Pharmacokinetic depletion phase of doxycycline in healthy and Mycoplasma gallisepticum-infected chicken broilers after coadministration of enrofloxacin traces
Abstract
The objective of this study was to investigate the influence of enrofloxacin (ENR) traces on doxycycline (DC) pharmacokinetic depletion phase parameters in plasma and lungs of healthy and Mycoplasma gallisepticum (MG)-infected chicken broilers. The multiple-dose oral administration of DC to chickens which were permanently exposed on ENR traces significantly increased concentration of DC in plasma and lung. It also prolonged the DC elimination time in both healthy and infected animals after final dose. The obtained result indicated that simultaneous administration of DC and ENR in chicken broilers therapy should be avoided.
INTRODUCTION
Most experiments which allow to investigate pharmacokinetic parameters are generally carried out on healthy animals (Castro et al., 2009; Ziółkowski et al., 2016). Moreover, interactions between medicines intentionally given to animals are generally known, but only for therapeutic doses (Elmas, Yazar, Uney, Er (Karabacak), & Traş, 2008; Devreese et al., 2012). The presence of infection as well as the other medicines traces can lead to higher concentration of the target compound in tissues (Ding, Wang, Shen, Zeng, & Zeng, 2013; Gajda, Bladek, Jablonski, & Posyniak, 2015). Chicken broilers time of breeding is very short (5–6 weeks) and sometimes the treatment of several infections is required, what can result with possible interactions between antimicrobial drugs. The probability of interactions increased, when substances are administered with water, what is the most common way of drug application on poultry farms. The DC and ENR are considered as one of the most effective antibacterial compounds, with a broad spectrum of antibacterial activity in poultry treatment. Moreover, results of our previous pilot study clearly indicated that ENR and DC were the most frequently detected antibacterial drugs in drinking water samples taken from probably not cleaned dispensers on poultry farms (Gbylik-Sikorska, Posyniak, Sniegocki, & Zmudzki, 2015). So, there is a strong need for providing study about drug traces in water and their possible interactions with target antibiotics. Additionally, the influence of infection for drug pharmacokinetic parameters after final dose in plasma and lungs as target organ was tasted. In this regard, this study is expected to provide necessary information about the evaluation of long-term chicken's exposure to trace amounts of ENR and its possible impact on the DC pharmacokinetic depletion phase.
Presented study results are continuation of previously reported research (Gbylik-Sikorska, Posyniak, Sniegocki, Sell, Gajda, Sawicka et al., 2016; Gbylik-Sikorska, Posyniak, Sniegocki, Sell, Gajda, Tomczyk et al., 2016). The experiment was carried out with the approval of the Local Ethical Committee in Lublin (Resolution No 59/2013). One-day-old Lohmann broiler chickens (n = 84) with mean bodyweight were divided into four different experimental groups (n = 21 each). Group I (DC): in the fifth week of the chickens’ life, birds received DC (doxycycline hyclate 500 mg/g, oral powder), at a dose of 20 mg/kg BW every 24 hr over 5 days. The aqueous solution of DC was administered through the gavage directly to the crop. Group III (ENR+DC): from the second day of life, birds received ENR via drinking water (500 μg/L) until the end of the experiment and then also received DC according to the same protocol as for group I. Group II (DC/MG): DC was administered the same protocol as in group I, and group IV (ENR+DC/MG): DC and ENR were administered as in group III, respectively, but in the second week of the chickens’ life, birds were experimentally infected with MG in both groups.
Chickens of the groups II and IV were infected by reference strain MG ATCC 19610 as described in our previous study (Gbylik-Sikorska, Posyniak, Sniegocki, Sell, Gajda, Sawicka et al., 2016; Gbylik-Sikorska, Posyniak, Sniegocki, Sell, Gajda, Tomczyk et al., 2016). Bacteriological examination was performed to ensure the infection presence. The rapid serum plate agglutination test with commercial antigen (MG RPATest) and the QuantiTect Probe PCR Master Mix (Raviv & Kleven, 2009) were used to identify MG.
Three birds from each group were euthanized at 2, 4, 8, 12, 24, 72 and 144 hr after final dose of DC. Lung and blood samples were collected at each time point. Blood samples were collected in the centrifuge tubes containing heparin and centrifuged (2930 × rcf for 5 min); plasma was harvested. Lung samples were collected in plastic container and homogenized. All samples were frozen at −80°C until the analyses.
Doxycycline (DC) concentration in chicken broilers plasma and lungs was determined using liquid chromatography–mass spectrometry (LC-MS/MS) based on our previous report (Gbylik-Sikorska, Posyniak, Sniegocki, Sell, Gajda, Sawicka et al., 2016; Gbylik-Sikorska, Posyniak, Sniegocki, Sell, Gajda, Tomczyk et al., 2016). The same sample preparation procedure was applied to the extraction of DC in plasma and lungs. Method was validated. The correlation coefficients for DC in both matrices were over R > 0.99. The coefficient of variation (CV) was 6.48% in plasma and 5.16% in lungs for repeatability. The within-laboratory reproducibility (CVs) was 6.79% and 7.11%, respectively. The average percentage recovery ranged from 86% to 113% for plasma and 92%–114% for lung. The method quantification limits (LOQ) were 1.0 ng g−1 or ml−1 and detection limits (LOD) were in the range of 0.25 and 0.37 ng g−1 or ml−1, respectively.
