Suspected aspirin resistance in individual healthy adult warmblood horses
Abstract
The reasons for this prospective experimental study were to determine a dosing scheme with loading and maintenance dose of aspirin inducing inhibition of platelet function measured by whole blood impedance aggregometry. Ten horses received aspirin orally in the morning with one loading dose of 4.7–5 mg/kg and maintenance doses of 1–1.3 mg/kg daily the following 4 days. Aggregometries (COLtest, ASPItest, ADPtest) and serum salicylic acid were measured. ASPItest showed significant difference in inhibition at 24 and 48 hr (p < .05) and 96 hr (p < .01). Significant change for ADPtest and COLtest couldn't be detected. Serum salicylic acid concentrations were significantly (p < .01) increased at 6 and 12 hr. Despite this, three horses failed any inhibitory effect of platelet function, suspecting an aspirin resistance. Regarding the other seven horses platelet aggregation induced by ASPItest was reduced between 37% and 100% from baseline at 6 and 12 hr and between 0 and 98% during the next 4 days. Correlations of serum concentration of salicylic acid and aggregometries couldn't be detected. It can be presumed that equine platelets are less susceptible to aspirin what may compromise eventually the anticoagulatory effects and efficacy in preventing and treating diseases with increased platelet activation as endotoxaemia or laminitis.
1 Introduction
In people, bleeding disorders and influences on hemostatic parameters during treatment with aspirin (acetylsalicylic acid) were first described in the late 1950s (Gast, 1964). The molecular mechanism of irreversible inactivation of the cyclooxygenase (COX) enzyme by aspirin resulting in inhibition of platelet function was identified 15 years later (Roth & Majerus, 1975). Oral intake of aspirin, often as dual therapy with a thienopyridine (e.g., clopidogrel) in preventing repeated occurrence of cardiovascular events (e.g., myocardial infarction) is widely recommended in human medicine (Baigent et al., 2009; Bonzel et al., 2008). In horses, activated platelets are associated with endotoxemia (Watts et al., 2014), laminitis (Bailey et al., 2009; Weiss, Evanson, McClenahan, Fagliari, & Jenkins, 1997), airway obstruction (Dunkel, Rickards, Page, & Cunningham, 2007), systemic inflammatory disorders (Segura et al., 2007), and inhibition of platelet function may therefore be a valuable adjunct therapy.
In humans, a loading dose of 4 mg/kg aspirin induces a full and irreversible inhibition of platelet function (Patrignani, Filabozzi, & Patrono, 1982), whereas maximal inhibition is reached 2 hr after oral ingestion (Siller-Matula, Gouya, Wolzt, & Jilma, 2009). Maintenance doses as low as 1–1.5 mg/kg once daily are sufficient to maintain the inhibition (Siller-Matula et al., 2009). In horses, aspirin is traditionally recommended in equine textbooks for thrombophylaxis with peroral (PO) dosages between 10 and 100 mg/kg at intervals of 12–48 hr (Reed, Bayly, & Sellon, 2010; Robinson & Sprayberry, 2009). In experimental studies, aspirin was administered orally once in three different dosages (5, 10 and 20 mg/kg; Baxter & Moore, 1987), once with 10 mg/kg (Segura et al., 2005), and daily for three (17 mg/kg; Kopp, Moore, Byars, & Brooks, 1985) respectively five (10 mg/kg; Brainard et al., 2011) consecutive days. The lowest dose of 5 mg/kg administered once induced an inhibition of platelet function determined by a 71%–87% reduction in serum TXB2 for 24–72 hr, and the inhibition was not further increased with the higher doses of 10 or 20 mg/kg (Baxter & Moore, 1987).
In some equine studies, the inhibitory effect of aspirin on platelets was indirectly determined with template bleeding time (Hagedorn, Bock, & Schulz, 1992; Judson & Barton, 1981), demonstrating an anticoagulatory effect of oral administered aspirin. However, template bleeding time has a poor reproducibility (Segura & Monreal, 2008) and therefore seems to be less suitable demonstrating inhibition of platelet function in horses. Measurement of TXB2 revealed a significant reduction in plasma concentrations demonstrating inhibition of platelet function after oral administration of aspirin in horses (Baxter & Moore, 1987; Brainard et al., 2011; Kopp et al., 1985). Only two equine studies measured platelet function after oral aspirin in whole blood (Segura et al., 2005) or platelet-rich plasma (Brainard et al., 2011), but with COX-1 unspecific agonists as ADP, collagen, and epinephrine.
