Pharmaco-parasitological evaluation of the ricobendazole plus levamisole nematodicidal combination in cattle
Abstract
The goals of the current study were to evaluate the potential pharmacokinetic (PK) interactions and the clinical efficacy occurring after the subcutaneous (s.c.) administration of ricobendazole (RBZ) and levamisole (LEV) given both separately and co-administered to calves naturally infected with susceptible gastrointestinal nematodes. The clinical efficacy was shown in two seasons, winter and spring, with predominance of different nematode populations. Groups of 15 calves were treated with RBZ alone, LEV alone and RBZ + LEV combination, and an untreated group was kept as a Control. RBZ and LEV plasma concentrations were quantified by HPLC. The clinical efficacy was determined by the faecal egg count reduction test. RBZ and LEV have similar plasma persistence, being detected in plasma over 24 hr post-treatment. No PK interactions were observed after the combined treatment, with similar PK parameters (p > .05) obtained for the single-drug and the combination-based strategy. In winter, the observed clinical efficacies were 96%, 99% and 100% for groups treated with RBZ, LEV and RBZ + LEV, respectively; however, in spring, the efficacies were 95%, 93% and 96% for the same groups. Remarkably, the combination was the only treatment that achieved 100% clinical efficacy against both Haemonchus spp and Ostertagia spp in winter; but the increased presence of Ostertagia spp. in spring (28% in untreated group) determined a tendency to reduced efficacies compared to winter time (only 10% of Ostertagia spp. in untreated group), even for the combined treatment. Overall, in a scenario where the nematode population is susceptible, the RBZ + LEV treatment may be a valid combination in cattle to delay the development of resistance, especially in winter when this combination achieved 100% of efficacy. Thus, selection of anthelmintic resistance will never occur. In fact, this is one of the greatest challenges for the whole cattle production system: to be one step ahead of anthelmintic resistance.
1 INTRODUCTION
Gastrointestinal (GI) parasitism is among the most important production-limiting diseases of grazing ruminants (Charlier et al., 2015). Inadequate anthelmintic drug uses, among other factors, have led to the current scenario of anthelmintic resistance, considered a main concern in veterinary medicine today (Kaplan & Vidyashankar, 2012). In the grazing systems of cattle production in Argentina, ivermectin (IVM) is definitely the most compromised anthelmintic. Resistance to IVM was diagnosed in 90% of the farms, while resistance to ricobendazole (RBZ) (the active sulphoxide derivative of albendazole—ABZ) was only diagnosed in 29% of the farms included in a survey carried out by Fiel et al. (2015). Resistance to levamisole (LEV) was not found in any farm (Fiel et al., 2015). In an attempt to manage anthelmintic resistance in ruminants, drug combination of two or more anthelmintic compounds has been proposed in some geographic regions as a strategy to delay the development of resistance (Anderson, Martin, & Jarret, 1988; Geary et al., 2012).
One of the most important prerequisite criteria to maximize the ability of multiple active formulations to slow the development of resistance is the pre-existing levels of resistance to each of the anthelmintics in the combination. Ideally, the use of nematodicidal combinations may be a valid strategy if the efficacy of each of the anthelmintic molecules approaches 100% (Bartram, Leathwick, Taylor, Geurden, & Maeder, 2012). This would be the ideal situation to use a nematodicidal combination.
As previously mentioned, in Argentina, as the RBZ resistance is only present in 29% of the farms, and the LEV resistance has not been shown yet (Fiel et al., 2015), a combination of both drugs would be the closest to the ideal nematodicidal combination to use in cattle farms in order to slow the development of resistance. RBZ is a broad-spectrum anthelmintic compound, which is effective against lungworms and GI nematodes (Campbell, 1990). In some Latin American countries, including Argentina, RBZ is formulated as an aqueous solution for subcutaneous (s.c.) injection to cattle, a route of administration that is widely accepted by veterinarians and farmers. LEV is an imidazothiazole compound, which is also effective against lungworms and GI nematodes (Courtney & Roberson, 1995). Additionally, the purpose of this combination is based on the different mechanism of anthelmintic action of each active ingredient. LEV causes a spastic paralysis by selectively gating acetylcholine receptor ion channels on nerve and muscles (Robertson & Martin, 1993), while the intrinsic anthelmintic action of RBZ is based on a progressive disruption of basic cell functions as a result of their binding to parasite β-tubulin and depolymerization of microtubules (Lacey, 1990).
