Association of recessive c.430G>A (p.(Gly144Arg)) thyroid peroxidase variant with primary congenital hypothyroidism in cats
Mario Van Poucke and Emilie Van Renterghem contributed equally to this study.
Abstract
Background
Primary congenital hypothyroidism (CH) is a rare endocrine disorder in cats with a largely unknown genetic cause.
Objectives
Describe the clinical presentation of CH in 11 affected cats and identify the causal genetic variant.
Animals
Eleven CH-cats from 10 unrelated families, 11 CH-free family members, 21 unrelated CH-free cats, and 155 unrelated nondiagnosed cats from different breeds.
Methods
Case control study of CH-cats and their siblings (2019-2021). Diagnosis was based on low to low-normal serum thyroxine (T4) concentrations, high thyroid-stimulating hormone (TSH) concentrations and clinical signs compatible with CH. We identified the causal variant using Sanger sequencing, genotyping via PCR-RFLP and variant interpretation using ACMG/AMP guidelines.
Results
All CH-cats (5 weeks-8 years) had disproportionate dwarfism. A goiter was not palpable in all. Thyroid scintigraphy with radiopertechnetate showed abnormally high uptake by thyroid glands, whereas scintigraphy with radioiodine showed abnormally low uptake, compatible with a defect in iodine organification by thyroid peroxidase (TPO). All cases were homozygous for TPO variant XM_006930524.4:c.430G>A(p.(Gly144Arg)), while none of the CH-free cats were. All sampled parents were heterozygous for this recessive variant. This variant was found in 15 cat breeds with an estimated allele frequency of 9%.
Conclusions and Clinical Importance
Disproportionate dwarfism, abnormally high TSH and abnormally low to low-normal T4 concentrations are diagnostic for CH in cats. All cases had dyshormonogenesis demonstrated by thyroid scintigraphy. This novel TPO missense variant (not described in humans) causes CH in cats and awareness of it can assist in diagnosis and breeding.
Abbreviations
-
- ACMG, AMP
-
- American College of Medical Genetics and Genomics, Association for Molecular Pathology
-
- CH
-
- congenital hypothyroidism
-
- 123I
-
- radioiodine
-
- LP/P
-
- (likely) pathogenic
-
- (L-)T4
-
- (levo)thyroxine
-
- 99mTcO4−
-
- radiopertechnetate
-
- TPO
-
- thyroid peroxidase
-
- T/S
-
- thyroid-to-salivary gland ratio
-
- TSH
-
- thyroid-stimulating hormone
1 INTRODUCTION
Primary congenital hypothyroidism (CH) is the most common congenital endocrine disorder in humans, with a reported prevalence of 1 in 2000 to 4000 live births, and is caused by a defect in the thyroid development (thyroid dysgenesis; 65% of the patients) or an impairment of the biosynthesis of hormones (dyshormonogenesis; 35% of the patients).1, 2 The etiology is identified in less than 5% of thyroid dysgenesis patients, whereas a genetic cause has been identified in 50% of dyshormonogenesis patients.1, 2 Dyshormonogenesis causing variants are found in genes involved in thyroid hormone synthesis and are generally recessively inherited.1-3 CH is described in multiple animal species, including cat, dog, pig, horse, sheep, goat and chicken.4 Because of its confirmed homology with its human counterpart on clinical, pathological, biochemical, and molecular levels, cats are considered as a clinically relevant animal model for CH.5 Typical clinical signs in cats include disproportionate dwarfism and mental dullness.6-11 The diagnosis of primary CH is based on the clinical presentation and a combination of low concentrations of serum total thyroxine (T4) and high concentrations of thyroid-stimulating hormone (TSH). Radiographic abnormalities are often found, showing epiphyseal dysplasia and delayed physeal closure.6, 8, 12 Scintigraphy using radiopertechnetate (99mTcO4−) and radioiodine (123I) can be used to differentiate between thyroid dysgenesis or dyshormonogenesis.13-16
Despite the detailed clinical description of patients, the extensive search for causal variants with improved sequencing technologies and the use of animal models over the past years, the pathogenesis of CH is still largely unknown (80% of the human patients have an unknown etiology) and involves more complex processes such as compound heterozygosity, monoallelic expression, and oligogenic causes combined with environmental influences.2, 17, 18 In cats, descriptions of clinical findings are sparse because the incidence is rare and underestimated as a proportion of cases die at birth or soon afterwards.6-11, 19 The only CH-causing variant in cats described so far is a missense variant in the thyroid peroxidase (TPO) gene in a family of Domestic Shorthair cats.20
The aim of this study was to expand the existing knowledge about CH in cats with a detailed description of the clinical findings of 11 cats diagnosed with CH, and to identify the causal genetic variant, useful for diagnostic and breeding purposes. The findings of this natural animal model can be extrapolated to other animal species and humans.