The use of the whole blood impedance multiple electrode aggregometer (MEA) Multiplate Analyzer (Roche) is well established in human platelet function testing, and the inhibitory effects of aspirin can be reliably and specifically detected via agonists arachidonic acid (AA) and collagen (Chen et al., 2011; Pedersen et al., 2009; Penz et al., 2010; Velik-Salchner et al., 2008). The method of whole blood impedance aggregometry has been applied in some equine studies (Jarvis & Evans, 1994; Kornreich, Enyeart, Jesty, Nydam, & Divers, 2010), and the Multiplate Analyzer is suitable detecting the inhibitory effects of clopidogrel in horses (Roscher, Failing, & Moritz, 2015).
The objective of this study was to validate a low-dosing scheme involving loading and maintenance doses of aspirin that was designed to induce the inhibition of platelet function, as measured by whole blood impedance aggregometry in healthy adult warmblood horses. We hypothesized that this dosing scheme would inhibit platelet aggregation within hours and maintain this inhibition over at least the next 4 days.
2 Materials and Methods
2.1 Study design
Ten healthy adult warmblood mares (n = 4) and geldings (n = 6) that had not received any medication for at least the preceding 14 days were used. Absence of pathologic abnormalities revealed by clinical examinations including ultrasound of both jugular veins justified the health status. The mean electronically determined bodyweight was 572.1 kg (range: 440–626 kg), and mean age was 15.2 years (range: 3–23 years). The horses were stabled in individual boxes with light daily exercise, fed with grass hay (≥1.5 kg/100 kg) and were provided with individualized amounts and varieties of concentrates. Horses received acetylsalicylic acid [Aspirin 500 mg and 100 mg, Bayer] PO at 9 a.m. on five consecutive days. To better simulate clinical settings, the horses were not fasted before the administration of medication, and individual weight-dependent dosing was replaced by dosing in weight categories of 50 kg, which resulted in single loading doses of 4.7–5 mg/kg on the first day and daily maintenance doses of 1–1.3 mg/kg the four consecutive days. The tablets were fed with the concentrate respectively crushed and mixed with molasses and administered via syringe. Medications and controls for complete intake were performed by one of the authors (KR). Horses underwent a physical examination every 12 hr throughout the study period. The prospective study was approved by the Ethics Committee for Animal Welfare of the district government of Giessen, Germany (GI 18/17-Nr. 102/2010).
2.2 Blood samples
Blood was collected by venipuncture of the jugular vein with an 18 G sterile needle and a vacuum system (S-Monovette, Sarstedt). Samples for platelet function testing and hematology were taken before medication (T0) and at time points (T) 6, 12, 24, 48, 72, 96, 144, 192, and 240 hr. Hematology (ADVIA 2120, Siemens) included complete cell counts (erythrocytes, leucocytes, and platelets), leucocyte differential counts, erythrocyte variables (hemoglobin, MCV, MCH, and MCHC), and platelet variables (MPV, MPC). At selected time points (T0, T24, T144, and T240), laboratory examinations were completed via clinical chemistry and coagulation panels. The clinical chemistry panel included urea, creatinine, ionized electrolytes (sodium, potassium, chloride, and anorganic phosphorus), total plasma protein, albumin, total bilirubin, direct bilirubin, enzymes (ALP, GLDH, GGT, AST, CK, and LDH), total bile acids (Pentra 400, Axon Lab), and ionized magnesium and calcium (Nova 8 Analyzer, Nova). Coagulation variables (STA Compact, Diagnostica Stago) included aPTT (Kaolin-activated, C.K. Prest, Diagnostica Stago), PT (STA-Neoplastine CI Plus, Diagnostica Stago), and fibrinogen (STA-Fibrinogen, Diagnostica Stago). All measurements were completed within 60 min after blood collection. At every time point except T192 and T240, serum samples were taken and stored at −20°C for analysis of the aspirin metabolite and degradation product salicylic acid (SA).
