Effects of aspirin dose escalation on platelet function and urinary thromboxane and prostacyclin levels in normal dogs
Funding information
Funded by the Mississippi State University College of Veterinary Medicine Internal Competitive Research Grant and Dr. Hugh G. Ward Endowment
Abstract
Established “low” aspirin dosages inconsistently inhibit platelet function in dogs. Higher aspirin dosages consistently inhibit platelet function, but are associated with adverse effects. The objectives of this study were to use an escalation in dosage to determine the lowest aspirin dosage that consistently inhibited platelet function without inhibiting prostacyclin synthesis. Eight dogs were treated with five aspirin dosages: 0.5 mg/kg q24h, 1 mg/kg q24h, 2 mg/kg q24h, 4 mg/kg q24h and 10 mg/kg q12h for 7 days. Utilizing aggregometry and a whole-blood platelet function analyzer (PFA-100), platelet function was evaluated before and after treatment. Urine 11-dehydro-thromboxane-B2 (11-dTXB2) and 6-keto-prostaglandin-F1α (6-keto-PGF1α), were measured. Compared to pretreatment, there were significant post-treatment decreases in the maximum aggregometry amplitude and increases in the PFA-100 closure times for all dosages expect 0.5 mg/kg q24h. There was no difference in amplitude or closure time among the 2 mg/kg q24h, 4 mg/kg q24h, and 10 mg/kg q12h dosages. Compared to pretreatment values, there was a significant decrease in urinary 11-dTXB2-to-creatinine and 6-keto-PGF1α-to-creatinine ratios, but there was no dose-dependent decrease for either metabolite. An aspirin dosage of 2 mg/kg q24h consistently inhibits platelet function without decreasing prostacyclin synthesis significantly more than lower aspirin dosages.
1 INTRODUCTION
Thromboembolism is a significant complication of many commonly encountered medical conditions in veterinary medicine, including immune-mediated hemolytic anemia (IMHA), protein-losing nephropathies, and hyperadrenocorticism (Johnson, Lappin, & Baker, 1999). Antiplatelet therapy, including low-dose aspirin, is one of the most affordable and widely used options for prevention of thrombus formation (thromboprophylaxis). Compared to other thromboprophylactic drugs, dogs diagnosed with IMHA and treated with low-dose aspirin had improved short- and long-term survival times (Weinkle et al., 2005). Low-dose aspirin has also been recommended as thromboprophylaxis for dogs with glomerular disease (Lunsford & Mackin, 2007; Subgroup et al., 2013).
Aspirin is a cyclooxygenase (COX) inhibitor that irreversibly inhibits platelet function. The COX enzyme converts arachidonic acid to biologically active eicosanoids, including prostaglandins, which are necessary for normal homeostasis and hemostasis (Ruan, So, & Ruan, 2011). By inhibiting COX, aspirin prevents synthesis of thromboxane A2 (TXA2) and prostaglandins such as prostacyclin. TXA2 is primarily produced by platelets, and triggers platelet activation and vasoconstriction (Majerus, 1983). Prostacyclin, in contrast, originates from the vascular endothelium and inhibits platelet activation and causes vasodilation (Ruan et al., 2011).
Based on our previous work, it was determined that anti-inflammatory or “high” dosages of aspirin (10 mg/kg twice daily) reliably inhibit COX function and prostaglandin production in all cells that express the COX enzyme, including platelets and vascular endothelial cells (Thomason et al., 2011). Unfortunately, high-dose aspirin may be associated with gastrointestinal and renal side effects (Data, Chang, & Nies, 1976; Johnston, Leib, Forrester, & Marini, 1995; Shaw, Burrows, & King, 1997), which make this dose less desirable for thromboprophylaxis (Thomason et al., 2011). In contrast to high-dose aspirin, a “low” dosage of aspirin inhibits platelet TXA2 synthesis in humans, without permanently inhibiting COX function within the vascular endothelium and other cells, thereby allowing prostacyclin synthesis and vasodilation to continue, and reducing the likelihood of gastrointestinal and renal side effects (Patrono et al., 1985). Unfortunately, low-dose aspirin (0.5–1 m/kg once daily), unlike high-dose aspirin, does not consistently inhibit platelet function in dogs (Dudley et al., 2013; Haines et al., 2016; Hoh, Smith, McMichael, & Byron, 2011). Patients that are poorly responsive to the antiplatelet effects of aspirin are termed “aspirin resistant” (Helgason et al., 1994). The incidence of aspirin resistance ranges in humans from 5% to 57% (Eikelboom, Hirsh, Spencer, Baglin, & Weitz, 2012; Grotemeyer, Scharafinski, & Husstedt, 1993; Gum et al., 2001; Helgason et al., 1994; Pappas, Westengard, & Bull, 1994; Sane, McKee, Malinin, & Serebruany, 2002) and, in healthy dogs, our group has previously estimated that the incidence ranges from 19% to 56% depending on the technique used to assess inhibition of platelet function (Dudley et al., 2013; Haines et al., 2016).
