No impact of polymorphism in the phosphodiesterase 5A gene in Cavalier King Charles Spaniels on pimobendan-induced inhibition of platelet aggregation response
Abstract
Background
A variant in the canine phosphodiesterase (PDE) 5A gene (PDE5A:E90K) is associated with decreased concentrations of circulating cyclic guanosine monophosphate (cGMP) and response to PDE5 inhibitor treatment. Pimobendan is a PDE inhibitor recommended for medical treatment of certain stages of myxomatous mitral valve disease (MMVD) in dogs.
Hypothesis
PDE5A:E90K polymorphism attenuates the inhibitory effect of pimobendan on in vitro platelet aggregation and increases basal platelet aggregation in Cavalier King Charles Spaniels (CKCS). Selected clinical variables (MMVD severity, sex, age, hematocrit, platelet count in platelet-rich plasma [PRP], and echocardiographic left ventricular fractional shortening [LV FS]) will not show an association with results.
Animals
Fifty-two privately owned CKCS with no or preclinical MMVD.
Methods
Using blood samples, we prospectively assessed PDE5A genotype using Sanger sequencing and adenosine diphosphate-induced platelet aggregation response (area under the curve [AUC], maximal aggregation [MaxA], and velocity [Vel]) with and without pimobendan using light transmission aggregometry. Dogs also underwent echocardiography.
Results
Pimobendan inhibited platelet function as measured by AUC, MaxA, and Vel at a concentration of 10 μM (P < .0001) and Vel at 0.03 μM (P < .001). PDE5A:E90K polymorphism did not influence the inhibitory effect of pimobendan or basal platelet aggregation response.
Conclusions and Clinical Importance
The PDE5A:E90K polymorphism did not influence in vitro basal platelet aggregation response or the inhibitory effect of pimobendan on platelet aggregation in CKCS. Dogs with the PDE5A:E90K polymorphism did not appear to have altered platelet function or response to pimobendan treatment.
Abbreviations
-
- ACVIM
-
- American College of Veterinary Internal Medicine
-
- ADP
-
- adenosine diphosphate
-
- AUC
-
- area under the curve
-
- BW
-
- body weight
-
- cAMP
-
- cyclic adenosine monophosphate
-
- cGMP
-
- cyclic guanosine monophosphate
-
- CHF
-
- congestive heart failure
-
- CKCS
-
- Cavalier King Charles Spaniel
-
- DBP
-
- diastolic blood pressure
-
- Emax
-
- early E wave transmitral peak velocity
-
- F
-
- female
-
- LA/Ao
-
- left atrial to aortic root ratio
-
- LV FS
-
- left ventricular fractional shortening
-
- LVIDDN
-
- left ventricular end-diastolic diameter normalized for BW
-
- LTA
-
- light transmission aggregometry
-
- LVIDSN
-
- left ventricular end-systolic diameter normalized for BW
-
- M
-
- male
-
- MaxA
-
- maximal aggregation
-
- MBP
-
- mean blood pressure
-
- MMVD
-
- myxomatous mitral valve disease
-
- MR
-
- mitral regurgitation
-
- NaCl
-
- sodium chloride
-
- PDE
-
- phosphodiesterase
-
- PRP
-
- platelet-rich plasma
-
- PPP
-
- platelet-poor plasma
-
- SBP
-
- systolic blood pressure
-
- Vel
-
- velocity
1 INTRODUCTION
Pharmacogenetics describes how variation in genes may affect an individual's response to medical treatment1 and is regarded as a fundamental step toward personalized medicine. In human medicine, it is an area of emerging interest, but limited research exists in veterinary medicine. The phosphodiesterase (PDE) 5A gene encodes a cyclic guanosine monophosphate (cGMP)-specific PDE. Platelets express both PDE5 and PDE3 and both are key enzymes involved in platelet signaling pathways ensuring the breakdown of cGMP and cAMP.2, 3 Polymorphisms in the PDE5A gene influence plasma cGMP concentrations under basal conditions and the response to vasodilatory stimuli in humans with heart failure.4 A specific polymorphism in the canine PDE5A gene (PDE5A:E90K) is associated with lower circulating cGMP in healthy dogs,5 possibly indicating an enhancement of PDE5A gene function and, in accordance, recent research indicates that this polymorphism decreases the response to the PDE5-inhibitor sildenafil in dogs with pulmonary hypertension.6
Myxomatous mitral valve disease (MMVD) is the most common cardiac disease in dogs, and Cavalier King Charles Spaniels (CKCS) are predisposed.7, 8 The pathophysiology of MMVD has not been fully elucidated but may be affected by altered platelet function.9
Standard cardiac medication regimens for MMVD in dogs target PDEs. Pimobendan is 1 of the most commonly used drugs for this condition. It is a calcium-sensitizing drug and an inhibitor of PDE3 and PDE510, 11 The beneficial effect of pimobendan is mainly thought to be a result of its inotropic and vasodilatory properties.12 In humans, where PDE inhibitors also play a role in treating cardiovascular disease, research suggests that these drugs have secondary effects on platelet aggregation and platelet inhibition.2, 13-20 Interestingly, a study of whole blood platelet aggregation indicated an in vitro inhibitory effect of pimobendan at high doses (10 μM) in blood samples from dogs.
