Polypoid Cystitis: A Retrospective Case-Series of 112 Dogs
Funding: The authors received no specific funding for this work.
ABSTRACT
Background
Polypoid cystitis (PoC) in dogs is associated with chronic inflammatory bladder conditions and is discovered during evaluation for signs of lower urinary tract disease, or incidentally.
Objective
To describe PoC in dogs evaluated in an academic practice.
Animals
Dogs with confirmed (n = 59) or presumptive (n = 53) PoC were evaluated between January 2004 and October 2020.
Methods
For this retrospective study, medical records were searched for PoC.
Results
The most common presenting signs of 112 dogs with PoC were hematuria (n = 42; 38%), stranguria (n = 28; 25%), and pollakiuria (n = 25; 22%). Polyps were found incidentally (n = 13; 12%). Urinary tract infection (UTI; n = 61; 54%) or urolithiasis (n = 38; 34%) was a common presumptive cause. Escherichia coli (n = 39; 53%), Enterococcus faecalis (n = 14; 19%) and Staphylococcus pseudintermedius (n = 5; 7%) were isolated from dogs with UTI. Ultrasonographic findings (n = 101) included polypoid structures (n = 44; 44%), broad-based masses (n = 16; 26%), and bladder wall thickening (n = 25; 25%); mostly in the cranioventral bladder apex (n = 56; 80%). Of 41 specimens tested, none had evidence of the BRAF V595E mutation. Urinary tract neoplasia was not reported in any dog during follow-up (range 1 month–8.4 years; median 8 months). Interventions included antibiotic or anti-inflammatory administration, and surgical or cystoscopic ablation. During follow-up, recurrent signs of lower urinary tract disease were reported in 23 (20%) dogs.
Conclusions and Clinical Importance
History of either UTI or urolithiasis, compatible imaging findings, and absence of detectable BRAF V595E mutation support the presumptive diagnosis of PoC in dogs. Affected dogs have a good prognosis, warranting differentiation from other urinary tract diseases.
Abbreviations
-
- CFU
-
- colony-forming units
-
- ddPCR
-
- droplet digital polymerase chain reaction
-
- hpf
-
- high-power fields
-
- PoC
-
- polypoid cystitis
-
- RBC
-
- red blood cells
-
- UTI
-
- urinary tract infection
-
- WBC
-
- white blood cells
1 Introduction
Polypoid cystitis (PoC), an important differential for dogs with urinary bladder masses, is the result of bladder mucosal inflammation leading to diffuse thickening, polyp formation, or development of masses without histopathologic evidence of neoplasia [1-5]. Polyps might result from inflammatory and hyperplastic reactions to chronic irritation of the bladder mucosa, with possible initiators including urinary tract infections (UTIs) and cystoliths. The precise pathogenesis remains to be determined. While not described in dogs, the most common condition resulting in bladder polyp formation in people is indwelling catheter-induced chronic irritation [6-11]. Dogs with PoC might present with signs of lower urinary tract disease including pollakiuria, stranguria, and hematuria, or PoC might be discovered incidentally [7-10]. Studies characterizing PoC in dogs have been limited to small case series.
It is vital to distinguish PoC from bladder cancer because their presentation and imaging findings can be similar, although their treatment strategy and prognosis are markedly different [1, 3, 4, 12-15]. PoC can be distinguished from bladder cancer by the location of the lesions and histological findings. PoC appear as polypoid to pedunculated masses either cranioventrally, cranioventrally to craniodorsally, or craniodorsally within the bladder wall during ultrasonography [1, 3, 4]. Conversely, urothelial carcinoma, the most common canine urinary tract neoplasia, tends to be found in the trigone region of the bladder [12, 13, 16]. Site predilection has not been documented for other reported tumor types of the urinary tract, which include adenocarcinomas, squamous cell carcinomas, undifferentiated carcinomas, leiomyosarcomas, leiomyomas, and lymphoma [12, 13]. Histopathology remains the gold standard for definitive diagnosis because some neoplastic conditions might have similar gross appearances to PoC [3, 4, 6, 7, 9, 17]. The identification of a BRAF V595E mutation in 85% of canine urothelial carcinomas provides opportunities for molecular detection of this cancer, either from tissue or urine specimens [18]. Chronic inflammatory conditions of PoC might represent a preneoplastic condition or predispose towards neoplastic transformation. The malignant transformation of PoC into urothelial carcinoma has been described, supporting this theory [19]. The paucity of information about PoC in veterinary literature and concerns related to neoplastic transformation warrant further study on this topic.
