Effects of trazodone administration on the neurologic examination in healthy dogs
Funding information: University of Wisconsin - Madison, School of Veterinary Medicine, Grant/Award Number: Start-up Funds
Abstract
Background
Trazodone is an anxiolytic used PO to decrease anxiety in dogs. Whether or not trazodone affects the neurologic examination in dogs has not been previously reported.
Objective
Investigate whether trazodone administration is associated with changes in the neurologic examination in healthy dogs.
Animals
Thirty-two healthy dogs between 1 and 6 years old with no previously diagnosed medical conditions and perceived by their owners as neurologically normal.
Methods
Baseline sedation and anxiety assessments and neurologic examination were performed on each dog, followed by trazodone administration (6.25-8.60 mg/kg PO). The sedation and anxiety assessments and neurologic examination were repeated 2.5 hours after trazodone administration. The examinations were performed by a single board-certified veterinary neurologist and were video-recorded. The videos were randomized and reviewed by a different neurologist, blinded to the previous evaluations, who scored the examinations.
Results
Seven of 32 (22%) dogs had worse scores on their neurologic examination after receiving trazodone, manifesting as new or progressive PR deficits. Although not clinically relevant, 18.7% of the dogs had consciousness levels that changed from bright, alert, responsive to quiet, alert, responsive after trazodone administration. No other changes were observed on neurologic examination. Sedation and anxiety scores were significantly different after trazodone administration compared to before (P < .001 and P < .001, respectively).
Conclusions and Clinical Importance
Most dogs did not have changes on neurologic examination after trazodone administration. However, approximately 20% of dogs had new or worsening PR deficits after receiving trazodone. Ideally, trazodone should not be given before neurologic examination in dogs.
Abbreviations
-
- BAR
-
- bright, alert and responsive
-
- PR
-
- postural reaction
-
- QAR
-
- quiet, alert and responsive
1 INTRODUCTION
A precise neurological examination is a crucial step in the diagnostic approach to patients with neurological problems. Accurate neurolocalization is essential for the development of a list of differential diagnoses, as well as for determining the correct area of the nervous system to be evaluated by further diagnostic testing. Abnormalities, or deficits, identified by the neurologic examination are used to determine if a patient has neurologic disease and, if present, to accurately localize the lesion.
For many dogs, the veterinary hospital setting induces stress and anxiety,1, 2 which can increase the risk of infection3-5 and prolong wound healing.6 To mitigate these adverse reactions and minimize patient distress, PO anxiolytics and sedatives are increasingly used in dogs during veterinary visits or hospitalizations.7, 8
Although numerous pharmaceutical agents used as anxiolytics have the potential to induce sedation, trazodone is an attractive initial agent because of its wide safety margin, low risk of adverse effects, and length of activity.9-11 Trazodone is a PO anxiolytic frequently prescribed by veterinarians to decrease situational anxiety in dogs9 and can be administered in-hospital as well as at home, with increasing usage before examinations with the advent of fear-free practice. Trazodone is classified as a serotonin 2A antagonist and receptor inhibitor (SARI) and functions by potentiating the activity of serotonin within the synapse by preventing its reuptake.12 In previous studies, trazodone administration resulted in fewer anxious behaviors and signs of distress in hospitalized dogs10 and decreased the level of anxiety in dogs diagnosed with anxiety disorders.9 Sedation after trazodone administration in dogs has been shown as a consistent adverse effect of the medication when administered PO.9, 13
The central nervous system is the target organ for anxiolytic medications,14, 15 but it is not currently known how the neurologic examination is affected by this class of medications and, if so, to what extent. To our knowledge, no previous studies have been published that evaluate the effects of trazodone on the neurologic examination in dogs. Therefore, our purpose was to investigate whether trazodone causes detectable changes in the neurologic examination in dogs considered neurologically normal by their owners. We hypothesized that trazodone administration would lead to a change (decrease) in the level of consciousness as well as mild changes in gait and proprioceptive deficits of the limbs.