Platelets express both PDE5 and PDE3, and their inhibition is thought to affect platelet signaling by decreasing the degradation of intraplatelet cyclic adenosine monophosphate (cAMP; particularly PDE3 inhibition) and cGMP (particularly PDE5 inhibition), thus leading to an increase in their concentrations. Because cAMP and cGMP are essential inhibitory intracellular second messengers, likely ensuring platelet inhibition, the net effect of PDE inhibition also might include platelet inhibition.2, 3, 21
Individual differences in age of onset, progression, survival, and clinical response to treatment have been described in dogs with MMVD.22, 23 Likewise, large individual variations in pharmacokinetic characteristics of pimobendan have been reported (McManamey, Anna K; DeFrancesco, Terese C; Keene, Bruce W, et al. 2021. Population Pharmacokinetics of Oral Pimobendan and its Metabolite in Dogs with Myxomatous Mitral Valve Degeneration. 2021 ACVIM Forum Abstract C27).24 Such variance might implicate polymorphisms in the PDE5A gene.
We aimed to investigate the presence and impact on basal platelet aggregation response of the PDE5A:E90K polymorphism in CKCS with or without preclinical MMVD. Additional objectives were to determine (1) the in vitro effect of pimobendan on adenosine diphosphate (ADP)-induced light transmission aggregometry (LTA) response at clinical treatment (0.03 μM) and high (10 μM) concentrations in platelet-rich plasma (PRP) and (2) if the potential effect of pimobendan on platelet aggregation was associated with PDE5A genotype and selected clinical variables (age, sex, severity of MMVD, hematocrit, platelet count, and echocardiographic left ventricular fractional shortening [LV FS]).
It was hypothesized that the PDE5A:E90K polymorphism would (1) be present in CKCS and increase basal platelet aggregation response because of a higher PDE5 activity and thus lower cGMP concentration and (2) attenuate the inhibitory effect of pimobendan on platelet aggregation response, because of the enhanced PDE5 activity. No association with the selected clinical variables was hypothesized.
2 MATERIALS AND METHODS
2.1 Dogs
Ours was a prospective study performed at the Department of Veterinary and Animal Sciences, University of Copenhagen from August 2020 to March 2021 and approved by the Danish Animal Experiments Inspectorate (license no. 2016-15-0201-01074).
No previous data were available on the frequency of the PDE5A:E90K polymorphism in CKCS or on the effect of pimobendan at a concentration of 0.03 μM (considered the clinical treatment concentration in our study) on platelet aggregation response in CKCS. Instead, sample size calculation was performed using in-house pilot data of ADP-induced (final concentration 20 μM) LTA aggregation response from 7 CKCS, adding pimobendan at a concentration of 10 μM. The mean ± SD of the paired difference (positive control reflecting the basal aggregation response of the dog [0 μM pimobendan] − pimobendan 10 μM response) in maximal aggregation (MaxA) was 35.4% ± 26.2. Thus, these data indicated that a minimal sample size of 8 CKCS was necessary to demonstrate a significant difference in MaxA response with an alpha of 0.05 and power of 0.80.
Client-owned CKCS with different preclinical stages of MMVD were recruited for the study. Written informed consent was given by all owners. All CKCS were unrelated at the parental level and further inclusion criteria included a minimum age of 4 years, absence of macrothrombocytopenia (defined as EDTA platelet count <100 × 106 PLT/mL25), and no vaccination or medical treatment within the last 30 days before examination. In addition, a period of at least 6 months since last blood collection must have passed.
Dogs with systemic or organ-related disease and cardiac disorders other than MMVD, unsuccessful blood collection, analytical error as well as dogs with considerable deviations from reference intervals on CBC and standard serum biochemistry profile were excluded.
A standardized protocol was used for examination including interview with the owner, venous blood collection, physical examination with focus on the cardiovascular system, blood pressure measurement, and echocardiography. The examination was performed within approximately 1 hour in the same consecutive order for all dogs. Murmur intensity was graded 1-6/6.26 Blood pressure was measured by high-definition oscillometry equipment (Vet HDO monitor [Memodiagnostic], S + B medVET GmbH, Babenhausen, Germany) on the proximal part of the tail and based on the mean of 5 repeated measurements as previously described.27
An in-house pilot study included 11 additional similarly examined client-owned CKCS recruited in order to make titration curves for pimobendan. Titration curves were created to confirm that pimobendan had a dose-dependent effect on platelet aggregation in CKCS. Description and results from this pilot study are included in Table S1 and Figure S1.
2.2 Blood sampling
Blood from dogs fasted 6 to 18 hours before sampling was collected from the jugular vein using a vacutainer system with a 21G butterfly catheter into 2 plastic tubes containing 3.2% sodium citrate (9 mL), 1 tube containing EDTA (4 mL), and 1 plain tube (4 mL). Tubes were mixed gently. Thirty minutes after sampling, plain blood was centrifuged at 3000×g for 10 minutes at 4°C and serum and EDTA-stabilized whole blood samples were analyzed at the Veterinary Diagnostic Laboratory at the University of Copenhagen for a standard serum biochemistry profile and CBC, respectively. The remaining EDTA-stabilized whole blood was used for (1) manual platelet count after adding 20 μL of EDTA-stabilized whole blood to 380 μL of stromatolytic solution,28 and (2) batched PDE5A:E90K genotype analyses after storage at −20°C and shipment to the Veterinary Genetics Laboratory at North Carolina State University.
