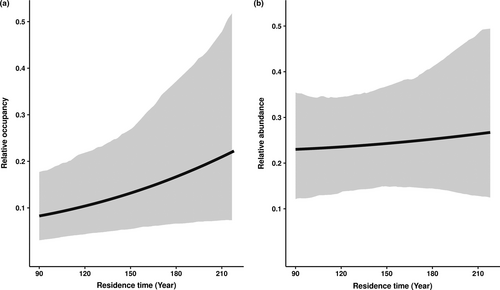
Alien woody species with longer residence time on the Hawaiian Islands were more widespread than those introduced more recently (βYear of first record = 0.009, 95% credible interval: 0.000– 0.018; Figure 3a). However, alien woody species with longer residence times were not more abundant locally (βYear of first record = 0.001, 95% credible interval: −0.008–0.011; Figure 3b).
3.4 Drivers of occupancy
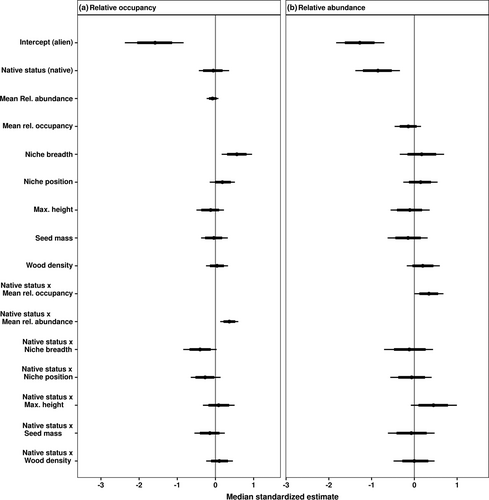
Our results showed that niche properties, and not functional traits, determined occupancy patterns of native and alien woody species (Figures 4 and 5). Occupancy of native and alien woody species increased strongly with niche breadth (Figure 4a). Yet the moderate interactive effect of native status and niche breadth (i.e. 80% credible intervals do not overlap with zero) suggests that occupancy of alien woody species increased more sharply with niche breadth than that of native woody species (Figures 4a and 5a). Similarly, the moderate interactive effect of native status and niche position on occupancy (Figures 4a and 5c) indicated a tendency for the occupancy of alien woody species to increase with a shift towards marginal niche positions (i.e. locations with higher MAT, aridity, PET, and VPD), while that of native woody species declined towards more marginal niche positions. Maximum height, seed mass, and wood density did not affect occupancy patterns of either native or alien woody species (Figures 4a and 5). In this model, there remained a strong, positive interactive effect between native status and mean relative abundance on occupancy (Figure 4a).
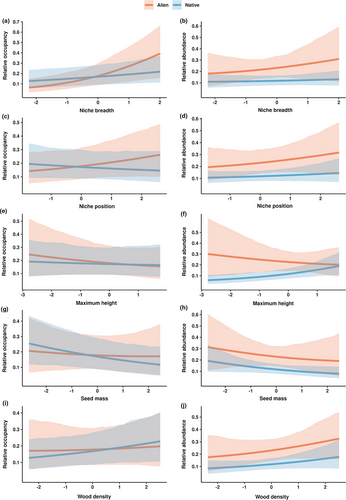
3.5 Drivers of mean relative abundance
In contrast with occupancy, niche properties and functional traits had limited impacts on the mean relative abundance of native and alien woody species (Figures 4b and 5). We observed a moderate positive interactive effect between maximum height and native status on mean relative abundance (80% credible intervals did not overlap with zero; Figures 4b and 5f), in which abundance of native woody species increased marginally with increasing maximum height and abundance of alien woody species declined with increasing maximum height. The sensitivity analysis that excluded species with imputed trait values exhibited qualitatively similar results as when the same models were fitted to the full data set (Appendix S12).
4 DISCUSSION
Our results provide evidence that primarily niche processes shape occupancy, but not abundance patterns of native and alien woody species across Hawaiian forests. While widespread native and alien woody species both have broad niches, species’ abundances were not associated with their niche properties. The longer residence times of widespread alien species suggest that historical legacies have shaped their dispersal across the archipelago to a greater extent than functional traits commonly associated with dispersal capacity, e.g. maximum height and seed mass. Together, our results indicate that the niche properties of alien woody species and invasion debts (Essl et al., 2011) contribute to the expansion of alien woody species across Hawaiian forests.
