Safety of TCMCB07, a melanocortin-4 antagonist peptide, in dogs with naturally occurring cachexia
Abstract
Background
The melanocortin 4 antagonist TCMCB07 is safe and effective in reversing cachexia caused by sepsis or cancer in rodents. The safety and pharmacokinetics of TCMCB07 are demonstrated in healthy beagle dogs.
Hypothesis/Objectives
The objectives of this study were to investigate the safety, peak plasma concentrations, and potential for efficacy of TCMCB07 in pet dogs with naturally occurring cachexia over a 4-week time period.
Animals
Fourteen dogs with cachexia of any underlying cause, except cancer of the oral cavity or gastrointestinal tract, were eligible for enrollment with informed client consent.
Methods
This study was a prospective, 1-armed open-label trial. Physical examination, complete blood count, chemistry panel, and owner-assessed quality of life surveys were checked at weeks 1, 2, and 4. Due to potential for bradycardia and hypotension, Holter monitoring and blood pressure evaluations were scheduled at pre-enrollment and week 4.
Results
Fourteen dogs completed the trial. Significant changes detected included increased mean body weight (18.6-19.5 kg, P < .02), increased body condition score (median Tufts 5-point thin dog scale score P < .004 and WSAVA muscle condition score P < .02) and increased mean blood urea nitrogen (21.79-30.43 mg dL−1, P < .004). On quality of life surveys, pet owners perceived their dog appeared to be panting less (P < .002) and that the general health improved (P < .03). Four dogs had a change in coat pigmentation. The peak plasma concentration of TCMCB07 in cachectic dogs was similar to that in healthy beagle dogs.
Conclusions and Clinical Importance
TCMCB07 was safe and has potential efficacy in pet dogs with cachexia.
Abbreviations
-
- ANOVA
-
- analysis of variance
-
- Cmax
-
- maximum peak plasma concentration
-
- MCR4
-
- melanocortin-4 receptor
-
- PK
-
- pharmacokinetic
-
- QOL
-
- quality of life
-
- SEM
-
- standard error of the mean
-
- WSAVA
-
- World Small Animal Veterinary Association
1 INTRODUCTION
Cachexia is the potentially deadly end result of several diseases, such as cancer, sepsis, or failure of heart, liver, or kidneys. It is a complex condition driven by inflammatory responses that result in increased energy requirements, decreased energy absorption, decreased nutritional intake, and altered metabolism.1-3 The resulting muscle wasting and tissue dysfunction diminish quality of life and reduce available treatment options.1, 4 For example, cachexia is a reliable predictor of poor outcome in human cancer patients and 20% will die due to cachexia rather than progressive cancer.4-6 Improved treatment for cachexia is critical to reduce the morbidity associated with these diseases. Melanocortin-4 receptor (MC4R) activation results in decreased food intake and increased expenditure of energy, therefore, MCR4 antagonism is an attractive area of study as it can cause increased appetite and lean body mass.7-11 However, MCR4 also regulates cardiovascular functions, and antagonism might result in hypotension and bradycardia.7, 9, 12
TCMCB07, with the structure Ac-Nle-cyclo[Asp-Pro-DNal(2′)-Arg-Trp-Lys]-DVal-DPro-NH2, is a cyclic substituted melanocortin antagonist13 which successfully treats cachexia in rodent models of cachexia.14 The MCR4 in dogs is fully functional,15 and TCMCB07 is a potent antagonist at the rat, human, and canine MC3 and MC4 receptors in terms of receptor binding and antagonism of cAMP activation by the α-MSH analogue [Nle4, DPhe7]-MSH (NDP-MSH; Melanotan-Ι).16 In addition, the pharmacokinetics and safety of TCMCB07 in normal beagle dogs are established.11 However, further studies are necessary to expand our knowledge of TCMCB07 in a real-world model of naturally occurring disease. Companion dogs represent an excellent model as conditions that cause cachexia in humans and their mechanisms of action are similar, so data can also be translated into human trials.17, 18
The objective of this study was to investigate the safety and preliminary efficacy of TCMCB07 in the treatment of naturally occurring cachexia in companion dogs. Our hypothesis was once daily subcutaneous administration of TCMCB07 in companion dogs with naturally occurring cachexia was safe and would result in weight gain, increased body condition, and improved quality of life.