Citrate tubes were allowed to stand for 15 minutes after sampling and PRP and platelet-poor plasma (PPP) were otherwise prepared as previously described.9 The PRP stood for 30 minutes after centrifugation and the platelets in PRP were counted manually.28
2.3 PDE5A:E90K genotype analysis
Extraction of DNA was performed using the standard protocol of the GeneJET Whole Blood Genomic DNA Purification Mini Kit (Thermo Fisher Scientific, Waltham, MA). Polymerase chain reaction (PCR) amplification primers were designed for the variant of interest, as described previously5 and located at chr32:38387073 of the University of California at Santa Cruz canFam3 reference sequence (https://genome.ucsc.edu/cgi-bin/hgGateway), using Primer 3 software (http://frodo.wi.mit.edu/) and the canine nucleotide sequences from the Ensembl genomic database (http://www.ensembl.org/index.html). A 330 base pair (bp) region flanking the single nucleotide polymorphism (SNP) of interest was amplified by PCR under standard reaction conditions on a BioRad S1000 thermal cycler with AmpliTaq Gold 360 Master Mix (Thermo Fisher Scientific) and the following primers: 5′-GCGATCAGCGGAGATAAGTC-3′ and 5′-CACGCAGAATCTTGCTCTTG-3′. The resulting PCR amplicon was sent to Genewiz LLC for Sanger sequencing.
2.4 Light transmission aggregometry
Platelet aggregation analyses were completed within 3 hours of blood collection using an optical 4-channel aggregometer (Thrombo-Aggregometer TA-V4, SD Medical, Frouard, France) with ThromboSoft software (Version 1.6.1.3). Aggregation was assessed by ADP (CHRONO-PAR ADP, Chronolog, Pennsylvania, USA; final concentration of 20 μM9) as agonist, with a temperature of 37°C and a stirring speed of 1100 rpm. The PRP was not adjusted to a standardized platelet count by dilution with PPP.29
Samples were analyzed based on the manufacturer's guidelines. Briefly, 450 μL of PRP for each channel was transferred into a glass cuvette containing a siliconized magnetic stir bar, and 500 μL PPP was transferred to a glass cuvette with no stir bar. Samples were incubated for 1 minute, and the 0% baseline and 100% baselines were calibrated by PRP and PPP, respectively (allowing 0% and 100% light transmittance, respectively). Recording of aggregation response started when 25 μL of pimobendan (Vetmedin vet, 0.75 mg/mL solution for injection, Boehringer Ingelheim Vetmedicia GmbH, Ingelheim/Rhein, Germany) at a final concentration of 0.03 or 10 μM, respectively (pimobendan channels), or 25 μL of NaCl 0.9% (control channels) was added. Platelet activation and aggregation were initiated by the addition of 25 μL of ADP in 3 channels (pimobendan channels and positive control channel; the latter reflecting the basal aggregation response of the dog) after 1 minute of incubation. In the last channel, an additional 25 μL of 0.9% NaCl was added and this channel, only containing PRP and NaCl, was used to check for auto-aggregation (negative control channel); a maximal drift of 10% was allowed (Table 1). Light transmission was monitored for 10 minutes after the addition of agonist and measured every 0.25 seconds. The following platelet aggregation outcomes were recorded: (1) area under the curve (AUC) in % × min calculated by GraphPad Prism (GraphPad Software Inc., Version 9, San Diego, CA), (2) MaxA in percentage, and (3) velocity (Vel) of aggregation in %/min calculated automatically by ThromboSoft as the steepest slope with the best regression coefficient during 30 seconds of the aggregation curve. Negative aggregation curve values, occurring after initial and expected ADP-induced platelet shape change, were changed to 0% on all LTA curves when calculating AUC. Samples were analyzed in 2 runs approximately 15 minutes apart. Setup for the second run was identical to that of the first run and used to confirm results from the first run. Thus, data from the second run was not used in data analysis unless an error in run 1 occurred.
| Negative control channel | Positive control channel | Pimobendan channel | Pimobendan channel | ||
|---|---|---|---|---|---|
| Run 1 | Incubation | NaCl | NaCl | Pimobendan [0.03 μM] | Pimobendan [10 μM] |
| Activation | NaCl | ADP [20 μM] | ADP [20 μM] | ADP [20 μM] | |
| Run 2 | Incubation | NaCl | NaCl | Pimobendan [0.03 μM] | Pimobendan [10 μM] |
| Activation | NaCl | ADP [20 μM] | ADP [20 μM] | ADP [20 μM] | |
- Note: Concentrations given are final concentrations.
- Abbreviations: ADP, Adenosine diphosphate; LTA, light transmission aggregometry; NaCl, sodium chloride.
2.5 Echocardiography
The echocardiographic examinations (VividE95 echocardiograph with a 5Sc transducer, GE Healthcare Denmark A/S) were performed by 2 experienced operators (MJR and LHO). Images were analyzed offline (EchoPAC PC. Version 202, GE Medical systems, Brøndby, Denmark) by a single operator (MJR) blinded to the identity of dogs and clinical characteristics. Standard transthoracic echocardiographic 2-dimensional, M-mode, and Doppler images were acquired in unsedated dogs from right and left parasternal positions and following a standardized protocol modified from previous studies.30, 31 As described, offline analyses were performed and severity of mitral regurgitation (MR) was estimated. In addition, left atrial and aortic root diameter ratio (LA/Ao), left ventricular (LV) end-diastolic and LV end-systolic diameter were measured and LV FS was calculated.
