Dissolution Characteristics of Split and Crushed Levetiracetam Extended-Release Tablets in Comparison With Immediate-Release Formulation
Funding: Funding for the study was provided by Emma's Fund of the North Carolina Veterinary Medical Foundation.
[Correction added on 15 May 2025, after first online publication: The first author’s first name was corrected.]
ABSTRACT
Levetiracetam (LEV) is a commonly used antiseizure medication in dogs, available in immediate-release (LEV-IR) and extended-release (LEV-XR) formulations. LEV-XR improves owner compliance with less frequent dosing, but its lowest concentration tablet (500 mg) often exceeds recommended doses for small dogs. This study evaluated how modifying LEV-XR tablets affects dissolution rates, comparing intact, split, and crushed LEV-XR tablets with intact LEV-IR tablets. Dissolution testing followed United States Pharmacopeia (USP) guidelines for LEV-XR tablets. Tablets were placed in a buffer solution (pH 6.0) and agitated at 100 rpm. Samples were collected at 0, 0.5, 2, 4, 6, and 8 h, then analyzed by high-pressure liquid chromatography (HPLC) using a USP reference standard. Results indicated that splitting LEV-XR tablets slightly increased drug release compared to intact tablets, while crushing eliminated extended-release properties, mimicking LEV-IR dissolution. These findings suggest that splitting LEV-XR tablets may be a viable strategy for dosing small dogs without compromising sustained release. Conversely, crushing LEV-XR tablets may be useful for rapid drug release in cluster seizure protocols. Future pharmacokinetic studies are needed to confirm if these in vitro results correlate with in vivo performance for both maintenance and emergency seizure management in dogs.
Levetiracetam (LEV) is an antiseizure medication (ASM) that works primarily by binding to synaptic vesicle protein 2A, a critical component in regulating neurotransmitter release in the nervous system (Lynch et al. 2004). While its exact mechanism of action is not fully understood, LEV is thought to have a multimodal effect, influencing neuronal excitability, cellular transporters, modulating gamma amino butyric acid (GABA) turnover, and potentiating GABA and glycine inhibition (Mastrocco et al. 2024). Levetiracetam has several advantages for the treatment of seizure disorders in dogs, including 100% oral bioavailability, minimal metabolism, a strong safety profile, not classified as a DEA controlled substance, and potential synergy with other ASMs (Patterson et al. 2008). Additionally, LEV's minimal drug–drug interactions and rapid onset of action further enhance its appeal as a treatment option for epilepsy in dogs, especially for those on polytherapy (Lyseng-Williamson 2011).
Levetiracetam is available in immediate-release (LEV-IR) and extended-release (LEV-XR) formulations. As a result of the short half-life of 3–4 h in dogs (Moore et al. 2010), LEV-IR is typically administered every 8 h, which can be inconvenient for many pet owners. LEV-XR, however, allows for reduced dosing frequency, which could improve owner compliance, as fewer daily doses have been associated with better treatment adherence (Booth et al. 2021).
According to the LEV-XR label (Keppra; UCB Inc. 2024), tablets must be swallowed whole, without breaking, chewing, or crushing, to preserve the extended-release properties. This presents a challenge when administering to small-sized dogs because the lowest concentration tablet is 500 mg, and this may exceed the recommended dose (30 mg/kg every 12 h; Beasley and Boothe 2015). Furthermore, in cases of cluster seizures, additional doses of LEV (in dogs administered it as maintenance medication) or as pulse therapy are recommended (Packer et al. 2015; Charalambous et al. 2024). When administered for this purpose, faster dissolution and absorption of the drug are desired for a rapid onset, and for dogs that are taking LEV-XR, veterinarians either prescribe LEV-IR to be used in the cluster protocol or anecdotally recommend breaking the LEV-XR tablets in half. Despite this, little is known about the effect of splitting or crushing LEV-XR tablets on their pharmacological properties, highlighting a need for evidence-based recommendations.