2 MATERIALS AND METHODS
2.1 Animals
This study was a multi-institutional, prospective, 1-armed open-label trial in client-owned dogs with naturally occurring cachexia from the years 2016 through 2021. All procedures were approved by an Institutional Animal Care and Use Committee (University of Florida IACUC protocol #201810174, University of Missouri IACUC protocol # 8441). The following institutions participated in recruitment: University of Florida, University of Missouri, VCA Animal Diagnostic Clinic, VCA West Los Angeles, VCA Family and Oahu Veterinary Specialty Center, VCA Northwest Veterinary Specialists, and VCA Animal Specialty and Emergency Center. Client-owned dogs with naturally occurring cachexia were recruited from the institutions’ presenting pet population. Dogs of any weight were eligible for enrollment after informed client consent if, on examination, there was clinical evidence of cachexia based on body condition and muscle condition scoring and at least 5% loss of body weight within the past 3 months. On biochemistry panel, the dog's serum activity of liver-derived enzymes must have been lower than 5 times the upper limit of reference range with normal plasma or serum bilirubin. Concurrent anti-emetic therapy and therapy for an underlying disease process was allowed. Glucocorticoids were permitted if therapy had been ongoing for greater than 14 days before enrollment, there was no change in dosage upon enrollment, and no increase in body weight or body condition score had occurred. Appetite stimulants were not allowed within 2 days of the first TCMCB07 treatment. Exclusion criteria included cancer of the oral cavity, cancer of the gastrointestinal tract, or other identified disease process(es) if that process led to an inability to consume food or caused a partial or complete gastrointestinal obstruction. Additionally, dogs with active cardiogenic pulmonary edema, bradyarrhythmias, or pathologic tachyarrhythmias were not included in the trial.
After informed client consent, screening was performed to confirm eligibility for trial. This included a physical examination, complete blood count, chemistry panel, urine analysis, abdominal ultrasound, Doppler blood pressure, and 24-hour Holter monitoring. At enrollment a standardized quality of life (QOL) assessment was completed by the client19; weight, photographs, and body condition score of the dog were recorded. Three body condition scoring systems were used. The trial was initiated using the Tufts Animal Care and Condition Scale, a 5 point thin dog scale that better reflected subtle changes in thin/muscle wasted dogs than more traditional body scoring systems.20 A score of 5 indicated emaciation and a score of 1 indicated an ideal body condition. As the trial progressed, 2 additional body condition scores were utilized, the 9-point Purina body condition score (score of 1-3 is too thin, 4-5 ideal, 6-9 too heavy)21 and the World Small Animal Veterinary Association (WSVA) muscle condition score (1 = normal 2 = mild muscle loss, 3 = moderate muscle loss 4 = severe muscle loss).22, 23 The screening Holter recording was reviewed and approved by a board certified veterinary cardiologist, and therapy commenced if no underlying arrhythmias occurred that would interfere with therapy.
2.2 Drug production
CPC Scientific Inc. (Sunnyvale, CA 94089) produced TCMCB07 using current Good Manufacturing Practice conditions. The active pharmaceutical component was dissolved in milliQ water at 10 mg mL−1 and sterile filtered using a 0.22 μm Millex®GP PES Membrane (Merck Millipore Ltd, Tullagreen IRL). Drug was stored at −20°C and shipped on ice overnight to participating institutions. Drug was stored protected from light and stored at 4°C once received at each institution and after prescribed to pets.
2.3 Treatment
On the first day of treatment (day 1) a weight, body condition score, and physical examination were performed in addition to the first treatment of TCMCB07 and collection of blood samples via jugular or peripheral venipuncture for pharmacokinetic evaluation at time points 0, 1, 4, and 24 hours post-treatment. Dogs were dosed at 0.75 mg kg−1 administered subcutaneously once every 24 hours based on results in a previous study in dogs.11
Clients were taught to administer TCMCB07 by subcutaneous injection and provided with a drug administration diary. Return visits were required at day 14 and day 28, at which time the following data were collected: weight, body condition score, dog photos, quality of life form, physical examination, complete blood count, chemistry panel, and 1-hour postdrug administration pharmacokinetic (PK) sample. At day 28, Doppler blood pressure (prior to drug administration) and a 24-hour Holter monitor (applied before or after drug administration on day 28) were also collected and applied, respectively.
Following the initial 28-day study period, if a good response was noted (such as increased appetite, activity level, weight gain, or any of these), clients were permitted to have their dog continue TCMCB07 treatment daily. These dogs were required to follow up monthly for 2 months and then once every 3 months for the following: physical examination, QOL form, weight, body condition score, dog photos, complete blood count, chemistry panel, urine analysis, and drug plasma concentration sample 1-hour post TCMCB07 administration. Dogs that showed improvement but had not returned to a normal body condition were allowed to continue at an increased dosage of 0.75 mg kg−1 every 12 hours if this was considered to be in the best interest of the dog in the opinion of the owner and treating clinician.
2.4 Plasma concentration determination
Plasma concentrations of TCMCB07 were assessed on days 1 (timepoints 0, 1, 4, and 24 hours post TCMCB07 administration), and 1-hour postadministration on days 14, 28, and any additional follow up visits for dogs on long-term treatment based on the Tmax in Beagle dogs of 1 hour.11 Whole blood (1–3 mL) was collected using jugular or peripheral venipuncture into lithium heparin tubes and kept on ice until centrifugation. Blood was centrifuged at 13 000 rpm at 4°C for 2.5 minutes, and plasma was extracted and stored at −80°C for batch analysis. Plasma concentrations of the active pharmaceutical ingredient was measured as previously described.11 The maximum, or peak concentration (Cmax) is described.