Although several pharmaceutical formulations combining either two or three chemical classes have been developed for small ruminants, the available information about drug interactions is rather limited in bovine livestock. It is necessary to determine the pharmacokinetic (PK) and pharmacodynamic (PD) behaviours, which may be altered when two or more anthelmintic drugs are administered simultaneously under natural field conditions. Therefore, pharmaco-parasitological studies are required before drug combinations are used for anthelmintic control in cattle.
The goals of this study were to evaluate the potential PK interactions and the clinical efficacy of RBZ and LEV given both separately and co-administered to calves naturally infected with susceptible GI nematodes. The clinical efficacy was shown in two seasons, winter and spring, with predominance of different nematode populations.
2 MATERIALS AND METHODS
2.1 Field Trial
This study was conducted in “Don Ernesto,” a cattle commercial farm with a grazing system of meat production representative of the Argentina bovine production. “Don Ernesto” is a 450-ha beef cattle grazing system located in the Humid Pampean Region, Argentina. The parasitological study was carried out in two phases, winter and spring, in the same commercial farm. The results of the parasitological trial in winter led to a subsequent study in spring aimed to evaluate the clinical efficacy with a different nematode population. The animals were kept under natural field conditions during the whole experimental period.
2.2 Animals
In both seasons, sixty male calves naturally infected with GI nematodes susceptible to RBZ and LEV were included in the trial. The selection of the animals was based on worm egg per gram (EPG) counts. On day -1, all calves were checked for EPG counts, ear tagged, and the individual body weights were recorded. In winter, animals with at least 300 EPG on day -1 were included in the study, while in spring, the lower limit was 100 EPG. Animals had an average of 863 EPG counts ranging from 320 to 1940 in winter, while the average in spring was 301 EPG ranging from 120 to 580. Animals had been grazing on the same festuca (Festuca arundinacea) pasture for 2 months before starting the study and during the experiment in both seasons, ensuring that their parasite load was native from “Don Ernesto” farm, and that they were infected by the same GI nematode strains during the whole trial. All animals had free access to water. Animal procedures and management protocols were approved by the Ethics Committee according to the Animal Welfare Policy (act 087/02) of the School of Veterinary Medicine, Universidad Nacional del Centro de la Provincia de Buenos Aires (UNCPBA), Tandil, Argentina.
2.3 Treatments
All parasitized animals (n = 60) were randomly assigned into four groups of 15 animals each according to their EPG counts. The mean EPG at day -1 was similar across all groups. Experimental animals received one of the following treatments by the s.c. route on day 0: RBZ (Bayverm PI®, 15% solution, Bayer, Argentina) administered at 3.75 mg/kg; LEV (Ripercol L Fosfato®, 18.8% solution, Zoetis, Argentina) administered at 8 mg/kg; or both RBZ (Bayverm PI®) and LEV (Ripercol L Fosfato®) administered at 3.75 and 8 mg/kg, respectively. For the efficacy trial, an untreated group was kept as Control.
2.4 PK trial
The PK trial was carried out in winter. Eight randomly selected animals from each RBZ, LEV and RBZ + LEV treated groups were used in the PK trial. Blood samples (10 ml) were taken from the jugular vein in heparinized Vacutainer® tubes (Becton Dickinson, NJ, USA) prior to treatments and at 2, 4, 6, 8, 10, 12, 16, 20 and 24 hr post-treatment. Plasma was separated by centrifugation at 3000 g for 15 min, placed into plastic tubes and frozen at −20°C until analysis by high-performance liquid chromatography (HPLC).
2.5 Clinical efficacy trial: faecal egg count reduction and coprocultures
Faecal samples were individually collected from the rectum of each calf during pretreatment (day -1) and on day 15 post-treatment. EPG counts were performed by a modified McMaster technique with a sensitivity of 10 EPG (Roberts & O'sullivan, 1950). Additionally, the genera and species of the nematodes recovered from parasitized calves were determined by the identification of the third-stage larvae (L3) recovered from individual and pooled faecal cultures obtained from each experimental group (MAFF, 1986).