Severity of MMVD was classified as MMVD stages modified from American College of Veterinary Internal Medicine (ACVIM) consensus statement guidelines as follows: stage A: CKCS with no auscultatory heart murmur and normal echocardiogram (no or minimal MR [MR < 20%]); stage B1: CKCS with auscultatory heart murmur or MR ≥20% but no cardiac enlargement; stage B2: CKCS with heart murmur ≥3 of 6 and echocardiographic evidence of cardiac enlargement but no current or previous clinical signs of CHF; stage C: CKCS with CHF.8, 32 Thus, the MMVD stage A included dogs with minimal MR, because such minimal valve leakage has little to no clinical or prognostic significance for MMVD in CKCS.33 Cardiac enlargement was defined as LA/Ao ≥1.6 and LV end-diastolic diameter normalized to body weight (LVIDDN) ≥ 1.7.32
2.6 Statistical analysis
Data were analyzed by statistical software (R version 4.2.1, R Core Team [2021]. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/). Unless otherwise stated, values of P < .05 were considered significant. Assessment for normal distribution was based on visual inspection of histograms, QQ plots, and the Shapiro-Wilk test.
All descriptive data are presented as median and interquartile ranges (IQR). Overall group differences were investigated using a nonparametric Kruskal-Wallis test (continuous data) or Fisher's exact test (categorical data) because some groups did not follow a normal distribution. Group differences for the variables LA/Ao, LVIDDN, and MR severity were not tested because these variables were used to allocate dogs into MMVD stages. To explore differences among MMVD stages, post hoc testing was performed by Wilcoxon rank sum testing (continuous data) with Bonferroni adjustment.
Linear regression analyses were used to determine the association of genotype (negative wild type, positive heterozygous or positive homozygous) and additional dog characteristics and clinical variables (MMVD stage, sex, age, hematocrit, platelet count in PRP, and LV FS) with basal aggregation response.
Because ADP-induced AUC and MaxA did not fulfill model assumptions of homoscedasticity and linearity, each explanatory variable was tested by nonparametric Kruskal-Wallis or Wilcoxon rank sum tests (categorical variables) or Spearman correlation (continuous variables). To select variables for multivariable analyses, univariable regression analyses were performed by ADP-induced LTA response (AUC, MaxA, and Vel) of the positive control channel as outcome variables and dog characteristics and clinical variables as explanatory variables. Variables with P < .2 in the univariable regression analysis or appropriate nonparametric analyses were included in a multivariable regression analysis. However, because the primary aim of our study was to evaluate the association between aggregation response and genotype, genotype was included as a fixed variable in all multivariable analyses regardless of P-value. Co-variance of the explanatory variables was assessed by visual inspection of scatter plots and calculation of the variance inflation factor. Visual inspection of residual plots, QQ plots, and the Shapiro-Wilk test were used to test model residuals for homogeneity of variation. Backward stepwise elimination was based on P values.
Paired differences of variables for each LTA response (AUC, MaxA, Vel) were defined by subtracting the aggregation response of each pimobendan channel from that of ADP (0 μM pimobendan) at each concentration of pimobendan: ΔPimo0.03AUC, ΔPimo10AUC, ΔPimo0.03MaxA, ΔPimo10MaxA, ΔPimo0.03Vel, and ΔPimo10Vel. A paired t-test (variables following a normal distribution) and Wilcoxon's signed-rank test (variables not normally distributed) were used to evaluate the inhibitory effect of pimobendan on aggregation response.
To identify variables associated with the effect of pimobendan, univariable analyses were performed as previously described by the paired difference variables (ΔPimo0.03AUC, ΔPimo10AUC, ΔPimo0.03MaxA, ΔPimo10MaxA, ΔPimo0.03Vel, and ΔPimo10Vel) in separate models as response variables and the previously mentioned dog characteristics and clinical variables as explanatory variables. Variables that did not fulfill model assumptions were tested by nonparametric Kruskal-Wallis or Wilcoxon rank sum tests (categorical variables) or Spearman correlation (continuous variables). Variables with P < .2 in the univariable regression analysis were included in a mixed multivariable linear model using the paired difference between the positive control (0 μM pimobendan) and pimobendan aggregation response for each LTA variable (ΔAUC, ΔMaxA, ΔVel) as response variable and taking repeated measurements (2 concentrations [0.03 μM and 10 μM] for each dog) into account.
Analyses were repeated including genotype (as a fixed variable) and the interaction between genotype and pimobendan concentration as explanatory variables in addition to the variables with P < .2 in the univariable regression analysis.
3 RESULTS
Seventy CKCS were recruited, but 18 were excluded because of macrothrombocytopenia (n = 13), serum biochemistry analysis indicating clinically relevant systemic disease (n = 2), lack of sufficient blood for analyses (n = 2), and error in sample handling (n = 1). A summary of descriptive data for the final population of 52 CKCS is provided in Table 2. All dogs were successfully genotyped and 12% of the dogs were negative wild type, 46% were positive heterozygous and 42% were positive homozygous for the PDE5A:E90K polymorphism. There were no differences in genotype among MMVD stages.
