Plasma pharmacokinetics of quinocetone in ducks after oral and intravenous administration
Abstract
Quinocetone (QCT), an antimicrobial growth promoter, is widely used in food-producing animals. However, information about pharmacokinetics (PK) of QCT in ducks still remains unavailable up to now. In this study, QCT and its major metabolites (1-desoxyquinocetone, di-desoxyquinocetone and 3-methyl-quinoxaline-2-carboxylic) in ducks were studied using a simple and sensitive UHPLC-MS/MS assay. Twenty ducks were divided into two groups. (n = 10/group). One group received QCT by oral administration at dose of 40 mg/kg while another group received QCT intravenously at 10 mg/kg. Plasma samples were collected at various time points from 0 to 96 hr. QCT and its major metabolites in duck plasma samples were extracted by 1 ml acetonitrile and detected by UHPLC-MS/MS, with the gradient mobile phase that consisted of 0.1% formic acid in water (A) and acetonitrile (B). A noncompartment analysis was used to calculate the PK parameters. The results showed that following oral dosing, the peak plasma concentration (Cmax) of QCT was 32.14 ng/ml and the area under the curve (AUCINF_obs) was 233.63 (h ng)/ ml. Following intravenous dosing, the Cmax, AUCINF_obs and Vss_obs were 96.70 ng/ml, 152.34 (h ng)/ ml and 807.00 L/kg, respectively. These data indicated that the QCT was less absorbed in vivo following oral administration, with low bioavailability (38.43%). QCT and its major metabolites such as 1-desoxyquinocetone and 3-methyl-quinoxaline-2-carboxylic were detected at individual time points in individual ducks, while the di-desoxyquinocetone was not detected in all time points in all ducks. This study enriches basic scientific data about pharmacokinetics of QCT in ducks after oral and intravenous administration and will be beneficial for clinical application in ducks.
1 INTRODUCTION
Quinocetone (QCT or 3-methyl-2-quinoxalinbenzenevinylketo-1,4-dioxide, Figure 1) is a new member of the national first-class veterinary drugs and has been approved as an animal growth promoter by Ministry of Agriculture in P. R. China for over 10 years. Olaquindox (OLA) and carbadox (CBX), the analogs of QCT, were banned or strictly limited for using in food-producing animals due to their genetic or other potential toxicities (Commission Regulation, 1998). In contrast, QCT showed lower toxicity and better growth-promoting activity than OLA and CBX (Yang et al., 2013). According to previous reports, its low toxicity was most likely due to the rapid excretion rate in animals (Chen et al., 2009; Wang et al., 2010; Zhang, He, Yan, Wang, & Fang, 2011; Zhang et al., 2012) and a lack of chemical modifications by intestinal bacteria (Li et al., 2003). Therefore, QCT, as the best substitute, has been widely used in food animals such as pigs, chickens, carps, and ducks, for growth promotion and prophylaxis of infections caused by Escherichia coli, Salmonella, Brachyspira hyodysenteriae (swine dysentery) and other gram-negative bacteria (Zhong et al., 2011).
In recent years, more and more food safety problems related to drug residues have been emerged. Therefore, it is indeed important to gain a better understanding and appropriate application for QCT through the studies of its absorption, distribution, metabolism and excretion in an animal model. The pharmacokinetics of QCT in food-producing animals have been elucidated, such as in pigs (Li et al., 2003; Li, Huang et al., 2014; Li, Liu et al., 2014; Wang, Fang, Fan, & Wang, 2012; Zhong et al., 2011), chickens (Li et al., 2003; Zhang et al., 2011), carps (Hu, Huang, & Yuan, 2008; Yang, Wang, Ai, Yuan, & Liu, 2010), channel catfish (Liu et al., 2009; Yang et al., 2010) and turbot (Li et al., 2011). The studies indicated that QCT had a low bioavailability because of its low absorption and rapid metabolism in vivo. However, there are no reports on pharmacokinetic studies in ducks, so the exact details of QCT metabolism are unknown.
Quinocetone has been widely used as a duck feed additive because it could significantly improve duck growth and slaughter performance (Chen, Deng, & Du, 2004; Xu & Zhu, 2012). The demand and raising quantity of food ducks have been gradually increasing. To preclude food safety problems that may be associated with long-term QCT use, we studied its absorption, distribution, metabolism, and excretion in ducks. The main objective of this study was to explore the pharmacokinetics and bioavailability of QCT in duck after oral and intravenous administration of QCT using UHPLC-MS/MS and to provide reference data for further in-depth study and clinical application.