Third-stage larvae (L3) were collected by the Baermann technique, and 100 larvae were differentiated from each sample. Thus, the relative participation of each genus per experimental group was determined.
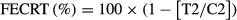
2.6 Analytical procedures
2.6.1 RBZ analysis
Plasma samples (1000 μl) were spiked with oxibendazole (OBZ) as internal standard. RBZ was extracted from plasma as previously described (Alvarez et al., 2008), using C18 SPE cartridges (Strata®, 100 mg, Phenomenex, CA, USA). Spiked samples were placed into preconditioned cartridges. Then the compounds were eluted with methanol and concentrated to dryness under a stream of nitrogen. Finally, samples were reconstituted with 100 μl of acetonitrile and 200 μl of water. Fifty microlitre of this solution was injected directly into the chromatographic system. RBZ plasma concentration was determined by HPLC (Shimadzu 10 A-HPLC System, Kyoto, Japan) with a UV detector set at 292 nm following a method previously developed (Alvarez, Sanchez, & Lanusse, 1999). Identification of RBZ was undertaken in comparison with the retention time of pure reference standard. Retention times for RBZ and OBZ were 4.32 and 9.32 min, respectively. There was no interference of endogenous compounds in the chromatographic determinations. Calibration curve in the range between 0.05 and 1 μg⁄ml was prepared. Plasma calibration curve had a correlation coefficient ≥ 0.998. Mean absolute recovery percentage for concentrations ranging between 0.05 and 1 μg⁄ml (n = 6) was 83.5% with coefficient of variation (CV) of 9.1%. Accuracy (expressed as the relative error) and precision (expressed as the coefficient of variation) were 7% and 4.4%, respectively, with a limit of detection (LOD) of 0.016 μg⁄ml. The limit of quantification (LOQ) was established at 0.05 μg⁄ml, which is the lowest concentration measured with a recovery higher than 70% and a CV < 20%.
2.6.2 LEV analysis
Plasma samples (1000 μl) were placed into C18 SPE cartridges (Strata®, 100 mg, Phenomenex, CA, USA) previously conditioned. They were sequentially washed with 1 ml of water, eluted with 1.5 ml of HPLC grade methanol and concentrated to dryness under a stream of nitrogen at 56°C in a water bath. The dried residue was reconstituted with 250 μl of mobile phase. Finally, 100 μl of this solution was injected into the chromatographic system. LEV concentrations were determined by HPLC using a Shimadzu HPLC system with autosampler (Shimadzu Corporation, Kyoto, Japan). HPLC analysis was undertaken using a C18 column (Phenomenex, 5 μm, 4.6 mm × 250 mm) and a phosphoric acid 85% in triethylamine/methanol/acetonitrile/water (0.32⁄0.5⁄15.5/83.36) mobile phase at a flow rate of 1.2 ml⁄min. There was no interference of endogenous compounds in the chromatographic determinations. Calibration curve was prepared in the range between 0.10 and 2 μg⁄ml. The linear regression lines for LEV analysed showed correlation coefficients of 0.999. The mean recovery percentage for concentrations ranging between 0.10 and 2 μg/ml (n = 6) was 77.3% with CV of 17.4%. Accuracy (expressed as the relative error) and precision (expressed as the coefficient of variation) were 5.9% and 7.1%, respectively, with a LOD of 0.02 μg⁄ml. The LOQ was calculated as described for RBZ. The LOQ was established at 0.10 μg/ml.
2.7 Pharmacokinetic analysis of the data
The concentration vs. time curves for RBZ and LEV in plasma for each individual animal after the different treatments were adjusted with the PK Solution 2.0 software (Summit Research Service, CO, USA). The peak concentration (Cmax) and time to peak concentration (Tmax) were displayed from the plotted concentration–time curve of each analyte. The absorption half-life (T½abs) and the elimination half-life (T½el) were calculated as ln2/kabs and ln2/λel, respectively, where kabs represents the first-order absorption rate constant and λel is the elimination rate constant. The area under the plasma concentration–time curve from zero up to the quantification time (AUC0–LOQ) was calculated by means of the trapezoidal rule (Gibaldi & Perrier, 1982) and further extrapolated to infinity (AUC0–∞) by dividing the last experimental concentration by the terminal elimination rate constant (λel). Statistical moment theory was applied to calculate the mean residence time (MRT) according to Perrier and Mayersohn (1982). PK analysis of the experimental data was performed using noncompartmental (area) and compartmental (exponential terms) methods without presuming any specific compartmental model.