| MMVD stage A (n = 10) | MMVD stage B1 (n = 29) | MMVD stage B2 (n = 13) | Overall P-value | |
|---|---|---|---|---|
| Sex (%) | ||||
| Female | 10 (100%) | 19 (66%) | 7 (54%) | .03 |
| Male | 0 (0%) | 10 (34%) | 6 (46%) | |
| Age (years) | 6.0 [5.6;6.7]B2 | 6.4 [4.6;8.9] | 8.1 [7.0;10.7]A | .02 |
| BW (kg) | 9.0 [8.2;10.1] | 9.0 [8.5;9.6] | 9.2 [8.5;10.7] | NS |
| SBP (mmHg) | 133 [126;144] | 138 [131;151]28 | 151 [143;161] | NS |
| DBP (mmHg) | 74 [64;82] | 80 [74;86]28 | 80 [74;87] | NS |
| MBP (mmHg) | 95 [85;104] | 100 [95;108]28 | 106 [102;111] | NS |
| MR severity | ||||
| 1 | 10 | 0 | 0 | Not tested |
| 2 | 0 | 22 | 0 | |
| 3 | 0 | 7 | 13 | |
| LA/Ao | 1.3 [1.2;1.3] | 1.3 [1.2;1.5] | 1.8 [1.6;2.0] | Not tested |
| LVIDDN (cm/kg0.294) | 1.4 [1.4;1.6] | 1.5 [1.4;1.7] | 1.8 [1.7;1.9] | Not tested |
| LV FS (%) | 32 [27;36]B2 | 35 [28;37]B2 | 42 [38;46]A,B1 | .001 |
| PLT EDTA (×106 PLT/mL) | 307 [223;339] | 258 [172;310] | 322 [283;356] | NS |
| PLT PRP (×106 PLT/mL) | 544 [408;669] | 360 [236;517]B2 | 626 [372;754]B1 | .02 |
| Hematocrit (%) | 0.43 [0.41;0.46]9 | 0.42 [0.40;0.44] | 0.41 [0.40;0.43] | NS |
| Plasma cGMP (pmol/mL) | 1253.1 [693.7;1754.9]9 | 900.8 [606.9;1319.7] | 797.0 [632.5;986.5] | NS |
| Genotype (%) | ||||
| Negative wild-type | 1 (10%) | 4 (14%) | 1 (8%) | NS |
| Positive heterozygous | 4 (40%) | 15 (52%) | 5 (38%) | |
| Positive homozygous | 5 (50%) | 10 (34%) | 7 (54%) | |
- Note: Values reported are median and interquartile ranges. Within each row, superscripts A,B1,B2,C represent the group from which there is statistically significant difference assessed by Wilcoxon rank sum test with Bonferroni adjustment. Superscript numbers refer to reduced number of observations. Categorical variables were tested using Fisher's exact test.
- Abbreviations: BW, body weight; cGMP, cyclic guanosine monophosphate; DBP, diastolic blood pressure; LA/Ao, ratio of left atrium-to-aortic root; LV FS, left ventricular fractional shortening; LVIDDN, left ventricular end-diastolic diameter normalized for BW; MBP, mean blood pressure; MR severity, mitral regurgitation severity assessed by jet area method where 1 = no or minimal MR (< 20%), 2 = mild MR (20-50%), 3 = moderate–severe MR (> 50%); PDE5A, phosphodiesterase 5A; PLT EDTA, platelet count in EDTA-anticoagulated blood; PLT PRP, platelet count in platelet-rich plasma; SBP, systolic blood pressure; TCP, thrombocytopenia defined as dogs with platelet count <100 × 106 PLT/mL in EDTA-anticoagulated blood.
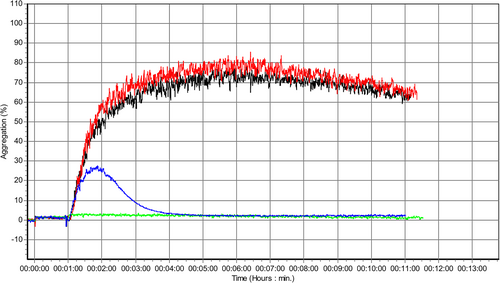
In 8 dogs, aggregation analyses were stopped after 9 minutes, and in 2 dogs, results only from run 2 were used in the analysis because of analytical errors in run 1. In 4 study dogs, ≥1 aggregation curve values fell below 0% and these were manually changed to 0 before calculation of AUC. Representative aggregation traces are given in Figure 1.
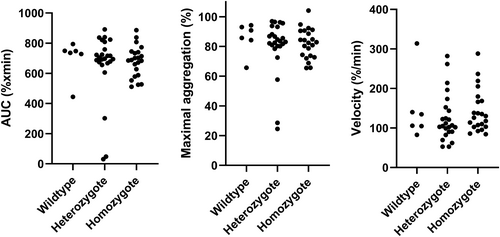
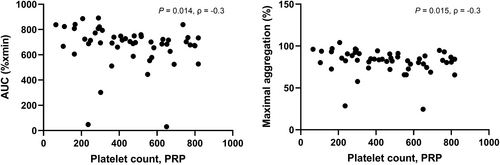
Association between basal platelet aggregation response and genotype (and additional dog characteristics and clinical variables): No association was found between genotype (or any other characteristics or clinical variable including MMVD stage, age, sex, hematocrit, and LV FS) and LTA aggregation response to ADP neither in univariable (Figure 2) nor multivariable analyses. However, a negative correlation was found between PRP platelet count and LTA aggregation response to ADP for AUC and MaxA, respectively (Figure 3). The results from univariable analyses are summarized in Table 3.
| AUC (% × min) | MaxA (%) | Vel (%/min) | |
|---|---|---|---|
| Genotype | 0.43 | 0.51 | 0.37 |
| MMVD stage | 0.92 | 0.93 | 0.76 |
| Sex | 0.23 | 0.083 | 0.82 |
| Age | 0.091 | 0.79 | 0.87 |
| Hematocrit | 1.00 | 0.72 | 0.53 |
| PLT PRP | 0.014 | 0.015 | 0.49 |
| LV FS | 0.72 | 0.31 | 0.84 |
- Abbreviations: AUC, area under the curve; LV FS, left ventricular fractional shortening; MaxA, maximal aggregation; PLT PRP, platelet count in platelet-rich plasma; Pimo, pimobendan; Vel, velocity.
