Pharmacokinetics of doxycycline after oral administration of single and multiple dose in broiler chickens
Abstract
As a semisynthetic tetracycline derivative, doxycycline (α-6-deoxy-5-hydroxytetracycline) is a time-dependent bacteriostatic agent. It is being widely used in the broiler husbandry in China. In this study, doxycycline was orally administered by gavage to 10 healthy broiler chickens at the dose of 20 mg/kg body weight every 24 hr for five total treatments. Plasma samples were collected from each animal at 5, 10, 20, and 30 min and 1, 2, 4, 6, 8, and 12 hr after the first dose, at 0.25, 0.5, 0.45, 1, 2, 3, 4, 6, 7, 13.5, 24, 36, 48, 60, and 72 hr after the last dose. Additional plasma samples were collected at a 24-hr interval during the dosing period (immediately prior to each oral administration). The doxycycline concentrations were determined by high-performance liquid chromatography with an ultraviolet detector and subjected to noncompartmental analysis. Then, the pharmacokinetics profiles were compared after the first and last oral doses. After the first dosing, the elimination half-life, area under the concentration–time curve from 0 hr to ∞, peak concentration, time to reach peak concentration, and volume of distribution per fraction absorbed were determined as 7.78 hr, 94.19 μg·hr/ml, 5.65 μg/ml, 3.50 hr, and 2,502.65 ml/kg, respectively, while the corresponding values of these parameters after the last dose were 19.90 hr, 121.08 μg·hr/ml, 5.71 μg/ml, 7.25 hr, and 5,285.28 ml/kg, respectively. After multiple oral doses, the absorption and elimination both became slower, while the distribution was more extensive than that following a single dose. However, after multiple oral doses, accumulation of doxycycline in plasma was not observed with an average accumulation factor of 1.11.
Doxycycline (α-6-deoxy-5-hydroxytetracycline) is a semisynthetic tetracycline derivative, which has a broad spectrum of activity including gram-positive and gram-negative bacteria, chlamydias, rickettsias, mycoplasmas, and spirochaetes (Yang, Li, Shan, & Zeng, 2014). It inhibits these micro-organisms in a time-dependent manner. As hyclate salt, it was approved to treat infections in poultry in China (Commission of Chinese Veterinary Pharmacopoeia, 2015), and its available dosage forms indicated in poultry included powder and water soluble powder (Commission of Chinese Veterinary Pharmacopoeia, 2015).
The pharmacokinetics of doxycycline have been studied in different bird species, such as broiler chickens (Anadon et al., 1994; Gbylik-Sikorska, Gajda, & Posyniak, 2018; Yang et al., 2013), layer chickens (Yang et al., 2016), ducks (Bratoev, Milanova, Pavlova, & Lashev, 2016; Yang, Sun, Zhao, Wang, & Wang, 2015), ostriches (Abu-Basha, Idkaidek, & Hantash, 2006), and quails (Pavlova, Lukanov, Ivanov, Petrova, & Genchev, 2018). In China, repeated doses of doxycycline were always administered to broiler chickens via medicated feed or drinking water. However, its pharmacokinetics profiles after multiple oral doses are very limited in broiler chickens. Most studies focused on its pharmacokinetics after single dose. In addition, there are currently no studies comparing the single- and multi-dose pharmacokinetics profiles of doxycycline in broiler chickens. Therefore, the primary objective of this study was to compare the pharmacokinetics characteristics of doxycycline following single and multiple oral doses in broiler chickens.
This study was approved by Institutional Animal Care and Use Committee at Henan University of Science and Technology. Ten healthy broiler chickens (Hubbard × Hubbard) of both sexes with age of 35–40 days and body weight (BW) ranging from 1.5 to 1.7 kg were enrolled in this study. Water soluble powder of doxycycline hyclate was obtained from Luoyang Huizhong Animal Medicine Co. Ltd (Luoyang, Henan, China) and diluted with 100 ml of sterile water to prepare oral solution at the final concentration of 50 mg/ml. Doxycycline solution was administered by oral gavage with an appropriate length plastic catheter at a dose of 20 mg/kg BW QD for a total of five doses. Blood samples were collected from each individual at 5, 10, 20, and 30 min and 1, 2, 4, 6, 8, and 12 hr after the first dose, at 0.25, 0.5, 0.45, 1, 2, 3, 4, 6, 7, 13.5, 24, 36, 48, 60, and 72 hr after the last dose. Additional samples were collected at a 24-hr interval during the dosing period (immediately prior to each oral administration). Blood samples (about 0.5 ml) were collected from the wing vein and collected into heparinized tubes using the syringe coated by heparin. Then plasma was collected by centrifugation at 2,000 g for 10 min and stored at −20°C until further analysis. In this study, another five chickens served as control group, and they were not treated with doxycycline. Plasma samples were collected from them simultaneously with the treatment group and then used for drug quantitation and method validation.