The objective of this study is to improve our understanding of the diagnostic features associated with PoC in dogs. We conducted a larger retrospective study than has previously been reported with a histologically confirmed or clinically supported diagnosis of PoC, integrating clinical, diagnostic, and outcome data from 112 dogs.
2 Materials and Methods
Client-owned dogs that received a diagnosis of PoC at North Carolina State Veterinary Hospital between January 2004 and October 2020 are described. Dogs were identified by searching medical records for the following terms: polypoid cystitis, polyp, bladder mass, bladder mucosal projections, bladder mucosal proliferations, pedunculated bladder mass, or apical bladder mass. Dogs were included if there was histopathologic evidence of PoC or imaging findings and other clinical features supportive of a presumptive diagnosis. These features included cranioventral bladder masses/projections found during ultrasonography, a history of UTIs or cystolithiasis, and a disease course inconsistent with neoplasia. Presumptive cases were included if imaging findings were supportive and if neoplasia was deemed unlikely; a definitive predisposing cause was not considered necessary. Dogs with histopathologic evidence of malignant neoplasia of the urinary bladder were excluded. Dogs without histopathologic evaluation of bladder biopsy specimens were included if they survived > 1 year following the initial diagnosis because we believed that dogs that survived beyond this timeframe without either therapy for or a definitive diagnosis of bladder neoplasia were unlikely to have bladder neoplasia [13, 14, 20, 21].
Collected data included: age at onset of clinical signs; sex; breed; body weight (kg); associated clinical signs; duration of clinical signs; prior treatments; prior diagnostic test results; urinalysis results; urine culture results; diagnostic imaging results; BRAF mutation screening; therapeutic interventions; histopathologic findings; and case follow-up when available.
Urine samples for urinalysis or aerobic bacterial culture were collected by cystocentesis, catheterization, or voiding based on clinician preference. Hematuria was defined as 3+ blood on urine dipstick or RBC/hpf in the urine sediment, while pyuria was defined as ≥ WBC/hpf in the urine sediment.
Bacterial cultures were considered consistent with bacteriuria when > 10,000 colony-forming units (cfu)/mL urine were isolated from samples collected by catheterization of male dogs, or when any bacterial growth was obtained from samples obtained by cystocentesis, or from bladder mucosa or bladder polyp samples obtained during cystotomy or cystoscopy [22-25].
Endoscopic and surgical biopsy specimens were fixed immediately in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 μm and stained with hematoxylin and eosin. Fixed tissue specimens from dogs suspected of having PoC based on histopathology were reviewed by board-certified veterinary pathologists. Histologic criteria used for the diagnosis of PoC based on previous studies included (1) microscopically detectable polypoid projections of mucosa and stroma into the lumen, (2) epithelial proliferation (thickening, mitotic figures, or down-growth of epithelial cords into the stroma), (3) stromal edema, (4) inflammation, (5) stromal hemorrhage, and hemosiderin accumulation [1]. Other common characteristics included intraepithelial lamina with proteinaceous secretions, ulcerations, erosions, granulation tissue, or epithelial atypia [1].
Genomic DNA extracted from fixed tissue specimens was screened with the NCSU BRAF Mutation Detection Assay, a droplet digital polymerase chain reaction (ddPCR) assay for the detection of the BRAF V595E mutation associated with canine urothelial carcinoma [15]. Samples were scored as either ‘mutation detected’ or ‘mutation undetected’ according to published criteria, using the same validated negative control (BRAF wild type) and two positive controls (BRAF V595E mutant specimens at both low and high fractional abundance) to establish uniform ddPCR thresholding for all test samples [18, 26].
3 Results
After initial screening, 135 dogs were identified for possible inclusion in the study, comprising 74 dogs with histopathology-confirmed PoC and 61 dogs with clinically supported PoC. Of these, 23 dogs were excluded, leaving 112 dogs in the study; 59 (52.7%) with confirmed PoC based on histopathology and 53 (47.3%) with presumptive PoC based on compatible clinical findings (Figure 1). Of the 23 excluded dogs, 3 were duplicate entries, 17 were samples submitted for histopathology without relevant clinical data, 1 had the BRAF V595E mutation detected in a urine sample and was subsequently diagnosed with urothelial cell carcinoma, and 2 had poor survival for reasons unrelated to the urinary tract, thus not meeting inclusion criteria.
