Investigating the Pharmacokinetics and Efficacy of Intramammary Ceftiofur Hydrochloride in Prevention of Udder Inflammation in Non-Lactating Dairy Heifers
Funding: The USDA National Institute of Food and Agriculture (grant no. 2020-41480-32520 and 2021-41480-35270; Kansas City, MO) supported this research, which funds the Food Animal Residue Avoidance and Depletion Program (FARAD).
ABSTRACT
Mastitis is the most burdensome concern for the dairy cattle industry. Antimicrobials are often prophylactically administered to dairy cows at dry-off to reduce the risk of intramammary infection during the dry period and subsequent lactation. Mastitis incidence has increased in dairy heifers after calving, leading to extralabel drug use of various dry cow products, including intramammary ceftiofur hydrochloride. However, the pharmacokinetics and efficacy of this application have yet to be studied. This study aimed to compare the pharmacokinetics and efficacy following no treatment, a non-antimicrobial teat sealant, or a single dose of intramammary ceftiofur given at 21 or 14 days before expected calving. We hypothesized that milk collected following dosing would contain drug residues below the FDA tolerance of 100 ng/mL by calving, and heifers within the ceftiofur treatment groups would have lower somatic cell counts (SCCs) than heifers in the teat sealant and nontreatment control groups. Following treatment or no treatment of 24 prepartum heifers, milk samples were collected until 21 days after calving. Somatic cell counts and ceftiofur concentrations were assessed utilizing a cell counter and UPLC/MS detection, respectively. Ceftiofur administration did not significantly reduce SCCs compared to other groups by days 7, 14, or 21. For heifers treated 14 and 21 days prior to calving, milk had a maximum ceftiofur concentration of 8.14 ± 6.24 and 4.20 ± 5.07 ng/mL 48 h into lactation, respectively. The minimal ceftiofur concentrations in milk collected from these heifers indicate that administration of ceftiofur 14 or 21 days before calving is unlikely to lead to violative residues. However, it is essential that regional regulations regarding the use of ceftiofur are adhered to.
1 Introduction
Mastitis is inflammation of the mammary gland, typically caused by bacterial infection, that poses a significant economic and welfare concern in dairy cattle, including prepartum heifers (USDA-ARS 2021). While it is commonly associated with lactating cows, mastitis can also affect heifers before their first calving. This condition often results in impaired mammary development, reduced milk production, and an increased risk of chronic infections throughout the animals' productive lifespan (Akers and Nickerson 2011; Oliver and Sordillo 1988; Puerto et al. 2021). Prior to calving, environmental pathogens are primary culprits, as young animals are often exposed to these bacteria in housing environments, making them more susceptible to bacterial colonization and infection (Fox 2009). Prepartum cows and heifers are most susceptible to mastitis in the weeks leading up to and following calving due to metabolic disorders, oxidative stress, and excessive lipid mobilization (Ayemele et al. 2021; Laliotis et al. 2020; Mezzetti et al. 2021). Following calving, postpartum heifers are additionally challenged by contagious pathogens spread from infected cows through contact with contaminated milking equipment (Fox 2009).
Preventing mastitis in dairy heifers involves a combination of management practices and environmental controls. Keeping heifer housing areas clean and dry reduces the environmental bacterial load and decreases the likelihood of exposure to mastitis-causing pathogens (De Vliegher et al. 2012). Proper nutrition is also essential for supporting a strong immune response in heifers, which aids in reducing their susceptibility to infection (Compton et al. 2007). Numerous studies have also shown varying degrees of efficacy for non-antimicrobial teat sealants used in prepartum heifers, as these create a physical barrier that protects the teat canal from bacterial invasion (Larsen et al. 2021; Nickerson et al. 2020; Parker et al. 2007). Vaccination against pathogens such as Staphylococcus aureus and Escherichia coli can be a proactive approach to preventing mastitis in heifers. While vaccines may not entirely prevent infection, they can reduce the severity and duration of clinical mastitis cases, thereby limiting the overall impact on milk production and animal welfare (Ismail 2017). Unfortunately, mastitis can still occur in herds that practice the highest standards of animal husbandry.