2 MATERIALS AND METHODS
Our study used a prospective, masked, clinical cross-over design. The protocol was approved by the Institutional Animal Care and Use Committee at the University of Wisconsin - Madison, School of Veterinary Medicine. Written informed consent was obtained from the dog owners before enrollment in the study.
2.1 Animals
Dogs owned by veterinarians, veterinary technicians, assistants, and veterinary students were recruited for the study. Dogs were eligible for enrollment if they met the following inclusion criteria: (a) were between the ages of 1 and 6 years, (b) had never been diagnosed with a chronic medical condition, (c) were perceived to be healthy and neurologically normal by their owners, and (d) were not receiving any medications. No laboratory diagnostic testing was required for participation. Dogs were excluded from the study if they were aggressive or resistant to restraint.
2.2 Study design
Before each comprehensive neurologic examination, the levels of anxiety and sedation of all dogs were assessed. This process was utilized for both the pre- and post-trazodone assessments. The Grint Sedation Scale16 (as previously validated17) and the Lincoln Canine Anxiety Scale (as previously validated18) were used for sedation and anxiety assessment, respectively. Immediately after these assessments, each dog was given a single standardized dose of trazodone (between 6.25 and 8.60 mg/kg) PO. Dogs were not fasted before presentation. Within 105 to 195 minutes after administration of trazodone, anxiety and sedation scales and neurologic examinations were repeated. All anxiety and sedation scoring was performed by the same clinician (LL), and all neurologic examinations were performed by the same single board-certified veterinary neurologist (SC). All neurologic examinations, pre- and post-trazodone administration, were recorded by video by the same investigator (LL) using a standard iPhone 11 video camera. The scores from each dog's pre- and post-trazodone anxiety and sedation scales were compared to confirm the efficacy of trazodone.13, 19, 20 All study participants were kept in a consistent environment throughout the duration of the study to minimize the impact of variations in environment. Consistently across each neurologic examination per dog, background noise and the presence of uninvolved individuals were both kept at a minimum. Owners were not present for the examinations aside from 1 dog, the owner of which was the neurologist performing the examination. In between neurologic examinations, each dog remained in the same cage it had been placed in before the first examination.
After all videos of the neurologic examinations had been recorded, the video files were randomized and given a numerical code before being blindly reviewed and scored by a second veterinary neurologist (NZ). The time and date stamps of each video were removed before providing the videos to this blinded clinician. For all videos except 1, the sound was muted when reviewed; for 1 dog, the before and after videos were specifically requested to be viewed with audio, because this component was important for interpretation. Each dog served as its own control throughout our comparisons between pre- and post-trazodone neurologic examination.
2.3 Neurologic examination
The order of the neurologic examination performed was as follows for each dog: mentation, cranial nerves, postural reactions (PR), reflexes, palpation, range of motion, and gait analysis. The videos were scored by the masked reviewer (NZ) using the preassigned numerical values per category (Table 1).
| Component of neurologic examination | Numerical score per exam component (maximum score: 39) | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| Mentation | BAR (0a) QAR (0b) | Obtunded | Stuporous | Comatose | |
| Cranial nerves | Normal (all) | Abnormal (any) | |||
| Postural reactions (1 score per limb) | Normal | Mildly delayed | Moderately delayed | Absent | |
| Gait | Ataxiaa | Normal | Mild | Moderate | |
|---|---|---|---|---|---|
| Paresis | Normal | Mild | Moderate | ||
| Spinal reflexes | Withdrawal (1 score per limb) | Normal | Reduced | Absent | |
| Patellar (1 score per limb) | Normal | Reduced | Exaggerated | Absent | |
| Perineal | Present | Absent | |||
| Cutaneous trunci | Present | Absent |
| Spinal palpation | Normal | Abnormal | |||
|---|---|---|---|---|---|
| Range of motion | Tail | Normal | Abnormal | ||
| Cervical | Normal | Abnormal | |||
- Note: Each category was tested and interpreted in identical sequence across all examinations. A total score of 0 would be considered a normal neurologic examination. The scores for each category were summated to yield an overall score for each neurologic examination (pre- and post-trazodone).
- a The masked clinician was instructed to characterize any ataxia appreciated as either proprioceptive, cerebellar, or vestibular.