Concentrations of doxycycline in plasma samples were determined by high-pressure liquid chromatography (HPLC) with an ultraviolet detector utilizing a previously validated method (Yang et al., 2012, 2013, 2015, 2016). Calibration standards were made by spiking blank plasma with doxycycline to concentrations of 0.1, 0.2, 0.5, 1, 2, 5, and 10 μg/ml. Samples (0.3 ml) underwent an extraction by adding 250 μl of buffer/EDTA (0.1 mol/L sodium phosphate, containing 0.1 mol/L disodium EDTA) and 50 μl of 20% perchloric acid. After vortexing for 3 min, samples were centrifuged at 12,000 g for 15 min. A total of 50 μl of supernatant were subjected to HPLC analysis. The mobile phase consisted of acetonitrile and 0.01 mol/L trifluoroacetic acid (3:7, v/v) at a flow rate of 1 ml/min. Separation was achieved with a C18 column (Hypersil BDS C18; 4.6 × 250 mm, 5 μm; Elite analytical instruments Co., Ltd, Dalian, China) maintained at 30°C. The wavelength was set at 350 nm for ultraviolet detector.
The HPLC assay method presented here was found to be linear and reproducible across the range from 0.1 to 10 μg/ml. In order to monitor the accuracy and precision of this method, three replicates of doxycycline at concentrations of 0.1, 1, and 10 μg/ml were detected for three consecutive days to evaluate the recoveries and coefficients of variation. The results showed that all recoveries were above 81.47% with an average value of 86.19%, and all interday and intraday coefficients of variation were below 7.13%. The limits of detection (LOD) and quantitation (LOQ) based on a signal-to-noise ratio >3 and >10 were 0.05 and 0.1 μg/ml, respectively. The HPLC method presented here met the requirements (Ministry of Agriculture and Rural Affairs of the People's Republic of China, 2009).
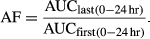
The mean concentrations of doxycycline after multiple oral doses are presented in Figure 1. And the pharmacokinetics parameters after single and multiple oral doses of doxycycline are summarized in Table 1. After the first and last doses, the parameters of Cmax and AUC0–24 hr shared similar values and had no significant differences, while tmax after last dose was later than first dose and Cl/F was slower than the first dose but both without significant differences for tmax and Cl/F. The values of t1/2λz, AUC0–∞, AUMC0–∞, Vz/F, and MRT after the last dose were all significantly greater than the corresponding values after the single dose, while the λz for Day 5 was significantly slower than that for Day 1. The mean accumulation factor for doxycycline was determined as 1.11, showing no accumulation was observed in plasma after five consecutive oral doses with an interval of 24 hr.