2 MATERIALS AND METHODS
2.1 Chemical and reagents
QCT raw material (purity ≥ 98.6%) was obtained from Wuhan Lullaby Pharmaceutical Chemical Co., Ltd. (Wuhan, P. R. China). QCT (purity ≥ 99.0%) and 3-methyl-quinoxaline- 2-carboxylic (MQCA) (purity ≥ 99.0%) standards were purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany). 1-desoxyquinocetone (1-DQCT, purity ≥ 98.2%) and di-desoxyquinocetone (BDQCT, purity ≥ 98.2%) were provided by Agricultural Bio-pharmaceutical Laboratory, Qingdao Agricultural University (Qingdao, P. R. China). Analytical HPLC-grade acetonitrile was from the Tedia Company and HPLC-grade formic acid was purchased from Tianjin Kemiou Chemical Reagent Co., Ltd. (Tianjin, P. R. China).
2.2 Instrumentation and analytical conditions
The UHPLC-MS/MS system consists of an Agilent 1290 UHPLC system (Agilent, Santa Clara, CA, USA), equipped with a binary pump solvent delivery system, an auto-sampler and an online degasser. The chromatographic separation was achieved on a ZORBAX RRHD Eclipse Plus C18 column (2.1 × 50 mm, 1.8 μm) obtained from Guangzhou Dexiang Science and Technology Co., Ltd. (Guangdong, P. R. China). The gradient mobile phase consisted of 0.1% formic acid in water (A) and acetonitrile (B) as follows: 0–2.0 min, linear change from 95% to 60% A; 2.0–2.4 min, linear change from 60% to 60% A; 2.4–3.6 min, linear change from 60% to 0% A; 3.6–5.4 min, linear change from 0% to 0% A; 5.4–5.41 min, linear change from 0% to 95% A; 5.41–7.2 min, linear change from 95% to 95% A, and then quickly returned to initial conditions. The flow rate was 0.3 ml/min, and the injection volume was 5 μL at a temperature of 30°C.
Mass spectrometry was performed using a triple quadrupole 6460 mass spectrometer (Agilent) with an electrospray ionization source interface set in positive ionization modes (ESI+). The multiple reaction monitoring (MRM) transitions were m/z 307→273 for QCT, 291→159 for 1-DQCT, 275→143 for BDQCT, 189→143 for MQCA, respectively. The optimal MS parameters were as follows: capillary voltage 3848 V (ESI+); fragmentor voltage 3 V; collision energy 24 eV; nebulizer gas (N2) pressure 25.0 psi with a flow rate of 8.0 L/min and a sheath flow rate of 7.0 L/ min. The desolvation gas was heated to 350°C, and the temperature of the sheath gas was set at 350°C. Nitrogen was served as the collision gas.
2.3 Preparation of solutions
2.3.1 Stock standard solutions and QCT raw material solutions
Stock standard solutions of QCT and its major metabolites (1.0 mg/ml) were prepared in dimethyl sulfoxide, and acetonitrile was then added to 90% v/v. The working solutions were prepared weekly by diluting the stock standard solutions with acetonitrile. All solutions were stored in brown containers for avoiding photochemical reaction at −20°C for 3 weeks.
QCT raw material was grinded, and the solution of QCT raw material (50 mg/ml) was prepared in 1% carboxymethylcellulose sodium.
2.4 Animals
Healthy Cherry Valley ducks (4 weeks, 1.5–2.0 kg, 50% of male) were obtained from Qingdao Flesh Duck Farms (Qingdao, P. R. China). The ducks were maintained under standard environmental conditions using the routine methods of animal husbandry. All the ducks were housed with free access to the diet and water for 1 week prior to the experiment for acclimation. Throughout the study period, feed was withheld for approximately 12 hr before and until 4 hr after QCT administration, while water was available ad libitum. All in-life experiments were performed complying with the care and use policies for laboratory animals. All animal experimental procedures followed the regulations of the Animal Ethics Committee of Qingdao Agricultural University.
2.5 Sample preparation
After thawing and equilibration at room temperature, a 500 μl aliquot of each plasma sample was placed in 2-ml Eppendorf tubes containing 1 ml acetonitrile. The mixture was blended by vortex-mixing for 60 s and centrifuged at 21,255 g for 10 min at 4°C. The supernatant was transferred to a new tube, and 5 μl was analyzed by UHPLC–MS/MS.
