Clinical outcome and Ki67 evaluation in dogs with nodal small cell B-cell lymphoma diagnosed by flow cytometry
Abstract
Background
Nodal small cell B-cell lymphoma subtypes in dogs cannot be distinguished by flow cytometry and information regarding treatment, prognosis, and outcome are limited.
Hypothesis/Objectives
Objectives were to describe outcome in dogs with nodal small cell B-cell lymphoma diagnosed by flow cytometry and correlate clinical and laboratory data with survival. We hypothesized that B-cell Ki67 expression measured by flow cytometry is associated with shorter progression free survival (PFS) and overall survival (OS).
Animals
Forty-nine dogs with nodal small cell B-cell lymphoma, defined by >80% CD21+ B-cells by flow cytometry and small-sized B-cells by forward scatter.
Methods
Retrospective study reviewing treatment and outcome data extracted from medical records. Percentage of Ki67-expressing B-cells was measured by flow cytometry. Clinical, laboratory, and flow cytometry data were assessed for association with outcome.
Results
Median percentage of B-cell Ki67 was 41% (range, 3%-97%). Median PFS was 119 days and median OS was 222 days (n = 49). Among cases treated with CHOP-based chemotherapy (n = 32), median PFS was 70 days, median OS was 267 days, and 50% of cases achieved complete response. Low percentage of B-cell Ki67 (≤11%) was associated with prolonged OS by univariable analysis. Greater age, substage B, high B-cell CD25 expression and low B-cell CD21 and class II major histocompatibility complex expression by flow cytometry were independently associated with shorter OS.
Conclusions and Clinical Importance
Most nodal small cell B-cell lymphoma cases had aggressive disease. Low Ki67 expression can help identify cases with better prognosis. Age, substage, and flow cytometry variables are useful prognostic factors.
Abbreviations
-
- CHOP
-
- cyclophosphamide, doxorubicin, vincristine, and prednisone
-
- CLL/SLL
-
- chronic lymphocytic leukemia/small lymphocytic lymphoma
-
- CR
-
- complete response
-
- DLBCL
-
- diffuse large B-cell lymphoma
-
- DSBCL
-
- diffuse small B-cell lymphoma
-
- FL
-
- follicular lymphoma
-
- FS
-
- forward light scatter
-
- MCL
-
- mantle cell lymphoma
-
- MHC
-
- major histocompatibility complex
-
- MST
-
- median survival time
-
- nMZL
-
- nodal marginal zone lymphoma
-
- OS
-
- overall survival
-
- PFS
-
- progression free survival
-
- PR
-
- partial response
-
- VCOG
-
- Veterinary Cooperative Oncology Group
-
- WHO
-
- World Health Organization
1 INTRODUCTION
Lymphoid malignancies are a heterogeneous group of tumors that are common in dogs. The World Health Organization (WHO) diagnostic guidelines classify subtypes of lymphoma based on phenotype, tumor architecture and cytomorphology, and these lymphoma subtypes have different prognoses and treatment indications.1-3 B-cell lymphomas are the most common phenotype diagnosed in dogs and diffuse large B-cell lymphoma (DLBCL) is the most common subtype.1, 2, 4 There are less common subtypes of B-cell lymphoma that are comprised of small to intermediate-sized B-cells, including diffuse small B-cell lymphoma (DSBCL), nodal marginal zone lymphoma (nMZL), follicular lymphoma (FL), mantle cell lymphoma (MCL), and B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).5 Less is known about the clinical presentation and outcome of these small cell B-cell subtypes compared to DLBCL.