2.8 Statistical analysis of the data
The PK parameters and concentration data are reported as arithmetic mean ± standard deviation (SD). Mean PK parameters for RBZ and LEV obtained after its administration both alone and co-administered were statistically compared using Student t test. Faecal egg counts (reported as arithmetic mean ± SD) were compared by nonparametric Kruskal–Wallis test. A value of p < .05 was considered statistically significant. The statistical analysis was performed using the Instat 3.0 software (Graph Pad Software, CA, USA).
3 RESULTS
3.1 Pharmacokinetic results
Figure 1 presents the mean (±SD) plasma concentration profiles for RBZ and LEV after their s.c. administration to parasitized calves. Similar plasma persistence was observed for both compounds, which could be detected for 24 hr post-treatment. The AUC0–LOQ for RBZ and LEV represents ≥95% of the AUC0–∞ for each compound, showing that the sampling time design was adequate. Both compounds were good-tolerated as no adverse events were observed in treated animals.
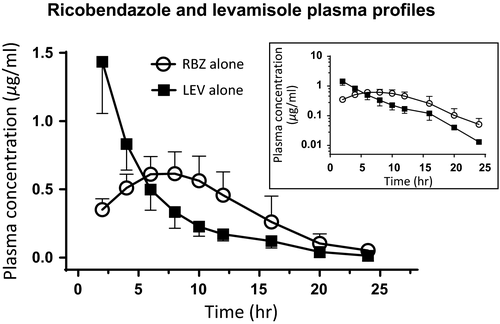
The mean (±SD) plasma concentration profiles for RBZ after the s.c. administration both alone and co-administered with LEV are shown in Figure 2. Table 1 summarizes the plasma PK parameters for RBZ obtained after those treatments. The presence of LEV did not affect the plasma disposition kinetics of RBZ after the s.c. administration. The AUC0–LOQ value of RBZ obtained after RBZ alone (8.20 ± 2.53 μg hr/ml) was similar to that obtained after the combined treatment (10.1 ± 4.01 μg hr/ml). Furthermore, no statistical differences between both treatments were observed for all PK parameters (p > .05).
| Pharmacokinetic parameters | Ricobendazole | Levamisole | ||
|---|---|---|---|---|
| RBZ alone | RBZ + LEV | LEV alone | LEV + RBZ | |
| Tmax (hr) | 7.43 ± 1.51 | 7.50 ± 1.41 | 2.00 ± 0.00 | 2.00 ± 0.00 |
| Cmax (μg/ml) | 0.64 ± 0.15 | 0.84 ± 0.32 | 1.43 ± 0.38 | 1.51 ± 0.55 |
| AUC0–LOQ (μg hr/ml) | 8.20 ± 2.53 | 10.1 ± 4.01 | 7.66 ± 2.12 | 9.07 ± 2.45 |
| AUC0–∞ (μg hr/ml) | 8.48 ± 2.61 | 10.4 ± 3.97 | 8.00 ± 2.26 | 9.30 ± 2.64 |
| MRT (hr) | 9.88 ± 0.91 | 10.5 ± 1.64 | 6.10 ± 1.49 | 6.50 ± 1.78 |
| T½abs (hr) | 1.79 ± 0.93 | 1.40 ± 0.50 | 1.07 ± 0.51 | 1.87 ± 1.44 |
| T½el (hr) | 3.81 ± 0.96 | 3.90 ± 1.20 | 6.74 ± 1.68 | 5.37 ± 2.05 |
- Tmax, time to peak plasma concentration; Cmax, peak plasma concentration; AUC0–LOQ, area under the plasma concentration vs. time curve from 0 to the quantification time; AUC0–∞, area under the concentration–time curve extrapolated to infinity; MRT, mean residence time; T½abs, absorption half-life; T½el, elimination half-life.
- For all pharmacokinetic parameters, p > .05.