2.5 Statistics
Continuous data sets (body weight, blood chemistry, CBC, blood pressure, heart rate, etc) were analyzed utilizing parametric tests, either Paired t test for before and after comparisons or repeated measures analysis of variance (ANOVA) followed by post hoc analysis when appropriate. Ordinal data sets (body condition score, quality of life, etc) were analyzed using either Wilcoxon sign ranked (before vs after) or Friedman analysis as appropriate. A probability level of ≤.05 was used for significance.
3 RESULTS
3.1 Clinical findings
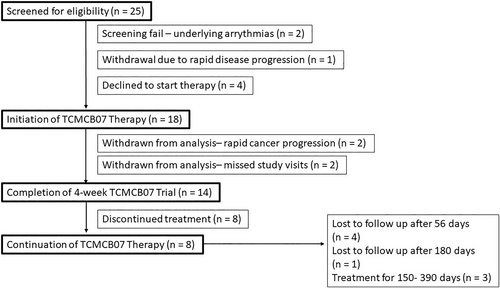
Twenty-five dogs were deemed eligible for enrollment and screening based on history, examination, and initial bloodwork. After informed client consent and the initial testing to confirm eligibility, 23 dogs were deemed eligible to start TCMCB07 therapy; 2 dogs were withdrawn by investigators due to underlying arrhythmias detected on the 24-hour Holter monitor. Five dogs did not start TCMCB07 therapy due to client withdrawal (rapid disease progression of neurologic disease—suspected forebrain lesion n = 1 or declined to start after screening n = 4; Table S1).
Eighteen dogs with cachexia started treatment in the 4-week open label trial. Sixteen were neutered males and 2 were spayed females. Dog breeds included: mixed breed (n = 8), Labrador retriever (n = 3), and 1 each of the following: American cocker spaniel, border collie, Bernese mountain dog, Chihuahua, Maltese, American pit bull terrier, and wolf hybrid. The reason for presentation and evaluation included cancer in 8 dogs: intermediate-to-large cell lymphoma (n = 3) and 1 each of the following: osteosarcoma, intestinal carcinoid with liver metastasis, thyroid and salivary carcinoma (in the same dog), metastatic prostate adenocarcinoma, and hepatobiliary neoplasm with intrahepatic metastasis. Other underlying diagnoses were as follows: suspected age-related cachexia without detectable underlying pain (n = 2) and 1 each of the following: chronic pain, congestive heart failure due to dilated cardiomyopathy, congestive heart failure due to degenerative valve disease (ACVIM stage C), inflammatory effusive disease of unknown origin manifesting primarily as pleural effusion, lymphoplasmacytic gastritis, intervertebral disc disease, generalized neurologic disorder of unknown etiology, and hepatopathy with chronic intervertebral disc disease.
Of the 18 dogs enrolled, 2 dogs suffered progressive lymphoma at day 7 (n = 1) and day 21 (n = 1) of trial, these dogs were removed from trial and humanely euthanized (neither dog received follow up therapy for lymphoma or palliative care). Two other dogs missed their week 4 visit by 6 and 19 days, and were removed from study evaluation for a total of 14 evaluable dogs (Table S2). Twelve of 14 dogs had concurrent medication lists completed; concurrent medications included metoclopramide (n = 1), maropitant (n = 3), ondansetron (n = 1), cyclosporine (n = 1), tramadol (n = 2), gabapentin (n = 4), nonsteroidal anti-inflammatory drugs (n = 3), diazepam (n = 1), intermittent capromorelin (n = 1), omeprazole (n = 1), phenylpropanolamine (n = 1), prazosin (n = 1), trazodone (n = 1), metronidazole (n = 1), prednisone (started prior to trial entry according to enrollment criteria, n = 2), furosemide (n = 1), spironolactone (n = 1), and pimobendan (n = 2). Eight dogs continued therapy beyond the 4-week trial period. Four dogs were lost to follow up after 56 days, 1 lost to follow up after 180 days, and the other dogs remained on drug for 150, 300, and 390 days (Figure 1).