Currently, there are no labeled treatment options available for mastitis in heifers, which leads to the extralabel use of antimicrobials, and this generates food safety risks for consumers. Blanket dry cow therapy (BDCT) is a commonly used strategy in which each cow is administered an intramammary antimicrobial at the end of lactation, regardless of disease status, to reduce the risk of mastitis during the dry period and subsequent lactation. Intramammary ceftiofur hydrochloride, a cephalosporin antibiotic, is used extensively in dairy cattle to treat existing clinical mastitis infections as well as for BDCT (USDA NAHMS 2014). While ceftiofur is not used in human medicine, cephalosporin drugs are critically important for treating various human diseases. This leads to concerns that their overuse may contribute to increased antimicrobial resistance (AMR) and reduced antibiotic effectiveness in livestock and human medicine. To combat this, the FDA limits the use of cephalosporin antibiotics in livestock and prohibits producers from using them in particular extralabel applications (21 CFR § 530.41, 2024). International politics also recommend reducing or prohibiting the overall use of antimicrobials in food-producing animals for disease prevention purposes (WHO 2017). For example, the EU banned BDCT under Regulation 2019/6 in 2022 to reduce the risk of AMR (European Parliament and the Council of the European Union 2019).
The Food Animal Residue Avoidance Databank (FARAD), a U.S. Department of Agriculture-supported national food safety program, has received numerous inquiries about the use of intramammary ceftiofur hydrochloride in heifers. FARAD's mission is to assist producers and veterinarians in preventing or mitigating illegal or harmful residues of drugs, pesticides, biotoxins, and other chemical agents that may contaminate foods of animal origin (Riviere et al. 2017). In addition to the risks posed by ceftiofur regarding AMR, the 2023 Milk and Dairy Beef Drug Residue Prevention Manual and the 2023 National Milk Drug Residue Database Annual Report state that it has the highest percentage of tissue residue violations in dairy cull cows, and beta-lactams, in general, have the highest percentage of milk residue violations in comparison to other antimicrobial classes. While there are several drugs used extralabel in heifers, this paper specifically focuses on the lactating cow formulation of ceftiofur hydrochloride (Spectramast LC, Zoetis Animal Health) due to its significant regulatory concern. Pretreating heifers to minimize the impact of subclinical mastitis (SCM) at calving is a relatively common practice, but not much is known about how close to calving antimicrobials can be administered without impacting food safety. Most studies assessing antimicrobial use in prepartum heifers have focused on formulations intended for lactating cows. We believe this is due to the fact that replacement heifers do not have a designated dry period, as they have not previously lactated. Additionally, dry cow therapies may only persist for 14–28 days into the dry period and fail to protect the udder during the late dry and early lactation periods (Petzer et al. 2009). To address this gap in coverage, producers may consider using lactating cow formulations closer to calving for their heifers, rather than dry cow formulations, such as the lactating cow formulation of ceftiofur hydrochloride. However, there is no published pharmacokinetic data for this application, and therefore, no data are available to establish an appropriate withdrawal interval for periparturient heifers. It is important to note that using the lactating ceftiofur hydrochloride formulation evaluated in this study or the dry cow formulation in prepartum heifers would be considered prohibited in the United States. Therefore, evaluating this treatment's efficacy and risk of violative residues is crucial.
Milk somatic cell count (SCC) measurements are used as an indicator of milk quality and as a marker to monitor the prevalence of mastitis in dairy herds (Alhussien and Dang 2018). An elevated level of milk SCC has a direct correlation to the severity of intramammary infection (IMI), reduces the quality of raw milk, and decreases profitability as it is less suitable for processing and sale (Sharma et al. 2011). In the European Union, China, New Zealand, Australia, Switzerland, and Canada, the legal bulk tank SCC limit is 300–400 × 103 cells/mL; in South Africa and Brazil, 500 × 103 cells/mL; and in the US, 750 × 103 cells/mL (Alhussien and Dang 2018). However, it is generally accepted that an SCC exceeding 200,000 cells/mL indicates cows suffering from a bacterial infection or SCM (Petzer et al. 2017).