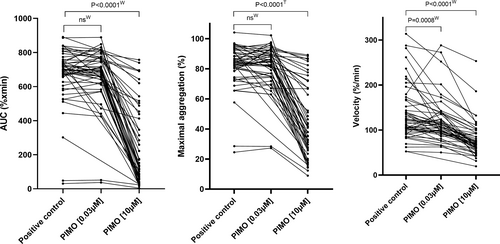
Effect of pimobendan on platelet aggregation response. Compared with the positive control (0 μM pimobendan) aggregation response, pimobendan inhibited platelet function as measured by AUC, MaxA, and Vel considerably at high concentrations (10 μM; Figure 4 and Table 4). At low concentration (0.03 μM) pimobendan only inhibited Vel (Figure 4).
| Positive control channel | Pimobendan 0.03 μM | Percent inhibition (%): Pimo 0.03 μM | Pimobendan 10 μM | Percent inhibition (%): Pimo 10 μM | |
|---|---|---|---|---|---|
| AUC (%xmin) | 702 [658;749] | 693 [631; 769]48 | 1.1% [−5.6;5.6]48 | 130 [59; 425] | 79.7% [45.4;89.5] |
| MaxA (%) | 84 [79;91] | 85 [75;91]48 | 0.4% [−5.4;4.4]48 | 38 [27;67] | 51.3% [26.2;66.9] |
| Vel (%/min) | 116 [101;159] | 107 [95;136]48 | 8.4% [0.5;18.5]48 | 69 [60;93] | 38.9% [32.0;51.2] |
- Note: Percent inhibition was calculated for each LTA variable (AUC, MaxA, and Vel) based on the difference between adenosine diphosphate (ADP)-induced aggregation with pimobendan channels or without pimobendan (positive control channel) using the following formula: Percent inhibition = ([positive control − pimobendan]/positive control) × 100.
- Abbreviations: ADP, adenosine diphosphate; AUC, area under the curve; MaxA, maximal aggregation; Pimo, pimobendan; Vel, velocity. Values reported are median and interquartile ranges. Superscript numbers refer to decreased number of observations.
Association between the inhibitory effect of pimobendan on platelet aggregation response and genotype (and additional dog characteristics and clinical variables): Genotype and platelet count in PRP were not associated with any of the LTA variables and therefore were not included in any multivariable models. The results from univariable analyses are summarized in Table 5.
| ΔPimo0.03AUC (%xmin) | ΔPimo10AUC (%xmin) | ΔPimo0.03MaxA (%) | ΔPimo10MaxA (%) | ΔPimo0.03Vel (%/min) | ΔPimo10Vel (%/min) | |
|---|---|---|---|---|---|---|
| Genotype | 0.27 | 0.36 | 0.44 | 0.49 | 0.30 | 0.32 |
| MMVD stage | 0.47 | 0.91 | 0.23 | 0.78 | 0.27 | 0.81 |
| Sex | 0.16 | 0.31 | 0.16 | 0.32 | 0.64 | 0.24 |
| Age | 0.054 | 0.14 | 0.061 | 0.24 | 0.38 | 0.81 |
| Hematocrit | 0.083 | 0.72 | 0.048 | 0.59 | 0.79 | 0.94 |
| PLT PRP | 0.60 | 0.39 | 0.45 | 0.57 | 0.49 | 0.93 |
| LV FS | 0.13 | 0.049 | 0.18 | 0.18 | 0.47 | 0.84 |
- Note: Paired differences of variables for each LTA response (AUC, MaxA, Vel) were defined by subtracting the aggregation response of each pimobendan channel from that of ADP (0 μM pimobendan) at each concentration of pimobendan: ΔPimo0.03AUC, ΔPimo10AUC, ΔPimo0.03MaxA, ΔPimo10MaxA, ΔPimo0.03Vel, and ΔPimo10Vel.
- Abbreviations: AUC, area under the curve; LV FS, left ventricular fractional shortening; MaxA, maximal aggregation; PLT PRP, platelet count in platelet-rich plasma; Pimo, pimobendan; Vel, velocity.
In all multivariable models, only pimobendan concentration (but no other explanatory variable) was associated with platelet aggregation response. For all LTA variables, the high concentration of pimobendan (10 μM) had a higher inhibitory effect compared to the low concentration (0.03 μM). Summary of the results from multivariable analyses is given in Table 6. No association was found between platelet aggregation response variables and genotype, and the interaction between genotype and pimobendan concentration was not significant when the analyses were repeated including these explanatory variables.
| ΔAUC (%xmin) | ΔMaxA (%) | ΔVel (%/min) | ||||
|---|---|---|---|---|---|---|
| Estimate | P | Estimate | P | Estimate | P | |
| Pimobendan concentration | 389.9 | <.0001 | 37.2 | <.0001 | 41.0 | <.0001 |
- Note: Positive estimates indicate the degree of inhibition of the high dose compared to the low dose.
- Abbreviations: AUC, area under the curve; MaxA, maximal aggregation; Vel, velocity.
