Occurrence and clinical relevance of postoperative hypernatremia in dogs undergoing cholecystectomy
Abstract
Background
Patients undergoing cholecystectomy have not been reported previously to develop clinically relevant postoperative hypernatremia.
Objectives
Describe the frequency of postoperative hypernatremia in dogs undergoing cholecystectomy and its clinical relevance (duration of hospitalization and survival).
Animals
Thirty-seven dogs undergoing cholecystectomy at 2 private referral hospitals.
Methods
Retrospective study of dogs undergoing cholecystectomy with available preoperative and postoperative serum sodium concentrations.
Results
Postoperative hypernatremia (>150 mEq/L) was common (56%; 95% confidence interval [CI], 40%-70%) and was associated with significantly higher mortality compared to nonhypernatremic patients (52%; 95% CI, 30%-70% vs 12.5%; 95% CI, 2%-40%; P = .02). Nonsurvivors had higher mean postoperative peak serum sodium concentrations (155 mEq/L; range, 146-172) than survivors (150 mEq/L; range, 142-156; P = .01). Dogs developing hypernatremia within 6 hours after surgery had 7.7 higher odds of nonsurvival (odds ratio [OR], 7.7; 95% CI, 5.9-9.4). A delta value (serum sodium concentration on admission [T0] − serum sodium concentration 6 hours postoperatively [T2]) of ≥10 mEq/L carried 3.3 higher odds of mortality (OR, 3.3; 95% CI, 1.6-5.1). All dogs with a postoperative peak sodium concentration >160 mEq/L did not survive.
Admission acute patient physiologic laboratory evaluation fast (APPLEfast) scores were not different between survivors and nonsurvivors or between postoperative hypernatremic and normonatremic patients. Hospitalization time was no different between hypernatremic and normonatremic patients (6 days vs 4.5 days; P = .15). Dogs with gallbladder mucocele were more likely to develop postoperative hypernatremia and have poorer outcomes.
Conclusions
Hypernatremia was a common and clinically relevant postoperative complication in dogs after cholecystectomy. Detection of hypernatremia within 6 hours after surgery may be associated with poorer outcomes.
Abbreviations
-
- APPLEfast
-
- acute patient physiological and laboratory evaluation
-
- NaCl
-
- sodium chloride
1 INTRODUCTION
Cholecystectomy is the treatment of choice for several gallbladder diseases including biliary mucocele, gallbladder neoplasia, gallbladder rupture, and, in some cases, cholelithiasis.1 This procedure is commonly performed by laparotomy or, less frequently, laparoscopy. However, concerns regarding postoperative complications and high mortality persist.
The high morbidity and mortality rates associated with surgery of the extrahepatic biliary tract are likely related to the complexity of the surgical approach and the pathophysiological consequences of hepatobiliary disease.2 Procedures involving biliary diversion have been reported to have higher mortality and complication rates than other biliary surgeries.3
Reported mortality rates of dogs undergoing cholecystectomy vary from 6% to 33.3%, with elective procedures carrying a better prognosis.4-6
Specific postoperative complications of dogs undergoing cholecystectomy have not been extensively investigated, but a recent retrospective study indicated a wide array of consequences ranging from mild to severe.5 Mild complications included pyrexia, gastrointestinal signs (eg, regurgitation, vomiting, diarrhea), and hypertension, and moderate to severe complications included hypoglycemia, azotemia, hypotension, seizures, aspiration pneumonia, thrombocytopenia, and bile peritonitis. Moderate to severe complications were observed more frequently in patients undergoing emergency cholecystectomy (59%) than in patients undergoing an elective procedure (35%).5
To the best of our knowledge, the only mention of hypernatremia in dogs with gallbladder disease in previously published veterinary literature is found in a recent retrospective study investigating the long-term survival of dogs treated for gallbladder mucocele.7 This study identified hypernatremia in 16.2% of cases, but the time of this finding was not specified, and it was not found to be associated with patient survival. At our institutions, hypernatremia is thought to be a frequent occurrence and an important postoperative diagnostic and therapeutic challenge, and anecdotally associated with worse outcomes. No other studies have reported hypernatremia as a clinicopathologic finding associated with gallbladder disease in dogs.13, 14
In human medicine, abnormalities of serum sodium concentration often are reported in patients who suffer from severe biliary disease, regardless of whether they require medical or surgical (cholecystectomy) treatment. Development of hyponatremia in this population is likely secondary to the syndrome of inappropriate antidiuretic hormone secretion (SIADH), whereas hypernatremia does not appear to be of concern in human patients.8-10
Our retrospective study aims to (1) describe the frequency of postoperative hypernatremia in dogs presented with gallbladder disease that underwent cholecystectomy, and (2) investigate potential relationships among hypernatremia, duration of hospitalization, and survival to discharge.