Although there are many proposed mechanisms of aspirin resistance, one potential mechanism is aspirin underdosage. Our group has previously reported that aspirin-associated platelet dysfunction in dogs appears to be highly dose-dependent (Haines et al., 2016; Thomason et al., 2011). While low-dose aspirin has inconsistent and variable effects in individual dogs, platelet function in dogs has been shown to be consistently inhibited by high doses of aspirin, regardless of the methods used to assess platelet function (Thomason, Archer, Wills, Press, & Mackin, 2016; Thomason et al., 2011). Using both PFA-100® and optical aggregometry, previous studies have demonstrated that 28%–57% of dogs treated with low-dose aspirin (1 mg/kg, q24h) were considered nonresponders, while all dogs were considered aspirin responders when treated with a much higher dose (10 mg/kg, q12h; Thomason et al., 2016). Furthermore, when canine platelets are exposed to aspirin in vitro, at a concentration that consistently inhibits activation, platelet function is consistently markedly inhibited (Haines et al., 2016). The pharmacokinetic profile of low-dose aspirin in dogs has not been evaluated, and a better understanding of drug pharmacokinetics would clearly be beneficial when exploring mechanisms for poor response to aspirin therapy. To date, only the antiplatelet effects of low and high doses of aspirin in dogs have been evaluated, and the wide range of potential doses between these two extremes have not been studied. Establishment of an optimal single “mid-range” aspirin dosage or a range of aspirin dosages which could reliably inhibit platelet function and TXA2 synthesis without inhibiting prostacyclin synthesis has the potential to overcome the impact of the aspirin resistance associated with standard dosages of low-dose aspirin.
The purpose of this study was to use incremental increases in aspirin dosages to precisely determine the dosage or dosages of aspirin that consistently inhibited platelet function and TXA2 synthesis without inhibiting prostacyclin synthesis in normal dogs. This study was designed to test the working hypotheses that small, incremental increases in aspirin dosages would lead to more consistent inhibition of platelet function and TXA2 synthesis while having minimal effects on prostacyclin synthesis.
2 MATERIALS AND METHODS
2.1 Study design, animals
Eight healthy adult research Walker Hound dogs, four females and four males, were used in this study. The dogs were not exposed to any medications or vaccines for at least 2 weeks prior to the initiation of the study. Normal health status was established by detection of no abnormalities on physical examination, complete blood count (including manual platelet count), serum chemistry, urinalysis, and testing for heartworm and tick-borne disease (SNAP 4Dx Plus Test; Idexx Laboratories Inc, Westbrook, ME, USA). The median age of the dogs was 1.5 years (range, 1–6.5 years), and their mean body weight was 27.4 kg (range, 25.6–30.5 kg). Body weight was obtained at the beginning of the study and used to calculate all subsequent drug doses. Animal use was approved by the Mississippi State University Institutional Animal Care and Use Committee and was in compliance with the requirements of a facility accredited by the American Association for Accreditation of Laboratory Animal Care.
In a five-way, randomized, crossover study, the dogs were separated into one of five groups, with each group given different oral dosages of aspirin (Aspirin; Major Pharmaceuticals, Livonia, MI, USA): 0.5 mg/kg q24h, 1 mg/kg q24h, 2 mg/kg q24h, 4 mg/kg q24h, or 10 mg/kg q12h. Aspirin doses were compounded into capsules by the Mississippi State University College of Veterinary Medicine Pharmacy. All drugs were administered orally for 7 days, followed by at least a 14-day washout period between dosing. After this washout period, the dogs switched groups, and the study was continued until all dogs had received each aspirin dosage. The dogs were not fasted prior to aspirin administration.