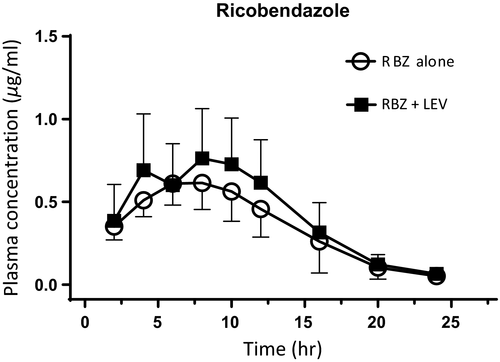
Figure 3 shows the mean (±SD) plasma concentration profiles for LEV after its s.c. administration both alone and co-administered with RBZ. Both treatments have similar PK profiles. Table 1 summarizes the plasma PK parameters for LEV both alone and co-administered with RBZ. Similar to what was observed for RBZ, the presence of RBZ did not affect the plasma disposition kinetics of LEV. Therefore, similar PK parameters were obtained for the single-drug and combination-based strategy (p > .05).
3.2 Parasitological results
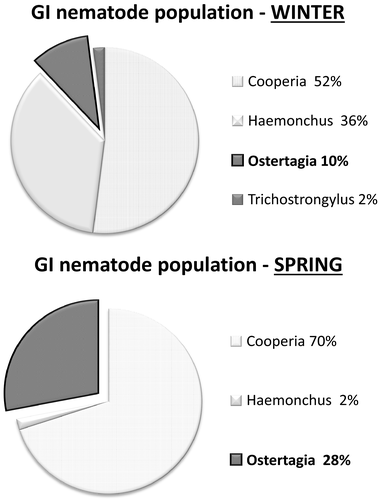
Figure 4a,b show some differences about nematode population between winter and spring in untreated animals. The most relevant variation was in terms of percentage of Ostertagia spp.; while only 10% of Ostertagia spp. was found in winter (Figure 4a), this genus increased up to 28% of the overall GI nematode population present in spring (Figure 4b). The mean EPG count of each control group demonstrates the high level of infection at day 0, showing differences in egg counts in both seasons. The EPG counts of the untreated animals at day 0 were 871 (320–1700) in winter, and dropped to 316 (120–460) in spring.
The overall faecal egg counts obtained for all experimental groups on day 15 after treatment in winter and spring are shown in Table 2, including the results of the FECRT and lower and upper confidence limits (95%). In winter, the faecal egg count reduction (FECR) at 15 days for all treatments was over 90%, demonstrating that the nematode population of this farm was susceptible to both drugs; but the combination was the only treatment that reached 100% clinical efficacy. However, in spring, the FECR for all treatments was high, but the combination did not reach 100% clinical efficacy as in winter. Regarding the EPG counts on day 15 post-treatment, no significant differences were found between the single-drug and combination-based strategy, neither in winter nor in spring (p > .05).
| Experimental group | Winter | Spring | ||||
|---|---|---|---|---|---|---|
| EPG counts (range) | FECRTa (CI) | EPG counts (range) | FECRTa (CI) | |||
| Day 0 | Day 15 | Day 0 | Day 15 | |||
| Control | 871a (320–1700) | 957a (140–2980) | – | 316a (120–460) | 202a (20–380) | – |
| RBZ alone | 864a (340–1940) | 37b (0–340) | 96% (83–99) | 298a (120–500) | 10b (0–40) | 95% (86–98) |
| LEV alone | 853a (340–1840) | 1.4b (0–20) | 99% (98–99) | 312a (120–480) | 14b (0–60) | 93% (80–97) |
| Combination RBZ+LEV | 865a (340–1760) | 0b (0–0) | 100% (100–100) | 280a (120–580) | 8b (0–40) | 96% (87–99) |
- EPG counts with different superscript letters are statistically different (p < .05).
- CI, lower and upper confidence intervals.
- a FECRT estimated according to Coles et al. (1992).