2.6 Method validation
This method was developed according to the Ministry of Agriculture of the People's Republic of China guidelines for validation of bioanalytical methods.
Method specificity was evaluated by comparing chromatograms of blank plasma with corresponding plasma samples spiked with QCT, 1-DQCT, BDQCT and MQCA, as well as real samples collected from treated ducks.
Standard curves were constructed using peak area ratios versus analyte concentrations of QCT, 1-DQCT, BDQCT in duck plasma in the concentration range of 0.05–500 ng/ml, MQCA in duck plasma in the concentration range of 5–500 ng/ml and described in the form of y = a + bx. The criteria for the calibration curve was a correlation coefficient (r) of >0.99. The limit of detection (LOD) of each analysis in duck plasma was determined using a signal-to-noise ratio (S/N) ≥3. The limit of quantification (LOQ) of each analysis in duck plasma was determined using a signal-to-noise ratio (S/N) ≥10.
The precision and accuracy of the assay were evaluated by analyzing the QC samples at three concentration levels on three consecutive days. The accuracy expressed as relative recovery (RR %) was between 80% and 120% at the LOQ and between 85% and 115% at all other concentrations. Interday and intraday precision was expressed as relative standard deviation (RSD, %). The allowed RSD was below 20% for LOQ and less than 15% for the others.
QCT and its major metabolites recoveries were determined by comparing processed samples with reference solutions in blank biological extracts at the same levels. The absolute matrix effect (ME) was assessed by comparing the peak areas obtained using the mobile phase spiked with low, middle, and high QCT concentrations (n = 5) and with postextraction blank spiked samples.
The stability profiles of QCT and its major metabolites were assessed by testing the stock solutions after storage at room temperature and 4°C for 4 days. Each analysis stability in duck plasma under the conditions that were encountered during sample collection, storage, sample preparation and analysis was profiled using QC samples. These investigations included short-term stability (4°C, 24 hr), long-term stability (−20°C, 15 days), stability after three freeze-thaw cycles (−20°C to room temperature) and postpreparation stability (4°C, 12 hr). All samples were stored in the dark. Each analysis stability was not considered an issue if its analytical response did not deviate by more than 15% from the corresponding freshly prepared samples.
2.7 Pharmacokinetic studies
Pharmacokinetic studies were performed in 20 ducks and randomly divided into two groups (n = 10/group). One group received a single oral administration of QCT at 40 mg/kg. Blood samples (1 ml) were collected at 0, 0.083, 0.167, 0.25, 0.5, 0.75, 1, 1.5, 2, 4, 6, 8, 12, 24, 36, 48, 60, 72, and 96 hr. Another group received a single intravenous administration of QCT at 10 mg/kg, and blood samples were collected at 0, 0.033, 0.083, 0.167, 0.25, 0.5, 0.75, 1, 2, 4, 6, 8, 12, 24, 36, 48, 60, 72, and 96 hr. Plasma samples were processed by centrifuging the blood at 1,519 g for 10 min at 4°C and stored at −80°C for analysis. QCT and its metabolites are not stable to light, so the specific protection measures have been taken in the preparation and detection process.
2.8 Data analysis
The noncompartmental analysis (NCA) was employed to calculate PK parameters using WinNonlin version 5.2 (Pharsight Corporation, USA). The Cmax (the maximum plasma concentration), Tmax (time of reaching maximum concentration), AUCINF_obs (the area under the plasma concentration–time curve from dosing_time extrapolated to infinity), Cl_obs (total body clearance for extravascular administration), Vss_obs (volume of distribution at steady-state) and MRTINF_obs (mean residence time (MRT) extrapolated to infinity) were estimated. Oral bioavailability was evaluated according to the following equation: F (%) = (AUCp.o. × Dosei.v.) / (AUCi.v. × Dosep.o.) × 100%. Data are presented as mean and standard deviation (SD).
3 RESULTS
3.1 Method validation
Under the optimized conditions, no endogenous substances interfered with the target peaks after oral and intravenous QCT administration (Fig. S1 to Fig. S4). The calibration curve showed good linearity over a range of 0.05–500 ng/ml for QCT, 1-DQCT, BQCT and 5–500 ng/ml for MQCA. The regression coefficient (r) of each analysis was >0.999.