Prior to aspirin therapy (day 0) and after one week of drug administration (day 7), blood samples were collected for platelet function analysis (optical aggregometry) and point-of-care platelet function analyzer), and urine samples were collected to measure urinary 11-dehydro-thromboxane B2 (11-dTXB2) and 6-keto-prostaglandin F1α (6-keto-PGF1α) concentrations (stable metabolites of TXA2 and prostacyclin, respectively). Blood and urine samples collected on day 7 were taken 1 hr after aspirin administration. Blood samples were collected via jugular venipuncture with a 20-gauge needle directly into a 4.5-ml Vacutainer tube containing 3.2% sodium citrate anticoagulant. Urine was collected via cystocentesis using a 22-gauge 1.5-inch needle, and samples were stored at −80°C for later analysis.
2.2 Optical aggregometry
To harvest platelet-rich plasma (PRP), whole blood collected into 3.2% sodium citrate was centrifuged at 1,200 g at room temperature for 3 min. The PRP supernatant was collected, and the remaining blood sample was centrifuged at 1,800 g at room temperature for 8 min to create platelet-poor plasma (PPP).
A two-channel light transmission (optical) platelet aggregometer (Chronolog 700 Whole Blood/Optical Lumi-Aggregometer; Chronolog Corporation, Haverton, PA, USA) that allowed for two samples to be evaluated concurrently was used to analyze platelet aggregation in PRP. Samples were analyzed based on the manufacturer's standard guidelines (Chrono-Log 700 manual; Chrono-Log Corp). Briefly, 450 μl of PRP was transferred into a glass cuvette containing a siliconized magnetic stir bar, and 500 μl of PPP was placed into a cuvette without a stir bar. Samples were incubated at 37°C for 5 min and then placed into the aggregometer, and stable baseline values for minimal (PRP) and maximal (PPP) aggregation were obtained (assigned values of 0% and 100% aggregation, respectively). Platelet numbers within the PRP were not adjusted to a standardized count by dilution with PPP prior to analysis, based on recommendations put out by the International Society of Thrombosis and Haemostasis Platelet Physiology and Scientific and Standardization Committee (Cattaneo, Lecchi, Zighetti, & Lussana, 2007; Linnemann, Schwonberg, Mani, Prochnow, & Lindhoff-Last, 2008; Mani, Luxembourg, Klaffling, Erbe, & Lindhoff-Last, 2005; van der Stelt, van Werkum, Seesing, Berg, & Hackeng, 2007). Collagen, 10 μg/ml, was then added to the PRP, and aggregation was monitored for 12 min. The maximal percentage aggregation was calculated and recorded using computer software (AGGRO/LINK 8; Chronolog Corporation). Platelet function analysis was performed within 4 hr of collection.
2.3 Platelet function analysis
A commercial point-of-care whole-blood platelet function analyzer (PFA-100®; Siemens Healthcare Diagnostics, Deerfield, IL, USA) that has previously been evaluated for use in dogs (Callan & Giger, 2001; Mischke & Keidel, 2003; Morales, Couto, & Iazbik, 2007; Nielsen, Zois, Pedersen, Olsen, & Tarnow, 2007) was used to analyze platelet function. This platelet function analyzer stimulates platelet function with several agonists under high shear forces and measures the closure time, in seconds, needed to form a platelet plug and inhibit blood flow. The manufacturer's cut-off time for the instrument is 300 s.
The instrument was used according to manufacturer's instructions. Briefly, blood samples were gently mixed and kept at room temperature without agitation until analysis. For analysis, 800 μl of whole blood was transferred into either a collagen/ADP cartridge (PFA Collagen/ADP Test Cartridge; Siemens Healthcare Diagnostics, Duluth, GA, USA; performed initially to establish normal platelet function) or collagen/epinephrine cartridge (PFA Collagen/EPI Test Cartridge; Siemens Healthcare Diagnostics; used throughout the study to assess aspirin-associated platelet dysfunction) and analyzed. Two collagen/epinephrine cartridges were analyzed at each time point for all dogs, and the closure times were averaged. Platelet function analysis was performed within 2 hr of collection.
2.4 Aspirin responsiveness
Based on our previously published criteria used for dogs (Haines et al., 2016), each dog was classified as either an aspirin responder or an aspirin nonresponder. For optical aggregometry, a dog was considered to be an aspirin responder if there was a >25% reduction in the percentage aggregation at maximum amplitude compared to day 0 values. For the whole-blood platelet function analyzer, a dog was considered to be an aspirin responder if the closure time was >300 s.