2.4 Statistical analysis
For our study population, findings were summarized as mean and SD for normally distributed data (time between examinations) and median with range for non-normally distributed data (age, body weight, dosage). The results of each dog's pre- and post-trazodone sedation and anxiety scores were compared using a 2-tailed paired t test to determine efficacy and level of effect of trazodone. A statistical analysis program (GraphPad Prism) was used for calculations. A P-value of <.05 was considered significant for all tests.
3 RESULTS
3.1 Study population
The study included 32 dogs (16 castrated male dogs, 15 spayed female dogs, and 1 intact female dog). The median age was 3 years (range, 1-6 years) and the median body weight was 22.85 kg (range, 4.0-37.7 kg). The mean time between administration of trazodone and performance of the second neurologic examination was 147 ± 28.9 minutes (2 hours, 27 minutes). The median dosage of trazodone administered was 7.60 mg/kg (range, 6.25-8.60 mg/kg). One dog was excluded from the study because of aggressive behavior elicited by attempts at restraint and neurological examination was not possible.
Breeds included Australian Shepherd, Beagle/Basset Hound Cross, Border Collie, Brittany Spaniel, Cattle Dog Mix, Chihuahua, Collie, Dachshund Mix, French Bulldog (n = 2), German Shorthair Pointer, Golden Retriever (n = 4), Irish Setter, Labrador/Golden Cross, Labrador Mix (n = 2), Labrador Retriever (n = 3), Miniature Poodle Mix, Mixed Breed, Pembroke Welsh Corgi (n = 2), Pit bull Terrier, Pointer Mix, Portuguese Water Dog, Pug, Shepherd Mix, Toy Australian Shepherd. Each dog breed represented 1 dog of that breed, unless otherwise specified.
3.2 Anxiety and sedation scales
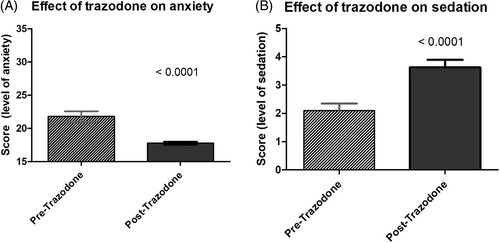
The level of sedation was significantly higher post-trazodone compared to pre-trazodone (P < .0001; Figure 1). The level of anxiety was also significantly lower post-trazodone compared to pre-trazodone (P < .0001; Figure 1a,b).
3.3 Neurologic examination
Eleven of 32 dogs had at least 1 neurologic abnormality identified on initial (pre-trazodone) neurological examination: 7 dogs had delayed PR deficits involving at least 1 limb, 1 dog had moderate cervical pain with delayed PR deficits of all 4 limbs, 2 dogs had decreased withdrawal reflexes bilaterally, 1 dog had an absent patellar reflex unilaterally, and 1 dog was obtunded. No cranial nerve abnormalities were identified on either the pre- or the post-trazodone neurologic examinations. One dog responded painfully to spinal palpation with no change in severity or location detected between pre- versus post-trazodone examination. One dog had mild ataxia post-trazodone. Two dogs had mildly reduced withdrawal reflexes on 1 hind limb in the pre-trazodone assessment, which were normal in the post-trazodone assessment.
3.3.1 Mentation
Mentation changes were noted in 9 of 32 dogs (28%) when comparing their pre- versus post-trazodone examinations (Table 2). None of the neurologic examination scores for any dog were altered by the changed mentation states on the post-trazodone examination. Twenty-four dogs were scored as bright, alert, and responsive (BAR) in the pre-trazodone examination, and 18 (75%) of these dogs remained BAR on the post-trazodone examination. The remaining 6 dogs (25%) were scored as quiet, alert and responsive (QAR) on the post-trazodone examination. Seven dogs (21%) were scored as QAR in the pre-trazodone examination. Of these, 4 (57%) remained QAR whereas 3 (43%) were scored as BAR in the post-trazodone examination. One dog (14%) was scored as obtunded in both the pre- and post-trazodone assessments.