This study aimed to determine ceftiofur hydrochloride elimination in milk in periparturient heifers and to evaluate its efficacy in antimicrobial-treated versus non-antimicrobial-treated groups. We hypothesized that milk collected from postpartum heifers after a single intramammary dose of 125 mg of ceftiofur hydrochloride in each quarter, administered 14 or 21 days before calving, would contain drug residues below the FDA's tolerance level by the time of calving or shortly thereafter. Additionally, we hypothesized that heifers in the antimicrobial treatment groups would demonstrate a decreased association with subsequent infection by measuring lower SCC values compared to those in the nontreatment and teat sealant control groups.
2 Materials and Methods
2.1 Animals and Housing
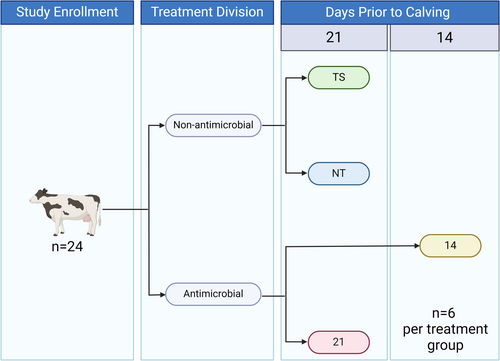
The study was approved by the North Carolina State University Institutional Animal Care and Use Committee (IACUC #21-172). The sample size was determined by the FDA's recommendation that at least 20 prepartum animals be enrolled in a milk residue study (FDA 2015) while maintaining an equal number of animals in each group and accounting for an anticipated 15% drop-out rate. Investigators were not blinded to the treatment groups of the heifers. Inclusion criteria for enrollment included prepartum heifers who had not been administered any antibiotic treatment 30 days prior to the start of the study, functional mammary quarters that passed udder inspection (redness, swelling), and palpation (firmness, pain, heat). Twenty-four prepartum Holstein–Jersey cross-bred heifers were enrolled for use in this study, each expected to calve 14 or 21 days from the initial treatment date. Using a parallel study design, all treatments were decided based on heifer and farm labor availability 14 or 21 days prior to calving, so heifers were not randomly allocated to treatment groups. The heifers were sourced and group-housed on pasture at the North Carolina Department of Agriculture (NCDA) Cherry Research Station. The heifers were fed a total mixed ration (TMR) twice daily, provided water ad libitum, and had pasture access for grazing throughout the duration of the study. All heifers received two coliform mastitis vaccines (J-VAC, Boehringer Ingelheim Animal Health) approximately 60 days prior to calving and 7 days post-calving. All heifers received two neonatal calf diarrhea vaccines (Scourguard 4KC, Zoetis Animal Health) approximately 60 and 21 days prior to calving. All heifers were dewormed (Cydectin (moxidectin) Pour-On, Elanco Animal Health) 7 days post-calving.
2.2 Dosing and Sample Collection
The heifers received one of the four following intramammary treatments (Figure 1): TS, teat sealant (n = 6); 14, ceftiofur hydrochloride (Spectramast LC, Zoetis Animal Health) and teat sealant 14 days prior to the expected calving date (n = 6); 21, ceftiofur hydrochloride (Spectramast LC, Zoetis Animal Health) and teat sealant 21 days prior to the expected calving date (n = 6); NT, no treatment (n = 6). Prior to treatment, all teats were scored on a 1–3 scale based on the outward appearance of the health of the teat. This scale was adapted from a previous study in prepartum heifers (Booth et al. 2016). A score of 1 was given for teats that appeared healthy with no abrasions or scabbing. A score of 2 was given for teats with residual damage from healing scabs. A score of 3 was given for teats that appeared unhealthy with fresh scabs. Lacteal secretions (2–3 mL) were also collected from each quarter of every heifer for future testing before treatment. Each teat end was scrubbed with a 70% alcohol-soaked pad starting on the teats farthest away from the administrator before lacteal secretion collection and dosing.