2.5 Urine 11-dehydro-thromboxane B2 analysis
Urinary 11-dTXB2 concentration was analyzed using an enzyme-linked immunosorbent assay kit (11-dehydro-thromboxane B2 EIA kit-Monoclonal; Cayman Chemical Co, Ann Arbor, MI, USA) that has been previously validated in the dog (Hoh et al., 2011). Prior to analysis, urine samples were thawed to room temperature and then handled according to the manufacturer's instructions. Samples were analyzed in triplicate on a plate reader (SpectraMax M5 Multi-Mode Microplate Reader; Molecular Devices, Sunnyvale, CA, USA). Results were averaged and reported as picograms per milliliter of urine. Urine creatinine concentration was measured using a biochemistry analyzer (ACE Alera® 201 Clinical Chemistry System; Alfa Wasserman, Inc.) to calculate a urinary 11-TXB2-to-creatinine ratio.
2.6 Urine 6-keto-prostaglandin F1α analysis
Urinary 6-keto-PGF1α concentration was analyzed using an enzyme-linked immunosorbent assay kit (6-keto-prostaglandin-F1α ELISA; Cayman Chemical Co) that has undergone extensive analytic validation by the manufacturer and has been used previously in dogs (Baltzer et al., 2012). Using a similar technique as previously described with urinary 11-dTXB2, urinary 6-keto-PGF1α was analyzed according to the manufacturer's instructions. Samples were analyzed in triplicate, averaged, and reported in picograms per milliliter of urine. Urine creatinine concentration was measured to calculate a urinary 6-keto-PGF1α-to-creatinine ratio.
2.7 Statistical methods
Mixed models using PROC MIXED in a computer software program (SAS for Windows 9.4; SAS Institute, Inc., Cary, NC, USA) were fit for each outcome that included dose, sample, and the dose*sample interaction as fixed effects. Dog identity within run and run were included as random effects with variance component covariance structure. An LSMEANS statement was used to make comparisons among levels of significant main effects. If the interaction term was significant, differences in least squares means between each of the levels of one variable were calculated for each level of the other variable in the interaction using an LSMESTIMATE statement. If there were significant main effects with more than two levels or significant interaction terms, the simulate option in the LSMEANS or LSMESTIMATE statements was used to adjust p-values to account for the effect of multiple comparisons. The distribution of the conditional residuals was evaluated for each outcome to ensure the assumptions of normality of the residuals and heteroscedasticity had been met. An alpha level of .05 was used to determine statistical significance for all methods.
3 RESULTS
3.1 Optical aggregometry
The mean optical aggregometry maximum amplitude for all aspirin dosages on days 0 and 7 is represented in Figure 1. There was no significant difference in the maximum amplitude for the five aspirin dosages on day 0 (pretreatment). When compared to day 0, there was a significant decrease in maximum amplitude on day 7 for 1 mg/kg q24h (p = .0259), 2 mg/kg q24h (p < .0001), 4 mg/kg q24h (p < .0001), and 10 mg/kg q12h (p < .0001). On day 7, the maximum amplitude for 0.5 mg/kg q24h was significantly greater than for 2 mg/kg q24h (p = .0004), 4 mg/kg q24h (p = .0007), and 10 mg/kg q12h (p = .0003). There was no difference in maximum amplitude among the 2 mg/kg q24h, 4 mg/kg q24h, and 10 mg/kg q12h dosages.
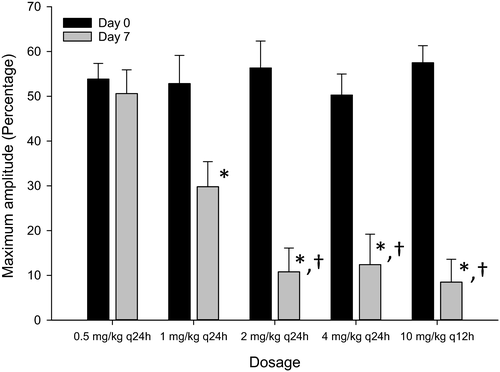
Two dogs were considered aspirin responders when treated with 0.5 mg/kg q24h. When treated with 1 mg/kg q24h and 4 mg/kg q24h, six dogs were considered to be aspirin responders. All dogs were considered to be aspirin responders when treated with 2 mg/kg q24h and 10 mg/kg q12h. One dog was classified as an aspirin responder with all five aspirin dosages.