4 DISCUSSION
The PDE5A:E90K polymorphism was present in CKCS with no or preclinical MMVD, without association with MMVD severity, and no association was seen between genotype and basal ADP-induced LTA response or the effect of pimobendan on platelet aggregation response. The study confirmed the previously reported inhibitory effect of pimobendan at high concentration (10 μM) on in vitro platelet aggregation but also indicated that pimobendan at clinical treatment concentration (0.03 μM) inhibited only ADP-induced aggregation Vel. Finally, platelet count in PRP showed a weak negative correlation with basal ADP-induced LTA response.
Our study confirms that findings from previous research indicating that the PDE5A:E90K polymorphism is highly prevalent in dogs also applies to CKCS.5, 6 Genotype-positive animals (46% heterozygous, 42% homozygous) outnumber wild-type dogs (12%). Although this polymorphism is not breed-specific, the prevalence likely reflects breed-specific selection pressures. Previous research has reported a possible functional relevance of the PDE5A:E90K polymorphism.5, 6 In 1 study of 15 healthy dogs, the PDE5A:E90K polymorphism was associated with a lower circulating cGMP concentration, possibly indicating enhancement of the PDE5A gene function, although cGMP protein function was not assessed.5 However, such an association was not identified in a subsequent study using a different study population.6 In theory, enhanced PDE5A gene function and increased PDE5 expression could lead to decreases in intraplatelet cGMP, which could increase basal platelet aggregation through cellular signaling pathways.21 However, in contrast to our hypothesis, the present polymorphism does not appear to increase basal ADP-induced LTA response or attenuate the inhibitory effect of pimobendan. Large individual variations were present in both the basal platelet aggregation response and the inhibitory response to pimobendan (Figure 4), and other genetic polymorphisms might be of importance. Furthermore, cGMP pathways are complex and may result in a variety of effects causing our simplified hypothesis not to apply.21 This situation might be the reason that, counterintuitively, higher plasma concentrations of cGMP are reported in dogs with congestive heart failure (CHF) compared to CKCS with preclinical MMVD.34 Measures of cGMP, PDE5 or novel downstream activity measures of cAMP/cGMP such as phosphorylation of platelet vasodilator-stimulated phosphoprotein (pVASP) were not performed in our study, and represent a future direction for further elucidation of our findings.35
A weak negative association between platelet count in PRP and basal LTA response (AUC and MaxA) was found. Previous research also has found associations between platelet count and platelet aggregation response in CKCS using various methodologies. Some found that low platelet count in CKCS was associated with a more hypercoagulable pattern,36 others found that platelet count was positively associated with aggregation response9 and no association between platelet count and aggregation response also has been reported.37, 38 Approximately one third of CKCS have inherited macrothrombocytopenia, which usually is not associated with clinical signs of disease.25, 28, 39 Such dogs were included in the previously mentioned research, but excluded in our study because of the reported alterations in platelet aggregation response. Therefore, results are difficult to compare. The discrepancy between previous and present findings may be a result of the different methods of assessing platelet function or methods of performing LTA or differences in presence or means of classifying dogs as having or not having thrombocytopenia. One difference in LTA performance includes PRP standardization. Although it is possible to adjust PRP platelet count with PPP to avoid differences in platelet count, this method was not applied in our study because it is no longer recommended.29 Yet, uncertainty remains about best practice when platelet count exceeds 600 × 106 PLT/mL because of risk of autoaggregation. In our study, samples with PRP platelet count above 600 × 106 PLT/mL (n = 15) were included because such a platelet count could reflect EDTA platelet counts within the normal range.
The reason for including the echocardiographic variable LV FS among variables with possible influence on basal aggregation and the effect of pimobendan on aggregation response is that a previous study indicated that increasing LV FS was associated with hypercoagulability based on thromboelastography in CKCS.36 Our study did not find a similar association, which may be a result of different methods of aggregation analysis. In accordance with previous findings,12 our study found an in vitro inhibitory effect of pimobendan at high concentrations (10 μM). In contrast, our study also found an inhibitory effect of pimobendan at clinical treatment concentration (0.03 μM), but only for the LTA variable Vel (and not AUC or MaxA). There are important differences between our study and the previous study12 that might explain such a difference. First, our study used LTA, which is considered the gold standard for testing platelet function and widely used for monitoring antiplatelet treatment and is a method also applied in dogs40, 41 whereas whole blood aggregometry and thromboelastography were used in the previous study.12 Second, an injectable pimobendan solution was used in our study for aggregometry, whereas a pimobendan tablet was dissolved in 50% methanol in the previous study.12 In humans, methanol has been found to affect platelet function.42 Third, findings in the previous study12 are based on 10 healthy dogs of different breeds whereas our study focused on CKCS with no or preclinical MMVD because such dogs constitute a relevant patient population for pimobendan treatment. Furthermore, the incubation period of pimobendan was 5 minutes versus 1 minute in our study. In vivo, the exposure of platelets to pimobendan would be much longer than both of these incubation periods. However, the short incubation period was chosen based on recommendations stating that longer incubation at 37°C will decrease aggregation response.43 Finally, the dose considered to reflect clinical treatment concentration of pimobendan in the 2 studies differed. In the previous study,12 0.01 μM (3.09 ng/mL) of pimobendan was considered the mean peak plasma concentration achievable in dogs after clinical dosing protocols (0.25 mg/kg PO) based on the Vetmedin vet package insert. Yet, in our study, a mean peak plasma concentration of 0.03 μM (10 ng/mL) was used to indicate clinical relevance (Boehringer Ingelheim Animal Health, personal communication). Variation in pharmacokinetics of pimobendan in dogs are reported in previous studies with mean peak plasma concentrations ranging from 0.022 μM (7.3 ng/mL) to 0.12 μM (39.4 ng/mL) after PO administration of pimobendan at recommended dosages.44-46 In addition, recent preliminary findings from 57 dogs with MMVD treated with pimobendan at a PO dosage of approximately 0.36 mg/kg indicated a mean peak plasma concentration of 0.095 μM or 31.8 ng/mL (McManamey, Anna K; DeFrancesco, Terese C; Keene, Bruce W et al. 2021. Population Pharmacokinetics of Oral Pimobendan and its Metabolite in Dogs with Myxomatous Mitral Valve Degeneration. 2021 ACVIM Forum Abstract C27). Thus, investigating the effect of pimobendan on platelets at concentrations in the 0.03-0.2 μM range could be relevant.