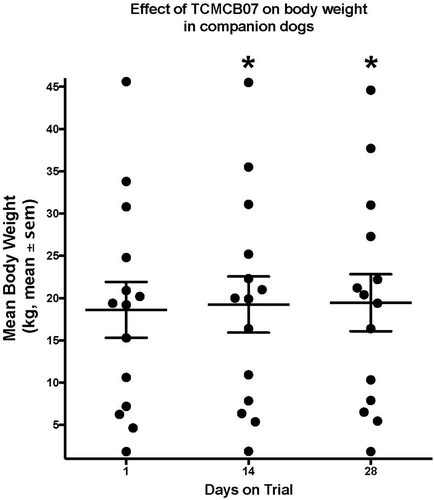
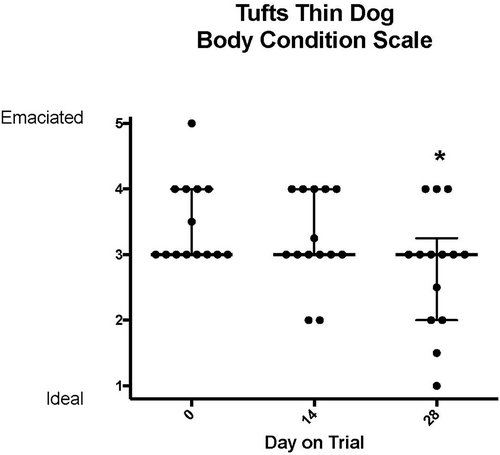
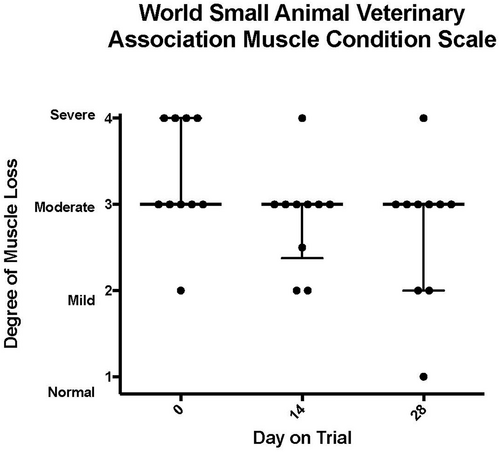
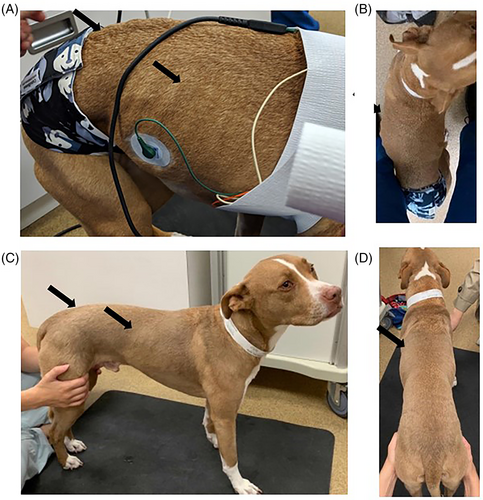
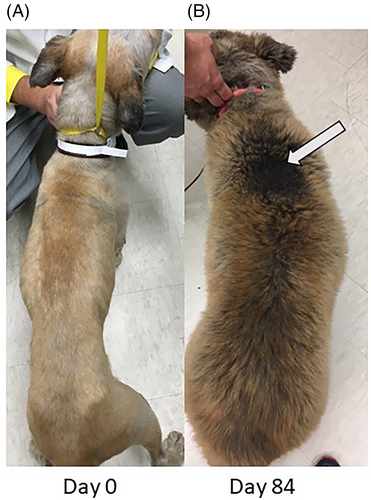
During treatment with TCMCB07, cachectic dogs had a significant increase in body weight, Tufts 5-point thin dog body condition score, and WSAVA 4-point muscle condition score. The mean body weight was significantly increased on days 14 and 28 compared to day 1: on day 1 of trial the mean body weight was 18.6 kg which increased to 19.2 kg on day 14 and 19.5 kg on day 28 (P < .02; Figure 2). The median Tufts 5-point thin dog scale score (n = 14 dogs) was 3 (thin) on all time points measured days 1 (score range 2–4), 14 (score range 2–4), and 28 (score range 1–4), however, there was a significant improvement on day 28 compared to day 1 (P < .004) as dogs' scores improved over time (Figure 3). Similar results were found with the WSAVA muscle condition score (n = 12), in which the median score was 3 (moderate muscle wasting) on days 1 (range 2–4), 14 (range 2–4), and 28 (range 1–4), but a significant improvement was noted on day 28 of trial (P < .02; Figure 4). No change was noted when using the Purina 9-point body condition scoring system (n = 9). The median body condition score using the Purina 9-point system was 3 (too thin) with a range of 2–4 on days 1, 14, and 28 of trial (P = .36). A representative dog in trial is provided in Figure 5 and Figures S1–S3. In addition, 4 of 14 dogs experienced a change of pigmentation to their coat at day 28 or later, when therapy was extended past the 28-day trial period (Figure 6). Tables S3 and S4 summarize weight and Tufts body condition score data in all 14 evaluable dogs.