The antimicrobial-treated heifers (groups 14 and 21) received 125 mg ceftiofur hydrochloride lactating-cow therapy (Spectramast LC, Zoetis Animal Health) intramammary from one 10 mL syringe/quarter. These heifers were treated using a partial-insertion technique starting on the teats closest to the administrator. Ceftiofur was massaged into each quarter to distribute the suspension into the milk cistern. Treated heifers then received 4 g of a sterile, non-antibiotic internal teat sealant (Lockout, Boehringer Ingelheim Animal Health) containing 60% bismuth subnitrate administered intramammary at one syringe/quarter. For group TS, this internal teat sealant was administered intramammary as the sole therapy. For sealant administration, the teat was pinched where it joined the base of the udder, and the teat sealant was applied starting on the closest teat to the administrator. All contents of the syringe were deposited into the teat, and careful attention was paid to not massage this into the udder. Heifers in the NT group only had their teats scored and lacteal secretion samples collected. TS and NT procedures were performed 21 days prior to the expected calving date. All heifers were moved to a close-up pen to be watched in the weeks leading up to calving.
Once the heifers calved, milk samples were collected every 12 h for the first 7 days and then once daily on days 14 and 21 post-treatment. Milk samples were collected for 21 days to measure treatment efficacy when the heifers were most susceptible to acquiring mastitis. Milk sample collection time points were taken based on the established milking frequency at the dairy unit. The heifers were ushered into a side-by-side herringbone parlor where they could be identified while in a milking stall. All teats were dipped in a germicidal solution allowing at least 30 s of contact time. Teats were then dried with a clean paper towel starting with teats farthest away from the collector. Each quarter had a foremilk sample stripped before an aliquot of approximately 3 mL was collected from each quarter. Approximately 15 mL was collected as a composite sample into a sterile 15 mL conical tube (Globe Scientific, Mahwah, NJ). The heifers were then milked as they usually would by a milking claw, and a post-dipping germicidal solution was applied before releasing them back to pasture. The antimicrobial-treated heifers (groups 14 and 21) were allowed to move out of the waste milk group and into the milking herd once each heifer received a negative antibiotic screening test (Delvotest SP-NT, dsm-firmenich). Milk samples were stored in cryovials. All samples were placed in a −20°C freezer until the study's completion and moved to a −80°C freezer until they could be analyzed.
2.3 Somatic Cell Count Analysis
In this study, the lacteal secretions, as well as a subset of all composite milk samples, were used for SCC analysis to determine the number of leukocytes present per mL of the sample. At each collection time point, milk was analyzed for SCC utilizing a DeLaval Cell Counter (DCC) (DeLaval International AM, Tumba, Sweden), an electronic quantitative cow-side cell counter. The analysis took place immediately after sample collection, and the measured SCC was recorded.
2.4 Drug Concentration Analysis
Milk samples were analyzed by ultra-performance liquid chromatography with mass spectrometry detection (UPLC/MS) to determine the active concentrations of ceftiofur hydrochloride and its metabolites as previously described (Smith et al. 2004). Ceftiofur is rapidly metabolized to the active metabolite, desfuroylceftiofur, in cattle. The assay converts all ceftiofur and desfuroylceftiofur conjugates to a single stable derivative, desfuroylceftiofur acetamide (DCA). The analytical method for determining DCA in milk was validated before use in this study.