Higher concentrations (0.1 μM, 1 μM) of pimobendan were evaluated in the previous study,12 but inhibition of platelet aggregation response only occurred at the highest concentration (10 μM). However, on the titration curves from the in-house pilot study, it appears that there may be an effect in some CKCS of pimobendan at even lower concentrations (Figure S1).
In vivo, pimobendan is rapidly metabolized in the liver to the more potent metabolite UDCG 212.24, 46, 47 Investigating only the effect of pimobendan in our study may not accurately reflect the substances to which canine platelets in vivo are exposed. Therefore, investigating the effect of UDCG 212 on platelet aggregation using a mixture of pimobendan and UDCG 212 or, perhaps even more relevant, blood from dogs treated with pimobendan would be relevant and could likely result in different findings than reported in our study.
Research also indicates an in vitro inhibitory effect of pimobendan on platelet aggregation in other species. In humans, platelet aggregation and thromboxane A2 production were inhibited by pimobendan,17, 19 and, in cats, pimobendan also inhibited platelet aggregation.18 Furthermore, in vivo research supports this finding, suggesting an antithrombotic effect of pimobendan in humans.20
Thus, our findings indicate that pimobendan exerts a considerable inhibitory effect on in vitro platelet LTA response at concentrations approximately 100 to 1000 times above clinical treatment concentration in dogs and a mild inhibitory effect on aggregation velocity at a clinical treatment concentration (0.03 μM). Pimobendan is generally well-tolerated in dogs.10, 32 However, in the European Medicine Agency's product summary of pimobendan characteristics it is stated that in very rare cases, signs of effects on primary hemostasis (petechiae on mucous membranes, SC hemorrhages) may be observed during treatment although a relationship with pimobendan has not been clearly established. Pimobendan is recommended for treatment of dogs with ACVIM stage B2, C, and D MMVD.8 In such ACVIM stages, dogs are commonly older and often suffer from comorbidities. Thus, administration of pimobendan in combination with other drugs that have antiplatelet properties or to dogs with hemostatic disorders could raise concern. Thus, the clinical importance of platelet inhibition mediated by pimobendan or the metabolite UDCG 212 and interaction with other drugs deserves further research.
Our study had some limitations. Only CKCS were included, which may limit the translational value to other dog breeds given the fact that CKCS have a different platelet aggregation response compared to other breeds.9, 48 On the other hand, CKCS represents a highly relevant patient population for pimobendan treatment. Different platelet counts in PRP theoretically could result in differences in platelet LTA response. As previously mentioned, PRP was not standardized to a specific platelet count because guidelines no longer recommend doing so.29 Furthermore, platelet count was included in the statistical analyses and did not influence the effect of pimobendan. Also, each dog was used as its own control and only the difference between aggregation response with and without added pimobendan was analyzed. Therefore, any alteration in platelet count would affect each of the aggregation responses of each dog equally and should not have affected the comparison of different pimobendan concentrations.
5 CONCLUSIONS
The PDE5A:E90K polymorphism was present in CKCS with and without preclinical MMVD, but in contrast to our hypothesis, this polymorphism did not appear to be associated with basal LTA response. As hypothesized, pimobendan inhibited in vitro platelet aggregation response at high (10 μM) and to some extent clinical treatment (0.03 μM) concentrations. The inhibitory effect of pimobendan on in vitro platelet aggregation response was not affected by PDE5A:E90K genotype, but a weak negative correlation was found between platelet count in PRP and basal ADP-induced LTA response.
ACKNOWLEDGMENT
The study was supported financially by research grants from the Independent Research Fund Denmark (Project no. 7017-00131B), Thure F and Karin Forsberg's Foundation (Sweden; Project no. 2020-03), University of Copenhagen Foundation for Scientific Studies in Companion Animals (Denmark; Project no. 2020-0002) and Sveland Foundation (Sweden). Preliminary results were presented at the European College of Veterinary Internal Medicine – Companion Animals (ECVIM-CA) 31st Annual Online Congress, 1-4 September 2021. Authors thank Marianne Kjestine Petersen, Silas Alexander Hovmand, and Carl Sichlau Bruun at the Department of Veterinary and Animal Sciences, University of Copenhagen, Denmark, and Nicole DeBruyne at the Department of Clinical Sciences, North Caroline State University, US for excellent technical assistance.
CONFLICT OF INTEREST DECLARATION
Maria J. Reimann's current affiliation is Boehringer Ingelheim Animal Health Nordics. Lisbeth H. Olsen participated in a scientific meeting in 2022 organized by Boehringer Ingelheim where accommodation was included. Joshua Stern serves as Associate Editor for the Journal of Veterinary Internal Medicine. He was not involved in review of this manuscript. No other authors declare a conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the Danish Animal Experiments Inspectorate (license no. 2016-15-0201-01074).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