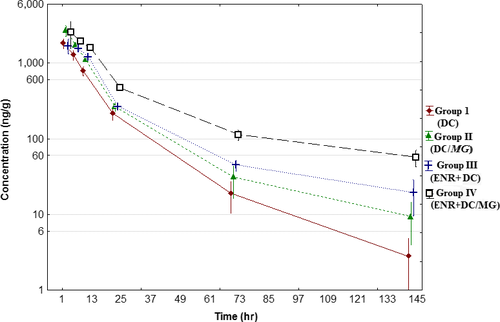
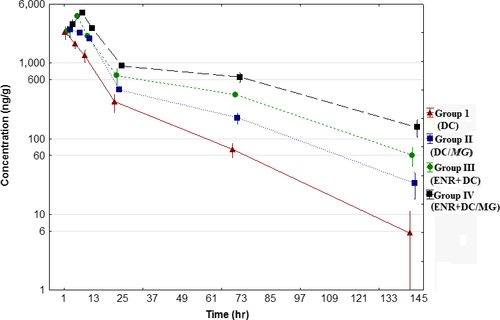
The DC mean depletion phase concentration in plasma and lungs of broiler chickens is shown in Figure 1 and Figure 2. The mean values of pharmacokinetic parameters of depletion phase are presented in Table 1. The differences of mean DC concentration in plasma and lungs after final dose between each group in each time point were found to be statistically significant p < .05. The highest Cmax of doxycycline was observed in plasma and lungs in group IV with concentration almost two times higher than that in groups I and II. Tmax of DC was found at 2 hr in healthy chickens (groups I and III) and at 4 hr in infected birds (groups II and IV), regardless of birds received an ENR or ENR was not administered. The same observation was found for Tmax value in lungs. The AUC(0-t), AUC(0-∞) and AUMC(0-t) were statistically significantly different for all experimental groups, whereas there were no significant differences in MRT(0-t) and kel.
| Parameter | Matrix | Group I (DC) | Group II (DC/MG) | Group III (ENR+DC) | Group IV (ENR/MG+DC) |
|---|---|---|---|---|---|
Cmax Mean + SD (ng g−1 or ml−1) |
Plasma | 2130.8 ± 235a | 2103.5 ± 211a | 3194.5 ± 394a | 3703.5 ± 467a |
| Lung | 3106.0 ± 441a | 3448.0 ± 481a | 4140.0 ± 508a | 4579.5 ± 527a | |
| Tmax (hr) | Plasma | 2 | 4 | 2 | 4 |
| Lung | 4 | 8 | 4 | 8 | |
| T1/2 (hr) | Plasma | 14.7 | 21.7 | 18.1 | 27.1 |
| Lung | 20.0 | 28.5 | 33.5 | 43.6 | |
|
AUC(0–t) Mean + SD (μg g−1or ml−1 hr) |
Plasma | 28,071 ± 3.7a | 36,061 ± 4.5a | 39,008 ± 50a | 55,809 ± 6.6a |
| Lung | 44,258 ± 8.5a | 66,790 ± 9.6a | 93,836 ± 125a | 129,747 ± 16.3a | |
| AUC(0–∞)Mean + SD (μg g−1or ml−1 h) | Plasma | 26,380 ± 3.1a | 36,962 ± 4.4a | 35,376 ± 5.0a | 56,036 ± 7.1a |
| Lung | 17,957 ± 1.1a | 33,544 ± 4.2a | 58,904 ± 6.5a | 96,123 ± 1.0a | |
|
AUMC(0–t) Mean + SD (μg g−1or ml−1 hr2) |
Plasma | 392,116 ± 33.5a | 676,848 ± 58.4 | 572,992 ± 43.0a | 1273,872 ± 98.7a |
| Lung | 781,680 ± 62.4a | 1590,740 ± 88.4a | 2839,812 ± 120.3a | 4687,896 ± 154.3a | |
|
MRT(0–t) (hr) Mean + SD |
Plasma | 13.9 ± 3.1 | 18.8 ± 1.6 | 14.7 ± 2.1 | 22.8 ± 3.8 |
| Lung | 17.7 ± 2.9 | 23.8 ± 3.7 | 30.3 ± 4.5 | 36.1 ± 4.2 | |
|
kel (hr−1) Mean + SD |
Plasma | 0.05 ± 0.01 | 0.04 ± 0.02 | 0.03 ± 0.01 | 0.03 ± 0.02 |
| Lung | 0.03 ± 0.02 | 0.02 ± 0.01 | 0.02 ± 0.02 | 0.01 ± 0.01 |
- Pharmacokinetics depletion phase parameters were calculated by Biokinetica 4.0 software package. Cmax, maximum drug concentration; Tmax, time to peak concentration; T1/2, elimination half-life; AUC(0-t), area under the concentration–time curve; AUC(0-∞), area under the concentration–time curve from 0 to infinity; AUMC(0-t), area under the thirst moment of curve; MRT(0-t), mean residence time from 0 to t; kel, elimination rate constant. For the statistical analysis, ANOVA was used.
- a p < .05 was regarded as significant.
The result confirms that DC should not be administered in combination with ENR, because even trace amounts of ENR caused statistically significant changes in the DC pharmacokinetic profile in depletion phase. The biggest differences were observed in distribution and elimination time. The MG infection has also influence on DC pharmacokinetic parameters after final dose, what definitely delayed the time to peak concentration. This kind of research reflects the situation that may occur in the chicken broiler farms, where drug traces present in water supply systems can be washed out and interact with target drug used to tread infection. In conclusion, not only products of animal origin should be controlled for antibiotics residues, but also water from water supply systems on farms should be analysed to avoid adverse interactions.
ACKNOWLEDGMENT
This work was financially supported by the Ministry of Science and Higher Education (NCN Project No. DEC-2013/11/N/NZ7/00434).
CONFLICT OF INTEREST STATEMENT
None of the authors of this study has a financial or personal relationship with other people or organization that could inappropriately influence or bias the content of the paper.




