Pharmacokinetics of marbofloxacin in freshwater crocodiles (Crocodylus siamensis) after intravenous and intramuscular administration
Abstract
To evaluate the fate and disposition of marbofloxacin (MBF) in freshwater crocodiles (Crocodylus siamensis), MBF was administered either intravenously (i.v.) or intramuscularly (i.m.) at a dosage of 2.0 mg/kg body weight. The concentrations of MBF in plasma were measured using high-performance liquid chromatography equipped with a fluorescence detector. The concentrations of MBF in the plasma were measurable up to 144 h after i.v. and i.m. administration. After the first 45 min, the mean pharmacokinetic profiles produced by the two administration routes were almost identical. No statistically significant differences in the pharmacokinetic parameters between the groups were observed. The half-life was long (about 2.5 days), the volume of distribution was large (about 1.44 L/kg), λz was small (0.01 h−1), and the clearance was slow (22.6 mL/h/kg). The absolute i.m. bioavailability (F%) was 105.36%. The dose of MBF administered in this study seems to produce appropriate PK-PD parameters that predict antibacterial success for disease caused by susceptible bacteria. More studies are warranted to evaluate the likely residues after administration of multiple doses.
Introduction
Siamese crocodiles (Crocodylus siamensis), a freshwater crocodile species, are found in Thailand. They are the most popular freshwater crocodile for commercial purposes. Freshwater crocodile farming has increased over the past decade in Asia including Thailand. Currently, freshwater crocodile (C. siamensis) skins attract a premium price either for export earning or to supply local demand in Thailand. In the breeding farms, crocodile mortality is often directly related to infectious diseases and conditions precipitated by stress (Huchzermeyer, 2002).
Marbofloxacin (MBF) is a secondary generation of fluoroquinolone. It has a broad-spectrum bactericidal activity and is approved by FDA for skin and soft tissue infections in dogs and cats and for urinary tract infections in dogs (Pfizer, 2004). MBF has demonstrated a significant postantibiotic effect for both gram-negative and some gram-positive bacteria and is active in both stationary and growth phases of bacterial replication (Plumb, 2015). MBF is considered the most active veterinary fluoroquinolone against Mycoplasma and Pseudomonas species (Brown, 1996; Plumb, 2015).
The treatment of bacterial infections should be based on a rational scientific approach. The most common pharmacokinetic/pharmacodynamic (PK/PD) approach for antimicrobial agents uses plasma concentration as the PK input value and minimum inhibitory concentration (MIC) as the PD input value (Liu et al., 2002).
Fluoroquinolones are considered to be well tolerated in both humans and animals; however, inappropriate use has led to a significant increase in antimicrobial resistance (Giguere & Dowling, 2013). In reptiles, there have been a number of pharmacokinetic studies of MBF in ball pythons, loggerhead sea turtles, and red-eared and yellow-bellied slider turtles (Coke et al., 2006; Hunter et al., 2007; Lai et al., 2007, 2009; Marin et al., 2009; Vercelli et al., 2016), but limited information is available in crocodiles including the freshwater crocodile, C. siamensis. The different physiology and metabolism of reptiles make dosage extrapolation from mammalian studies questionable (Hunter & Isaza, 2008; Hunter et al., 2008). Therefore, this study was conducted to evaluate the pharmacokinetic characteristic of MBF in the freshwater crocodile, C. siamensis following single i.v. or i.m. administration.
Materials and methods
Animals
Twelve freshwater crocodiles, Crocodylus siamensis (males, 1–1.2 years of age, body weight of 5.9–8.1 kg, length of 124–142 cm), were sampled. All twelve experimental crocodiles were housed in cement ponds at Wongveerakit Farm, Kanchanaburi province, Thailand. All experimental procedures were performed according to the Guideline for Animal Experiments and approved by the Animal Ethics Research Committee of the Faculty of Veterinary Medicine, Kasetsart University, Bangkok, Thailand.
Drugs and chemicals
MBF pure standard was purchased from Sigma (St. Louis, MO, USA). Other reagents and chemicals were of analytical grade. Purified water was produced using the Milli-Q water purification system from Millipore, Inc. (Bedford, MA, USA). MBF was dissolved with 0.01 m phosphate saline buffer, pH 7.4 to the final concentration of 20 mg/mL for injection.