A 500 μL aliquot of raw milk was added to 5 mL acetonitrile and centrifuged for sample cleanup. The supernatant was evaporated to dryness, reconstituted in 2 mL of ultra-pure water, and 5 mL of 0.4% w/v dithioerythritol in borate buffer (pH, 9.0; prepared by dissolving 19 g of boric acid and 3.7 g of potassium chloride in 1 L of ultra-pure water) was added. The samples were incubated in a 50°C water bath for 30 min and then cooled in room-temperature water. Three milliliters of 14% w/v iodoacetamide in phosphate buffer (pH, 9.0; prepared by dissolving 3.4 g of potassium phosphate in 1 L of ultra-pure water) was added, and the samples were incubated in the dark at room temperature for 30 min. The pH was adjusted to between 2.5 and 2.6 with a 5% phosphoric acid solution, placed in the refrigerator for at least 1 h, and then loaded onto Oasis PRiME HLB 3 cc (60 mg) Extraction Cartridges (Waters Corporation, Milford, MA) in a positive pressure manifold (Biotage). The samples were washed with 2 mL of 95:5 water: methanol, eluted with 2 mL of 90:10 acetonitrile: methanol, and evaporated to dryness. The sample was then reconstituted in 250 μL 15:85 ACN:H2O, filtered with an EZFlow hydrophilic PVDF 13 mm 0.22 μm syringe filter (Foxx Life Sciences) into a max recovery vial (Waters Corporation, Milford, MA), and 5 μL was injected into the instrument for analysis.
Milk concentration analysis was performed on a Waters Acquity Ultra Performance Liquid Chromatography coupled to a Waters Acquity QDa mass spectrometer detector. The instrument was set to Single Ion Recording (SIR) of 487.0528 m/z using electrospray ionization in the positive ion mode (ESI+). The cone and capillary voltages were 15 and 0.8 V, respectively. A Waters Acquity UPLC BEH C18 1.7 μm (2.1 × 100 mm) column with a corresponding VanGuard Pre-Column (2.1 × 5 mm) (Waters Corporation, Milford, MA) was used for the separations. A gradient was used for the mobile phase. Solvent A was 0.1% formic acid in water, and solvent B was 0.1% formic acid in acetonitrile. The flow rate was 0.4 mL/min. The gradient was programmed as follows: from 0 to 1 min, the mobile phase composition was 90% A and 10% B; from 1 to 2.5 min, the composition changed linearly to 5% A and 95% B and then held there until 3.5 min; finally, back to 90% A and 10% B from 3.51 to 5 min.
The chromatograms were integrated with a computer program (MassLynx Software V4.1, Waters Corporation, Milford, MA), which quantified the unknown concentrations in all samples. The DCA concentration range was 2.5–100 ng/mL with an average correlation coefficient, R2, of 0.99. The limit of detection (LOD) and the limit of quantification (LOQ) for DCA in milk was 2.5 and 5 ng/mL, respectively. Intraday precision and accuracy were obtained on the same day using three different concentrations (2.5, 5, and 10 ng/mL) repeated four times each. Intraday precision was 1.5% to 15.2%, with an accuracy of 97.4% to 115.2%. Interday precision and accuracy were obtained by measuring six different concentrations (2.5, 5, 10, 25, 50, and 100 ng/mL) across 10 days. Interday precision was 4.4%–16.3%, with an accuracy of 84.8%–108.4%.
2.5 Statistical Analysis
Repeated measures ANOVA with the Geisser–Greenhouse non-sphericity assumption followed by Tukey's multiple comparisons test was used to compare the mean ACCs between treatment groups. Fisher's exact test was used to compare the prevalence of SCM over time for all treatment groups. All tests were chosen based on the normality of data distribution. All statistical analyses were performed using GraphPad Prism Software (V10.4.0 for Windows, Boston, MA).
3 Results
No adverse reactions were observed in the heifers after intramammary ceftiofur hydrochloride administration. The median time (and range) from study enrollment to calving for groups TS, NT, 14, and 21 was 20.5 days (14–29), 17 days (12–27), 13 days (9–16), and 19.5 days (15–22), respectively. Upon calving, four heifers received dexamethasone for the treatment of udder edema. Of the four, three heifers belonged to the non-antimicrobial treatment groups (TS, 1; NT, 2) and one belonged to the antimicrobial treatment group, 21. The heifer belonging to group 21 needed assistance during parturition. Two cases of clinical mastitis were briefly observed from one heifer in the NT group and one in the 14 group. Both heifers had visible changes in milk appearance (clotted and discolored milk). The heifer's milk in the NT group presented this way for only one milking the day after calving while the heifer's milk in the 14 groups presented poor milk appearance for three consecutive days before spontaneously recovering. All 24 heifers were used for milk analysis.