2 MATERIALS AND METHODS
A search of the electronic medical records of 2 private referral hospitals was performed, identifying any patient that had undergone cholecystectomy for diagnosed gallbladder disease between March 2016 and May 2022.
Gallbladder diseases include gallbladder mucocele, cholelithiasis, cholecystitis, cholangitis, common bile duct rupture, traumatic gallbladder rupture, or other causes of biliary obstruction.
Dogs undergoing cholecystectomy, either electively or as an emergency, were included. Dogs were excluded if cholecystectomy was performed at a different institution than that of the authors, if insufficient medical records were available for review, or if IV fluid treatment with a solution containing >145 mEq/L of sodium had been administered, including normal saline (0.9% NaCl), hypertonic crystalloid solutions, or sodium bicarbonate.
Water deprivation was not recommended in preparation for surgery and preoperative fasting protocol followed the 2020 American Animal Hospital Association (AAHA) Anesthesia and Monitoring Guidelines for Dogs and Cats.11
Because of the retrospective nature of the study, care was not standardized. Treatment decisions were made by the attending veterinarian who was either an emergency and critical care (ECC) diplomate or a veterinarian in training working under the supervision of an ECC diplomate. As a standard of care at our institutions, all dogs received balanced isotonic crystalloids (Hartmann's solution), opiate analgesia, and early enteral nutrition or, if enteral nutrition was not tolerated, total parenteral nutrition. Administration of other analgesics to provide multimodal analgesia, antiemetics, gastroprotectants, pro-kinetics, and hepatic support supplements was dependent on the clinician's judgment and the patient's clinical status and progress. Data collected for each patient included signalment (age, sex, reproductive status, and breed), physical examination findings, and blood analysis including packed cell volume, total protein concentration, serum biochemistry, venous blood gas analysis, and serum electrolyte, blood glucose, and lactate concentrations. Preoperative abdominal ultrasound findings also were reviewed and used as the main diagnostic tool for confirming the presence of gallbladder disease.
Preoperative and postoperative serum sodium concentrations were evaluated. Time points included serum sodium concentration at admission (T0), immediately before surgery (T1), and postoperatively (T2-T5), with T2 being the first result obtained postsurgery (within 6 hours postoperatively) and T5 the last sample obtained before discharge or death. A delta value, defined as the difference in serum sodium concentration between T0 and T2, was calculated for each patient. The T2 result was chosen over the other postoperative results because it was the most consistent result available in 100% of patients within a reliable timeline (ie, within 6 hours after surgery).
Because of the retrospective nature of the study, no standardized time frame for repeated venous blood gas analysis was followed, and as many as 4 analyses were included for each patient postoperatively. Serum sodium concentration was measured using either an ABL800 FLEX blood gas analyzer (ABL800 FLEX blood gas analyzer, Radiometer Limited, UK; serum sodium concentration reference interval, 139-150 mEq/L) or EPOC Veterinary Blood Gas, Electrolyte and Critical Care Analyzer (EPOC Veterinary Blood Gas, Electrolyte and Critical Care Analyzer, Woodley Equipment Company Ltd, UK; serum sodium concentration reference interval, 140-150 mEq/L). The same analyzer was used at all time points for individual patients. Postoperative hypernatremia was defined as a serum sodium concentration > 150 mEq/L at T2, T3, T4, or T5.
Acute patient physiological and laboratory evaluation scores12 on admission were retrospectively calculated based on clinical notes in the medical record and laboratory results.
The nature of surgical intervention characterized as emergency surgery or elective surgery, and the perioperative medical strategies including administration of opioid analgesia, pro-kinetics, antiemetics, and gastroprotectants were reviewed. Additional variables collected included duration of hospitalization, tolerance of enteral nutrition (clinically assessed as the ability of patients to receive enteral nutrition through a feeding tube or to eat voluntarily without regurgitation or vomiting), development of gastrointestinal signs (regurgitation, vomiting, diarrhea) and outcomes (euthanasia, in-hospital cardiopulmonary arrest, survival to discharge). A negative outcome was defined as nonsurvival to discharge because of either natural death or euthanasia.