The LOD of QCT, 1-DQCT and BQCT was 0.025 ng/ml, and the LOQ of QCT, 1-DQCT and BQCT was 0.05 ng/ml in duck plasma. The LOD of MQCA was 2.5 ng/ml, and the LOQ of MQCA was 5 ng/ml in duck plasma. The relative recovery (RR %) for intraday and interday analyses was between 88.0% and 95.4%, and the relative standard deviation (RSD, %) was less than 15%. The recoveries of QCT, 1-DQCT, BQCT and MQCA were not interfered by absolute matrix effect, ranging from 88.6% to 94.5%.
The results of stabilities of QCT and its major metabolites showed that there were no significant changes of their concentrations under different storages. The accuracy of each analysis ranged from 86.2% to 94.9%. It is indicated that our handling and storage methods were applicable for routine analyses.
3.2 Pharmacokinetics
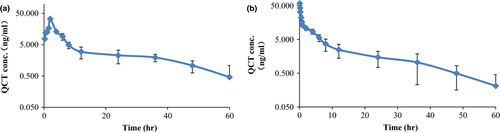
In this study, QCT and its major metabolites such as 1-DQCT and MQCA were detected at individual time points in individual ducks, while the BDQCT was not detected at all time points in all ducks. The concentrations of 1-DQCT and MQCA in duck plasma were ranged from 0.230 to 0.334 ng/ml and 3.995–54.223 ng/ml after oral administration, respectively, and 0.243–0.483 ng/ml and 7.466–262.218 ng/ml after intravenous administration, respectively. Plasma QCT concentration was below the LOD for all ducks at 72 hr, with the concentration range of 0.515–32.135 ng/ml after oral administration, and 0.211– 96.687 ng/ml after intravenous administration.
The plasma concentration–time relationship of QCT following a single 40 mg/kg oral dose and a single 10 mg/kg intravenous dose is depicted in Figure 2, and relevant pharmacokinetic variables are summarized in Table 1. Plasma QCT concentration was below the LOD for all ducks at 72 hr, Cl_obs was rapid at 69.00 L/h/kg and Vss-obs was 807.00 L/kg after intravenous administration. The Tmax after oral administration was 2 hr, indicating rapid absorption. The observed Cmax after oral and intravenous administration was 233.63 and 152.34 (hr ng) ml−1, respectively. The MRTINF_obs was 18.75 hr for oral administration and 12.39 hr for intravenous administration. The calculated bioavailability for orally administered QCT was 38.43%.
| Parameter | Unit | Oral administration (40 mg/kg) | Intravenous administration (10 mg/kg) |
|---|---|---|---|
| HL_Lambda_z | hr | 16.38 ± 5.02 | 10.88 ± 3.20 |
| Lambda_z | hr | 0.047 ± 0.017 | 0.069 ± 0.022 |
| T max | hr | 2.00 ± 0.00 | – |
| C max | ng/ml | 32.14 ± 2.25 | 96.70 ± 7.56 |
| AUCINF_obs | (hr ng) ml−1 | 233.63 ± 38.10 | 152.34 ± 38.39 |
| Cl_obs | L/hr/kg | 69.00 ± 16.00 | |
| Vss _obs | L/kg | 807.00 ± 146.00 | |
| MRTINF_obs | hr | 18.75 ± 5.69 | 12.39 ± 4.04 |
4 DISCUSSION
4.1 UHPLC-MS/MS method development and optimization
The MS method, which has been shown in the materials and methods in detail, was further optimized based on previous reports (Li, Huang et al., 2014; Li, Liu et al., 2014; Liu et al., 2014; Yan et al., 2013). Furthermore, the HPLC separation was optimized and started with previously published condition (Li, Huang et al., 2014; Li, Liu et al., 2014; Liu et al., 2014; Yan et al., 2013). The final mobile phase conditions were 0.1% formic acid in water and acetonitrile using a gradient elution program (see 2.2 Instrumentation and analytical conditions) and achieved excellent peak separation and symmetry. This method had a short running time (7.2 min), high sensitivity (LOD 0.025 ng/ml for QCT, 1-DQCT, BDQCT and 2.5 ng/ml for MQCA) and economical sample pretreatment steps.