2.3 Measurement of platelet function
After blood collection, tubes containing hirudin (20 μg/ml, Dynabyte) were stored at rest at room temperature for 30 min. Platelet function was measured by whole blood impedance aggregometry (Multiplate Analyzer, software version V2.03.11) according to the instructions of the manufacturer (Dynabyte GmbH, 2010) as previously described (Roscher et al., 2015). Briefly, 300 μl of blood was added to 300 μl saline (0.9%) preheated to 37°C in the test cell. After 3 min, aggregometry was induced by the addition of 20 μl of standardized agonists: ADP (6.5 μM final concentration, ADPtest, Roche), arachidonic acid (0.75 mM final concentration, ASPItest, Roche), and collagen (1.6 μg/ml final concentration, COLtest, Roche). Aggregation was measured over 12 min. The activation of platelets was continuously registered and transformed into arbitrary aggregation units and plotted against time. Two curves were assessed by two independent sensors in each test cell. The parameters calculated by the software and used for statistical analysis were the mean values of the parameters determined with both curves. Repeated measurements were advised when the coefficient of correlation was less than 98% or when differences in measurements of more than 20% of the mean were observed. Without advisement of repeated measurement each test was performed once. The area under the aggregation curve reflected the overall platelet activity, which was displayed in units (U). All measurements were completed within 60 min after blood collection.
2.4 Quantitative analysis of serum salicylic acid
Serum samples were prepared according to a validated direct injection method (Buntenkötter, 2012). Briefly, 0.5 ml serum was diluted with 0.5 ml of Milli-Q-water and the internal standard d4-SA. Protein precipitation was conducted by addition of 1.5 ml acetonitrile. After centrifugation, samples were basified in order to hydrolyze acetylsalicylic acid to SA. 200 μl aliquots were used for liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. LC-MS/MS analyses were performed on a Series 1200 liquid chromatograph (Agilent) coupled to a API 3200 triple quadrupole mass spectrometer (AB Sciex) equipped with an electrospray ionization (ESI) interface. The column was a Nucleodur C18 Pyramid with dimensions of 4 × 70 mm and particle size of 5 μm (Macherey und Nagel) protected by a Gemini C6 phenyl column guard (Phenonemex). The LC-MS/MS conditions were as follows: mobile phase A = ammonium acetate buffer (5 mM ammonium acetate, 0.1% acetic acid, pH 3.5), mobile phase B = acetonitrile, flow rate 0.8 ml/min with a linear gradient B 15%→100% in 7 min, and injection volume of 10 μl. Samples were measured in the negative ionization mode at an interface temperature of 450°C with an ion spray voltage of −4500 V. Diagnostic ions were generated by collision-induced dissociation with nitrogen at 2.9 × 10−3 Pa. For quantitation purposes, multiple reaction monitoring (MRM) experiments were performed on the most abundant ion transitions. The ion transitions m/z 137-65 and m/z 141-69 were used as quantifier ions for SA and the internal standard d4-SA, respectively. Data acquisition and analysis were accomplished with Analyst® software (Version 1.4.2, AB Sciex). The level of quantification of SA was 0.2 μg/ml.
2.5 Statistical analysis
Statistical analyses were performed with the BMDP program package (Dixon, 1993). For the majority of variables, data descriptions represent the arithmetic mean and standard deviation (SD). After testing for deviations from the normal distribution using the normal probability plot of the residuals (Q-Q-plot), one-way repeated measures ANOVAs were performed after logarithmic respectively square root transformation of the data if required. When the ANOVAs showed significant changes over time, post hoc Dunnett-tests were used to compare each time point to T0. Comparison of plasma levels of SA and aggregometry percentage values from baseline was determined by correlation analysis. For each variable, the level of statistical significance was set at p ≤ .05.
An a priori power analysis based on data from Baxter and Moore (1987) using the program BiAS. for Windows (Version 9.08) revealed a sample size of 10 horses needed to detect an expected mean reduction of 70% in AA-induced platelet aggregation 24 hr after the loading dose of aspirin with a probability of .95 and an accuracy of ±10%.
3 Results
All horses remained clinical healthy.