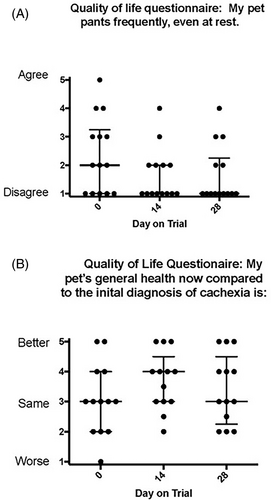
Fourteen clients completed the quality of life questionnaires at all timepoints; however, not all clients answered all questions. Quality of life questionnaires were evaluated and quality of life ratings were compared between days 1, 14, and 28 (Table 1). Two variables were statistically significant over the course of the study period. Pet owners perceived their dog appeared to be panting less over the course of the trial (P < .002) and that the general health of their dog had improved (P < .03; Figure 7).
| Question | Day 1 | Day 14 | Day 28 | P value |
|---|---|---|---|---|
| Median (range) | Median (range) | Median (range) | ||
| 1. My pet wants to play | 3 (1-5) | 4 (1-5) | 4 (1-5) | .22 |
| 2. My pet responds to my presence | 5 (3-5) | 5 (4-5) | 5 (3-5) | .17 |
| 3. My pet enjoys life | 4 (2-5) | 5 (2-5) | 4 (1-5) | .09 |
| 4. My pet has more good days than bad days | 4 (1-5) | 5 (1-5) | 5 (1-5) | .10 |
| 5 My pet sleeps more, is awake less | 3 (3-5) | 3 (1-5) | 3 (1-5) | .22 |
| 6. My pet seems dull or depressed, not alert | 3 (1-4) | 2 (1-4) | 2 (1-4) | .45 |
| 7. My pet is in pain | 2 (1-5) | 2 (1-5) | 2 (1-5) | .17 |
| 8. My pet pants frequently, even at rest | 2 (1-5) | 1 (1-4) | 1 (1-4) | .002 |
| 9. My pet shakes or trembles occasionally | 3 (1-5) | 3 (1-5) | 1 (1-5) | .21 |
| 10. My pet eats the usual amount of food | 3 (1-5) | 4 (1-5) | 4 (1-5) | .06 |
| 11. My pet acts nauseous or vomits | 1 (1-4) | 1 (1-4) | 1 (1-4) | .95 |
| 12. My pet keeps him/herself clean | 5 (2-5) | 5 (2-5) | 4 (1-5) | .46 |
| 13. My pet smells like urine or has skin irritation | 2 (1-4) | 2 (1-4) | 1 (1-4) | .62 |
| 14. My pet's hair is greasy, matted, rough looking | 1 (1-5) | 1 (1-4) | 2 (1-4) | .16 |
| 15. My pet drinks adequately | 5 (1-5) | 5 (2-5) | 5 (2-5) | .85 |
| 16. My pet has diarrhea | 1 (1-4) | 2 (1-4) | 2 (1-3) | .79 |
| 17. My pet is urinating a normal amount | 4 (1-5) | 5 (2-5) | 5 (1-5) | .33 |
| 18. My pet moves normally | 4 (1-5) | 4 (1-5) | 3 (1-5) | .26 |
| 19. My pet lays in 1 place all day long | 3 (1-5) | 2 (1-3) | 2 (1-5) | .44 |
| 20. My pet is as active as he/she has been | 3 (1-5) | 3 (1-5) | 4 (1-5) | .34 |
| 21. General health compared to last evaluation: worse (1) to better (5) | 3 (2-5) | 4 (3-5) | 4 (2-5) | .28 |
| 22. General health compared to initial diagnosis of cancer: worse (1) to better (5) | 3 (1-5) | 4 (2-5) | 3 (2-5) | .031 |
3.2 Clinical pathology data
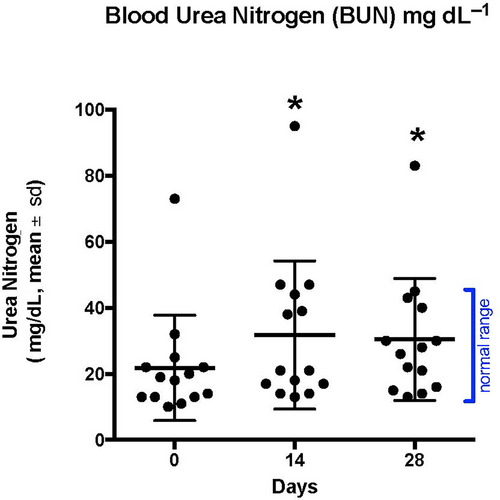
Hematology on all 14 dogs revealed no significant differences between days 1, 14, and 28 (Table 2). There were also no significant differences between any of the chemistry panel values between days 1, 14, and 28 with the exception of blood urea nitrogen (Table 3). Blood urea nitrogen was increased at days 14 and 28 when compared to day 1 (day 1 mean 21.79 mg dL−1, day 14 mean 31.79 mg dL−1, and day 28 mean 30.43 mg dL−1, P < .004; Figure 8). The increases were not correlated with increases in creatinine, decreases in red blood cell count, or other clinical variables.