4.1 Contrasting abundance–occupancy relationships
We found contrasting AORs for native and alien woody species, providing evidence that widespread native species are usually locally abundant, whereas widespread alien species may be either locally abundant or rare. Our results for native species in an island setting are consistent with previous studies on plants from temperate and tropical regions (Blackburn et al., 2006; Arellano & Macía, 2014; Díaz et al., 2020; Marino et al., 2020), which are largely drawn from continental contexts (but see Buckley & Freckleton, 2010). The decoupled relationship between abundance and occupancy that we observed for alien woody species, however, appears to be an exception to the general rule that AORs are positive, even for alien species (Holt & Gaston, 2003; Buckley & Freckleton, 2010; Rigal et al., 2013; Sarabeev et al., 2018; Miranda & Killgore, 2019). Alien bird and mammal species in Great Britain, for example, exhibited similar AORs as native species, suggesting that alien species are likely in equilibrium and respond to environmental conditions in similar ways as native species, possibly due to longer average residence times (Holt & Gaston, 2003). Of the studies reporting positive AORs for alien species, only one was performed on oceanic islands (arthropods; Rigal et al., 2013) and none examined woody species, which have longer generation times and may take longer to establish in potentially suitable habitats than the more vagile focal taxa of the previously mentioned studies. In contrast, our finding that occupancy and abundance of alien species are decoupled indicates that ecological factors differentially influence the occupancy and abundance patterns of native and alien woody species.
4.2 Expansion of alien woody species
Differences in invasion history among species may be one explanation for the decoupled relationship between occupancy and abundance of alien woody species. Our analysis revealed that occupancy, but not abundance, of alien woody species increases with residence time. This suggests that alien woody species, at least those introduced to the Hawaiian archipelago since the 19th century, progressively expand their non-native range and occupy new locations (e.g., Daehler, 2005; Ibanez et al., 2020). Indeed, four of the five alien woody species with the highest occupancy across the archipelago in our data set, Psidium cattleianum, Leucaena leucocephala, Psidium guajava, and Aleurites moluccanus were first introduced before 1900. The two alien species introduced by Polynesians before the 19th century, Aleurites moluccanus and Morinda citrifolia, were also among the ten most widespread alien species. In contrast, two of the most abundant alien woody species in our data set, Acacia confusa and Schinus terebinthifolia, were introduced to the Hawaiian archipelago after 1900. Thus, it appears that the decoupled relationship between occupancy and abundance of alien woody species arose because locally abundant species may not have had sufficient time to occupy all locations within their fundamental niches as they continue to expand into new habitats (Brown, 1995), or that pathogen accumulation over time may reduce the abundance of widespread alien woody species (Flory & Clay, 2013). Our results substantiate the idea that invasion debts (Essl et al., 2011), i.e. the legacies of historical human activities, play a determinant role in shaping the spatial distribution of alien woody species. Given sufficient time, it is possible that positive AORs of alien species may emerge (e.g., Holt & Gaston, 2003; Buckley & Freckleton, 2010; Rigal et al., 2013; Sarabeev et al., 2018). However, the continuing influx of alien plant species in the Pacific (Seebens et al., 2017; Wohlwend et al., 2021), human- and other animal-mediated dispersal of alien woody species within and across islands, and ongoing habitat modification and/or degradation by humans and alien mammals, such as feral pigs, goats, and other ungulates (Nogueira-Filho et al., 2009; Weller et al., 2011; Murphy et al., 2014), act synergistically in facilitating the expansion of alien invasions. Thus, it seems more likely that the abundance–occupancy relationship of alien woody species will remain decoupled.
4.3 Drivers of occupancy and abundance
We found that niche properties had stronger impacts on occupancy patterns than functional traits associated with dispersal capacity and competitive processes. In line with our expectations, we found that widespread native and alien woody species have broad niches (Figures 4 and 5; Slatyer et al., 2013; Heino & Tolonen, 2018; Díaz et al., 2020; Marino et al., 2020). Additionally, there was a tendency for widespread alien woody species to occur in marginal environmental contexts while widespread native woody species tended to occur in non-marginal environmental contexts. These findings suggest that the impacts of species’ niche properties on occupancy may percolate across ecological scales to the community level, where spatial variation in species composition of Hawaiian forests has been linked with abiotic factors (Craven et al., 2019). The niche properties of widespread alien species are consistent with previous studies on the Hawaiian Islands showing that alien plant species are habitat generalists (Ainsworth & Drake, 2020) capable of colonizing empty niche space in marginal environmental contexts (Henn et al., 2019). While there are hundreds of non-native woody species present on the Hawaiian Islands, our results indicate that there is only a small number of widespread species that are invading and restructuring the species composition of the islands forests (Craven et al., 2018). Yet, niche properties of native and alien woody species did not confer a competitive advantage within forest communities, nor did wood density, which is associated with competitive ability (Lasky et al., 2014; Kunstler et al., 2016). The moderate positive interactive effect of maximum height and native status on relative abundance suggests that alien woody species may have a competitive advantage in forest understories, as small-statured alien woody species achieved higher relative abundances than low-statured native woody species. Relative abundance, therefore, is likely to be mediated more strongly by local-scale factors, such as the phylogenetic or functional distinctiveness of alien species to the native community, that better reflect competitive processes or other biotic interactions (Carboni et al., 2018; Levin et al., 2020; van der Sande et al., 2020).