3.2 Platelet function analysis
The mean closure times for all aspirin dosages on days 0 and 7 are represented in Figure 2. There was no significant difference in the closure time for the five aspirin dosages on day 0 (pretreatment). When compared to day 0, there was a significant increase in closure times on day 7 for 1 mg/kg q24h (p = .0002), 2 mg/kg q24h (p = .0002), 4 mg/kg q24h (p < .0001), and 10 mg/kg q12h (p < .0001). On day 7, the closure times for 2 mg/kg q24h (p = .0388), 4 mg/kg q24h (p = .0002), and 10 mg/kg q12h (p = .0006) were significantly greater than 0.5 mg/kg q24h. There was no difference in closure times among the 2 mg/kg q24h, 4 mg/kg q24h, and 10 mg/kg q12h dosages.
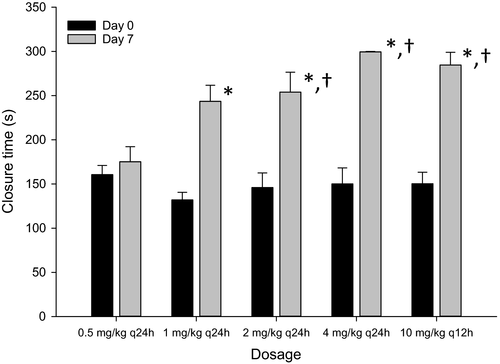
None of the dogs treated with 0.5 mg/kg q24h were considered to be aspirin responders. Two dogs were considered to be aspirin responders when treated with 1 mg/kg q24h, and five dogs were classified as aspirin responders when treated with 2 mg/kg q24h. When treated with 4 mg/kg q24h and 10 mg/kg q12h, seven dogs were considered to be aspirin responders.
3.3 Urine 11-dehydro-thromboxane B2 analysis
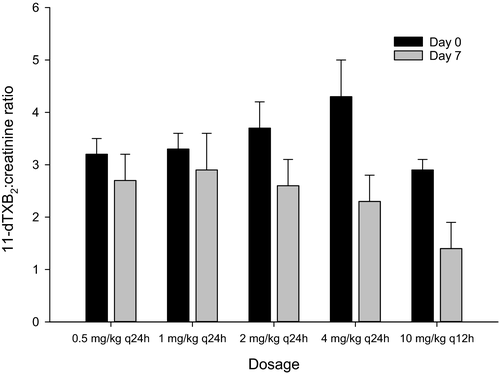
The urine 11-dTXB2-to-creatinine ratio for all aspirin doses on days 0 and 7 are represented in Figure 3. Compared to day 0, there was a significant (p = .0003) decrease in the 11-dTXB2-to-creatinine ratio on day 7 for all aspirin dosages. However, there was no significant dose-dependent decrease in the 11-dTXB2-to-creatinine ratio for all doses.
3.4 Urine 6-keto-prostaglandin F1α analysis
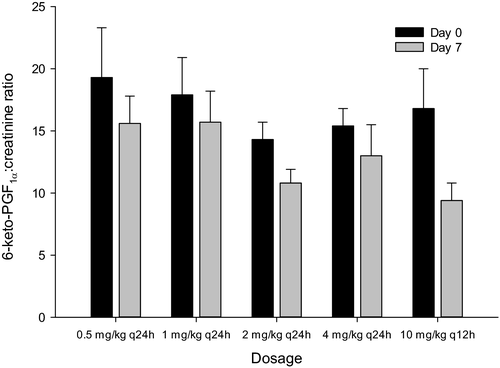
The urine 6-keto-PGF1α-to-creatinine ratio for all aspirin doses on days 0 and 7 are represented in Figure 4. Compared to day 0, there was a significant (p = .0075) decrease in the 11-dTXB2-to-creatinine ratio on day 7 for all aspirin dosages. However, there was no significant dose-dependent decrease in the 6-keto-PGF1α-to-creatinine ratio for all doses.