2 MATERIALS AND METHODS
2.1 Animal selection and data collection
Between January 2019 and February 2021, we diagnosed primary CH in 11 client-owned cats, based on the presence of low or low-normal serum T4 concentrations, high TSH concentrations, and clinical signs compatible with hypothyroidism. Of these 11 cats, 6 cats were from Belgium and 5 were from the United States. From each case the following data were collected: signalment, history, reason for presentation, known comorbidities, physical examination, laboratory results, imaging results (thyroid scintigraphy, MRI, CT, abdominal ultrasound, echocardiography, radiographs), treatment, and follow-up. All 3 siblings from case 7 underwent scintigraphy with 99mTcO4−. From all family members, serum T4 and TSH concentrations were measured with assays validated in cats.21, 22
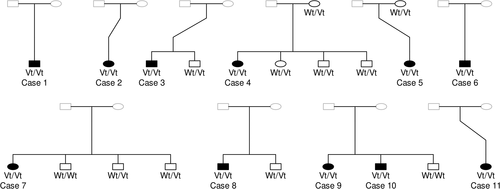
EDTA blood samples were gathered from the 11 CH-cats (belonging to 10 unrelated families: 6 from Belgium and 4 from the United States) and 11 family members with owner consent (Figure 1). Left-over EDTA blood samples from 21 unrelated cats, presented at the Small Animal Department at Ghent University (Belgium) for consultations, with normal clinical presentation and normal T4 concentrations,21, 22 were selected as samples from CH-free cats. Another 155 left-over EDTA blood samples from unrelated, nondiagnosed cats from different breeds in the Belgian cat population were used for variant frequency determination. Approval was granted by the local ethical (Faculty of Veterinary Medicine, Ghent University, Belgium) and deontological (Federal Public Service Health, Food Chain Safety and Environment, Brussels, Belgium) committees (EC2017/86) for the use of all left-over samples in this study.
2.2 Thyroid scintigraphy
Thyroid imaging was performed using the radionuclides 99mTcO4− and 123I. 99mTcO4− was injected (1-5 mCi) IV and imaging was performed 20 to 60 minutes after injection. The thyroid-to-salivary gland ratio (T/S) was calculated in all cats.13-16 123I was injected (1-1.32 mCi) IV and thyroid imaging was performed at 8 to 24 hours after injection. The percentage of the injected dose that accumulated in the thyroid gland (radioactive iodine uptake, RAIU) was calculated.13-16
2.3 Genetic analysis
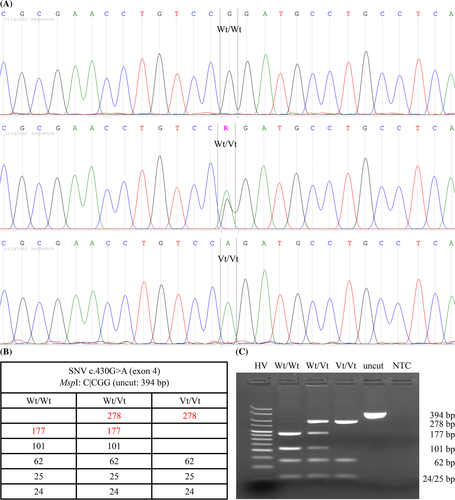
DNA was isolated from EDTA blood by performing a proteinase K digestion. Based on the clinical phenotype of the cases and literature data, TPO was chosen as the strongest candidate gene in the search for a CH-causing variant (see section 4). The already described TPO CH-causing variant NC_018725.3(XM_006930524.4):c.1333G>A(p.(Ala445Thr)) was genotyped as described by Giger and colleagues.20 To analyze the complete coding TPO sequence, the predicted feline gene sequence was first compared to its reviewed human ortholog. Primers amplifying all potential coding exons were designed, based on the feline genomic reference sequence. PCR amplicons were generated, Sanger sequenced and sequences were compared with the feline reference sequence. All variants were evaluated for potential causality based on population, disease-specific and sequence database data, and computational predictive programs (both feline and human). The most likely causal variant was genotyped in all other sampled cats via PCR-RFLP (validated via Sanger sequencing; Figure 2). Variant pathogenicity was determined after adding the segregation and population data evidence from this study, according to the ACMG/AMP guidelines.23 For detailed information see Data S1, Supporting Information.