Subclassification of lymphoma by the WHO scheme requires a lymph node biopsy, however lymph node histology often is not pursued6 because of the invasiveness, cost and time associated with collecting a biopsy and lack of subtype-specific therapies. Lymphoma is commonly diagnosed by a combination of cytology and flow cytometry on a lymph node fine-needle aspirate. Flow cytometry is useful in obtaining a diagnosis of lymphoma and identifying the phenotype, as well as providing subclassification and prognosis based on cell size or antigen expression or both in some, but not all cases.7, 8 Ninety percent of B-cell lymphoma cases with a B-cell size (as determined by flow cytometry forward light scatter [FS]) above a specific cutoff are histologically diagnosed as DLBCL.9 A subsequent study focused on small cell B-cell subtypes, defined by having a flow cytometry FS below the cutoff for DLBCL, found that the majority of cases were a provisional histologic subtype DSBCL, which has an aggressive clinical course (median survival time [MST] 140 days), suggesting some forms of small cell B-cell lymphoma have poorer outcomes than DLBCL.5 This candidate DSBCL subtype does not correlate well with an established WHO classification and requires additional characterization and consensus as a diagnosis. Another study demonstrated that non-DLBCL B-cell lymphoma cases had significantly shorter survival (MST 114 days) and poorer response rates to treatment compared to DLBCL cases (MST 325 days).9
The goal of this study was to examine outcome in a larger cohort of dogs with small cell B-cell lymphoma diagnosed by flow cytometry. Small cell B-cell lymphomas account for 12% of nodal B-cell lymphoma in dogs diagnosed by flow cytometry through our laboratory.5 We aimed to evaluate response to treatment, survival and prognostic factors in these cases, and assess the utility of measuring cell proliferation by Ki67 expression in B-cells by flow cytometry, which is useful in discriminating low-grade and high-grade lymphoma10 and showed prognostic significance in high-grade B-cell lymphoma11 and B-cell CLL in dogs.12 We hypothesized that Ki67 expression could offer prognostic information, particularly for cases where histologic subtyping is not pursued.
2 METHODS
2.1 Study sample
Dogs with nodal small cell B-cell lymphoma diagnosed by flow cytometry were identified through the Colorado State University-Clinical Hematopathology Laboratory. Cases had flow cytometry performed on a lymph node aspirate and an expansion (>80% of total cells) of small-intermediate sized CD21+ B-cells with a median B-cell FS <500. Criteria rationale and a figure depicting B-cell FS in a case compared to a healthy dog lymph node and DLBCL case are provided in the Supporting Information S1. As a size comparison, the mean FS of canine neutrophils in the peripheral blood is 620 (SD, 61).5 From July 27, 2017 to September 27, 2018, we measured the percentage of Ki67-expressing B-cells by flow cytometry for cases with adequate sample remaining after routine immunophenotyping. Ki67 expression was measured for research purposes and not reported, so these results did not change treatment decisions. Veterinarians submitting samples for all cases with Ki67 expression data were contacted to provide medical records.
2.2 Flow cytometry
Routine immunophenotyping was performed on a fresh lymph node aspirate as previously described, using the antibody panel in Table 1.13 Samples were analyzed on a 3 laser Coulter Gallios instrument (Beckman Coulter Inc., Brea, California) and data were analyzed using Kaluza Analysis Software (Beckman Coulter Inc.). Cell size was approximated by median linear FS. Expression of CD21 and class II major histocompatibility complex (MHC) on B-cells was measured by median fluorescence intensity and the percentage of CD25-expressing B-cells was determined as previously described.14 Control CD4 