The clinical efficacies against Cooperia spp., Haemonchus spp., Ostertagia spp. and Trichostrongylus spp. for the different treatments in winter and spring are shown in Table 3. Although all the treatments achieved effective control against all the GI nematode genera, there were some relevant differences between the single-drug and the combination-based strategy. In winter, while some Haemonchus spp. specimens survived after RBZ alone treatment (90% FECR), some Ostertagia spp. specimens survived after the treatment with LEV alone (98% FECR). In contrast, the combination was the only treatment that achieved 100% clinical efficacy against Cooperia spp., Haemonchus spp., Ostertagia spp. and Trichostrongylus spp. However, in spring, the RBZ + LEV combination failed to control Ostertagia spp., showing a clinical efficacy of 89% against this genus. Definitely, in this season, the combined treatment did not kill all parasites as in winter (100% FECR). However, as the egg count allocated to this genus was low, these results should be interpreted with caution (the efficacy value against Ostertagia spp. does not mean anthelmintic resistance).
| Experimental group | FECRTa day 15 | |
|---|---|---|
| Winter (%) | Spring (%) | |
| Cooperia spp. | ||
| RBZ alone | 100 | 97 |
| LEV alone | 100 | 100 |
| RBZ + LEV | 100 | 100 |
| Haemonchus spp. | ||
| RBZ alone | 90 | 80b |
| LEV alone | 100 | 100 |
| RBZ + LEV | 100 | 100 |
| Ostertagia spp. | ||
| RBZ alone | 100 | 94 |
| LEV alone | 98 | 80b |
| RBZ + LEV | 100 | 89b |
| Trichostrong. spp. | ||
| RBZ alone | 100 | – |
| LEV alone | 100 | – |
| RBZ + LEV | 100 | – |
- a FECRT estimated according to Coles et al. (1992). FECRT (%) for all genera, p > .05.
- b The low egg counts associated with Ostertagia spp. and Haemonchus spp. could be determined that the efficacy values against this genus do not be anthelmintic resistance.
4 DISCUSSION
The principal aim of this study was to assess the pharmacokinetic and pharmacodynamic interactions after the combined use of RBZ and LEV in cattle under natural field conditions. The results of the parasitological trial in winter led to a subsequent study of the clinical efficacy in spring.
Due to the low levels of resistance to RBZ and LEV in Argentina (Fiel et al., 2015), these drugs are two of the most widely used anthelmintics in the country, and therefore, they may be combined to control GI nematodes in cattle. When these drugs are administered simultaneously under natural field conditions, it is necessary to determine the PK behaviours, which may be altered. Mean plasma concentration–time profiles obtained in the current study for both RBZ and LEV were similar to the results of previous studies in cattle (Dorn & Federmann, 1976; Lanusse et al., 1998). After s.c. administration of RBZ (3.75 mg/kg), a Cmax value of 0.64 ± 0.15 μg/ml and an AUC∞ of 8.48 ± 2.61 μg hr/ml were obtained. These results agree with those previously reported by Lanusse et al. (1998). The plasma Cmax observed after s.c. administration of LEV (8 mg/kg) was similar to that previously described by Dorn and Federmann (1976). In relation to the PK profiles of the drugs included in a combination, one prerequisite condition to slow resistance development is that anthelmintics in the combination have similar duration of persistent action (Bartram et al., 2012). The rationale behind this condition is to ensure that both anthelmintics are present together throughout the duration of efficacy of the combination. In this sense, RBZ and LEV have similar duration of action, and they could be detected during 24 hr post-treatment in cattle (Figure 1). Geary et al. (2012) reported that an adequate overlap of the time-to-kill curve for each component of a combination must be observed to ensure that they are present simultaneously in sufficient concentrations for sufficient duration to attain co-incident lethal exposures. Moreover, a major advantage of short-acting drugs compared to long half-life anthelmintics (e.g., macrocyclic lactones) is their lower impact on the development of anthelmintic resistance. Short-acting drugs prevent the exposure of the parasite population (including adult worms and L3 larvae ingested by treated animals) to the anthelmintic for extended periods, which results in a lower selection pressure compared to a drug with a long half-life (Dobson, Lejambre, & Gill, 1996; Prichard, 2002).