| Mentation level noted on pre- and post-trazodone neurologic examinations | Number of dogs | Change in neurologic exam score post-trazodone |
|---|---|---|
| BAR → BAR | 18 | 0 |
| BAR → QAR | 6 | 0 |
| QAR → QAR | 4 | 0 |
| QAR → BAR | 3 | 0 |
| Obtunded → obtunded | 1 | 0 |
| Total dogs | 32 |
- Note: Despite changes noted in several dogs amongst bright, alert and responsive (BAR) and quiet, alert and responsive (QAR) levels, the score for level of consciousness (mentation) did not change for any case. Each arrow symbol (→) represents the transition made from the pre-trazodone mentation status (preceding the arrow) to the post-trazodone mentation status (following the arrow).
3.3.2 Postural reactions
Proprioception was assessed in all dogs by way of the paw replacement test, and results are shown in Table 3. Because of patient temperament or inconsistent results on paw replacement, several dogs required additional testing of proprioception using the hopping response. Seven dogs (22%) developed new or worsening PR deficits on the post-trazodone assessment. Six of those dogs had PR deficits post-trazodone in limbs that were initially normal; 1 dog had initially mild PR deficits in all limbs that progressed to moderate deficits post-trazodone. Seven of 32 dogs (22%) showed PR deficits on the pre-trazodone assessment. Of those, 3 dogs experienced normalization (improvement/resolution) of those initial delays on their post-trazodone assessment. Another 3 dogs had PR deficits that were unchanged in the pre- and post-trazodone examinations, and 1 dog had a mild unilateral PR deficit in one hind limb pre-trazodone but mild PR deficits in both hind limbs on the post-trazodone assessment. The latter dog therefore also was included with the 6 dogs with new PR deficits.
| Changes in postural reactions (PR) observed following trazodone administration | |||||
|---|---|---|---|---|---|
| New PR Deficits | Progressive PR Deficits | Normalized PR | Normal PR-Static | Abnormal PR-Static | |
| Number of dogs | 6 | 1 | 3 | 21 | 3 |
| Ratio of dogs affected | 6/32 | 1/32 | 3/32 | 21/32 | 3/32 |
| Percentage of total dogs | 18.8% | 3.1% | 9.4% | 65.6% | 9.4% |
- Note: Normal PR-Static indicates the dogs who had consistent normal PRs across both assessments, while Abnormal PR-Static indicates the dogs who had consistently abnormal PRs across both assessments. Progressive PR includes dogs who had PR deficits noted on the pre-trazodone assessment that worsened post-trazodone. New PR Deficits includes dogs who had normal PRs pre-trazodone but abnormal PRs post-trazodone, in one or more limbs. Percentages do not summate to 100% because some dogs were represented more than once for variations across their limbs.
3.3.3 Neurologically normal dogs
Of the 31 dogs that participated in the study, 22 had completely normal neurologic examinations before administering trazodone (71%). Four of these 22 dogs (18%) had changes on post-trazodone neurologic examination, all of which manifested new PR deficits. Two of these dogs developed mild PR deficits in a single limb. One dog developed a mild PR deficit in 2 limbs, and 1 dog developed moderate PR deficits in 2 limbs. Additionally, 3 of these 22 dogs were initially QAR but became BAR post-trazodone; 2 were initially BAR but became QAR post-trazodone.
4 DISCUSSION
Our findings indicate that most dogs do not experience clinically relevant changes or progression of abnormalities in neurologic examinations after PO trazodone administration. However, 20% of dogs did exhibit alterations in their neurologic examinations, but these changes were mild. Of the dogs that were neurologically normal before trazodone and developed neurologic deficits post-trazodone, the changes were limited only to new PR deficits affecting 1 or 2 limbs. The clinically relevant changes identified were in the form of new or worsened PR deficits when considering the entire sample collectively. Overall, these changes were mild in nature and, in most cases, involved only 1 limb. Nonclinically relevant changes in mentation were appreciated in <20% of dogs.
Seven dogs (of 32) in the study displayed new or worsened paw replacement deficits after trazodone administration. This change is perhaps explained by trazodone's cortical effects. Given the involvement of the cerebral cortex in the pathway for conscious proprioception,21 it is possible that the effects of trazodone on the telencephalon precipitated the PR deficits newly identified on the post-trazodone examinations.