3 RESULTS
3.1 Signalment, history, and clinical presentation
Two unrelated Domestic Shorthairs and 1 Russian Blue (cases 1-3) were presented to the Small Animal Department at Ghent University (Belgium), 2 Domestic Shorthairs, and 1 British Shorthair (cases 4-6) were presented to their primary care veterinarian located in Belgium, and 3 Domestic Shorthairs, 1 Domestic Medium Hair, and 1 Domestic Longhair (cases 7-11) were presented to the Animal Endocrine Clinic in New York. The cases were between 5 weeks and 8 years old (median: 6 months). There was no sex predisposition (5 males and 6 females).
Nine cats were presented for signs of mental dullness and growth retardation, whereas cases 2 and 3 had other major clinical signs. All cats had disproportionate dwarfism. Besides this, the presence of a goiter (n = 6/9), constipation (n = 4/11), and delayed tooth eruption (n = 3/11) were noticed on physical examination. Growth retardation was either very obvious or more subtle in some cats, as can be seen in Figure 3. CBC and chemistry were performed in 10 cats and showed no severe abnormalities except in case 2. The latter was initially presented at the age of 8 years old for inappetence and lethargy, and had a history of epileptic seizures, which had been treated with phenobarbital (Phenoleptil [Dechra]) at 3.5 mg/kg twice a day for the past 5 years. Serum biochemistry showed azotemia (creatinine 8.4 mg/dL, ref.: 0.8-2.4 mg/dL). The cat was diagnosed with acute-on-chronic kidney disease, was treated accordingly and discharged with 2.12 mg/dL of creatinine. Serum creatinine concentration was stable after 1 month, but no follow-up after supplementation of L-T4 was available. Case 3 was presented for nonambulatory tetraparesis. The cat was diagnosed with a subarachnoid diverticulum at the level of C2 and hemilaminectomy was performed. A heart murmur was present on auscultation. Echocardiography showed the presence of a mild systolic dysfunction. The clinical findings of case 3 have recently been reported Hermans et al.24 A summary of all clinical investigations and results of each case before supplementation with L-T4 can be found in Data S2.

3.2 Serum T4 and TSH concentrations
Nine of the hypothyroid cats had abnormally low serum concentrations of total T4. Two cats (cases 6 and 8) had low-normal T4 concentrations. All cats had abnormally high serum TSH concentrations (Data S2), ranging from 1.59 to >12 ng/mL (median 4.47 ng/mL). Serum T4 and TSH concentration in the nonaffected family members were within normal limits. T4 concentration for control cats was within normal limits. At least 1 or more follow-up concentrations of serum T4 and TSH 3 to 4 hours after administration of L-T4 were available for all cases except case 2. Each case responded well to treatment with an increase of serum TT4 and decrease of serum TSH concentrations over pretreatment values. Persistence of high serum TSH concentrations were present when serum T4 concentrations remain within the lower end of reference interval. Normal or only slightly high TSH concentrations were measured when serum T4 was within the high-normal range or just above the reference interval. High serum T4 concentrations and signs of hyperthyroidism were present in 1 cat (case 5).