As a secondary analysis, the frequency of hypernatremia and its impact on outcome was compared between dogs presented with gallbladder mucocele and those presented with other gallbladder diseases. This additional analysis was carried out because of the high number of dogs presented with gallbladder mucocele within the recruited population.
2.1 Statistical methods
Continuous data (age in years, body weight, serum biochemistry results, hospitalization time) were recorded and values for median and range were calculated for each variable. Categorical data (including clinical presentation, APPLEfast scores, urgency of surgical treatment, implemented medical treatments, frequency of regurgitation, outcomes, and reasons for euthanasia) were anonymized and recorded, with each category presented descriptively.
Because of the small size of our population and the nonnormal distribution of most of the data, nonparametric 2-tailed Mann-Whitney U tests were performed to compare serum sodium concentrations and hospitalization times. Relationships between continuous and noncontinuous variables were investigated by Chi-squared tests.
Odds of mortality were calculated by Fisher Exact test with confidence interval (CI) set at 95%.
All statistical analyses were performed by freely available online software (https://www.socscistatistics.com). Results were considered significant if P < .05.
3 RESULTS
One hundred cases were identified, of which 63 were excluded because of either incomplete medical records with insufficient preoperative or postoperative serum sodium concentrations or because cholecystectomy was performed at an institution other than that of the authors. Ultimately, 37 dogs met the inclusion criteria and were included.
Of these, 17 out of 37 (46%) were Border Terriers and they all were presented with gallbladder mucocele. The remaining 20/37 dogs (54%) were of other breeds including 4 crossbreed dogs, 3 Border Collies, 2 Pomeranians, 3 Beagles, 2 Shetland Sheepdogs, and 1 each of Dandie Dinmont Terrier, Cavalier King Charles Spaniel, Labrador Retriever, Cairn Terrier, Bichon Frise, and Yorkshire Terrier. These patients underwent cholecystectomy for management of gallbladder mucocele in 24 of 37 cases (64.9%), and otherwise, patients were presented with a range of different gallbladder diseases, including cholecystitis (5 of 37, 13.5%), ruptured gall bladder (3 of 37, 8.1%), cholelithiasis (2 of 37, 5.4%), gall bladder mass (1 of 37, 2.7%), extrahepatic common bile duct obstruction secondary to a mucous plug (1 of 37, 2.7%), and extrahepatic common bile duct rupture (1 of 37, 2.7%).
Median age was 11 years (range, 2-17 years) and median weight was 10.9 kg (range, 2.5-30.5 kg).
Overall, 21/37 (56%; 95% CI, 40%-70%) patients developed postoperative hypernatremia. The median duration of hospitalization for the population was 6 days (range, 3-10 days). No statistical difference in hospitalization times was found between hypernatremic dogs (6 days; range, 3-10 days) and dogs that never became hypernatremic (4.5 days; range, 3-9 days; P = .15; Figure 1).

The mortality rate was 35.1% (95% CI, 20%-50%), with hypernatremic patients having significantly higher mortality rate (11/21; 52%; 95% CI, 40%-70%) than nonhypernatremic patients (2/16; 12.5%; 95% CI, 2%-40%; P = .02).
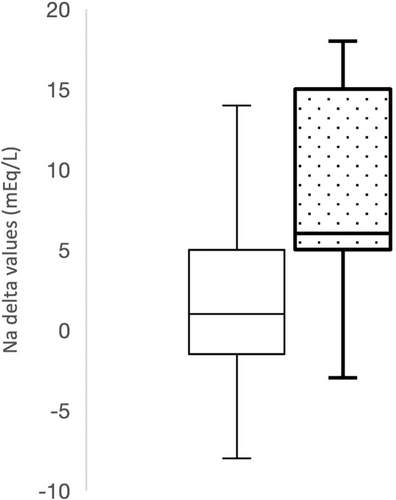
The difference in serum sodium concentration between T0 and T2 (defined as T0-T2 delta) also was considered, and a significant difference was found between delta values of survivors (1 mEq/L; range, −8 to 17 mEq/L) and nonsurvivors (6 mEq/L; range, −3 to 18 mEq/L; P = .04; Figure 2). Furthermore, dogs with delta values of ≥10 mEq/L had 3.3 times higher odds of poor outcome (OR, 3.3; 95% CI, 1.6-5.1). Additionally, dogs that were hypernatremic (>150 mEq/L) at T2 had 7.7 times higher odds of dying than dogs that were not hypernatremic (≤150 mEq/L) at T2 (OR, 7.7; 95% CI, 5.9-9.4).