After PO trazodone administration, 18.8% of the dogs experienced change in attitude from BAR to QAR, remaining alert and responsive. This finding is consistent with previous studies showing a decrease in overall level of excitation and increased calmness.6, 13, 22 No mentation changes occurred that altered the neurologic examination score because none involved progression from a state of alert and responsive to that of obtundation, stupor, or comatose. The 3 dogs that were scored QAR on the pre-trazodone examination and BAR on the post-trazodone examination emphasize the subjectivity of this specific assessment. Alternatively, these dogs may have relaxed and become more comfortable in their surroundings once the anxiolytic properties of trazodone had taken full effect. This interpretation would explain the subsequent increase in fear-free behaviors appreciated in each of these dogs, because all 3 dogs experienced decreases in their anxiety scores after trazodone administration. Entering a veterinary hospital has been shown to induce stress in dogs,1 with more stress being experienced in the examination room than in the waiting room.23-25 The neurologic examinations were in large part performed in an examination room. It is possible that, for the 3 dogs in our study that later became BAR (from QAR), sufficient time had passed for these dogs to relax and feel more comfortable, despite receiving trazodone.
None of the dogs in our study were fasted, and the average time from PO trazodone administration to repeat neurologic examination was 147 minutes. Fasted compared to non-fasted PO trazodone administration may affect plasma concentrations, especially related to the timing of the postadministration neurologic examination. However, because none of our dogs had been fasted before the study, our cohort was uniform in this regard. In addition, we used a dosage range of 6.25 to 8.60 mg/kg for the PO trazodone with the goal of approximating 7.50 mg/kg using the tablet sizes available (i.e., not compounding any dose) according to recommendations based on previously published studies in dogs.8, 13
A major limitation of our study is that despite our inclusion criterion that all dogs be normal and healthy according to their owners, several dogs (n = 11) were found to have abnormalities on baseline (pretrazodone) neurologic examination. However, we chose to include these dogs because the situation is representative and clinically relevant for the population of dogs seen by veterinarians. Subtle deficits detected on a neurologic examination performed by a neurologist are easily overlooked by clients and practicing veterinarians who are not performing neurologic examinations regularly. Additionally, each dog received a single neurologic examination for each assessment, which increased the possibility of spurious findings or abnormalities that may not actually be abnormal or consistent.
Future studies should assess the effects of trazodone on the neurologic examination for dogs with neurologic abnormalities such as intracranial, neuromuscular or myelopathic conditions. This approach may help characterize how trazodone alters the neurologic examination based on prerecognized neurolocalizations. Additionally, it would be prudent to investigate whether trazodone alters the neurologic examination in dogs that are systemically or critically ill. Such dogs are more likely to warrant hospitalization and subsequent anxiolytic treatment, in addition to being more prone to the development of neurologic abnormalities as a result of their systemic disease. Having a basis on which to gauge the likelihood that new-onset neurologic signs are a result of trazodone versus progression of the disease may be helpful in these circumstances.
In conclusion, for situations in which a dog urgently requires a neurologic examination but has received PO trazodone, the findings of our study are not sufficient to preclude the clinician from performing and interpreting a complete neurologic examination. However, in these circumstances, caution should be exercised when interpreting any mild PR deficits found on neurologic examination. We did not find evidence to suggest dogs experience any clinically relevant change in mention after trazodone administration. If the clinician needs to discern whether any mild deficits identified on the neurologic examination were the result of the trazodone versus true manifestations of a neurologic lesion, it is recommended that the neurologic examination be repeated at a later time when the patient has not received trazodone.
ACKNOWLEDGMENT
This study was funded by the University of Wisconsin-Madison, School of Veterinary Medicine. The authors thank Lauren Trepanier, DVM, PhD, DACVIM for her contributions in the data analysis of this study.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This study protocol was approved by the IACUC of the University of Wisconsin - Madison, School of Veterinary Medicine (IACUC-V006493-A02).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