3.1 Teat Scores
All heifers had four functional mammary quarters at the time of enrollment. With the exception of one heifer in the NT group, all animals received teat scores of 1 (healthy appearance with no abrasions or scabbing). The heifer in the NT group had one teat that scored a 2 (residual damage from healing scabs). Due to the consistency of teat appearance, teat scores were not evaluated for their effect on the efficacy of treatment or antimicrobial milk residues.
3.2 Somatic Cell Count
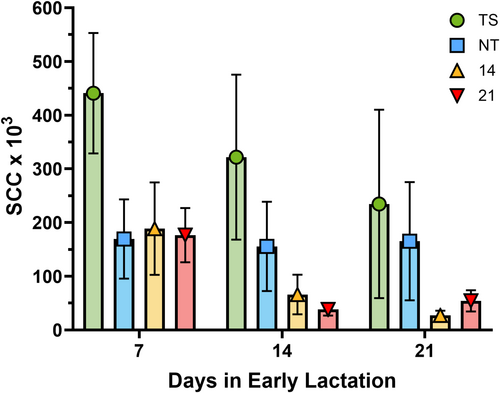
Unfortunately, all pretreatment lacteal secretions and some milk samples collected soon after calving could not be analyzed as they were too thick to flow through the DCC properly. The DCC had a measuring range of 10,000 to 4,000,000 cells/mL. No milk sample exceeded 4,000,000 cells/mL, but samples that measured below the lower LOQ were given a fixed SCC value of 10,000 cells/mL. Figure 2 compares mean SCC measurements at 7, 14, and 21 days post-calving. There may be apparent treatment efficacy differences, but the analysis suggests there were no statistically significant differences (p < 0.05) in SCC measurements among the different treatment groups or over time within the same treatment groups. Table 1 depicts the prevalence of SCM among heifers in all treatment groups out to 21 days post-calving. SCM was confirmed based on SCC measurements exceeding 200,000 cells/mL. There were no statistically significant differences (p < 0.05) in SCM prevalence among the different treatment groups.
| Time (days) | NT, % (n) | TS, % (n) | 14, % (n) | 21, % (n) |
|---|---|---|---|---|
| 1 | 100 (1/1) | 100 (1/1) | 66 (2/3) | 100 (3/3) |
| 2 | 100 (5/5) | 80 (4/5) | 60 (3/5) | 33 (2/6) |
| 3 | 50 (3/6) | 83 (5/6) | 83 (5/6) | 50 (3/6) |
| 4 | 50 (3/6) | 83 (5/6) | 50 (3/6) | 50 (3/6) |
| 5 | 33 (2/6) | 66 (4/6) | 33 (2/6) | 66 (4/6) |
| 6 | 33 (2/6) | 66 (4/6) | 33 (2/6) | 33 (2/6) |
| 7 | 33 (2/6) | 66 (4/6) | 33 (2/6) | 33 (2/6) |
| 14 | 17 (1/6) | 50 (3/6) | 17 (1/6) | 0 (0/6) |
| 21 | 17 (1/6) | 17 (1/6) | 0 (0/6) | 0 (0/6) |
- Abbreviations: 14, heifers received intramammary ceftiofur hydrochloride and internal teat sealant 14 days prior to expected calving (n = 6); 21, heifers received intramammary ceftiofur hydrochloride and internal teat sealant 21 days prior to expected calving (n = 6); n, milk samples collected from each heifer that could properly flow through the DCC; NT, heifers received no treatment (n = 6); TS, heifers received only internal teat sealant (n = 6).
- a Subclinical mastitis (SCM) confirmed based on SCC measurements exceeding 200,000 cells/mL.