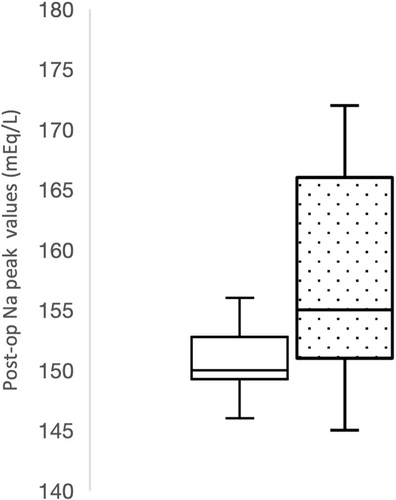
Postoperative peak serum sodium concentrations were also significantly higher in nonsurvivors compared to survivors (P = .01) with a median peak serum sodium concentration of 155 mEq/L (range, 146-172 mEq/L) in nonsurvivors compared to a median of 150 mEq/L (range, 142-156 mEq/L) in survivors (Figure 3). Additionally, all dogs with a postoperative peak sodium concentration >160 mEq/L (n = 6) did not survive.
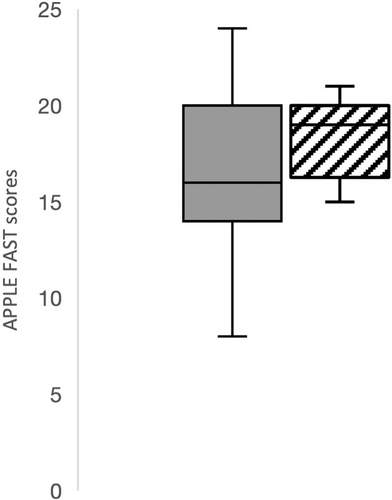
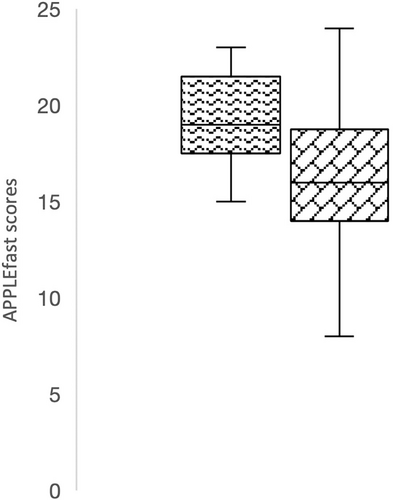
The APPLEfast scores on admission were not significantly different between patients that developed postoperative hypernatremia compared to those that did not (P = .14; Figure 4). Similarly, APPLEfast scores on admission were not significantly different (P = .07) between survivors (mean, 19; range, 8-30) and nonsurvivors (mean, 16; range, 10-24; Figure 5).
Postoperative frequency of gastrointestinal signs also was investigated. No dogs were reported to vomit or suffer from clinically relevant diarrhea postoperatively that required additional treatment (eg, adjustments of fluid treatment) after surgery. Regurgitation or intolerance to enteral feeding occurred in 16 of 37 (43.2%) patients. Of these, only 1 required administration of total parenteral nutrition, whereas the others were managed using alternative enteral feeding protocols, including intermittent food boluses or continuous feeding via nasogastric tubes (starting at a lower percentage of resting energy requirements with increasing volumes over time). No significant correlation was found between patients developing postoperative regurgitation and the frequency of hypernatremia (P = .09).
When the frequency of postoperative hypernatremia (>150 mEq/L) was compared between dogs that were presented with gallbladder mucocele (18 of 24; 75%) and those with other gallbladder diseases (3 of 13; 23%), hypernatremia was found to be significantly more frequent in dogs with gallbladder mucocele (P = .004). The mortality rate in dogs presented with gallbladder mucocele was significantly higher than in dogs presented with other gallbladder diseases (P = .01). Specifically, 12 of 24 (50%) dogs with gallbladder mucocele died. Of these, 10 of 12 (83%) were euthanized and 2 of 12 (17%) suffered cardiopulmonary arrest; only 1 of 13 (7%) dogs with another gallbladder disease died by euthanasia (Figure 6).