| Parameter | Unit | After the 1st dose | After the last dose |
|---|---|---|---|
| λz | hr−1 | 0.09 ± 0.02 | 0.04 ± 0.02a |
| t1/2λz | hr | 7.78 ± 1.37 | 19.90 ± 8.46a |
| tmax | hr | 3.50 ± 2.06 | 7.25 ± 4.48 |
| Cmax | μg/ml | 5.65 ± 1.33 | 5.71 ± 2.11 |
| AUC0–24 hr | μg·hr/ml | 80.38 ± 17.57 | 87.29 ± 28.86 |
| AUC0–∞ | μg·hr/ml | 94.19 ± 22.30 | 121.08 ± 33.45a |
| AUMC0–∞ | μg·hr2/ml | 1,246.25 ± 402.27 | 2,540.90 ± 503.68a |
| Vz/F | ml/kg | 2,502.65 ± 637.05 | 5,285.28 ± 3,166.99a |
| Cl/F | ml·hr−1/kg | 229.38 ± 76.40 | 175.14 ± 38.25 |
| MRT | hr | 13.02 ± 2.07 | 21.51 ± 3.39a |
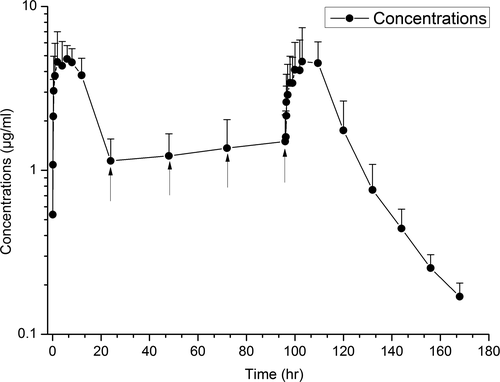
| AF | Unitless | NA | 1.11 ± 0.32 |
Notes
- λz: apparent elimination rate constant; t1/2λz: apparent elimination half-life; tmax: time to reach peak concentration; Cmax: the peak concentration; AUC0–24 hr: area under the concentration–time curve from 0 to 24 hr; AUC0–∞: area under the concentration–time curve from 0 to ∞; AUMC0–∞: area under the first moment curve from 0 to ∞; Vz/F: volume of distribution per fraction absorbed; Cl/F: clearance per fraction absorbed; MRT: mean residue time; AF: accumulation factor.
- a Significantly different from the corresponding parameter after the first dose (p < 0.05).
To our knowledge, the pharmacokinetics profiles were compared for the first time for doxycycline after single or multiple oral doses in broiler chickens. After multiple administration, doxycycline was absorbed more slowly than the first dose. And the average tmax was extended from 3.5 to 7.25 hr (Table 1). We did not know the real reasons for this discrepancy. However, the intake process might have some effects on the absorption of doxycycline. After both first and last dosing, there were large differences in the tmaxs among different individuals (data not shown), which ranged from 1 to 6 hr and from 2 to 13.5 hr, respectively. These big differences of tmaxs among individuals might result from the food effect. With the exception of the time of dosing and blood sampling, all animals used in the present study had free access to feed throughout the experiment. Therefore, their intake time and/or amount might differ greatly. The food effect on the oral pharmacokinetics profile of doxycycline has been indicated in broiler chickens by a previous study (Laczay, Semjen, Lehel, & Nagy, 2001), which showed that the feed significantly reduced and extended the absorption of doxycycline. Therefore, in order to find the true cause of the discrepancy in tmaxs after the first and last doses, pharmacokinetics study should be further carried out in broiler chickens fed at fixed times.
After multiple dosing, the Vz/F was expanded from 2.5 to 5.3 L/kg (Table 1), indicating that the distribution after multiple doses was more extensive than that following a single dose. We did not know the real cause to this difference. However, the possible changes of protein binding of doxycycline in plasma and tissues would have effects on its distribution after multiple doses. If its free fraction in plasma increases or its free fractions in the tissues become smaller after multiple dosing, the value of Vz/F will increase significantly. Additional studies should be performed to clarify this hypothesis about the possible changes of protein binding in plasma and tissues.
After multiple oral doses, the elimination of doxycycline became significantly slower. And the value of λz was slowed from 0.09 to 0.04 hr−1 (Table 1). According to the report of EMA (1996), doxycycline was mainly eliminated by hepatic metabolism and bile excretion. Up to 40% of doxycycline was metabolized to inactive forms, and the others were largely excreted in feces. The differences between the elimination rates might be due to the possible hepatic enzyme saturation after the multiple oral administration. However, this possible reason was not validated in the present study.
The pharmacokinetics of doxycycline following single oral administration at the same dose of 20 mg/kg BW has been published in broiler chickens (Anadon et al., 1994). The t1/2λz calculated here (7.78 hr) was close to that reported by Anadon et al. (1994); however, the values of Cmax and AUC0–∞ determined here (5.65 μg/ml and 94.19 μg·hr/ml, respectively) were both smaller than the corresponding values in the previous study (54.58 μg/ml and 214.21 μg·hr/ml, respectively). The reason for these differences might be due to the food effect mentioned above: Food was withheld in the previous study (Anadon et al., 1994).