3.3 Milk Residues
Figure 3 displays milk concentration–time curves for groups 14 and 21 following antimicrobial treatment with intramammary ceftiofur hydrochloride at one syringe/quarter. Ceftiofur concentrations, measured as DCA, were not present above the FDA milk tolerance for cattle of 100 ng/mL in any milk sample. Drug concentration analysis showed that 87% and 94% of milk sample concentrations fell below our LOQ of 5 ng/mL for groups 14 and 21, respectively. A noncompartmental analysis (NCA) of ceftiofur concentration versus time profiles for the antimicrobial-treated heifers performed with analytical software (Phoenix Win-Nonlin 8.0; Certara Inc.) was attempted. However, not enough values were measured above our LOQ to accomplish this. Due to the unreliability of our data, the NCA was not performed, and only approximate concentration values were reported. The mean peak milk concentration (Cmax ± SD) for groups 14 and 21 in this study reached 8.14 ± 6.24 and 4.20 ± 5.07 ng/mL 48 h into lactation (Tmax), respectively.

4 Discussion
This study is the first to report on the efficacy and milk residue profile of 125 mg intramammary ceftiofur hydrochloride lactating cow formulation in prepartum dairy heifers. On average, 87% and 94% of all milk samples fell below our LOQ of 5 ng/mL for groups 14 and 21, respectively, demonstrating that the risk of violative residues from this practice is low (Figure 3). Milk had a maximum DCA (ceftiofur equivalents) concentration of approximately 8.14 ± 6.24 and 4.20 ± 5.07 ng/mL 48 h into lactation for groups treated 14 or 21 days prior to expected calving, respectively. We did not find significant differences in SCC among the different treatment groups or within the same treatment groups over time. It is important to note that a cow's SCC in milk will typically be higher immediately following calving due to the production of colostrum (Barkema et al. 1999). The mean SCC for groups 14, 21, and NT was below the SCM threshold of 200,000 cells/mL at days 14 and 21 post-calving, but group TS exceeded this.
Prior research has evaluated the efficacy of intramammary lactating cow therapy administered to prepartum heifers. Oliver et al. (1992, 2003) observed significant cure rates in heifers treated with sodium cloxacillin or cephapirin sodium compared with untreated controls after infusion at 7 to 14 days prior to calving. They also found a significant reduction in SCC and an increase in milk production over the first lactation. Middleton et al. (2005) observed a greater cure rate for heifers treated with pirlimycin 10–14 days prior to calving compared with untreated controls, but a reduction in SCC was not consistent, and no effect on milk yield was observed. Borm et al. (2006) observed greater cure rates in heifers treated with cephapirin sodium 10–21 days prior to calving compared with controls but found no effects on SCC or milk production.
Similarly, others have evaluated the efficacy of intramammary dry cow therapy administered to prepartum heifers. Nickerson et al. (2020) administered the dry cow formulation of ceftiofur hydrochloride (500 mg) 2 months before the heifers were expected to calve. They concluded that no treatment was just as beneficial as administering dry cow therapy, teat sealant, or a combination of the two if the quarters were uninfected at the time of treatment. However, most heifers had at least one infected quarter at the time of enrollment. For these infected quarters, dry cow therapy was most successful (100%) at curing the infection upon entering early lactation, followed by combination therapy (96.1%) and sole teat sealant therapy (85.7%) in comparison to no treatment (p < 0.001).