3.1 Signalment
Of the 112 identified dogs, 72 (64.3%) were female (64 spayed, 8 intact) and 40 (35.7%) were male (34 neutered, 6 intact; Table 1). Purebred dogs accounted for 103 (92%) of the cases, with the remaining nine dogs being considered mixed breed. Represented breeds included Labrador retriever (14 dogs), American cocker spaniel (6), bichon frisé (5), shih tzu (5), golden retriever (4), Shetland sheepdog (4), Maltese (4), Lhasa apso (4), Chihuahua (3), Yorkshire terrier (3), beagle (3), border collie (3), Airedale terrier (2), American Staffordshire terrier (2), Australian shepherd (2), Bernese mountain dog (2), Cardigan Welsh corgi (2), Jack Russell terrier (2), miniature poodle (2), rat terrier (2), rottweiler (2), schnauzer (2), chow (2), hound dog (2), and 1 dog each of miniature schnauzer, Boykin spaniel, Brussels griffon, toy poodle, American Eskimo dog, English cocker spaniel, American bulldog, Doberman pinscher, English bulldog, keeshond, Norwegian elkhound, Pomeranian, poodle, pug, beagle, papillon, Russell terrier, Basset hound, Great Dane, and a West Highland white terrier. Nine dogs were identified as mixed breed, including one labradoodle and one schnoodle. The median age of affected dogs at the time of diagnosis was 8.5 years (range 6 months–15 years). The median weight of affected dogs was 15.5 kg (range 1.7–60.8 kg).
| Sex | Male castrated | Male intact | Female spayed | Female intact |
|---|---|---|---|---|
| n = 34 | n = 6 | n = 64 | n = 8 | |
| Polyp location | Cranioventral | Trigone | Multifocal | Location not described |
| n = 56 | n = 9 | n = 1 | n = 4 | |
| Comorbditiy | UTI | Urocystolithiasis | UTI + Urocystolithiasis | Unknown |
| n = 61 | n = 38 | n = 14 | n = 16 |
3.2 Clinical Findings
All 112 dogs had been evaluated at primary care veterinary practices prior to presentation, with clinical signs ranging in duration from 1 to 1344 days (median 84 days). Presenting complaints of the identified dogs were macroscopic hematuria (37.5%), stranguria (25.0%), pollakiuria (22.3%), recurrent UTI (14.3%), urolithiasis (6.3%), inappropriate urination in the house (6.3%), further investigation of a bladder mass (3.5%), acute kidney injury (1.8%), new onset UTI (1.8%), vulvar discharge (1.8%), polyuria/polydipsia (2.7%), and one dog each for dribbling urine and dysuria. The discovery of a bladder polyp was an incidental finding in 13 dogs (11.6%) being presented for reasons entirely unrelated to the urinary tract, none of which were reported to have signs of lower urinary tract disease.
3.3 Laboratory Findings
Urine was collected for urinalysis at admission from 105/112 (93.8%) of dogs; the median urine specific gravity was 1.023 (range 1.001–1.050). Hematuria was present in 68 (64.7%) of these samples and pyuria in 37 (35.2%), while bacteriuria was noted in only 29 (27.6%). Twenty-six (24.8%) dogs had crystalluria (calcium oxalate, 11 dogs; struvite, 5; amorphous, 10).
Within 2 weeks before presentation, 35 (30.3%) dogs had been administered antibiotics. During the initial evaluation, urine samples from 80/112 dogs (71.4%; cystocentesis, 71; urethral catheterization, 9) were submitted for microbial culture and susceptibility testing; 26/80 (33%) were positive for bacterial growth. When considering historical records provided by referring veterinarians, a total of 61/112 (54.5%) of dogs had bacterial growth in urine samples. An additional 118 urine samples from 51 dogs were submitted for microbial culture and susceptibility testing during follow-up evaluations (cystocentesis, 111; urethral catheterization, 7). Urine yielded bacterial growth in 65 (55.1%) of these samples collected from 13 dogs. Results of positive urine cultures from any point were reviewed to determine the most common isolates. A total of 74 bacterial isolates were obtained from the 198 total cultures performed. Escherichia coli was the most common (n = 39; 19.7%). Other isolates included Enterococcus faecalis (n = 14; 7.1%), Staphylococcus pseudintermedius (n = 5; 2.5%), Enterobacter aerogenes (n = 4; 2.0%), Proteus spp. (n = 3; 1.5%), Pseudomonas aeruginosa (n = 3; 1.5%), Streptococcus spp. (n = 2; 1.0%), Staphylococcus epidermidis (n = 2; 1.0%), Staphylococcus hemolyticus (1; 0.5%), and Enterococcus faecium (n = 1; 0.5%). Of the positive urine cultures from initial presentation to this institution onward, seven (3.5%) were polymicrobial.