4 DISCUSSION
The ideal dose of aspirin that consistently inhibits platelet function in dogs, while minimizing any drug-associated side effects, is unknown. Currently used standard low or antiplatelet dosages (0.5–1 mg kg−1 day−1) of aspirin, however, do not reliably inhibit platelet function (Dudley et al., 2013; Haines et al., 2016; Hoh et al., 2011). Platelet function in dogs will, in contrast, be consistently inhibited by high dosages of aspirin (Thomason et al., 2016), suggesting that, if dogs are treated with high enough doses of aspirin, there is an increased likelihood of inhibition of platelet function. Unfortunately, as the aspirin dosage increases, so does the risk of undesirable inhibition of prostacyclin, and of aspirin-induced side effects. The data reported in the current study suggest that a modified “low” dosage of aspirin (2 mg/kg q24h) will consistently inhibit canine platelet function in normal dogs, without the need to resort to much higher standard anti-inflammatory aspirin dosages of 10 mg/kg twice daily.
In humans, optical aggregometry is considered to be the gold standard method for evaluating aspirin-induced platelet dysfunction (Kalbantner, Baumgarten, & Mischke, 2010; Lordkipanidze et al., 2007; Nielsen et al., 2007). Recently, our group demonstrated that optical aggregometry was the most reliable test of platelet function that consistently identified dogs that responded to an antiplatelet dosage of aspirin (Haines et al., 2016). In the current study, compared to pretreatment values, all aspirin dosages except the very lowest dosage (0.5 mg/kg q24h) significantly decreased platelet aggregation after 7 days of drug administration. On day 7, there was no significant difference in the mean platelet amplitude among the aspirin dosages, except at the lowest dosage. Additionally, on day 7, compared to the 0.5 mg/kg q24h dosage, there was a significant decrease in platelet aggregation for the 2 mg/kg q24h, 4 mg/kg q24h, and 10 mg/kg q12h dosages, but not for the 1 mg/kg q24h dosage. These results are consistent with a dose-dependent decrease in platelet aggregometry that reaches maximal effect at an oral aspirin dosage of 2 mg/kg once daily.
Recently, using optical aggregometry, canine aspirin responsiveness was defined as a >25% reduction in the percentage aggregation at maximum amplitude following aspirin treatment, compared to pre-aspirin values (Haines et al., 2016). Using this definition, our group had demonstrated that, when aspirin was administered to dogs at an oral dosage of 1 mg/kg once daily for 7 days, ~80% of the dogs were classified as aspirin responders. These previous results were similar to the results reported here, in that when dogs were administered the same dose of aspirin for 7 days, 75% were considered to be aspirin responders. In contrast, in this current study, when treated with a 2 mg/kg once-daily aspirin dosage, all dogs were considered to be aspirin responders, a rate of aspirin responsiveness that was identical to that seen with the high anti-inflammatory dosage of aspirin (10 mg/kg q12h). These results suggest that an aspirin dosage of 2 mg/kg once daily will provide consistent aspirin-induced platelet dysfunction in normal dogs and that higher dosages of aspirin may not provide additional antiplatelet benefits. Although the 2 mg/kg q24h and 10 mg/kg q12h dosages achieved 100% aspirin responsiveness, only six of eight (75%) of the dogs receiving the 4 mg/kg q12h dosage were considered aspirin responsive. In the two dogs considered non-responsive at this dose, platelet function was inhibited (by 7% and 20%) compared to pre-aspirin values, but did not achieve the 25% reduction required for classification as aspirin responders. When treated with the 10 mg/kg q12h dose, these same two dogs had the least reduction in aggregometry amplitude (73% and 43%) compared to an average of 98% reduction for the other six dogs. These findings could suggest that additional pharmacodynamic effects, other than dosage, could influence the antiplatelet effects of aspirin.
Similar to previous studies in dogs (Dudley et al., 2013; Mischke & Keidel, 2003; Nielsen et al., 2007), this study demonstrated that aspirin-induced platelet dysfunction could be determined using a commercial whole-blood point-of-care platelet function analyzer (PFA-100) with a collagen/epinephrine cartridge. Our group has previously reported that, compared to optical aggregometry, the PFA-100 is less reliable at determining drug responsiveness in dogs receiving aspirin (Haines et al., 2016). In our previous studies using the PFA-100 in dogs treated with aspirin at a dosage of 1 mg/kg once daily for 7 days, only 33%–44% of dogs were classified as aspirin responders (Dudley et al., 2013; Haines et al., 2016) and, when the same aspirin dosage was used in the current study, 25% of dogs were considered to be aspirin responders. Also in this study, at the same aspirin dosage of 1 mg/kg daily, 75% of the dogs were considered to be aspirin responders based on aggregometry results. This discrepancy was not unexpected because the PFA-100, although it is often used to clinically evaluate platelet function in dogs receiving aspirin, has been previously reported by this group to markedly overestimate the degree of aspirin resistance compared to optical aggregometry (Haines et al., 2016). When receiving aspirin at a dosage of 2 mg/kg once daily, ~63% of the dogs in this study were classified as aspirin responders by the PFA-100, which is only slightly below the 88% of patients that were classified as aspirin responders when treated with the higher two aspirin dosages (4 mg/kg once daily and 10 mg/kg twice daily). Based on PFA-100 results alone, an aspirin dosage of 4 mg/kg once daily would appear to be sufficient to maximize drug antiplatelet effects. However, when PFA-100 results are considered concomitantly with aggregometry results, an aspirin dosage of 2 mg/kg may be sufficient to overcome aspirin resistance, especially considering that aggregometry is more sensitive than the PFA-100 to the antiplatelet effects of aspirin.