3.3 Thyroid scintigraphy
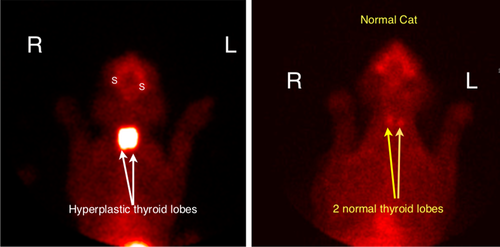
Thyroid imaging was performed using the radionuclide 99mTcO4− (cases 1, 2, 7, and 9) and 123I (case 1). Thyroid scintigraphy with 99mTcO4− showed abnormally high uptake (T/S > 0.6-1.03)13-16 of the radionuclide by the thyroid glands in cats with CH, but not in those from their siblings (Figure 4). Scintigraphy with 123I showed abnormally low uptake after 24 hours, compatible with an organification defect.
3.4 Radiographs
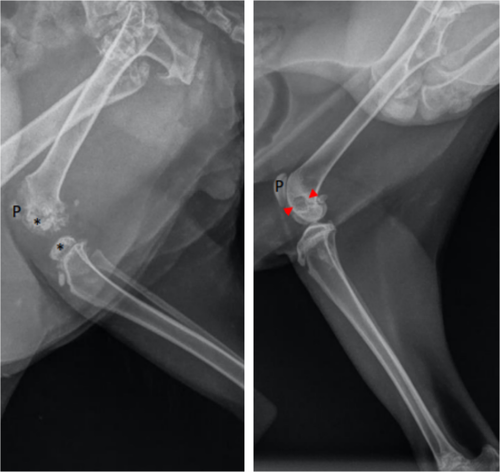
Radiographs of the vertebral column, and front and hind limbs were performed in 3 cats (cases 1, 3, and 6; Data S2). Epiphyseal dysplasia and delayed physeal closure were seen in all radiographs. An example of radiographs of the stifle of case 3 before and after thyroid hormone supplementation is shown in Figure 5.
3.5 Treatment with thyroid hormone
All cats with CH received thyroid hormone replacement therapy. Cats were treated with levothyroxine (L-T4) sodium (Leventa [MSD Animal Health]; Forthyron [Eurovet Animal Health]; or Thyrotabs [Lloyd]), at a starting dosage of 50 to 200 μg/cat. Disproportionate dwarfism persisted in our cats despite treatment, but there was some growth compensation after supplementation of L-T4. Dosage was increased in most cases after follow-up thyroid testing.
3.6 Genetic analysis
Based on the clinical phenotype of the cases and literature data, TPO was chosen as the strongest candidate gene in the search for a CH-causing variant (see section 4). Cases were first checked to carry the TPO CH-causing variant NC_018725.3 (XM_006930524.4):c.1333G>A(p.(Ala445Thr)) described by Giger et al.20 Because this variant was not observed in any of the cases, the complete TPO coding sequence was further investigated in the search for a novel CH-causing variant.
Sequencing all coding TPO exons revealed 11 SNVs (compared to the feline TPO mRNA reference sequence XM_006930524.4). They were all deposited in the EVA database,25 described in Table 1 and shown in the protein and amplicon sequence (Data S1). No variants were found at the splice site regions.
| Descriptiona | Exon | Type | PredictSNP |
|---|---|---|---|
| c.49G>A(p.(Ala17Thr)) | 1 | Missense | Deleterious |
| c.430G>A(p.(Gly144Arg)) | 4 | Missense | Deleterious |
| c.798G>A(p.(Glu266Glu)) | 6 | Silent | NA |
| c.843T>C(p.(Cys281Cys)) | 6 | Silent | NA |
| c.990T>C(p.(Asn330Asn)) | 7 | Silent | NA |
| c.1074G>A(p.(Ala358Ala)) | 7 | Silent | NA |
| c.1154C>T(p.(Thr385Met)) | 7 | Missense | Neutral |
| c1182C>T(p.(Arg394Arg)) | 7 | Silent | NA |
| c.2346T>C(p.(His782His)) | 12 | Silent | NA |
| c.2377C>G(p.(Pro793Ala)) | 12 | Missense | Neutral |
| c.2594C>T(p.(Ala865Val)) | 14 | Missense | Neutral |
- Note: All variants were submitted to the EVA database (Project: PRJEB44876, Analyses: ERZ2176294).