3.4 Diagnostic Imaging Findings
Survey abdominal radiographs were performed in 26/112 (23.2%) dogs. Radiopaque cystic calculi were identified in 16/26 (61%). Contrast cystography revealed irregular bladder margination in two dogs and a vesicourachal diverticulum in one dog. Abnormalities were not detected in the remaining radiographic studies.
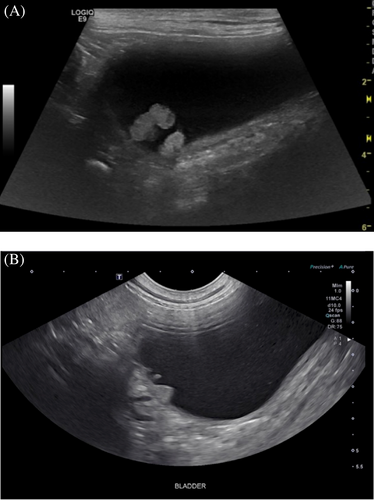
Abdominal ultrasonography was performed in 101/112 (90.1%) dogs (Figure 2). Abnormal findings included polypoid structures in 44 (43.6%) dogs, broad-based masses in 26 (25.7%) dogs, bladder wall thickening in 25 (24.8%) dogs, and bladder wall irregularity in two (2.0%) dogs. In the 70 dogs with polypoid structures or broad-based masses, 56 (80%) of these lesions were in the cranioventral apex, while nine (13%) were in the trigone; the mass was described as multifocal in one dog, while the location was not described in four (6%) dogs. Twenty-six (25.7%) of the 101 dogs had evidence of cystic calculi on abdominal ultrasound, while echogenic material was present in 12 (11.9%).
3.5 Cystoscopic Findings
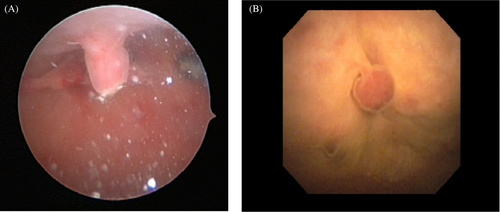
Overt polypoid masses were identified in 43 of the 48 (89%) dogs that had cystoscopy (Figure 3). A focal area of decreased vascularity was seen in the bladder apex of one dog that did not have an appreciable mass. Another dog that did not have a mass present during cystoscopy had this procedure postoperatively after the cystotomy for polyp and cystolith removal. The remaining three dogs without overt polypoid masses had nodular mucosal irregularity and hyperemia in the cranioventral apex. Polyps were assessed as multiple in 34 (79%) of these dogs and singular in nine (21%). The location of the masses was cranioventral apex (n = 31, 72%), trigone (n = 2, 4%), bladder neck (n = 2, 4%) or midway between the apex and trigone (n = 1, 2%); the location was not specified in the remaining seven (6%) dogs. Other abnormalities identified during cystoscopy included excessive perivulvar skin folds (n = 17), hyperemic bladder mucosa (n = 10), cystic calculi (n = 10), nodular bladder mucosal irregularity (n = 8), bladder mucosal hemorrhage (n = 5) and vestibulovaginal remnant (n = 2).
3.6 Histopathology Findings
Histopathologic evaluation was performed on biopsy specimens obtained from 70 dogs; 38 (54%) obtained via cystoscopic biopsy, 27 (39%) via surgical biopsy, and five (7%) via post-mortem tissue collection. Histopathologic evaluation revealed mixed findings, including inflammatory infiltrates (n = 46, 65%), hyperplastic epithelium (n = 42, 60%), submucosal hemorrhage (n = 35, 50%), submucosal edema (n = 29, 41%), papillary projections (n = 22, 31%), Brunn's nests (n = 12, 17%), epithelial ulcerations/erosions (n = 8, 11%), mucoid metaplasia (n = 5, 7%), and epithelial dysplasia (n = 2, 2%). There were five dogs that had histopathologic findings suggestive of polypoid cystitis, without prior evaluation identifying a mass/polypoid structure. A single sample was nondiagnostic because of marked crush-handling artifact.