T-cell size, B-cell size and B-cell CD21, class II MHC and CD25 expression data from lymph nodes of 37 healthy dogs with no evidence of lymphoproliferative disease by a combination of histology, flow cytometry or PCR for antigen receptor rearrangements were used as comparison. CD25 expression data were available for 23/37 healthy control dogs. For all lymphoma cases, intracellular Ki67 (SolA15 clone, eBioscience, San Diego, California) was measured in CD21+ B-cells by flow cytometry on the same lymph node aspirate used for routine immunophenotyping as previously described.12 We gated on CD21+ B-cells, excluding CD5+ T-cells and CD18+ neutrophils, to measure the percentage of B-cells positive for Ki67 (Ki67%). We previously measured Ki67 expression on control B-cells from healthy dog blood and lymph node samples (n = 12).12
| Antibody tube | Antibody specificity and fluorochrome |
|---|---|
| Routine immunophenotyping antibody panel | |
| 1 | No antibodies/Propidium iodide |
| 2 | CD3-FITCa/CD25-PEb/CD5-APCb/CD8-Alexa 700a/CD4-Pacific Blueb/Propidium iodide |
| 3 | Class II MHC-FITCb/CD34-PEb/CD21-Alexa 647a/Propidium iodide |
| 4 | Class II MHC-FITCb/CD18-PEa/CD5-APCb/CD14-Alexa 700a/CD4-Pacific Blueb/Propidium iodide |
| 5 | CD5-FITCa/CD45-PEa/CD21-Alexa 647a/Propidium iodide |
| Ki67 antibody panel | |
| 1 | Isotype control-FITCb/CD18-PEa/CD5-PerCP-e710b/CD21-Alexa 647a/Zombie Violet |
| 2 | KI67-FITCb/CD18-PEa/CD5-PerCP-e710b/CD21-Alexa 647a/Zombie Violet |
- Note: Clones are as follows: CD45, YKIX716.13; CD18, YFC118.3 (human CD18); CD4, YKIX302.9; CD8, YCATE55.9; CD5, YKIX322.3; CD21, CA2.1D6; CD3, CA17.2A12; CD14, TUK4 (human CD14); class II MHC, YKIX334.2; CD34, 1H6; CD25, P4A10; KI67, SolA15; KI67 isotype control, eBR2a. In the routine immunophenotyping antibody panel, the percentage of CD25-expressing B-cells was determined by gating on lymphocytes in antibody tube 2, excluding cells expressing CD4, CD8, CD3 and CD5, and calculating CD25% on remaining cells.
- a Antibodies were purchased from Bio-Rad (Hercules, California).
- b Antibodies were purchased from eBioscience/Thermo Fisher Scientific (Waltham, Massachusetts).
2.3 Clinical variables
Signalment, physical exam and imaging findings, and hematologic and biochemistry data were extracted from the Colorado State University-Clinical Hematopathology Laboratory submission form and medical record. Laboratory abnormalities were identified based on the reference intervals for the laboratory performing the CBC or biochemistry panel. Thrombocytopenia was defined as a platelet count below the reference interval without evidence of platelet clumping on blood film review. Staging was performed at the discretion of the supervising clinician and not all dogs were fully staged. Minimum stage was assigned based on the supervising clinician's assessment in the medical record; if no stage was assigned, a minimum stage was assigned by the author JHB based on recorded physical examination findings and review of diagnostics performed. Determination of whether dogs with lymphocytosis or circulating blasts were stage V or not was variable between supervising clinicians and for consistency, all dogs with a lymphocytosis or circulating blasts were assessed as stage V. Substage was determined by the supervising clinician or the author JHB based on recorded clinical signs at the time of diagnosis.
2.4 Cytology and histology
All available lymph node cytology and histology reports that were performed by a board-certified pathologist were reviewed. Cytology slides were not available for re-evaluation. For lymph node cytology reports, the diagnosis, lymphocyte cell size, size determination method, chromatin pattern, nucleoli description and cytoplasm description were recorded by author EDR. For histology reports, the tumor architecture pattern, cytomorphologic features and mitotic rate were recorded by author KLH.