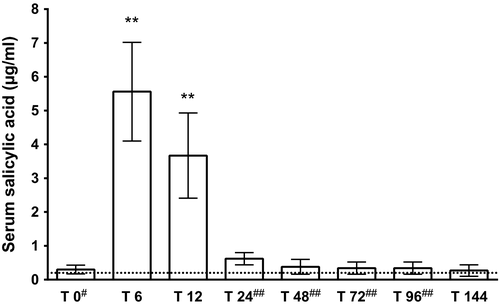
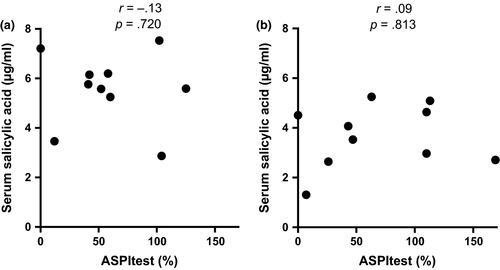
ANOVA for serum concentrations of SA showed significant change over time (p < .0001) where concentrations were significantly (p < .01) increased at T6 (5.56 ± 1.46 μg/ml) and T12 (3.67 ± 1.26 μg/ml) (Figure 1). Significant correlation of serum concentrations of SA and aggregometry percentage values from baseline (ASPItest [Figure 2], COLtest, ADPtest) was not detectable. ANOVA for ASPItest showed significant change over time (p < .001). Significant inhibition could not be detected at T6 and T12. Only T24 (p < .05), T48 (p < .05), and T96 (p < .01) demonstrated a significant difference (Table 1). There was no significant change in platelet aggregation induced by ADPtest and COLtest at any time point (Table 1).
| Test | T0a | T6 | T12 | T24b | T48b | T72b | T96b | T144 | T192 | T240 |
|---|---|---|---|---|---|---|---|---|---|---|
| ASPItest | ||||||||||
| U | 107 ± 45 | 76 ± 71 | 86 ± 83 | 66c±62 | 68c±58 | 78 ± 56 | 56d±48 | 109 ± 58 | 101 ± 59 | 90 ± 44 |
| % | 100 | 59 ± 40 | 69 ± 55 | 54c±35 | 57c±29 | 73 ± 35 | 51d±30 | 107 ± 45 | 97 ± 65 | 84 ± 12 |
| COLtest | ||||||||||
| U | 344 ± 74 | 302 ± 70 | 322 ± 71 | 310 ± 67 | 316 ± 70 | 310 ± 76 | 317 ± 64 | 348 ± 66 | 318 ± 47 | 329 ± 47 |
| % | 100 | 89 ± 10 | 93 ± 15 | 96 ± 23 | 91 ± 16 | 95 ± 23 | 94 ± 21 | 102 ± 28 | 98 ± 24 | 96 ± 13 |
| ADPtest | ||||||||||
| U | 193 ± 64 | 186 ± 72 | 195 ± 71 | 169 ± 53 | 186 ± 64 | 167 ± 48 | 165 ± 50 | 174 ± 39 | 164 ± 49 | 149 ± 34 |
| % | 100 | 99 ± 31 | 102 ± 23 | 90 ± 23 | 97 ± 15 | 88 ± 13 | 88 ± 16 | 94 ± 17 | 95 ± 29 | 84 ± 14 |
- a 4.7–5 mg/kg aspirin PO.
- b 1–1.3 mg/kg aspirin PO.
- c p < .05 significant difference from T0 (Dunnett-test).
- d p < .01 significant difference from T0 (Dunnett-test).
Looking at the individual values of the 10 horses, three of them (horse 5, 8, and 10) failed any inhibitory effect of AA-induced platelet function at T6 and T12, where significant higher levels of mean serum concentration of SA were measured (Table 2). During the 4 days of low dose maintenance dosing (T24–T96) AA-induced platelet function in these three horses showed a maximal decrease to 78% from baseline (horse 8) corresponding to a reduction of 22% (Table 2). The other seven horses demonstrated decreases in AA-induced platelet function to 0%–60% from baseline at T6, to 0%–63% from baseline at T12 and during the 4 days of low dose maintenance dosing decreases to 2%–100% from baseline. This equates a reduction of 40%–100% at T6, of 37%–100% at T12 and of 0%–98% during the maintenance dosing (Table 2).