A previous study (Sun et al. 2016), using one of the generic formulations of LEV-XR, compared the dissolution rate of intact, split, and crushed tablets, and found that the split tablets dissolved faster than the intact tablets and that crushing the tablets eliminated the extended-release properties, allowing for complete drug release in approximately 60 min. However, this study did not compare the dissolution of the LEV-XR tablets with the LEV-IR tablets. Our study aims to fill this gap by comparing the dissolution rates of intact, split, and crushed LEV-XR tablets with those of intact LEV-IR tablets and to provide a reference for future pharmacokinetic studies in dogs.
Levetiracetam-XR 500 mg tablets and LEV-IR 500 mg tablets were obtained from an FDA-approved source (Solco Healthcare US). Levetiracetam analytical reference standard was acquired from the United States Pharmacopeia (USP). Dissolution studies were conducted in accordance with Dissolution Test 2 described in the USP monograph for LEV-XR tablets (USP Monographs 2020), with the following exceptions: We used an absorbance wavelength of 205 nm (as described for Test 1), our mobile phase was 90% buffer and 10% acetonitrile instead of 95%/5%, and we used a flow rate of 1 mL/min instead of 0.8 mL/min. Test 2 of the USP monograph calls for USP Apparatus 1 for dissolution testing (USP Monographs 2020; United States Pharmacopeial Convention 2011a). We did not have access to this apparatus and instead, we used a magnetic stirring plate set to 100 rpm. While this may have affected absolute dissolution values, all tablets were tested under identical conditions, allowing valid comparisons between formulations. The studies were performed in triplicate for intact, split, and crushed LEV-XR tablets, as well as for intact LEV-IR tablets. The crushed tablets were thoroughly pulverized to a powder using a mortar and pestle. Following the USP monograph's specifications, a buffer solution at pH 6.0 was prepared, and dissolution testing was carried out in 900 mL of this buffer. Dissolution testing was performed by placing the beakers containing tablets on a magnetic stirring plate set to a speed of 100 rpm, with testing performed at room temperature. One milliliter samples of the dissolution media were obtained at time 0, 0.5, 2, 4, 6, and 8 h. The LEV concentration was determined by high-pressure liquid chromatography (HPLC). Briefly, the HPLC system (Agilent 1200 series system, Agilent Technologies, Wilmington, DE) consisted of a quaternary solvent delivery system, autosampler, and ultraviolet (UV) detector. The data were collected with chromatogram integration software (Agilent Technologies OpenLAB CDS software, Agilent Technologies, Wilmington, DE).
An analytical reference solution was obtained by dissolving the USP pure analytical reference standard to a concentration equivalent to the maximum concentration of dissolved tablets in the buffer solution. A 500 μL aliquot of the test solution was loaded into a Whatman syringeless vial (Whatman UN503NPEAQU syringeless filter device, pore size 0.2 μm) and injected. Calculation of the percent release was from the formulas available in the LEV-XR USP monograph (Table 1).
| Calculate the concentration, C, of levetiracetam in Medium (mg/mL) after time point: |
| Result = (rU/rS) × CS; where |
| rU = peak response from the Sample solution |
| rS = peak response from the Standard solution |
| CS = concentration of the Standard solution (mg/mL) |
| Standard solution: L/900, where L is table size (500 mg). |
| Calculate the percentage of the labeled amount of levetiracetam dissolved at each time point: |
| Result 1 = C1 × V × (1/L) × 100 |
| Result 2 = [(C2 × V) + (C1 × Vs)] × (1/L) × 100 |
| Result 3 = {(C3 × V) + [(C2 + C1) × Vs]} × (1/L) × 100 |
| Result 4 = {(C4 × V) + [(C3 + C2 + C1) × Vs]} × (1/L) × 100 |
| Result 5, 6, 7 = repeat pattern as above. |
| V = volume 900 mL |
| L = label claim of tablet (500 mg) |
| Vs = Volume of sample withdrawn (1 mL) at each sample point |
Dissolution results are reported as mean ± standard deviation (SD) for each time point (n = 3 per group). As shown in Figure 1, the rate of drug release from the split LEV-XR tablets showed a similar, but more rapid pattern to the intact LEV-XR tablets. At the 8-h time point, 72.4% ± 2.36% and 88.8% ± 5.8% were released from the intact and broken tablets, respectively. While the split tablets met the USP acceptance criteria of not less than 80% release, the intact tablets fell slightly below this threshold. Despite this, the release profile of the split tablets was similar in pattern to intact tablets, suggesting that splitting does not dramatically alter extended-release characteristics. In contrast, crushing the tablet resulted in the loss of XR properties, with drug release closely resembling that of IR tablets. The percentage release of these tablets was within the USP acceptance criteria of 90%–110% release (Figure 1) and within the acceptance criteria for dissolution of LEV-IR of not less than 80% release at 30 min (United States Pharmacopeial Convention 2011b).