| Value | Day 1 | Day 14 | Day 28 | P value |
|---|---|---|---|---|
| Mean (range) | Mean (range) | Mean (range) | ||
| Platelet (×103 μL−1) | 391 (183-683) | 388 (87-824) | 407 (136-674) | .77 |
| Lymphocyte (×103 μL−1) | 1.305 (0.17-2.4) | 1.253 (0.18-2.8) | 1.245 (0.48-3.4) | .80 |
| Segmented neutrophil (×103 μL−1) | 7.282 (1.71-17.56 | 7.318 (1.32-16.2) | 7.237 (2.3-19.83) | .983 |
| Monocyte (×103 μL−1) | 0.639 (0.17-2.28 | 0.576 (0.02-1.44) | 0.528 (0.137-1.102) | .68 |
| Eosinophil (×103 μL−1) | 0.333 (0.119-0.912) | 0.37 (0-1.8) | 0.277 (0-0.648 | .692 |
| Red blood cell (×103 μL−1) | 6.16 (5.39-7.5) | 5.72 (4.3-7.1) | 5.96 (4-6.9) | .12 |
| Hemoglobin (g dL−1) | 14.54 (12.4-17.5) | 13.7 (10.8-16.9) | 14.06 (12.2-15.8) | .17 |
| Hematocrit (%) | 43 (37-53) | 40.07 (33-50) | 41.79 (35-50) | .132 |
| Mean corpuscular volume (fL) | 69.9 (63.6-76) | 69.7 (64-76) | 70.3 (64-79) | .58 |
| Mean corpuscular hemoglobin (pg) | 23.7 (21.4-26.2) | 23.8 (21.5-26.1) | 23.6 (21.1-27.6) | .69 |
| Mean corpuscular hemoglobin concentration (g/dL) | 34 (31-36.8) | 34.3 (32-37) | 33.8 (32-36.6) | .46 |
- Note: No significant differences were found in any variable between days 1, 14, and 28.
| Value | Day 1 | Day 14 | Day 28 | P value |
|---|---|---|---|---|
| Mean (range) | Mean (range) | Mean (range) | ||
| Cholesterol (mg dL) | 217 (131-342) | 230 (144-354) | 247 (143-452) | .06 |
| Total bilirubin (mg dL) | 0.14 (0.1-0.2) | 0.14 (0.1-0.2) | 0.14 (0.1-0.3) | .71 |
| Alanine aminotransferase (U L) | 74 (20-187) | 79 (31-242) | 81 (22-282) | .56 |
| Alkaline phosphatase (U L) | 174 (5-963) | 299 (9-1385) | 366 (7-2223) | .26 |
| Phosphorus (mg dL) | 3.96 (3.3-4.8) | 4.4 (2.4-6.6) | 4.4 (2.7-6.1) | .09 |
| Albumin (g dL) | 2.98 (1.8-3.6) | 2.95 (1.9-3.5) | 2.97 (1.7-3.5) | .78 |
| Total protein (g dL) | 5.9 (4.2-6.9) | 5.8 (4.2-7) | 5.8 (3.5-6.8) | .3 |
| Globulin (g dL) | 2.9 (2.1-3.9) | 2.9 (2.1-4) | 2.8 (1.8-3.8) | .13 |
| Sodium (mEq L) | 146.9 (139-161) | 147.2 (142-150) | 146.9 (141-151) | .94 |
| Potassium (mEq L) | 4.5 (3.5-5.6) | 4.5 (3.4-5.6) | 4.5 (3.7-5.9) | .95 |
| Calcium (mg dL) | 9.8 (8.3-11.5) | 9.8 (8.4-10.8) | 9.7 (7.8-10.5) | .82 |
| Chloride (mEq L) | 111.5 (104-117) | 111.3 (104-117) | 111.5 (104-121) | .89 |
| Glucose (mg dL) | 91 (40-119) | 96 (76-115) | 93 (63-117) | .6 |
| Creatinine (mg dL) | 0.96 (0.4-2.6) | 1.06 (0.5-3.1) | 1.05 (0.5-2.6) | .32 |
| Blood urea nitrogen (mg dL) | 22 (10-73) | 32 (13-95) | 30 (13-83) | <.004 |
- Note: No significant differences were found between days 1, 14, and 28 with the exception of blood urea nitrogen. The blood urea nitrogen was increased on days 14 and 28 when compared to day 0 (P < .004).
3.3 Cardiovascular data
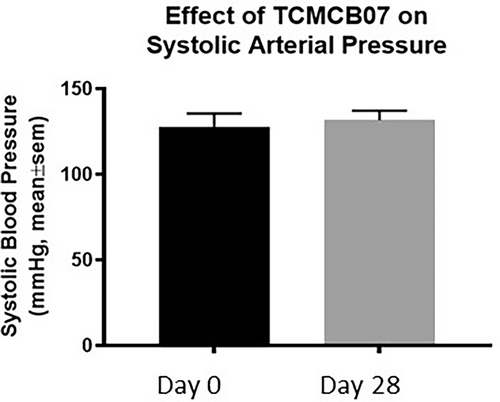
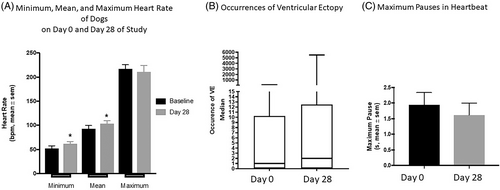
There were no significant changes in systolic blood pressure between screening and day 28 of trial (Figure 9). When comparing the screening and day 28 Holter monitor, there was no difference in the maximum heart rate. There was an increase in the mean and minimum heart rate between day 0 and day 28 of the study (P ≤ .03). There were no increases in the occurrence of ventricular ectopy or maximum pauses in heartbeat between days 0 and 28 (Figure 10).