4.2 Pharmacokinetics
So far, there have been some studies about QCT and its metabolites in animals, such as in pigs (Li et al., 2003; Li, Huang et al., 2014; Li, Liu et al., 2014; Wang et al., 2012; Zhong et al., 2011), chickens (Li et al., 2003; Zhang et al., 2011), carps (Hu et al., 2008; Yang et al., 2010), channel catfish (Liu et al., 2009; Yang et al., 2010) and Turbot (Li et al., 2011). QCT, 1-DQCT and BDQCT were detected in pig plasma following a single 40 mg/kg oral dose by HPLC-MS/MS (Zhong et al., 2011). QCT and MQCA were detected in chicken plasma after oral administration at 30 mg/kg, while QCT and 1-DQCT were detected in chicken plasma after intravenous administration at 2.5 mg/kg by HPLC-MS/MS (Zhang et al., 2011). QCT, 1-DQCT, BDQCT, and MQCA were detected in pig plasma following a single 40 mg/kg oral dose by HPLC-MS/MS (Wang et al., 2012). In our study, QCT, 1-DQCT, and MQCA were all detected at individual time points, while BDQCT was not detected in all time points after oral and intravenous administration by UHPLC-MS/MS.
Plasma QCT concentrations increased rapidly after oral administration and reached the Cmax within 2.0 hr, indicating that it was orally active and quickly absorbed. The Cmax after oral administration was lower than the value after injecting the QCT. It maybe due to its low absorption from the gastrointestinal tract, or widely distribution in the tissues, or transformation into other metabolites.
As the reported literatures, the Tmax, Cmax, AUC in pigs after oral single dose at 40 mg/kg were 2.2 hr, 260 ng/ml, 1540 (hr ng) ml−1, respectively (Wang et al., 2012). The Cmax and AUC in chickens after oral single dose at 30 mg/kg were 100 ng/ml, 860 (hr ng) ml−1, respectively (Zhang et al., 2011). The Cmax in carp and channel catfish blood after oral single dose at 50 mg/kg was 452 ng/ml and 29 ng/ml, respectively (Liu et al., 2009). The Tmax in chickens after oral single dose at 31.15 mg/kg was 1.8 hr (Li et al., 2003) and 2 hr in carps after feeding 30 mg/kg QCT (Liu et al., 2009). In this study, the Tmax, Cmax, AUCINF_obs in duck plasma after oral administration at 40 mg/kg were 2 hr, 32.14 ng/ml, and 233.63 (hr ng) ml−1, respectively. The Cmax and AUC in duck plasma were lower than that in pig plasma after oral administration with the same dose. These studies showed that QCT was absorbed less by oral administration in animal's body. These differences in the Cmax, AUC values among ducks, and other animals may be caused by differences in plasma protein binding, distribution of metabolites, dosage regimes, as well as detection method.
The calculated bioavailability for orally administered QCT was 38.43% for a 40 mg/kg dose. Previous studies have reported much lower QCT bioavailability such as 21.47% in chickens (Zhang et al., 2011), 0.5% in pigs, and 3.0% in another chicken's study (Li et al., 2003). These values were all significantly low. The bioavailability of a drug given orally was subject to metabolic or bioconversion, microflora influences as well as modification by metabolic enzymes in the gastrointestinal tract. The results indicated that QCT was absorbed less by oral administration, most of QCT was excreted from the gastrointestinal tract and QCT played a part in growth promoting mainly through animal's gastrointestinal tract.
5 CONCLUSIONS
A fast, simple, selective, and sensitive UHPLC-MS/MS method was developed and validated for investigating pharmacokinetics of QCT and its major metabolites in ducks. This method showed reproducible and consistent recoveries with minimal matrix effects. It was concluded that QCT and its major metabolites were stable in solution as well as in plasma samples. The pharmacokinetic parameters indicated that QCT in ducks was not easy to be absorbed by oral administration, leading to low blood drug concentrations and low bioavailability. The low bioavailability indicated that QCT in duck's gastrointestinal tract was similar to that in other animals. To our knowledge, this is the first study on bioanalysis and pharmacokinetics of QCT in ducks. This study offers a scientific basis for the further research of QCT in ducks and could be useful as the references for further clinical application of QCT in ducks.
ACKNOWLEDGMENT
This work was supported by National Natural Science Foundation of China (31402256), International Advanced Agricultural Science and Technology Foundation of P. R. China (2016-X28), and High-level Talent Research Foundation of Qingdao Agricultural University (No. 631206).