- a According to the feline mRNA reference sequence XM_006930524.4.
Six of the variants were silent (excluded) and 5 were missense variants (Table 1). The latter 5 were analyzed by PredictSNP, a consensus classifier for disease-related variants combining 6 prediction methods based on evolutionary, physico-chemical and structural characteristics, supplemented by experimental annotations from databases.26 Three of them were predicted to be neutral (excluded), while the other 2, namely c.49G>A(p.(Ala17Thr)) and c.430G>A(p.(Gly144Arg)), were predicted to be deleterious (Data S1). None of the deleterious SNVs were already described in cats (dbSNP).27 In humans (orthologous amino acids differ in number; Data S1), an identical variant was described for c.49G>A(p.(Ala17Thr)) as a very rare but neutral variant (dbSNP #rs369441749:NP_001193673.1:p.Ala5Thr),27 with no entry in the ClinVar database.28 In contrast, no identical variant for c.430G>A(p.(Gly144Arg)) has been described in humans, and the hypothetical orthologous variant (NP_001193673.1:p.Gly132Arg) is considered as deleterious by Condel.29 According to I-Mutant2.0,30 a predictor of protein stability changes upon mutations, the TPO p.Gly144Arg variant decreases protein stability (reliability index = 6).
As a consequence, the c.430G>A(p.(Gly144Arg)) variant was prioritized and genotyped in all CH family members, 21 unrelated CH-free cats and 155 unrelated nondiagnosed cats from different breeds in the Belgian cat population. All cases were homozygous, all sampled parents were heterozygous, and none of the sampled healthy family members were homozygous for this variant (Figure 1 and Table 2). The segregation data shows that this variant follows a recessive mode of inheritance and cosegregates perfectly with CH in the affected families (from Belgium and the United States). Because none of the unrelated CH-free cats were homozygous for the variant (Table 2), the odds ratio is infinitive, and the variant can be considered as fully penetrant. From the 155 unrelated nondiagnosed cats, 129 did not carry the variant, 23 were heterozygous, and 3 were homozygous for the variant, resulting in an estimated allele frequency of 9% (Table 2). The variant was found in 15 breeds, namely the Balinese, Birman, British Longhair, British Shorthair, Chartreux, Domestic Shorthair, Domestic Medium Hair, Domestic Longhair, Exotic Shorthair, Main Coon, Norwegian Forest Cat, Oriental Shorthair, Russian Blue, Siberian, and Sphynx (Data S1).
| c.430G>A genotype | Wt/Wt | Wt/Vt | Vt/Vt | Total | Vt-frequency (%) |
|---|---|---|---|---|---|
| CH-cases | 0 | 0 | 11 | 11 | 100 |
| CH-free family members | 2 | 9 | 0 | 11 | 41 |
| CH-free unrelated Belgian cats | 20 | 1 | 0 | 21 | 2 |
| Nondiagnosed unrelated Belgian cats | 129 | 23 | 3 | 155 | 9 |
4 DISCUSSION
In this study, most CH-cats had signs of disproportionate dwarfism, mental dullness, constipation and delayed tooth eruption, which is consistent with other reports.6-11 Most affected cats had bilateral enlargement of both thyroid lobes, as is also seen in humans with dyshormonogenesis.12 In 2 of the 11 cats (cases 6 and 8), serum T4 concentration was in lower half of the reference interval, but all cats had high serum TSH concentrations. In cats, dogs, and man, the combination of low to low-normal serum T4 and high TSH concentrations is diagnostic for primary hypothyroidism. Most, but not all, of our cats with dyshormonogenesis had a palpable goiter (presumably because of thyroid hyperplasia).