The identified inflammation (n = 46) was described as predominantly mild (n = 25, 54%), moderate (n = 18, 39%) or marked (n = 3, 6%). In nine (12%) samples, an inflammatory infiltrate was not described. The samples predominantly consisted of lymphoplasmacytic infiltrates (n = 34, 73%), with fewer numbers containing neutrophilic inflammation (n = 12, 26%) and eosinophilic inflammation (n = 4, 8%). Further description of the cell type of the inflammatory infiltrate was not provided in specimens from nine (26%) dogs.
3.7 BRAF Mutation Detection
Molecular testing for the BRAF V595E mutation was performed in 31 of the 112 (27.7%) dogs using DNA recovered from formalin-fixed biopsy/necropsy samples. No detectable V595E mutations were evident in any of the 31 dogs. All BRAF V595E ddPCR data were associated with a detection threshold of ≤ 0.1%.
3.8 Treatment and Follow-Up
Antimicrobial agents were the most common treatment administered, given to 73/112 (65.2%) dogs and included clavulanic acid/amoxicillin (n = 48, 65%), enrofloxacin (n = 10, 13%), cephalexin (n = 8, 11%), cefpodoxime (n = 7, 9%), ciprofloxacin (n = 6, 8%), and marbofloxacin (n = 4, 5%). Of the 112 dogs, surgical intervention was the next most common treatment, utilized in 36 (32.1%) dogs, with cystotomy for polyp removal alone (11, 9.8%), cystotomy with concurrent stone removal (n = 21, 18.8%), partial cystectomies (n = 1.8, 2%), ureteroneocystostomy (n = 1, 0.9%), and unilateral nephrectomy with concurrent bladder polyp removal (n = 1, 0.9%). Ureteroneocystotomy was performed in one dog due to a large bladder polyp contributing to right ureteral obstruction. The unilateral nephrectomy in another dog was performed due to a combination of obstructive ureterolithiasis and suspected severe pyelonephritis with marked purulent material within the renal pelvis. Cystoscopy was used to remove polyps from 15 dogs via biopsy snares (n = 10) or laser ablation (n = 5). Of the dogs that underwent cystoscopy for polyp removal, 8 had concurrent cystoliths present, which were removed during cystoscopy. Anti-inflammatory agents were given to 23 dogs, including piroxicam (n = 9), carprofen (n = 8), meloxicam (n = 3), prednisone (n = 3), prednisolone (n = 1), and firocoxib (n = 1). Other assorted treatments for concurrent problems included cranberry products (n = 8), episioplasty (n = 4), dietary changes for management of uroliths (n = 12), and diethylstilbesterol (n = 1). There was no treatment recorded for 15 dogs.
Based on clinical findings, the most common suspected cause of PoC was chronic or recurrent UTI (n = 61, 54.5% dogs) and the second most common was cystolithiasis (n = 38, 33.9%). There were 14 dogs (12.5%) that had both UTI and cystoliths. In 16 of the 112 (14.3%) dogs, a clear cause of the polyps could not be identified; most of these dogs (13/16, 81%) were presented for reasons unrelated to the urinary tract.
Median duration of follow-up in all dogs was 8 months (range 1 months–8.4 years). There were 23 dogs that experienced recurrent signs of lower urinary tract disease, infections, or stone recurrence at a median of 8 months (range 3 months–4.2 years) after the initial treatment. Initial treatments for these dogs included surgical polypectomy (n = 6), cystoscopic polypectomy (n = 4) or medical management only (n = 13). Persistence of a urinary bladder polyp was documented in three dogs, each of which had been treated with either antibiotics (n = 2) or a combination of antibiotics and anti-inflammatory drugs (n = 1). Recurrent UTI was documented in 11 of these dogs; four of whom had recurrent cystolithiasis.
Follow-up abdominal ultrasonography was performed in 50 (49.5%) of the 101 dogs that originally had an ultrasonographic study at a median of 2 months (range: 4 days–44 months) following initial evaluation and treatment. At that time, PoC was believed to be resolved in 22 dogs, improved in 6 dogs, and static in 22 dogs. Improvement was characterized by a reduction in number, size, or both, of polypoid structures or masses. None of the dogs were described as having a worsening appearance on abdominal ultrasound. Of the dogs with persistent evidence of PoC, only 3 were clinical during this available follow-up period.