When an anthelmintic combination is administered, unfavourable PK interactions between constituent actives or excipients are possible and must be considered. In the current study, no adverse PK interactions were observed after the combined subcutaneous administration of RBZ and LEV in calves. The lack of interaction was demonstrated by the results of the PK trial in which there were no statistical differences for all PK parameters between the single-drug and combination-based strategy (Table 1). Furthermore, the plasma concentration vs. time curves obtained after both treatments (alone and combined) were almost identical (Figures 2 and 3). In contrast, some studies showed that the interaction between co-administered anthelmintic may induce changes in the PK behaviour of either molecule in lambs: a drug–drug PK interaction was found between ABZ and IVM (Alvarez et al., 2008) as well as between IVM, ABZ and LEV (Suarez et al., 2014) after their co-administration to lambs. Although limited information is available on the PK interactions between nematodicidal drugs in cattle, Leathwick et al. (2016) found that abamectin (ABM) bioavailability was significantly greater after the oral ABM + LEV combination than after the single-active oral administration in cattle. In contrast, the same study did not find any difference in LEV plasma concentrations between single and combined treatments (Leathwick et al., 2016). Similarly, no significant PK changes were observed in the current study for LEV after their s.c. co-administration with RBZ in calves. Furthermore, a similar plasma disposition was observed after the co-administration of ivermectin–closantel compared to that described after the treatment with each anthelmintic compound alone in cattle (Cromie, Ferry, Couper, Fields, & Taylor, 2006). Likewise, no PK interactions were observed after the combined s.c. administration of IVM and RBZ in cattle (Canton et al., 2017). Therefore, these previous reports of other anthelmintic combinations in cattle are in agreement with the findings of the present study reporting no adverse interactions between RBZ and LEV after their combined treatment. The observed PK data demonstrate that the co-administration of both anthelmintics did not modify the plasma PK behaviour of either drug in cattle.
In agreement with the results of the PK assessment, no significant differences in the overall clinical efficacy (FECR) were found between the single-drug and combination-based strategy. Although the co-administration of RBZ-LEV did not significantly improve the FECR, the combination was the only treatment that achieved 100% clinical efficacy in winter. This observed clinical efficacy for the combined treatment was almost as expected, that is additive anthelmintic effects between the two drugs (Bartram et al., 2012). An additive effect occurs when the combined effect of two drugs equals the sum of their independent activities measured separately (Prescott, 2000). This effect has been demonstrated in some trials performed with sheep and treated with an anthelmintic combination (Anderson, Martin, & Jarret, 1991; Anderson et al., 1988; Entrocasso et al., 2008; Mckenna, 1990b). Although published information in cattle is scarce, some preliminary results indicate that the combination of macrocyclic lactones and LEV was highly effective in minimizing the survival of resistant nematodes (Leathwick et al., 2016; Mason & McKay, 2006; Smith, 2014). Furthermore, the combination IVM + RBZ obtained significantly higher efficacy against IVM-resistant Haemonchus spp. than RBZ alone in cattle (Canton et al., 2017). Unlike these trials, which were performed in anthelmintic resistance scenarios, the current study was carried out in a susceptible scenario. In fact, the significant reduction in the total nematode egg counts 15 days after treatment supports the high efficacy of RBZ and LEV after their administrations alone in winter (96% and 99%, respectively). A scenario where the nematode population is susceptible represents the ideal situation for implementing drug combinations to be one step ahead of anthelmintic resistance. Indeed, one of the most important prerequisite criteria to maximize the ability of multiple active formulations is the pre-existing levels of resistance to each of the anthelmintics in the combination (Bartram et al., 2012). As indicated in modelling studies (Dobson et al., 2011; Leathwick, 2012; Leathwick, Waghorn, Miller, Candy, & Oliver, 2012), the key to successful use of anthelmintic combinations would be their administration before significant resistance (efficacy < 70%), to one or more of the active components, is developed. The use of nematodicidal combinations may be a valid strategy if the efficacy of each of the anthelmintic molecules approaches 100% (Bartram et al., 2012), as it did in the present work. Unlike the results obtained in winter, the overall clinical efficacy for the combined treatment in spring dropped to 96%. Similarly, FECR of RBZ and LEV alone also declined to 95 and 93%, respectively. The increased presence of Ostertagia spp. in spring (Figure 4b) may be a likely explanation for the reduced efficacies in this season compared to winter time. This interpretation is based on the lower activity of LEV against Ostertagia spp. than that against all other GI nematodes in cattle (Hart, James, & Curr, 1969). Furthermore, LEV is widely known to be ineffective against inhibited fourth-stage larvae of Ostertagia spp. (Williams, 1991), which is the predominant stage in naturally infected cattle during spring (Fernández, Fiel, & Steffan, 1999). The findings of this study are also consistent with those from a field trial in the United States, in which the overall efficacy of LEV, against all stages of Ostertagia ostertagi, was consistently low when inhibited fourth-stage larvae were predominant (Williams, Knox, Marbury, Swalley, & Eddi, 1991). The efficacy of RBZ against Ostertagia spp. could also be less than 100%. In fact, Steffan, Fiel, Ferreyra, and Monfrinotti (2002) found 95.7 and 50% absolute efficacy after the s.c. administration of RBZ at 7.5 mg/kg (double dose compared with the current study) and at 3.5 mg/kg (same dose as the current study), respectively. Overall, the increased presence of Ostertagia spp. in spring determined a tendency towards reduced efficacies compared to winter, even for the combined nematodicidal treatment.