The diagnosis of CH in cats is usually made at young age (6-8 weeks).6-11 Similarly, all of our cats were diagnosed as kittens, except case 2, which was not diagnosed until the age of 8 years. In cat 2, palpation of a cervical goiter was an important clue for the diagnosis of CH. This cat was also azotemic, which have been described in CH in cats and humans,31, 32 and also had a long history of epilepsy, which was treated with phenobarbital. Seizures have already been described in 2 littermate kittens with thyroid dysgenesis.33 Although it is unknown if epilepsy was caused by dyshormonogenesis in our cat, thyroid hormones are thought to play an important role in this pathology.34
Phenobarbital decreases serum T4 and increase TSH in different species such as humans, rats and dogs and is partially explained by the increased metabolism of thyroid hormones.35, 36 A small prospective study of cats treated with phenobarbital showed that cats were not likely to be misdiagnosed as hypothyroid, with serum T4 and TSH concentration remaining within the respective reference intervals.37 In our phenobarbital-treated cat, the serum TSH concentration was high (7.49 ng/mL), which was unlikely to be caused by the chronic administration of phenobarbital. Scintigraphy with 99mTcO4− showed a marked, higher uptake of the radionucleotide compatible with an organification defect. Unfortunately, because of the retrospective nature of the study, scintigraphy with 123I was not available to confirm an organification defect. This study however, confirms the presence of dyshormonogenesis because of the presence of a causal variant.
Case 3 was diagnosed with a subarachnoid diverticulum at the level of C2, which led to nonambulatory tetraparesis. After surgery, the cat made a complete recovery. To the authors' knowledge, this is the first CH-cat to suffer from this pathology, and its relationship to CH, if any, is unclear.
Radiographs in our CH-cats showed epiphyseal dysplasia and delayed physeal closure, as previously described in cats and humans with CH.6, 8, 12
Thyroid scintigraphy is useful in confirming CH and in differentiating thyroid dysgenesis from dyshormonogenesis.13-16 Either 123I or 99mTcO4− can be used for thyroid imaging. 123I, like stable iodine, is trapped and concentrated within thyroid follicular cells by the iodine pump. Once within the cell, 123I is oxidized and incorporated into tyrosine groups of thyroglobulin. Coupling of these iodotyrosyl groups forms T4 and T3 (triiodothyronine), which are then stored within the follicular colloid. Like 123I, 99mTcO4− is trapped and concentrated within thyroid follicular cells. Uptake of 99mTcO4−, however, reflects only the trapping mechanism of the thyroid gland. Unlike 123I (and stable iodine), 99mTcO4− is neither organically bound to thyroglobulin nor stored in the thyroid gland. In humans and cats with dyshormonogenesis (which is most commonly caused by a defect in the organification of iodide13-16, 38), high uptake of 99mTcO4− by both thyroid lobes is a characteristic finding, because there is no defect in the iodine trapping in dyshormonogenesis and the high circulating TSH concentrations associated with primary hypothyroidism increase the thyroid uptake of the radionuclide. In contrast, scintigraphy with 123I (which was done at 24 hours, much later than the 20-60 minutes when imaged with 99mTcO4−) shows lower uptake, because the 123I cannot be stored in the thyroid gland because of its defect in iodine organification.
All cats for which follow-up was available showed clinical improvement, as well as an increase in serum T4 and decrease in TSH concentrations after supplementation with L-T4. Thyroid testing was performed 3 to 4 hours after administration of L-T4 since this is the peak concentration measured in dogs.39 Cats that had persistent increases of serum TSH concentrations with T4 concentrations within the reference interval responded well to increased dosages of L-T4, as shown by improvement of clinical symptoms and follow-up thyroid testing. Normal or only slightly increased serum TSH concentrations combined with serum T4 within the high-normal range or slightly above the reference interval was seen as a good response to L-T4 treatment. Iatrogenic hyperthyroidism was rare and responded well to a lowered dosage of L-T4. Because hypothyroidism is often diagnosed at early age, adjustment of the dosage was needed with increasing body weight.