According to the clinical records available for the patient population, the decision to euthanize consistently was based on clinical deterioration rather than financial considerations.
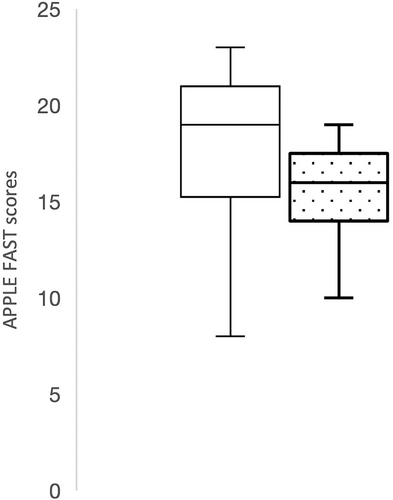
The APPLEfast scores at admission were found to be significantly lower in dogs with gallbladder mucoceles (median, 16; range, 8-24) than dogs presented with other gallbladder diseases (median, 19; range, 15-30; P = .008; Figure 7).
4 DISCUSSION
We observed a high frequency of postoperative hypernatremia in dogs undergoing cholecystectomy. In our population, 56% of dogs developed this electrolyte disturbance. Furthermore, hypernatremia was found to be associated with a significantly higher mortality rate.
To the best of our knowledge, the only mention of hypernatremia in dogs with gallbladder disease in previously published veterinary literature was found in a recent retrospective study investigating the long-term survival of dogs treated for gallbladder mucocele, either medically or surgically.7 This study identified hypernatremia in only 16.2% of cases, however, the timing of this finding was not specified, and it was not found to be relevant to patient survival. Other studies have evaluated clinicopathological abnormalities in dogs presented with gallbladder disease, but none have reported hypernatremia as a relevant finding. A previous study that investigated several clinicopathological variables in dogs with gallbladder mucocele found no significant abnormalities in serum sodium concentrations.13 However, these results were obtained within the first 72 hours of gallbladder mucocele diagnosis and no specific information regarding postcholecystectomy variables was presented. In another study, pertaining to gallbladder disease in Shetland Sheepdogs, mean serum sodium concentrations were reported to be within normal limits and not different between survivors and nonsurvivors.14 Again, these results were obtained on admission and were not followed up after surgical intervention.
Sodium disorders have long been recognized as common and clinically relevant electrolyte abnormalities in critically ill patients both in human and veterinary medicine.15, 16 Specifically, the prevalence of hypernatremia is reported to be between 6% and 26% in human intensive care unit patients17 and 5.7% in critically ill dogs.16 Furthermore, even if mild, increases in serum sodium concentrations have been associated with increased fatality rates, with hypernatremic dogs showing a 5.7 odds ratio of nonsurvival compared to those with serum sodium concentrations within the reference interval.16 Interestingly, a previous study found that 17.9% of hospitalized hypernatremic dogs had hepatobiliary disease recorded as their primary disease process, but the authors did not specify the nature of the hepatobiliary disorder and whether or not these patients underwent cholecystectomy.16
The nature of the link between hypernatremia and poor outcomes is still unclear, and it is likely multifactorial. Hypernatremia could be considered simply a marker of disease severity, but the hyperosmolar state that ensues, especially with moderately to severely increased serum sodium concentrations, has marked effects on cellular functions which could contribute to metabolic, neurological, and cardiovascular complications.18
From a pathophysiological point of view, hypernatremia reflects a decrease in the amount of total body water because of loss of hypotonic fluid, or may occur secondary to an increase in the amount of total body sodium. In critically ill patients, solute gain is uncommon unless iatrogenic in nature with the administration of sodium-rich IV fluid products. The most common reason for hypernatremia in hospitalized patients is hypotonic fluid losses (eg, excessive gastrointestinal losses, renal losses) or free water loss (eg, central or nephrogenic diabetes insipidus, fever, hyperthermia, primary hypodipsia).18 A previous study identified gastrointestinal fluid losses as the most common underlying cause of hypernatremia in critically ill dogs, followed by central diabetes insipidus.16
Because of the retrospective nature of our study, it was not possible to accurately determine the cause of the postoperative hypernatremia in our patients. Gastrointestinal losses via regurgitation, vomiting, or diarrhea were potential causes explored, but no significant correlation was found between the development of postoperative regurgitation and the frequency of hypernatremia. Third space losses, such as those occurring in peritonitis, were not recorded in the dogs included in our study. Alternatively, we also considered that normovolemic hypernatremia might have developed during the postoperative period. Acquired central and nephrogenic diabetes insipidus have been reported previously in humans with inflammatory diseases causing either decreased production or peripheral resistance to vasopressin19 and a similar mechanism might be involved postsurgically in dogs. Future prospective studies are needed to investigate this hypothesis further.