| Horse | Test | T0a | T6 | T12 | T24b | T48b | T72b | T96b | T144 | T192 | T240 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ASPItest (%) | 100 | 52 | 47 | 69 | 38 | 91 | 48 | 192 | 225 | 81 |
| SA (μg/ml) | 0.35 | 5.58 | 3.53 | 0.58 | – | – | <0.2 | 0.24 | – | – | |
| 2 | ASPItest (%) | 100 | 60 | 63 | 26 | 31 | 23 | 16 | 28 | 41 | 68 |
| SA (μg/ml) | 0.29 | 5.25 | 5.25 | 0.65 | – | – | <0.2 | 0.21 | – | – | |
| 3 | ASPItest (%) | 100 | 41 | 113 | 58 | 64 | 62 | 44 | 113 | 99 | 101 |
| SA (μg/ml) | 0.25 | 5.76 | 5.09 | 0.81 | – | – | 0.54 | <0.2 | – | – | |
| 4 | ASPItest (%) | 100 | 58 | 0 | 21 | 21 | 16 | 2 | 60 | 89 | 85 |
| SA (μg/ml) | 0.34 | 6.20 | 4.51 | 0.79 | – | – | 0.34 | <0.2 | – | – | |
| 5 | ASPItest (%) | 100 | 125 | 169 | 106 | 79 | 100 | 81 | 128 | 174 | 96 |
| SA (μg/ml) | <0.2 | 5.59 | 2.71 | 0.48 | – | – | 0.26 | <0.2 | – | – | |
| 6 | ASPItest (%) | 100 | 42 | 43 | 37 | 78 | 77 | 54 | 120 | 116 | 68 |
| SA (μg/ml) | 0.31 | 6.15 | 4.07 | 0.64 | – | – | 0.33 | 0.26 | – | – | |
| 7 | ASPItest (%) | 100 | 0 | 26 | 31 | 61 | 60 | 35 | 128 | 57 | 69 |
| SA (μg/ml) | 0.37 | 7.21 | 2.64 | 0.51 | – | – | 0.38 | 0.26 | – | – | |
| 8 | ASPItest (%) | 100 | 102 | 110 | 104 | 88 | 78 | 89 | 112 | 106 | 85 |
| SA (μg/ml) | <0.2 | 7.53 | 2.97 | 0.45 | – | – | 0.41 | 0.34 | – | – | |
| 9 | ASPItest (%) | 100 | 12 | 7 | 9 | 16 | 113 | 42 | 68 | 63 | 85 |
| SA (μg/ml) | 0.22 | 3.46 | 1.31 | 0.36 | – | – | <0.2 | 0.20 | – | – | |
| 10 | ASPItest (%) | 100 | 104 | 110 | 81 | 99 | 108 | 94 | 125 | 99 | 97 |
| SA (μg/ml) | 0.61 | 2.87 | 4.64 | 0.95 | – | – | 0.70 | 0.73 | – | – |
- a 4.7–5 mg/kg.
- b 1–1.3 mg/kg PO.
ANOVA and ad hoc Dunnett-test revealed the following changes in hematology and coagulation: Leucocytes at T6 (p < .05); Lymphocytes at T6 and T12 (p < .05); MCV at T48 (p < .05); MPC at T12 (p < .01); and aPTT at T240 (p < .05). Significant changes in clinical chemistry could not be detected.