During this study, mild transient gastrointestinal side effects were noted in some dogs with the administration of the anti-inflammatory high dosage of aspirin, 10 mg/kg twice daily, but no side effects were noticed with any of the lower aspirin dosages. This study was, however, not designed to specifically monitor for aspirin-induced gastrointestinal lesions such as gastric ulceration or erosions, and additional studies would be warranted to further explore the potential gastrointestinal side effects of using aspirin at dosages >0.5–1 mg/kg daily. Furthermore, as low-dose aspirin is commonly administered concurrently with glucocorticoids to dogs with IMHA, additional studies would be necessary to determine the gastrointestinal side effects of a slightly increased “low” aspirin dosage, such as 2 mg/kg once daily, during concurrent glucocorticoid administration.
In previous studies in humans and dogs, the stable TXA2 metabolite 11-dehydro-thromboxane B2 has been used as an indicator of aspirin-induced inhibition of thromboxane synthesis (McConnell et al., 2001; Perneby et al., 1999). A second stable TXA2 metabolite, 2,3-dinor-thromboxane-B2 (2,3-dinor-TXB2), is also present in the urine of dogs and can also serve as a surrogate marker of thromboxane synthesis (Hoh et al., 2011). A previous study demonstrated that, compared to 11-dTXB2, canine urinary 2,3-dinorTXB2 concentrations were a more sensitive indicator of aspirin-induced thromboxane inhibition (Hoh et al., 2011). Unfortunately, an assay for 2,3-dinorTXB2 in dogs was unavailable at the time of this study. However, although the urine TXA2 metabolite assay used in this study has been published to not be the most sensitive indicator of aspirin-induced thromboxane inhibition, our previous work has shown that this assay is still sufficiently sensitive to detect a decrease in thromboxane synthesis during aspirin administration (Dudley et al., 2013). The results of the current study revealed that the administration of aspirin at any dosage caused a significant decrease in thromboxane synthesis, but there was no detectable dose-dependent relationship between the aspirin dose and thromboxane synthesis. The results reported here were similar to results from our previous work performed in dogs, in that aspirin-induced reductions in thromboxane synthesis were not dependent on drug dosage (Dudley et al., 2013; Haines et al., 2016; Thomason et al., 2011, 2016). The current study suggests that the inhibition of thromboxane synthesis seen with an aspirin dosage of 2 mg/kg once daily is similar to that seen with the traditional lower antiplatelet dosages of 0.5 and 1 mg/kg once daily and that an increase in aspirin dosages beyond 2 mg/kg once daily will not cause additional inhibition in thromboxane synthesis. However, it is conceivable that a more sensitive assay, such as the 2,3-dinorTXB2 assay, might be able to detect more subtle variations in thromboxane synthesis, particularly considering that the current study detected a possible subtle (and not statistically significant) dose-dependent decrease in urine 11-dTXB2 concentrations at higher aspirin dosages (Figure 3).