Experimental design
Twelve crocodiles were weighed and then divided into two groups (n = 6) using a randomization procedure according to a parallel study design. Each group was administered i.v. or i.m. with MBF at a dosage of 2 mg/kg b.w. The i.v. and i.m. dosing was delivered by 22-gauge, 1.5 inches long needles, into the subdural sinus and left biceps, respectively. Injection sites were cleaned with ethyl alcohol prior to injection. The i.m. injection site was selected to reduce the renal portal system effect. Blood samples (2.5 mL) were collected by alternating between the tail veins of each animal with heparinized syringes at 0, 5, 15, and 30 min and at 1, 2, 6, 12, 24, 36, 48, 72, 96, 120, 144, and 168 h after administration. The plasma was separated by centrifugation (1986 g) for 15 min and immediately stored at −20 °C for 2 weeks before analysis. The dose of MBF used in this experiment was based on those reported in earlier studies in loggerhead sea turtles and red-eared and yellow-bellied slider turtles (Lai et al., 2007; Marin et al., 2009; Vercelli et al., 2016). No adverse effects at the point of injection and no behavioral or health alterations were observed in the experimental animals during or after the study.
Sample extraction and cleanup
The extraction of MBF in plasma was performed as described previously (Mahmood et al., 2012), after revalidation in crocodile plasma. Briefly, plasma samples were extracted using liquid–liquid extraction. A 100 μL of 20% perchloric acid (HClO4) was added to 200 μL in a tube then shaken for 15 s. The mixture was centrifuged at 1474 g for 10 min, and the supernatant solution was then collected. After passing it through a Ministart® RC filter (pore size 0.45 mm, Sartorious AG, Gottingen, Germany), the sample (50 μL) was subjected to high-performance liquid chromatography (HPLC).
HPLC conditions
The analytical method was performed in accordance with Mahmood et al. (2012) with slight modifications. The HPLC analysis was performed using an Agilent 1260 series system consisting of a binary pump, an automatic sample injector, a column thermostat, and a fluorescence detector (Agilent Technologies, Waldbronn, Germany) with excitation and emission wavelength of 294 and 500 nm, respectively. Separation was achieved using a PLRP-S column (5 μm, 4.6 × 150 mm) with an RP18-E guard column (5 μm, 4 × 40 mm) (Polymer Laboratories Inc., Church Stretton, UK). The column was maintained at a temperature of 35 °C using a column oven. The mobile phase consisted of 14% acetonitrile and 86% orthophosphoric acid. The flow rate was 1.2 mL/min. The limit of detection (LOD) and the lower limit of quantification (LLOQ) of MBF in plasma were 0.005 μg/mL and 0.01 μg/mL, respectively. HPLC assay was conducted in duplicates on each sample.
Fortification and validation procedures
The calibration standard concentrations were prepared by spiking the working standard solution into crocodile control plasma to yield final concentrations of 0.01, 0.05, 0.1, 0.5, 1.0, 5.0, and 10 μg/mL. Crocodile control plasma (for quality control samples) was collected some days before commencement of the treatment protocol. Linearity of the regression curve in the range 0.1–10 μg/mL was assessed on the basis of residual plot, the lack of fit test and back calculation (within 20% of known amount). Five replicates of the quality control samples at concentrations of 0.1, 1, and 5 μg/mL were prepared and used to determine the recoveries, intraday and interday precision, and accuracy of the method. The procedure was repeated five times within the same day to gain intraday run precision and accuracy and five times for each concentration over five different days to determine interday run precision and accuracy. The extraction recoveries were 84.63 ± 3.77, 86.86 ± 3.23, and 85.56 ± 2.79% for 0.1, 1, and 5 μg/mL, respectively. The intraday precision and accuracy ranged from 1.36–4.86% and from 99.45–102.6%, respectively.