4 Discussion
The loading dose of 4.7–5 mg/kg aspirin failed to induce an inhibition of platelet function within hours measured with AA-induced whole blood aggregometry. Looking at the individual horses, seven of the ten horses demonstrated a reduction in inhibition between 37% and 100% at T6 and T12. In the remaining three horses, an aspirin resistance was suspected based on complete failure of inhibition during the first 12 hr where maximal inhibitory effects are observed in people (Siller-Matula et al., 2009). Furthermore, these three horses demonstrated a maximum reduction of AA-induced aggregation of 22% on the four consecutive days of low maintenance dosing and until the end of the study. Data for intra-individual and intra-assay imprecision in horses are lacking, but an intra-individual day-to-day variation of 4.9%–22.9% and intra-assay imprecision of 7.0%–19.6% in humans (Seyfert, Haubelt, Vogt, & Hellstern, 2007) and intra-assay imprecision of 6.0%–17.6% in dogs (Kalbantner, Baumgarten, & Mischke, 2010) have been demonstrated in AA-induced platelet function measured with the Multiplate Analyzer. Likewise, it can be presumed that the low reduction demonstrated in the three horses is based on test imprecision or intra-individual day-to-day variation. The low maintenance dose was not fully suitable for preserving this inhibition over the course of therapy because the inhibition of platelet function measured with the ASPItest did not reach a significant level on day four (T72). Regarding the seven horses responding to the loading dose, only three of them (horse 2, 3, and 4) preserved the inhibition from T24 to T96 (Table 2). In people, a low dose of 100 mg aspirin (corr. 1–1.5 mg/kg) for seven consecutive days induced a reduction of 91%–100% in platelet function measured with the ASPItest with the Multiplate (Pedersen et al., 2009; Penz et al., 2010), whereas our dosing regimens failed to reach this levels in nearly all measurements (Table 2). Differences between people and horses in the bioavailability of aspirin after oral administration are a possible explanation, but determination of serum SA in humans showed concentrations of about 6 μg/ml 6 hr after oral 325 mg (corr. 5 mg/kg) aspirin (Patrick, Dillaha, Armas, & Sessa, 2015) when a concentration of 5.56 ± 1.46 μg/ml was measured in our horses at T6. So it can be speculated that horses are less susceptible to the effects of aspirin. This consideration is supported by the results of other equine studies using measurement of serum concentration of TXB2 after treatment with aspirin once (5, 10, and 20 mg/kg; Baxter & Moore, 1987), or on five consecutive days (10 mg/kg, Brainard et al., 2011) where a maximal reduction of serum concentrations of 90% were demonstrated, whereas in people more than 99% were determined (Pedersen et al., 2009).
The a priori power analysis in the present study revealed a sample size of 10 horses needed to detect an expected mean reduction of 70% in AA-induced platelet aggregation at T24. The study failed to demonstrate this reduction. The analysis based on data from an equine study (Baxter & Moore, 1987) where the inhibitory effects of 5 mg/kg aspirin were determined using a different method (serum concentrations of TXB2). In people, a low dose of 1–1.5 mg/kg aspirin induces a mean reduction of 91% in AA-induced aggregation measured with the Multiplate Analyzer, whereas serum concentrations of TXB2 are decreased to less than 1% comparing to baseline values (Pedersen et al., 2009). These varying results in percental reduction using different methods might be an explanation for the failure in the present study.
The term aspirin “resistance” is widely used in human medicine, but resistance to aspirin should be limited to situations in which aspirin is unable to inhibit COX-1-dependent synthesis of Thromboxane (TX) A2 (Cattaneo, 2007). There is no consensus about a reliable and specific laboratory method to determine this so-called resistance, but methods using stimulation with thrombin, adenosine diphosphate (ADP), collagen, or epinephrine can activate platelets in the presence of complete inhibition of the COX-1 and are therefore not useful to determine an aspirin resistance (Gurbel & Tantry, 2013). COX-1-specific methods are the measurement of serum TXB2 (metabolite of TXA2), determination of agonist-induced TXB2 in platelet-rich plasma (PRP), and arachidonic acid (AA)-induced platelet aggregation tests (Gurbel & Tantry, 2013). Aspirin resistance is described in 5%–45% of human patients, and this wide variation can be explained to a large extent by lack of standardized definition and validated laboratory methodology (Floyd et al., 2014). Compliance plays a crucial role in humans for the occurrence of aspirin resistance, and lack of biochemical assessment of compliance (e.g., measurement of serum salicylic acid concentration) has a major intrinsic bias in most of the studies (Patrono, 2013). To the authors knowledge aspirin resistance in horses is not described previously in the literature.
We measured efficacy of aspirin in horses with AA-induced aggregometry and defined resistance with failure of inhibition in the first 12 hr. To verify adequate absorption of aspirin, serum SA was measured, and the three horses with failure of inhibition showed no difference in serum concentrations compared with the other seven horses (Table 2). Furthermore, one of these horses (horse 8) showed the highest SA (7.53 μg/ml at T6) in all measurements without any reduction of AA-induced aggregation. This was also demonstrated in healthy people where all volunteers with aspirin resistance had detectable levels of serum salicylate measured by an ELISA (Floyd et al., 2014). Overall serum concentrations did not correlate with the change of AA-induced aggregation measurement leading to the suspicion of high inter-individual variability of the aspirin effect in horses. Correlation of serum salicylate and aggregation was not determined in people (Floyd et al., 2014). In most human studies, at least two different methods are used to determine true aspirin resistance (Floyd et al., 2014; Schwartz et al., 2005). Our suspected resistance in horses was not verified with a second COX-1-specific method as measurement of serum TXB2 considering a limitation of the study. Other equine studies determined aspirin effect with also only one method (measurement of TXB2), and aspirin resistance was not described (Baxter & Moore, 1987; Brainard et al., 2011; Kopp et al., 1985).