There are several limitations to this study. Four individuals administered treatment and scored teats; this introduced potential variability. Heifers were also not randomly allocated to treatment groups, as this would have required a large number of dosing days. Instead, heifers were grouped based on proximity to calving and allocated to treatment groups that aligned best with the availability of farm staff on dosing days. This introduced potential selection bias. A randomized controlled trial is preferred. Because there was substantial variability present in SCC measurements within each treatment group, more animals would have to be enrolled in future studies to detect a significant difference between them, if one exists. The sample size for this study was selected to address the risk of violative residues, which likely led to too small a sample size to demonstrate differences in SCC. To prevent this from moving forward, power analyses should be conducted prior to the start of a study to adequately detect meaningful differences in treatment efficacy and residue concentrations. Additionally, our cell counter could not quantify the pretreatment lacteal secretions collected from each heifer due to their high viscosity. It would have been beneficial to compare pre-calving and post-calving SCC across the different treatment groups. The lacteal secretion samples should be diluted in future studies with a suitable buffer to measure SCC accurately. This study was conducted at a small-scale, pasture-based dairy, which was beneficial for evaluating the effect of these environmental conditions but may not represent heifer housing at large-scale dairies. It would also be preferable to collect representative samples of the total milking volume at each collection time point, but the dairy did not have a way to do this. Instead, quarter milk samples had to be collected, which may not be the most accurate representation of residues present at that time.
In the future, conducting a bacteriological or metagenomic analysis to detect causative and potentially resistant pathogens would be beneficial. Identifying these pathogens could aid in creating tailored control and treatment strategies for mastitis in prepartum heifers. In 2008, the FDA placed prohibitions on the use of cephalosporins in food animals due to concerns about the movement of foodborne bacteria between livestock and humans and evidence of cross-resistance among drugs in the cephalosporin class (Davis et al. 2009). Assessing AMR after the use of ceftiofur in this application would be beneficial. It may also be appropriate to evaluate the efficacy and pharmacokinetics of the dry cow formulation of ceftiofur hydrochloride, which contains 500 mg per syringe, instead of the 125 mg used in this study at various time points prior to calving. Due to the higher dose in the dry cow formulation, additional residue studies should be performed.
In the United States, the extralabel use of third-generation cephalosporins is prohibited (Davis et al. 2009) with some exceptions under the Animal Medicinal Drug Use Clarification Act (AMDUCA). It is important to note that the use of the lactating ceftiofur hydrochloride formulation evaluated in this study or the dry cow formulation in prepartum heifers would be considered prohibited in the United States. There are other countries where the use of third-generation cephalosporins in food-producing animals is completely prohibited (i.e., the Netherlands), while in other countries, extralabel use is freely permitted. It is imperative that veterinarians and dairy producers follow the drug use laws of their country to ensure that antimicrobials are being used in a safe and legal manner. FARAD does not encourage prohibited extralabel drug use and conducted this research to explore the potential for violative residues in milk collected from treated heifers. With this pharmacokinetic data, FARAD is now able to improve its guidance for U.S. veterinarians and producers in establishing evidence-based milk withdrawal recommendations, specifically for accidental exposures. Additionally, this research serves to encourage veterinarians on a global scale to make educated and legal treatment decisions for their dairy herds based on their local regulations. For the sake of antimicrobial stewardship, it is encouraged that antimicrobials other than third-generation cephalosporins be considered as primary interventions with supported justification (susceptibility testing, SCC, California Mastitis Test (CMT), etc.) wherever possible if prophylactic treatment is desired for use in prepartum heifers. Third-generation cephalosporins are classified as the highest priority among the critically important antimicrobials; therefore, it is essential to use them sparingly and intentionally only in situations that necessitate their use (WHO 2024).
In conclusion, intramammary ceftiofur hydrochloride administered at least 14 days before the anticipated calving date poses little to no food-safety risks. Rules and regulations concerning legal and prohibited drug use should be followed in each country.
Author Contributions
G.W.S., R.E.B., D.M.F., J.L.H., and R.A.M. conceived and designed the study. R.A.M. collected samples and data. R.A.M. developed the analytical method and performed sample analysis. R.A.M. performed pharmacokinetic modeling. All authors were involved in the drafting and revising of the manuscript.
Acknowledgments
The authors thank the North Carolina Department of Agriculture—Cherry Livestock Research Program for providing the facilities and animals used in this study. We also thank Laura Neumann for her assistance with sample collection.
Ethics Statement
The study was approved by the North Carolina State University Institutional Animal Care and Use Committee (IACUC #21-172).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