In addition, none of the dogs included in the study were noted to develop malignant neoplasia within the urinary tract during available follow-up evaluation at the primary institution or based on primary care follow-up (presumptive diagnosis range 12 months–8.4 years; median 1.8 years; definitive diagnosis 1 month–6.7 years; median 5 months).
4 Discussion
This case series represents a large collection and comprehensive description of dogs with histopathologically or presumptively diagnosed PoC. PoC is an uncommon manifestation of chronic bladder inflammation that leads to the development of mass-like projections of the mucosa. Histopathologic evaluation of polyps reveals epithelial proliferation with a core of proliferative connective tissue, with stromal edema, stromal hemorrhage, and hemosiderin accumulation. Since dogs with PoC might present with clinical signs and imaging findings like those seen with bladder cancer, it is important to consider the condition as a differential for these malignancies. Previous case series on PoC included a relatively small number of dogs (n = 17), limiting our overall understanding of the condition [1, 3].
[Studies of PoC demonstrate a strong sex predisposition, with 88%:12% of the female:male dogs [1]. In the present study, a weaker sex predisposition was noted, with 64.3% of the 112 dogs being female and 35.7% being male; most dogs were spayed or neutered. Of the 72 female dogs, 23% had evidence of excessive perivulvar skin folding, which might have predisposed these dogs to, or been a result of, recurrent or chronic UTI and led to an increased risk of polyp formation 1, 3, 4, 27].
Our aim was to raise awareness of PoC, to inform our understanding of the diagnostic features associated with this condition, and to provide additional evidence for determining whether PoC is a common precursor to UC.
A large variety of dog breeds were diagnosed with PoC, with 47 different breeds represented. The most represented was the Labrador Retriever, followed by the American Cocker Spaniel and mixed breed dogs. These represent popular dog breeds, and there is no indication from our study of a breed predisposition to the development of PoC. Dogs of all sizes and ages were represented, although the median age was 8.5 years, suggesting that it might be more common in middle-aged dogs.
The most common presenting complaint was macroscopic hematuria, noted in 37.5% of the affected dogs. The timing of hematuria in relation to urination was not well documented. The frequency of hematuria is lower than previous reports (82%). In the present study, 14.3% of dogs were presented for further investigation of chronic or recurrent UTI without reporting the dog's clinical signs at the time of presentation. Several dogs were presented for investigation of a known bladder mass, which might represent more widespread availability of ultrasonographic imaging in general practice. The percentage of dogs that presented for reasons unrelated to the urinary tract and had PoC incidentally identified was similar in this study (11.6%) compared with the previous study (11.7%) [1]. The median duration of clinical signs before presentation was 84 days, supporting the chronic nature of the disease.
A definitive cause of polyp formation in dogs is unknown but is believed to be a result of chronic inflammation leading to hyperplastic proliferation of the bladder mucosa. The most common causes of chronic bladder inflammation in dogs are chronic or recurrent UTI or cystic calculi. In the present study, 33% of dogs that had urine samples submitted for microbial culture at the time of presentation had confirmed bacteriuria. However, 31.3% of dogs were receiving antibiotics within the 2 weeks before presentation, which could have led to an underestimation of UTI. Review of referral veterinarian records supports this suspicion, with an additional 35 dogs having prior positive urine cultures. Cystic calculi or echogenic debris were present in 33.9% of dogs with PoC, and 12.5% had both UTI and cystic calculi. An obvious underlying cause could not be identified in 14.3% of dogs, with a majority (81%) of these dogs presenting for reasons unrelated to the urinary tract.
In humans, PoC is associated with the use of indwelling urinary catheters, with up to 80%–100% of chronically catheterized people developing polyps [8, 11]. In the study herein and in prior reports of PoC in dogs, no dogs had a history of indwelling urinary catheters before noted presence of a polyp [1-6]. Other conditions noted to be associated with the formation of PoC in humans include colovesical fistulas, calculi, urinary tract obstruction, and a history of radiation therapy. UTI does not seem to influence the frequency of PoC in humans [8]. Based on the frequency with which UTI is noted in the patient population of this report and the absence of reported dogs with prior indwelling urinary catheters, it is possible that the pathogenesis of polyp formation is distinct between dogs versus humans.