Prostacyclin (prostaglandin I2), after being synthesized by the vascular endothelium, has a short half-life, and will quickly be converted into stable metabolites, including 6-keto PGF1α and 2,3 dinor-6-keto PGF1α, which can be used as surrogate markers of prostacyclin synthesis (Baltzer et al., 2012; Dusting, Moncada, & Vane, 1977; Rosenkranz, Fischer, Weimer, & Frolich, 1980). One proposed benefit of low-dose aspirin is that lower dosages will irreversibly inhibit platelet thromboxane synthesis without permanently inhibiting COX function in the vascular endothelium, allowing prostacyclin synthesis to continue. Anti-inflammatory high dosages of aspirin, in contrast, are purported to inhibit COX function and prostaglandin synthesis in both platelets and the vascular endothelium (Patrono et al., 1985; Rackear, 1988). The ideal antiplatelet dosage of aspirin would reliably inhibit platelet function but would not inhibit prostacyclin synthesis. The results reported here, however, demonstrated that the administration of aspirin to dogs at any dosage leads to a decrease in prostacyclin synthesis. Similar to the findings with urinary thromboxane metabolites, there was no detectable dose-dependent relationship between aspirin dose and urinary prostacyclin metabolites. These results suggest that, although prostacyclin synthesis is reduced somewhat even at the lowest evaluated dosage of aspirin, the incremental increases in aspirin dosages needed to progressively inhibit platelet function will not further reduce the beneficial properties of residual prostacyclin synthesis. A slightly increased “low” aspirin dosage of 2 mg/kg once daily, therefore, would be expected to consistently inhibit platelet function while still maintaining prostacyclin concentrations comparable to those seen with traditional lower antiplatelet dosages of aspirin. Again, as with urine 11-dTXB2, this study detected a possible subtle (and not statistically significant) dose-dependent decrease in urine 6-keto-PGF1α concentrations at higher aspirin dosages. Therefore, the inclusion of a larger number of dogs in the study could conceivably have elucidated a statistically significant dose-dependent decrease in 6-keto-PGF1α concentrations.
One limitation of the study is that there are no standard techniques that are consistently used to evaluate aspirin responsiveness. The platelet function assays and classification system used in this study are similar to previous studies that have evaluated aspirin-induced platelet dysfunction (Haines et al., 2016; Lordkipanidze et al., 2007). However, other studies have used different methodologies and protocols to assess aspirin-induced platelet dysfunction (Sharpe et al., 2010), and this variability in methods does not allow for ready comparison between studies. Additionally, the dogs used in the study were healthy, and these results may not be directly applicable to prothrombotic patients. Finally, the dogs used in this study were only given aspirin for one week, whereas low-dose aspirin is commonly administered long term, and it is as yet unknown how canine platelet function and eicosanoid synthesis will respond to chronic aspirin administration.
The role of aspirin therapy for thromboprophylaxis is controversial in veterinary medicine. In patients that are at risk of venous thromboembolism, anticoagulants such as low-molecular-weight heparin are commonly used for thromboprophylaxis and might arguably be more effective than antiplatelet therapy because platelets are not the primary facilitator of venous thrombus formation. However, platelets can still contribute to the formation and acceleration of venous thrombosis and act as a source of procoagulant factors such as phosphatidylserine. For IMHA, for example, Weiss & Brazzell (2006) demonstrated that dogs with IMHA will have an increase in circulating activated platelets, and Kidd et al. (2015) demonstrated an increase in phosphatidylserine-associated procoagulant activity in canine IMHA patients. Antiplatelet drugs can therefore still provide effective thromboprophylaxis in conditions such as IMHA. Additionally, dogs with IMHA treated with 0.5 mg/kg q24h of aspirin had better short- and long-term survival times compared to other anticoagulant therapy (Weinkle et al., 2005). Finally, in a study evaluating the safety of aspirin, clopidogrel, or a combination of both medications in dogs with IMHA, only two of 24 dogs developed a suspected thromboembolism (Mellett, Nakamura, & Bianco, 2011).
As discussed, low-dose aspirin is commonly used in an attempt to prevent thrombus formation in prothrombotic dogs but, at traditional antiplatelet dosages of 0.5–1 mg/kg once daily, platelet function appears to be inconsistently inhibited. Higher aspirin dosages may therefore be needed to provide appropriate platelet inhibition. As the aspirin dosage increases, however, so do the likely risks of aspirin-induced side effects and inhibition of prostacyclin. The results of this study suggests that, in healthy dogs, an oral aspirin dosage of 2 mg/kg once daily will consistently and adequately inhibit platelet function without decreasing the degree of prostacyclin synthesis significantly more than lower aspirin dosages. Additional studies will be required to determine whether this higher aspirin dosage is associated with an increased risk of adverse drug effects.
ACKNOWLEDGMENTS
The authors thank Matthew Raby, Cyndi Dunaway, Ben Lee, Emily Belt, and Melanie Barnett for their assistance.