The dosing regimes in our study failed to induce a decrease in platelet function measured with collagen-induced platelet aggregation (Table 1). In people, a low dose of 100 mg ASA (corr. 1–1.5 mg/kg) induced a decrease of 30%–50% in platelet function measured with the COLtest (Can et al., 2010; Velik-Salchner et al., 2008). In contrast to the studies in people where the COLtest was performed with the recommended collagen concentration of 3.2 μg/ml, we used a lower concentration of 1.6 μg/ml within the recommended range listed in the guidelines for humans published by the Clinical and Laboratory Standards Institute (Christie, 2008). In a small pilot study, we found high aggregation values in horses with the recommended collagen concentration (data not shown). The lower collagen concentration was applied because detection of platelet function inhibition is more sensitive near the threshold concentration (Mischke & Schulze, 2004). Furthermore, a concentration of 1 μg/ml collagen results in nearly 100% aggregation of equine platelets in PRP (Pelagalli et al., 2002).
Likewise, higher doses of aspirin (10 mg/kg on 5 consecutive days) had no influence on collagen-induced aggregometry performed with PRP in horses (Brainard et al., 2011). Hence, the COLtest and other collagen-induced platelet function testing cannot be recommended to assess aspirin-induced platelet inhibition in horses. Moreover, the failure of inhibition of platelet function after contact with collagen may again indicate differences in platelet activation regarding horses and humans. There was no aspirin effect on ADP-induced aggregometry (ADPtest) as demonstrated in people (Penz et al., 2010) and horses (Brainard et al., 2011; Segura et al., 2005).
This is the first study using the MEA Multiplate Analyzer for detection of the inhibitory effect of aspirin on platelet function in horses. The device is established to detect the effects in people to be as good or better as other methods (Siller-Matula et al., 2009; Velik-Salchner et al., 2008). The principle of whole blood impedance aggregometry has previously been applied in equine studies (Jarvis & Evans, 1994; Kornreich et al., 2010), and the Multiplate is suitable detecting the inhibitory effects of clopidogrel on platelet function in horses (Roscher et al., 2015).
All statistically detected changes in hematology and coagulation were clinically not relevant.
In conclusion, the loading dose of aspirin induced a rapid but only moderate inhibition of platelet function within hours in seven horses, but the maintenance doses were not fully adequate to retain inhibition levels over 4 days of therapy. In three horses, an aspirin resistance was suspected. The Multiplate Analyzer was suitable for detecting the effects of aspirin but only with the agonist arachidonic acid. Comparing the inhibitory effects of aspirin in horses to that determined in humans by AA- and collagen-induced whole blood aggregometry, it can be assumed that equine platelets are less susceptible to aspirin what may compromise the efficacy in preventing and treating diseases with increased platelet activation as endotoxemia or laminitis. It has been demonstrated in horses that even a higher loading dose of 10 or 20 mg/kg had no additional inhibitory effect (Baxter & Moore, 1987), but further studies are needed to confirm these results using the AA-induced aggregation (ASPItest) with the Multiplate. Likewise, more studies are needed to get more information on probably equine specific platelet functions regarding the susceptibility of aspirin.
Acknowledgments
The majority of the horses used in the study were provided courtesy of the Institute of Animal Nutrition, Nutrition Diseases and Dietetics, Faculty of Veterinary Medicine, University of Leipzig, Germany, and the Clinic for Obstetrics, Gynecology and Andrology of Large and Small Animals, Justus Liebig University Giessen, Germany.
Competing Interests
Material for the testing of platelet function with the Multiplate was provided free of charge from the former manufacturer Dynabyte. This company had no influence on the design of the study, the collection, management, analysis, or interpretation of the data or the preparation of the manuscript. None of the authors of this manuscript has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the manuscript.