3.4 Plasma concentration data
On day 1 of drug therapy, the plasma Cmax of TCMCB07 was a mean of 1.52 ± 0.2 μg mL−1 standard error of the mean (SEM) 1 hour after drug administration. At 4 hours, plasma concentrations were 1.23 ± 0.3 μg mL−1 (mean ± SEM) and in most subjects, by 24 hours after injection, TCMCB07 was below the level of detection. On day 28, plasma levels again peaked at 1 hour after injection at 1.21 ± 0.2 μg mL−1 (mean ± SEM).
4 DISCUSSION
TCMCB07, a peptide melanocortin 3/4 antagonist, was safely administered to 14 pet dogs with naturally occurring cachexia from various underlying diseases in this study. The most notable physical examination changes detected throughout the trial period were an increase in body weight and body condition, and most pet owners detected an improvement in the quality of life of their pets during treatment. Coat color pigmentation altered following long-term administration in 4 dogs. No clinically relevant changes were detected on chemistry panel or complete blood count other than a mild increase in blood urea nitrogen, which was not associated with a decreased red blood cell count, increased creatinine, or other clinical variables. No change in blood pressure was detected between the day 0 and day 28 blood pressure measurements and no bradycardic arrhythmias were detected on day 0 or 28 24-hour Holter monitors. Peak plasma concentrations were reached 1 hour after subcutaneous administration. Improvement in the body and muscle condition of dogs enrolled in the trial suggests efficacy and offers promise for future study.
The overall incidence of cachexia in dogs is unknown.17, 18, 24 We suspected that a combination of the low quality of life caused by cachexia, lack of current targeted treatments for cachexia in dogs, and a disease process that is at the end-stage caused pet owners to decline referral to a specialty institution for advanced diagnostics and therapy. These factors likely contributed to the unexpectedly low number of 25 dogs deemed eligible for screening in a period of 5 years at 7 specialty institutions. Of the initial 25 dogs screened, 18 dogs started therapy with 7 dogs excluded for various reasons (Figure 1). The delay between screening and first treatment likely played a role in the 4 dogs that completed screening and were eligible to start treatment, but did not begin trial. Holter monitor results took an average of 2–3 weeks, which for those dogs might have been too long of a delay for intervention before quality of life declined to the point that no further treatment or euthanasia was elected. Furthermore, of the 18 dogs that started treatment, only 14 completed the 28-day trial; 2 dogs were withdrawn early due to cancer progression and 2 dogs missed study visits and were withdrawn for lack of compliance. While the investigators anticipated the potential of disease progression interfering with dogs successfully completing of trial, only 56% of screened dogs completed study. The chronicity and lack of treatment for a primary disease might also have contributed to pet owners lack of follow up in trial. For future study, a Holter monitor is likely not indicated and thus treatment can start much sooner. A quicker turnaround time between screening and initiation of therapy, reduction in screening tests, and promising preliminary results on efficacy reported here might alleviate some of the challenges with dog accrual and client compliance.
On serial physical examinations, the most prominent findings included weight gain, increased body condition score, and increased muscle mass. The melanocortin 4 receptor (MCR4) is a G protein-coupled receptor expressed in the brain and central nervous system, and is very important in regulating food intake and energy homeostasis. When the MCR4 is activated, energy expenditure increases and food intake decreases.9, 10 Chronic inflammatory diseases result in the production and release of proinflammatory cytokines such as IL-1β; when IL-1β stimulates type I IL-1 receptors on the opiomelanocortin neurons in the arcuate nucleus, α-MC is released which stimulates MCR4. Chronic MCR4 stimulation results in cachexia and several investigations demonstrate that MCR4 antagonism is helpful in cachexia therapy by improving appetite and muscle condition.3, 4, 7-10, 14 Based on the physical examination findings in this study, TCMCB07, a peptide MC4R antagonist, might be helpful in improving weight gain and muscle mass in dogs with cachexia. Although dogs in this study were not cured of the underlying disease that caused cachexia, body weight and body and muscle condition score increased when using the Tufts Animal Care and Condition scale for thin dogs and World Small Animal Veterinary Association muscle condition scale, respectively. Weight gain might have improved due to improved caloric intake, improved energy homeostasis, or both. Client questionnaires showed no significant increase in the amount of food intake; however, this variable was subjective in nature and caloric intake/food intake was not measured in this study. As anorexia is an integral component of cachexia syndrome, improvement in caloric intake via antagonism of MC4 can assist in improved energy homeostasis and body condition.2, 4, 7 No statistical difference was noted in the Purina 9-point body condition scoring; however, this is likely due to difficulty differentiating subtle changes in thin dogs using the 9-point scale compared to the thin-dog scale and smaller number of dogs evaluated by the 9-point scale in this study. While this data is preliminary in nature, it underlines the importance of further study of TMCB07 in cachectic conditions and in combination with definitive therapies.