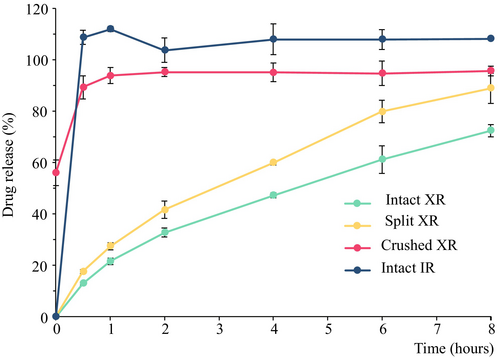
These results agree with the previous study by Sun et al. (2016) which used a different generic brand of LEV-XR. In addition to this, we showed that the crushed XR behaves very similarly to the intact IR tablets. These findings suggest that, based on dissolution tests, LEV-XR tablets could be split in half for administration to smaller dogs without compromising the XR properties. Future studies are aimed at confirming this hypothesis using pharmacokinetic studies in dogs administered intact and broken LEV-XR tablets. If pharmacokinetic studies are confirmatory, this could enhance treatment compliance by reducing the number of administrations per day.
Furthermore, this study demonstrates that breaking the tablet in half does not accelerate drug release. As a result, splitting the tablet may not be effective for cluster seizure protocols where a rapid onset is needed. However, crushing the tablet could be a viable approach in such scenarios if IR tablets are not available. This modification of the LEV-XR tablets may mimic the drug release profile of IR tablets. However, it is important to consider that crushing LEV-XR tablets may pose a risk of inhalation exposure to aerosolized drug powder for individuals handling the medication, including veterinarians, technicians, or pet owners. When pulverizing tablets, appropriate precautions, such as the use of masks, should be considered.
A limitation in our methodology was that the intact LEV-XR tablets did not meet the USP criterion of no less than 80% release at 8 h. We attribute this to deviations from standard testing conditions (e.g., not using Apparatus 1), which may have influenced the results. Nevertheless, since identical test conditions were applied to all the tablets, the comparative analysis remains valid. The goal of this study was not to establish compliance with USP dissolution thresholds, but to compare relative differences in drug release across tablet preparations using a consistent, controlled method. In addition to this, the concentrations of LEV-IR were 110% of the reference standard for some samples; this, however, is within the USP acceptance criteria of 90–110%. Furthermore, this study was designed as a descriptive dissolution analysis rather than a statistical comparison of formulations. As such, no inferential statistical tests were performed, and conclusions are based on observed trends rather than statistical significance. Future pharmacokinetic studies in dogs are needed to confirm whether these dissolution patterns translate to meaningful pharmacokinetic differences in vivo.
Author Contributions
A.M., J.A.N., M.G.P., and K.R.M. conceived and designed the study. A.M. acquired the data with assistance from J.A.N., M.G.P., and K.R.M. M.G.P. developed the analytic methods and performed the analysis. A.M. and M.G.P. interpreted the data. All authors have read and approved the final manuscript.
Acknowledgements
Funding for the study was provided by Emma's Fund of the North Carolina Veterinary Medical Foundation.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