Our results revealed that interspecific variation in occupancy patterns was not determined by functional traits commonly associated with dispersal capacity for either native or alien woody species. This finding further supports the idea that occupancy patterns of native species are more strongly shaped by niche-related processes than neutral ones, but does not preclude the possibility that they may have been shaped by infrequent, long-distance dispersal across and within islands (Nathan, 2006; Price & Wagner, 2018) or historical legacies (Essl et al. 2011). In the case of alien woody species, the lack of a relationship between occupancy and functional traits associated with dispersal capacity indicates that our analyses failed to capture novel mutualistic seed-dispersal interactions between alien woody species and alien mammals (Nogueira-Filho et al., 2009; Bullock & Pufal, 2020; Vizentin-Bugoni et al., 2021), and how such interactions may further modify biodiversity patterns (Rogers et al., 2017; Fricke & Svenning, 2020). One possible explanation for the weak trait–occupancy relationships of alien woody species observed in this study is that human-mediated dispersal was more important than other biotic (native or alien) or abiotic dispersal vectors in determining occupancy patterns. Dispersal distances of seeds dispersed by humans are likely greater than those dispersed by other dispersal vectors (Wichmann et al., 2009), although this would need to be corroborated for species in Hawaii. If indeed long-distance dispersal (Nathan, 2006; Price & Wagner, 2018) drives occupancy patterns of alien woody species, it is perhaps not surprising that traits associated with dispersal capacity were weakly associated with occupancy, as these traits have been shown to exhibit stronger relationships with mean dispersal distance than with maximum dispersal distance (Thomson et al., 2011). Our results highlight that species-specific dispersal capacity, as inferred by functional traits, largely fails to provide insights to interspecific variation in occupancy patterns, suggesting that occupancy patterns of alien woody species have been shaped more directly by historical drivers of invasion to a greater extent than contemporary ones, i.e. invasion debt (Essl et al., 2011), or by landscape-scale processes such as habitat loss (Fahrig, 2003; Watling et al., 2020).
4.4 Potential statistical artefacts
In our analyses, we accounted for phylogenetic relationships and used null models to address two ways in which statistical artefacts may influence the magnitude of AORs (Gaston et al., 1997; Holt & Gaston, 2003). Similar to recent studies (e.g., Díaz et al., 2020; Marino et al., 2020), our null models indicate that the AORs of native and alien woody species may be attributable to sampling artefacts associated with the undersampling of species with low abundances. However, our sensitivity analysis (Appendix S11) shows that the AORs of native and alien woody species were robust to the exclusion of low-abundance species, suggesting that the reported AORs emerged from ecological processes. One such ecological process is the disproportionate impact of biological invasions on rare native species (Powell et al., 2011); by restricting the spatial distribution and reducing the abundances of rare native species, biological invasions could strengthen AORs of native species. Our results indicate that contrasting AORs between native and alien woody species and the impacts of residence time and niche properties are robust to the shared evolutionary history among species, which was a particular concern in our study because a large number of native and alien woody species are concentrated in the Myrtaceae family (Craven et al., 2018).
5 CONCLUSIONS
Integrating ecological factors is considered a fundamental step towards a more mechanistic understanding of occupancy and abundance patterns of native and alien species alike (Blackburn et al., 2006). Here, we show that the spatial distribution of native and alien woody species across Hawaiian forests are largely shaped by niche processes, and not by traits associated with dispersal capacity as often assumed. The strong, positive impacts of residence time on the occupancy patterns of alien woody species highlight how invasion debts shape their spatial distribution and, perhaps more importantly, that the impacts of alien woody invasions in Hawaiian may take a considerable amount of time to fully manifest. Our results also show that abundance patterns are weakly associated with deterministic factors, e.g. niche properties or niche-related functional traits, and stochastic factors, e.g. dispersal processes, suggesting that local-scale biotic interactions may be important in determining community structure. In conclusion, our results highlight the fragility of native insular forest biodiversity and the threat posed by biological invasions to native forest biodiversity on oceanic islands.
ACKNOWLEDGEMENTS
We would like to thank Hanno Seebens for providing first record data.
AUTHOR CONTRIBUTIONS
DC, PW and HK conceived of the study, DW provided seed mass data, DC compiled and analysed the data with input from PW, and all authors wrote and edited the paper.
Open Research
DATA AVAILABILITY STATEMENT
The forest plot data used to estimate mean relative abundance are available at https://doi.org/10.5061/dryad.1kk02qr. The processed data used to perform all analyses are deposited at FigShare (https://figshare.com/s/20d580d76e582d99b6fd).