The most common bacterial isolate in the present study was E. coli, accounting for 53% of all isolates. Proteus spp., identified as the most common isolate in prior reports of dogs with PoC, was isolated from 23% of the dogs in the present study. In those prior studies, a possible relationship between Proteus spp. and the development of PoC was suggested [1]. However, in the present study, Proteus spp. accounted for only 4% of all bacterial isolates, making a relationship seem less likely. To date, no clear role for specific bacteria in the pathogenesis of PC has been established.
Bladder masses were identified in 70% of the dogs in this report during abdominal ultrasonography and were described as polypoid structures (44%) or broad-based (26%) masses. Like previous reports, the cranioventral apex was the most common site for these masses, with 80% of polyps identified in this location; a small number (13%) were in the trigone. These findings, which agree with previous reports, suggest that there is a predilection for polyps to develop in the apical bladder wall, but they can form in other sites [1, 3, 4]. Similarly, in humans, polyps tend to form in the bladder apex but might also form in the trigone region [8, 9, 11, 28].
Most dogs (43/48; 89%) that underwent cystoscopy had overt polypoid masses identified during the procedure. Like the findings during abdominal ultrasound, polyps were most commonly identified in the cranioventral apical region of the bladder during cystoscopic examination. In the dogs that had histopathologic findings consistent with PoC in the absence of an overt mass, areas of thickened mucosa and nodular mucosal irregularity were found. These dogs might represent an earlier point in the disease spectrum before significant polypoid changes have occurred, and it cannot be ruled out that they might have gone on to form masses. Alternatively, folding of the bladder mucosa might have led to a failure to identify the masses during cystoscopy.
Treatments varied markedly depending on clinical features and clinician preference. The most common treatment administered was an antimicrobial agent, administered to 65% of dogs, which was supported because 54% of dogs in this report had a UTI as the suspected cause for their PoC. The most prescribed antibiotic was amoxicillin-clavulanic acid, consistent with current ISCAID guidelines for the treatment of UTI [24]. Other components of medical management varied, with the most common treatment being NSAID class drugs. Piroxicam was the most used NSAID, likely due not only to its anti-inflammatory properties but also to reports of suspected anti-neoplastic properties in the treatment of urothelial carcinoma, the most relevant differential diagnosis for dogs with bladder masses [13, 29]. The remainder of medical treatments used were interventions aimed at reducing the recurrence of UTI or cystolithiasis, which could in turn reduce the risk of PoC recurrence.
Results of the present study support surgical intervention as an effective treatment for the management of PoC. Surgical treatment was used in 32% of dogs. Most of these dogs underwent a cystotomy for polyp removal as well as removal of cystoliths when present. Of the 48 dogs that underwent a surgical procedure for polyp/mass removal, only six had recurrent signs of lower urinary tract disease, infection, or cystoliths after their procedure. Polyp recurrence was not noted in any of the dogs that had surgery followed by recheck imaging evaluation.
Our results support that polyp removal during cystoscopy is also an effective treatment. Although four of the 17 dogs that had polyps removed at the time of cystoscopy had recurrent signs of lower urinary tract disease, infections, or stone recurrence, none had evidence of polyp persistence or recurrence.
Due to the lack of standardization between treatments and follow-up, it is not possible to determine the most effective treatment for PoC. Of the 23 dogs that experienced recurrent signs of lower urinary tract disease, infections, or stone recurrence, only three of these dogs had evidence of persistent PoC. Although the largest portion of these dogs received only medical management, the numbers are too small to draw meaningful conclusions. The median time until recurrence of signs in the dogs that received only medical management was 8 months, suggesting that initial treatment was adequate for the management of that episode. It is possible that the recurrence of clinical signs was associated with the presence of UTI or cystoliths rather than specifically PoC. Due to the report of presumed malignant transformation in a young dog, either surgical or cystoscopic removal for treatment and histopathologic evaluation might be prudent [19]. Attempted medical management might be pursued, although close monitoring for the recurrence of clinical signs or imaging progression is warranted.