Pharmacokinetic parameter calculation
The concentration vs. time curves of MBF in experimental crocodiles were described by a noncompartmental model using WinNonlin software (version 5.3.1, Pharsight Corporation, Suite, NC, USA). The first order rate constant associated with the terminal portion of the concentration vs. time curve (λz) was estimated by log-linear regression of time vs. log concentration using at least the four plasma concentrations closest to the LLOQ for each animal. The terminal phase half-life (t1/2λz) was calculated by ln 2/λz. Maximum plasma concentrations (Cmax) were taken as the maximum observed concentration occurring at the corresponding times (Tmax) taken directly from the individual animal concentration vs. time curves after i.v. dosing. The area under the curve (AUC) to the last measured time point was calculated by the trapezoidal rule and was extrapolated to infinity by adding the area from Clast to infinity (AUC0→tlast + Clast/λz). In all the subjects, the % of the AUC that was extrapolated to infinity was lower than 20%. The volume of distribution based upon the terminal phase (Vz) after the i.v. bolus dose was determined from the i.v. dose/(λz•AUC0→∞), and the steady state volume of distribution for the i.v. bolus dose was calculated from Vss = (i.v. dose/AUC0→∞)•MRT0→∞. The total clearance of MBF after i.v. dosing was estimated by i.v. dose/AUC0→∞. The area under the first moment of the concentration vs. time curve (AUMC) from time of dosing to infinity was calculated using the product of concentration × time vs. time. Mean residence time (MRTi.v.) was calculated by MRT = 1/λz. Plasma concentration vs. time data from the i.m. dose were individually analyzed in a similar manner. Maximum plasma concentration (Cmax) and time to maximum plasma concentration (Tmax) were taken directly from the individual plasma concentration vs. time curves; terminal elimination rate constants (λz), terminal phase half-life (t1/2λz), AUClast, AUC0→∞ (extrapolated to infinity), AUMC (extrapolated to infinity), and MRT (extrapolated to infinity) were calculated as described above. The intramuscular bioavailability (F%) was calculated using the following equation: F% = (mean AUC i.m.)/(mean AUC i.v.) × 100.
Pharmacokinetic parameters were tabulated for each animal by dose route and reported as geometrical mean ± standard deviation, while the t1/2λz was reported as harmonic mean.
Results
The semilogarithmic plots of the mean (±SD) plasma concentration–time curves of MBF at a dosage of 2 mg/kg b.w. in Siamese crocodiles following i.v. and i.m. administrations are shown in Fig. 1. MBF was measurable from 5 min to 144 h after i.v. and i.m. administrations. Excluding the first part of the curve (0–0.75 h), the mean pharmacokinetic profiles of the two administrations were almost identical.
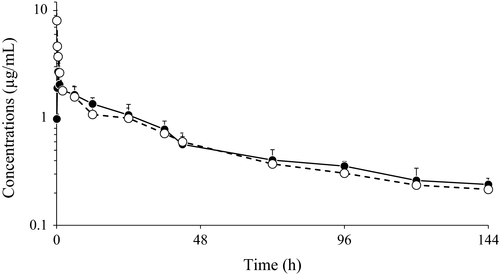
The averaged pharmacokinetic parameters of MBF are reported in Table 1. No statistically significant differences between the groups were observed.
| Pharmacokinetic parameter (Unit) | Marbofloxacin | |
|---|---|---|
| Intravenous | Intramuscular | |
| λz (h−1) | 0.01 ± 0.001 | 0.012 ± 0.002 |
| t1/2λz (h) | 57.51 ± 8.51 | 57.61 ± 11.79 |
| Tmax (h) | – | 0.54 ± 0.25 |
| Cmax (μg/mL) | – | 2.70 ± 0.52 |
| AUC0–24 (h*μg/mL) | 81.96 ± 10.43 | 86.38 ± 10.91 |
| AUC0−∞ (h*μg/mL) | 97.29 ± 15.08 | 102.91 ± 15.07 |
| CL (mL/h/kg) | 22.62 ± 4.10 | – |
| Vdss (mL/kg) | 1449.40 ± 244.48 | – |
| AUMC0−∞ (h*h*μg/mL) | 7145.10 ± 2087.42 | 7787.78 ± 1849.08 |
| MRT0−∞ (h) | 72.50 ± 10.92 | 75.06 ± 8.98 |
| F (%) | – | 105.39 |
- λz, terminal phase rate constant; t1/2λz, terminal half-life; Cmax, peak plasma concentration; Tmax, time of peak concentration; AUC0–24, area under the curve from 0–24 h; AUC0−∞, area under the curve from 0 h to infinity; CL, plasma clearance; Vdss, volume of distribution; AUMC0−∞, area under the first moment curve; MRT0−∞, mean residence time; F, bioavailability.
The half-life was long (about 2.5 days), the volume of distribution was large (about 1.44 L/kg), λz was small (0.01 h−1), and the clearance was slow (22.6 mL/h/kg). The i.m. bioavailability (F%) of MBF was complete with an average value of 105.36.
Discussion
The present study was designed to describe the disposition of MBF after i.v. or i.m. administration at a dosage of 2 mg/kg b.w. in a species of freshwater crocodile, Crocodylus siamensis. Previously reported studies described the pharmacokinetics of MBF in a number of reptiles including, ball pythons, loggerhead sea turtles, and red-eared and yellow-bellied slider turtles (Coke et al., 2006; Hunter et al., 2007; Lai et al., 2007, 2009; Marin et al., 2009; Vercelli et al., 2016).