Dyshormonogenesis causing variants are found in genes involved in thyroid hormone synthesis (eg, TG, TPO, SLC26A6, SLC5A5, DUOX2, DUOXA2, IYD).1-3 Depending on the site of the synthesis defect, inheritance patterns and associated features are different.1-3 In the search for a causal variant, TPO was chosen as the strongest candidate gene because of the clinical phenotype of the cases and literature data. Thyroid scintigraphy studies of the CH-cases showed a defect in organification of iodide, most commonly caused by TPO defects.1-3, 40, 41 In literature, decreased TPO activity leading to dyshormonogenesis in a cat has been confirmed with biochemical studies.42 In addition, variants in this gene were already described in cats20 and dogs43-47 with similar clinical features, and in many human patients40, 41 with a similar defect. In humans, severe CH with a total iodide organification defect is typically caused by variants in TPO (thyroid peroxidase, involved in iodide organification and thyronine coupling), while mild CH with a partial iodide organification defect is typically caused by variants in DUOX2 (dual oxydase, involved in peroxide production required for iodide organification), DUOXA2 (accessory protein for DUOX2) and SLC26A4 (pendrin, an anion transporter involved in apical iodide efflux).1-3
Genetic analysis identified the TPO c.430G>A(p.(Gly144Arg)) variant, that perfectly cosegregates with primary CH in multiple affected families from Belgium and the United States, with a recessive mode of inheritance. Because all CH-cats were homozygous for the variant and none of the CH-free cats were, odds ratio is infinitive. The variant was also absent in both feline and human population, disease-specific and sequence databases. PredictSNP,26 Condel,29 and I-Mutant2.030 predicted a deleterious effect for this variant, that is located in a part of the extracellular domain with a yet unknown function.41 It is well documented that changing a highly conserved Gly, a small nonpolar amino acid with huge conformational flexibility, into an Arg, a large polar (positively charged) amino acid that typically resides on the protein surface at active or binding sites, can have a drastic impact on the proteins function.48 In the humsavar database,49 more than 60% of all human Gly>Arg variants are classified as (likely) pathogenic (LP/P) and more than 70% of all human TPO missense variants (including a Gly>Arg variant) are LP/P for CH. Taking into account the strong pathogenic evidence from the segregation and population data, and the supporting evidence from the computational/predictive and functional data, we can, based on the ACMG/AMP guidelines (Data S1),23 consider the TPO c.430G>A(p.(Gly144Arg)) variant as pathogenic for feline primary CH with nonanomalous clinical features and a recessive mode of inheritance with full penetrance.
Because the variant was already found in 15 breeds with an estimated allele frequency of 9%, it indicates that this variant is widespread and supports the hypothesis that the incidence of CH is underestimated as a proportion of cases die at birth or soon after.6-11, 19 This underestimation might even be enhanced by the fact that not all cases might be reported, as 3 from the 155 randomly chosen nondiagnosed cats from the Belgian cat population were homozygous for the causal variant. Unfortunately, it was not possible to trace back these cats. Genotyping this variant would therefore be useful in breeding schemes or for confirmation of diagnosed cats, especially if a goiter cannot be palpated or serum T4 concentrations remain in the low-normal reference interval.
Although variant identification nowadays is shifting towards more affordable next-generation sequencing technologies, causal variant identification via the classical candidate gene approach can still be successful in complex endocrine disorders, as we reported in this study. We did this by integrating our cats' detailed history, clinical features and thyroid hormone tests, together with genetic databases from other species, especially humans. In addition, we described in this report a novel CH-causing variant in cats, not described in any other species, within a yet undefined but apparently functionally important region of the extracellular domain of the TPO protein.
ACKNOWLEDGMENT
No funding was received for this study. We thank Dominique Vander Donckt, Carolien Rogiers, Linda Impe, and Ruben Van Gansbeke for technical assistance, Stephanie Carmody, the clinical researcher coordinator from the Animal Endocrine Clinic, as well as the breeders, owners, and veterinarians who collaborated to this study.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Owner consent was given to perform bloodwork and thyroid imaging of the 11 family members of cats diagnosed with congenital hypothyroidism, in purpose of health screening. Approval was granted by the local ethical (Faculty of Veterinary Medicine, Ghent University, Belgium) and deontological (Federal Public Service Health, Food Chain Safety and Environment, Brussels, Belgium) committees (EC2017/86) for the use of all left-over samples in this study.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