The same dose and administration method have been applied in broilers by Gbylik-Sikorska et al. (2018); however, only the pharmacokinetics parameters after the last dose were calculated. As reported by them, the t1/2λz (14.7 hr) was shorter than that reported here (19.90 ± 8.46 hr); the tmax (2 hr) and Cmax (2.13 μg/ml) were later and smaller than those in the present study, which are 7.25 hr and 5.71 μg/ml, respectively. These inconsistencies might stem from the differences in drug preparations and/or broiler species. In another study (Hsiao, Chang, Hsu, Li, & Chou, 2016), doxycycline was administrated for 5 consecutive days via drinking water at a high dose of approximately 40 mg/kg BW. And a similar MRT (22.7 hr) was reported; however, the t1/2λz (14.9 hr) was shorter than that in the present study (19.90 hr).
The pharmacokinetics profiles of doxycycline were also reported in other bird species. The t1/2λz (7.78 hr) reported here after one single oral dose was shorter than those in ducks and ostriches (17.65 and 19.25 hr, (Yang et al., 2015) and (Abu-Basha et al., 2006), respectively). The tmax (3.5 hr) observed here in broilers was later than those in laying hens (1.73 hr; Yang et al., 2016), ducks (2 hr; Yang et al., 2015), and ostriches (3.03 hr; Abu-Basha et al., 2006). The Cmax (5.65 μg/ml) reported here was similar with that in layer chickens (5.88 μg/ml) after the same oral dose, however, lower than that in ducks (17.57 μg/ml; 20 mg/kg BW; Yang et al., 2015) and higher than that in ostriches (0.3 μg/ml; 15 mg/kg BW; Abu-Basha et al., 2006).
In the previous pharmacokinetics studies of doxycycline in broilers (Anadon et al., 1994; Atef, Youssef, El-Eanna, & El-Maaz, 2002; El-Gendi, Atef, Amer, & Kamel, 2010; Gutierrez, Zermeno, Alcala, & Sumano, 2017; Ismail & El-Kattan, 2004), compartmental models were used to determine the pharmacokinetics parameters. Initially, the compartmental method was also used in the current study. However, we found that the models that were most suitable for different individuals’ concentration and time data on days 1 and 5 were not the same. On Day 1 or Day 5, the best model was one compartment for some individuals, but for others it was two-compartment model. The main objective of this study was to compare the pharmacokinetics parameters of doxycycline after single and multiple oral administration, and if the compartmental models were used, different parameters would be determined for different chickens, and these parameters could not be further compared between different individuals or on Days 1 and 5. Therefore, we eventually used a noncompartmental model to obtain the same type of parameters on the first and fifth days. Then, the pharmacokinetics comparison was further performed for doxycycline.
In China, doxycycline was commonly applied in broiler chickens via drinking water. However, the main purpose of this study was to compare the pharmacokinetics profiles of doxycycline following single and multiple oral doses. The multiple doses of administration via drinking water would provide sustained exposure of doxycycline, and it was impossible to compare the pharmacokinetic differences between single and multiple doses. Another common route of administration for doxycycline is through medicated feed. Therefore, the current study can be expanded to perform the pharmacokinetics comparisons in broilers fed at fixed time using medicated feed.
In conclusion, after multiple oral doses, the rates of absorption and elimination were both slower, and the distribution was more extensive than that following a single dose. However, after multiple oral doses, accumulation of doxycycline in plasma was not observed in the current study.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (grant No. U1604107 and 31402253) and China Scholarship Council (grant No. 201608410275).
CONFLICT OF INTEREST
All authors have no conflict of interest.
AUTHOR CONTRIBUTION
Fan Yang conceived the presented idea. Fang Yang and Guoyong Wang performed the pharmacokinetics experiments in broilers. Tao Kong and Fan Yang determined the concentrations of doxycycline in plasma samples. Fan Yang performed the noncompartmental analysis and wrote this manuscript with support from Guoyong Wang. All authors have read and approved the final manuscript.