No dogs had evidence of malignant transformation during their available follow-up. One dog that had been excluded from the study, a 13-year-old female spayed Scottish terrier, had a low level V595E BRAF mutation detected in its urine and also in DNA extracted from biopsies of three masses detected during a subsequent cystoscopy several months later: two from the bladder and one from the urethra. The initial histopathological diagnosis was PoC, based on one of the bladder mass biopsies, but the diagnosis of the second bladder mass and the urethral mass were left open due to small size and significant handling artifact. However, when clinical signs were noted to be progressive 5 months after the initial diagnosis, the masses were biopsied again, revealing numerous small blood vessels within the sections directly opposing islands of mildly pleomorphic epithelium, but without evidence of mitotic activity or cellular atypia. While the samples were small and there was notable cautery and tissue handling artifact, a diagnosis of UC was established. While this case could represent malignant transformation, given the underlying breed, detection of an initial low level urinary BRAF mutation, and multiple masses with urethral involvement, it is possible that the definitive diagnosis was not achieved at the outset due to sampling limitations, or there was co-occurrence of neoplasia and PoC. This case supports the need for ongoing monitoring of dogs initially diagnosed with PoC and emphasizes that dogs with a detectable low level BRAF mutation in their urine should have continued surveillance for emerging clinical signs of UC.
The BRAF V595E mutation has been implicated in the pathogenesis of UC [18, 30]. This mutation might lead to constitutive activation of the MAPK pathway, causing abnormal cell proliferation and differentiation. Molecular testing allows for highly sensitive detection of this mutation in dogs. Using sophisticated ddPCR, standardized controls, and a stringent analytical algorithm developed at NCSU, 85% of dogs with UC demonstrate this V595E mutation, whether the cells are recovered from biopsy material or from urine specimens [18]. The use of ddPCR overcomes the challenges posed by the limited yield of genomic material and the presence of PCR inhibitors associated with formalin-fixed tissues and urine samples, which can often impede other PCR platforms. The present study highlights the value of the use of rigorous BRAF V595E mutation detection as an aid to support the diagnosis of a neoplastic or an inflammatory condition when a bladder mass is noted on diagnostic imaging. Results of BRAF genetic testing captured by this study population showed no evidence of the mutation in biopsied tissue from any of the 31 dogs with PoC that were tested. However, this study does reinforce that dogs with an initial diagnosis of PoC and that are also BRAF positive would benefit from ongoing mutation surveillance and clinical monitoring. The BRAF V595E Mutation Detection Assay [18] was first described in 2015, and so was not yet available at the time of diagnosis for most of the period covered by the present study. Screening data were therefore generated retrospectively from archival tissue biopsy specimens, and matched urine samples were not available. Now that molecular analyses are becoming more accessible in veterinary medicine, it would be prudent to consider data from urine-based testing for this mutation in tandem with traditional clinical features of disease to optimize opportunities for advancing patient care.
The main limitation of this study was its retrospective nature. Diagnostic evaluation, treatment protocols, and follow-up evaluation were not standardized between dogs, and comparisons between these factors could not be assessed. Many of the dogs in which PoC was suspected were being evaluated for a different primary problem, and the finding of PoC was incidental. In these dogs, diagnostic evaluation and further monitoring were limited compared to those dogs presenting primarily for signs of lower urinary tract disease. These dogs likely had a limited history related to the urinary tract recorded, thereby limiting the information available within their medical records. This would lead to the underappreciation of dogs with a known and potentially predisposing cause, whether that be cystolithiasis or UTI.
In conclusion, PoC is an inflammatory condition in dogs and an important differential diagnosis for urinary bladder masses. This report supports that PoC might be presumptively diagnosed and effectively treated in dogs with an appropriate history, evidence of an underlying inflammatory condition, and characteristic diagnostic imaging. Appropriate use of BRAF mutation testing might further support the diagnosis of PoC in dogs with bladder masses. This retrospective study should raise awareness of PoC as a consideration in dogs with bladder masses, and that such findings do not necessarily carry a poor prognosis. Furthermore, there was no evidence from these dogs examined that PoC led to the future development of bladder neoplasia. The present study confirms that PoC is a manageable condition, with resolution or improvement noted in over half of the dogs that had repeat abdominal ultrasound examinations.
Disclosure
The authors declare no off-label use of antimicrobials.
Ethics Statement
Authors declare no Institutional Animal Care and Use Committee or other approval was needed. Authors declare human ethics approval was not needed.
Conflicts of Interest
All BRAF V595E mutation detection was performed at North Carolina State University using testing that was developed there. Shelly L. Vaden serves as Associate Editor for the Journal of Veterinary Internal Medicine. She was not involved in the review of this manuscript. The other authors declare no conflicts of interest.