The significantly higher odds of nonsurvival in hypernatremic dogs do not seem to be related to a more severe initial clinical presentation, because APPLEfast scores at admission were not found to be significantly different between patients that developed postoperative hypernatremia and those that did not. The APPLEfast scores also were not significantly different between survivors and nonsurvivors.
Interestingly, but not surprisingly, a delta value ≥10 mEq/L was strongly associated with higher likelihood of poor outcome, even in the patients that did develop hypernatremia. This observation might be explained by the clinical relevance and severity of clinical signs associated with rapid changes in serum sodium concentrations.18
As a secondary analysis, we found postoperative hypernatremia to be most common in patients undergoing cholecystectomy for the management of gallbladder mucocele.
Previously reported mortality rates for dogs undergoing cholecystectomy are between 7% and 40%, which is similar to our overall mortality rate of 35.1% in our study. No association between mortality rate and reason for cholecystectomy (eg, gallbladder mucocele compared to other gallbladder diseases) has been reported.3, 4, 20 On the contrary, our study found a much higher mortality rate in dogs undergoing cholecystectomy for gallbladder mucocele (50%) compared to other gallbladder diseases (7%; P = .01).
Severity of the initial clinical presentation also did not impact mortality rates in our population, as it was found that dogs presented with gallbladder mucocele had significantly lower APPLEfast scores compared to those presented with other gallbladder diseases.
Given all of our findings, we suspect that the poorer outcome found in patients with gallbladder mucocele might be related to their tendency to develop postoperative hypernatremia. However, we recognize that the strength of this conclusion is limited by the higher number of cases affected by gallbladder mucocele in the recruited population.
Our study had some limitations, mainly related to its retrospective nature. First, the patients' treatment plans were not standardized or blinded and although the 2 institutions followed similar clinical approaches regarding preoperative and postoperative supportive care (eg, IV fluid treatment choices, antiemetic administration, implementation of multimodal analgesia, early enteral or parenteral feeding), variability in clinician preferences could not be avoided. Lack of consistency in the recorded data in terms of daily water intake, urine output, and urinalysis prohibited the formulation of any plausible hypothesis regarding the pathophysiology of the hypernatremia noted in the population. It is also possible that our findings might not be representative of a wider population because our study was conducted in referral institutions, and inclusion may have been biased towards more critically ill patients.
Finally, given the retrospective nature of our study, there was no control on the timing and number of blood samples taken, and it is plausible that clinically worse patients had more frequent assessments of serum sodium concentration compared to patients that did not have a complicated postoperative period. This factor potentially would have led to missed hypernatremic episodes in patients with less problematic postoperative periods.
Ultimately, our study can only establish an association between cholecystectomy and the development of postoperative hypernatremia, which seems to have a higher frequency in dogs with gallbladder mucocele. However, a causative relationship supported by a plausible pathophysiological mechanism is difficult to identify with a retrospective study design.
In conclusion, we found that postoperative hypernatremia was common in dogs undergoing cholecystectomy, occurred most frequently in patients presented for management of gallbladder mucocele, and was associated with euthanasia or death in this population.
Because the development of postoperative hypernatremia seems to be a potential negative prognostic factor, it is a variable clinicians should monitor closely during the first 6 hours (T2) after cholecystectomy. Patients presented with gallbladder mucocele also might have a higher likelihood of developing this electrolyte abnormality and consequently might have a worse prognosis. Additional prospective studies are needed to confirm these findings and investigate the possible underlying pathophysiological mechanisms for the development of postoperative hypernatremia in this subset of patients.
ACKNOWLEDGMENT
Funding provided by IVC Evidensia Research Fund. The authors thank Dr. Pauline Jamieson and Dr. Aimee Hope for their help with statistical analysis and proofreading. The authors are also grateful to IVC Evidensia which generously covered the article publication charge for this paper.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