Gastrointestinal parasitism in cattle always involves different parasite genera. Treatment with different anthelmintics administered simultaneously could be expected to lead to effective parasite control, because parasites that survive one active compound included in the combination could be killed by the activity of the other active compound (Geary et al., 2012; Lanusse, Lifschitz, & Alvarez, 2015). In fact, in the current study, the efficacy against Haemonchus spp. was 90% (RBZ), 100% (LEV) and 100% (RBZ + LEV), while the efficacy against Ostertagia spp. was 100% (RBZ), 98% (LEV) and 100% (RBZ + LEV). Remarkably, the combination was the only treatment that achieved 100% clinical efficacy against both parasite genera, Haemonchus spp. and Ostertagia spp. in winter. This level of efficacy would be the ideal situation given that selection of anthelmintic resistance will never occur if an anthelmintic treatment reaches 100% of efficacy against all nematode genera (Lanusse, Alvarez, & Lifschitz, 2014). In this context, the administration of combinations of anthelmintics with a similar spectrum of activity and different mechanisms of action, such as RBZ and LEV, has been suggested as an effective strategy to delay the development of resistance (Bartram et al., 2012). In contrast, as mentioned above, the increased presence of Ostertagia spp. in spring prevented the anthelmintic treatments from reaching 100% clinical efficacy against all nematode genera as in winter. These findings show that GI parasite genera involved in naturally infected calves are not negligible and should be taken into account.
This study has demonstrated that the RBZ + LEV treatment may be a valid combination in cattle because no adverse PK interactions were observed after the combined treatment. Additionally, this combination includes two short-acting anthelmintics and therefore has low selection pressure for the development of anthelmintic resistance. More importantly, although a high clinical efficacy was observed when RBZ and LEV were administered alone, the combination was the only treatment that achieved 100% clinical efficacy in winter. In this sense, in a scenario where the nematode population is susceptible, the use of the combination could be useful for quarantine treatments or in treat-and-move strategies. Indeed, Dobson et al. (2001) indicated that the principal key for slowing the emergence of anthelmintic resistance in a real field situation is to achieve the highest possible efficacy in treated animals. However, the results of the FECRT in spring demonstrated the importance of knowing the epidemiology of the different GI parasite genera in naturally infected calves. To conclude, the potential therapeutic advantages of combined anthelmintic treatment should be cautiously assessed, especially considering the involved nematode population and the potential PK interactions between the drugs included in a combination. In cattle production systems where some individual molecules, such as RBZ and LEV, still maintain their highest efficacy, the combined use of anthelmintics may be an important tool to delay resistance. In an ideal situation, if an anthelmintic treatment reaches 100% of efficacy, like in the present study, selection of anthelmintic resistance will never occur (Lanusse et al., 2014). In fact, this is one of the greatest challenges for the whole cattle production system, namely to be one step ahead of anthelmintic resistance.
ACKNOWLEDGMENTS
This study was funded by Agencia Nacional de Promoción Científica y Técnica (ANPCyT) (PICT 2012-1342) from Argentina. The authors would like to thank the farmer for collaborating with this study.
CONFLICT OF INTEREST
There is no potential conflict of interests associated with this study.