The t1/2λz value of almost 60 h indicated that the overall rate of elimination of MBF in this animal species is slow. MBF at 2 mg/kg is usually administered in multiple doses, every 24 h in cats and dogs (Boothe, 2006). If this dose interval (τ) was also used for crocodiles, the accumulation ratio (AUCss/AUC1st dose) would be 4, meaning that concentrations at steady state would be 400% greater than plasma concentrations obtained following the first administration (Toutain & Bousquet-Mélou, 2004). Hence, τ is a key value to reduce accumulation of residues in this reptile species. The mean t1/2λz value obtained in this study was longer than that previously reported in other reptiles including ball pythons, loggerhead sea turtles, and red-eared and yellow-bellied slider turtles (Coke et al., 2006; Hunter et al., 2007; Lai et al., 2007, 2009; Marin et al., 2009; Vercelli et al., 2016). This difference might result from species specific differences or different environmental temperatures at the time of the experiment. The long t1/2λz reported in this study is also in line with the small value of λz (around 0.01 h−1, a figure not easy to conceptualize). However, if this rate constant is multiplied by 100, it will mean that during the terminal phase of elimination, about 1.0% of the residual amount of MBF is eliminated per hour (Toutain & Bousquet-Mélou, 2004). Further supporting the long t1/2λz value is the quite large volume of distribution (1494 mL/kg) and the slow clearance rate (22.6 mL/h/kg). However, a limitation for the t1/2λz value reported in this study is that to have a full acceptance of this value, the animals should be sampled least 3× the half-life after Tmax. Further studies need to confirm this data.
The average i.m. F% was complete with a value of 105.4%. This finding fits well with those for enrofloxacin, another veterinary fluoroquinolone that has shown similar overall pharmacokinetic behavior to MBF in crocodiles. Indeed, it has been characterized by rapid absorption with an almost complete bioavailability and a prolonged elimination half-life in American alligators (Helmick et al., 2004) and in Crocodylus porosus (Martelli et al., 2009).
Although there are no reports on MBF minimum inhibitory concentrations (MICs) for most susceptible micro-organisms in freshwater crocodiles, MIC values are reported for several gram-negative and gram-positive bacteria in the range 0.016–0.56 μg/mL (Meunier et al., 2004). Fluoroquinolones are concentration-dependent antimicrobials. Either the PK/PD parameters Cmax/MIC ratio, or the AUC/MIC have been used to predict their antibacterial success (Papich, 2014). Most studies agree that a Cmax/MIC of 8–10, or a AUC/MIC of greater than 100-125 h are associated with a cure (Wright et al., 2000; Liu et al., 2002; Mckellar et al., 2004). There is a generally held assumption that, when the Cmax/MIC ratio cannot be maximized, the AUC/MIC ratio may be a better index of therapeutic success (Drusano et al., 1993; Ambrose & Grasela, 2000). In the present study, the Cmax/MIC ratio was >10 only with bacteria with MIC value of ≤0.25 μg/mL. In contrast, the AUC/MIC ratio was >125 h with bacteria with MIC >0.56 μg/mL. A dose of 2 mg/kg administered each two days might be a good compromise in obtaining antibacterial activity and to reduce accumulation of residues. However, more studies are warranted to verify this data.
In conclusion, this is the first time that the plasma concentrations of MBF have been measured in the freshwater crocodile species, Crocodylus siamensis, after i.v. or i.m. administration at a dosage of 2 mg/kg b.w. Based on the pharmacokinetic data derived from the present study, administration of MBF at a dosage of 2 mg/kg b.w. (i.v and i.m.) appears to be suitable for the treatment of susceptible bacterial diseases in freshwater crocodiles. However, further PK/PD studies are warranted to confirm these findings and trials of multiple dose treatments indicated to evaluate the drug residues in this animal species.
Acknowledgments
This study was partially supported by the Faculty of Veterinary Medicine, Kasetsart University, Bangkok, Thailand. The authors sincerely thank to Dr. Tara Wongwaipairote, Wongveerakit Crocodile Farm, Kanchanaburi province, Bangkok, Thailand, for providing the crocodiles. The English editing of the manuscript is kindly acknowledged to Dr. H. Owen (University of Queensland, Australia).