Four of 14 dogs developed a change in the pigmentation of their coat at day 28 during continuation of treatment after the initial 28-day trial period. This is similar to that seen in healthy beagle dogs in a previous study of TCMCB07.11 TCMCB07 can stimulate melanocortin receptor (MCR) 1 which results in increased melanin production.10, 13, 14, 25 Thus, it is likely that the darkening of the coat color is due to the MCR1 stimulation caused by TCMCB07.
In quality of life questionnaires, pet owners perceived their dogs to be panting less and that their dogs' general health improved over the course of the trial. Panting in dogs can be caused by many different factors, making this particular change difficult to interpret. Stress is a common cause of panting in dogs that are not exercising or overheated. In people, cachexia will result in stress.26, 27 While not proven in this study, it is possible that the decreased panting noted by pet owners might be a reduction in stress associated with the clinical improvement. Pet owners also perceived their dogs' general health to have improved over the course of the study—which could be related to actual improvement in health, muscle, and body condition vs performance bias in an open-label trial aimed at improving quality of life.
Administration of TCMCB07 caused no changes to complete blood count variables over the 28-day study period. There were also no significant differences between any of the chemistry panel values with the exception of BUN. The BUN change was mild and not correlated with increases in creatinine, decreases in red blood cell count, or other clinical variables. The increase in BUN might have been due to increased food intake, in particular increased protein intake.28
Although not noted in the previous study of normal dogs,11 monitoring for potential cardiovascular side effects was undertaken as a part of this safety study in cachectic dogs. Activation of MCR4 can cause increased blood pressure and antagonism of MCR4, using other pharmacologic agents, has resulted in hypotension and bradycardia.7-10 TCMCB07 is a cyclic MCR4 antagonist that reduces the potential cardiovascular effects of MCR4 antagonism by the inclusion of degradation impervious N- and C-terminal extensions, which prevents the exposure of the free RFamide pharmacophore.13 In this study, there were no significant changes in systolic blood pressure, maximum heart rate, maximum pauses in heartbeat, or increases in the occurrence of ventricular ectopy between the day 0 and day 28 timepoints. There was a statistically significant increase in the mean and minimum heart rate between screening and day 28 of study, but this was not a clinically relevant increase. The expected arrhythmogenic side effect of TCMCB07 would involve a decline in heart rate, so we hypothesize that the increased heart rate is not related to drug administration. It is possible that increased activity level might have played a role in the increased heart rate, as many dogs were eating more (subjectively, and not statistically significant, based on the client questionnaire) and clinically improving. However, activity level was not tracked in this study and should be considered in future quality of life studies.
Peak plasma concentration data in pet dogs with naturally occurring cancer is similar to that seen in healthy beagle dogs. In a previous study on purpose bred, intact male and female Beagles, plasma TCMCB07 also peaked at 1 hour after injection on day 1.11
One limitation of this study was that it was open-labeled without a placebo control. The design of the study was preliminary in nature, and given the end-stage of disease at presentation for dogs with cachexia the investigators elected a 1-armed open-label trial. In addition, measures of efficacy included body weight, muscle condition scoring, and body condition scoring. While body weight is objective, muscle and body condition scoring systems have inherent subjectivity and variability between users. This was minimized by using systems that were descriptive, previously evaluated, published, and utilized in practice. In addition, treatment of the primary disease was allowed and it must be acknowledged that this and concurrent supportive medications could have affected results. Lastly, food intake was not objectively measured. Based on the mechanism of action of MCR4, increased appetite was anticipated with treatment. Future study should quantify food intake and correlate to increased body and muscle condition score, as improvement in anorexia is considered a key component to recovery from cachexia.2, 4
In conclusion, TCMCB07 is safe in pet dogs with naturally occurring cachexia caused by various disease processes and potential efficacy is noted.
ACKNOWLEDGMENT
Supported by National Institutes of Health, National Cancer Institute, 1R43CA150703, 2R44CA150703, R44CA210763-02, and R44CA210763-03. The authors acknowledge the assistance of Erin Roof, Megan Bucker, Kirstie Barrett, Catherine McDonald, and Lynn Harbinson in their assistance in recruiting patients for this study.
CONFLICT OF INTEREST DECLARATION
Michael F. Callahan and Kenneth A. Gruber are shareholders in Tensive Controls, Inc. Tracy Mills, Sean K. Yoshimoto and Philip J. Bergman are employees of VCA, owned by Mars, Inc and were paid on a fee for service basis supporting this clinical study. No other authors declare a conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the University of Florida IACUC, protocol #201810174, and the University of Missouri IACUC, protocol # 8441.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