2.5 Statistical analysis
Clinical and flow cytometry data were summarized. For continuous variables, normality was assessed by a Shapiro-Wilk test. Continuous data were expressed as median and range or mean and SD and categorical data were summarized by percentages. Flow cytometry data were compared between lymphoma cases and control B-cells using a Mann-Whitney test. Treatments were categorized based on the induction protocol used. Response to treatment was evaluated by the attending clinician or author JHB using available data in the medical record. Treatment responses were classified as complete response (CR), partial response (PR), stable disease or progressive disease. Veterinary Cooperative Oncology Group (VCOG) nodal response criteria were used when lymph node measurements were available.15 Progression free survival (PFS) was calculated from the date that treatment was initiated to the date of progressive disease. Overall survival (OS) was calculated from the date of lymphoma diagnosis to the date of death. In cases with a definitive lymphoma diagnosis by cytology, the date of cytologic diagnosis was used. In cases without cytology or an equivocal cytologic diagnosis, the date of flow cytometry diagnosis was used. Cases alive at the time of data collection or lost to follow up were censored. Median PFS and OS were calculated by the Kaplan-Meier method. Clinical variables and flow cytometry variables were tested for association with PFS and OS by univariable analysis using Kaplan-Meier log rank tests. For continuous risk factors, X-Tile Software was used to predict the best cutoffs to divide cases based on outcome and these cutoffs were used for survival analysis.16 Multivariable Cox proportional hazards regression analysis was performed to investigate variables associated with PFS and OS. Four multivariable models were performed: (a) PFS model with treatment type (CHOP-based protocol including cyclophosphamide, doxorubicin, vincristine and prednisone vs no treatment or other treatment) included as a variable; (b) PFS model with treatment type excluded; (c) OS model with treatment type included; (d) OS model with treatment type excluded. Variables with a P-value < .25 testing association with PFS and OS across all cases by univariable analysis were passed into the PFS and OS multivariable models, respectively. Variables were added into the model backward; first all variables with P < .25 were added and then variables with a P-value > .1 were removed sequentially. Statistical analysis was performed in GraphPad Prism version 9.1.2 (San Diego, California) and MedCalc Software version 20.015 (Ostend, Belgium) and 2-sided P-values < .05 were considered significant.
3 RESULTS
3.1 Study sample
Ki67 expression was measured in 103 cases of nodal small cell B-cell lymphoma from July 27, 2017 to September 27, 2018 and cases were contacted for follow up information. Medical records were obtained for 54 cases, but 5 cases were omitted from the study because of inadequate follow up or a prior diagnosis of lymphoma and chemotherapy treatment. Specifically, 2 cases had no follow up data, 1 case had a prior diagnosis of lymphoma treated with COP (cyclophosphamide, vincristine, and prednisone) chemotherapy and flow cytometry was performed at relapse, 1 case had a history of splenectomy and subsequent lymphoma treated with doxorubicin and chlorambucil, and 1 case had a history of lymphocytosis and was undergoing chlorambucil and prednisone treatment when generalized lymphadenopathy developed and flow cytometry was pursued. The remaining 49 cases were included in the study.
3.2 Clinical presentation
Information including signalment, stage, substage, hematologic data and biochemical data for dogs enrolled in this study are summarized in Table 2. The median age at diagnosis was 10.3 years (range, 2.1-14.8 years) and median body weight was 19.2 kg (range, 2.7-62.2 kg). Most cases (96%) were assessed as minimum stage III or higher. Eighteen cases (37%) had stage V disease, which was attributed to lymphocytosis or circulating atypical lymphocytes in 16 cases, pulmonary involvement in 1 case, and presence of a mediastinal mass in 1 case. Eleven cases (22%) were assessed as substage B. Among the anemic cases (n = 15), the median hematocrit was 32% (range, 25%-37%). Among the thrombocytopenic cases (n = 13), the median platelet count was 138 000/μL (range, 3000-176 000/μL). Four cases had concurrent anemia and thrombocytopenia. One case had pancytopenia without lymphocytosis. Nine of 10 dogs with neutrophilia had mild neutrophilia while 1 case had 45 600 neutrophils/μL. Among cases with lymphocytosis, 12/14 had a lymphocyte count <35 000/μL and 2 cases had a marked lymphocytosis >100 000/μL.
| Variable (na with available data) | n (%) affectedb or median (IQR; range) |
|---|---|
| Age (years) (n = 49) | 10.3 (7.5-12.0; 2.1-14.8) |
| Body weight (kg) (n = 49) | 19.2 (10.1-34.0; 2.7-62.2) |
| Sex (n = 49) | |
| Female intact | 1 (2%) |
| Female spayed | 20 (40.8%) |
| Male intact | 7 (14.3%) |
| Male castrated | 21 (42.9%) |
| Breedc (n = 49) | |
| Mixed breed | 11 (22.4%) |
| Labrador retriever | 5 (10.2%) |
| Boxer | 2 (4.1%) |
| French bulldog | 2 (4.1%) |
| Bull terrier | 2 (4.1%) |
| Minimum stage (n = 49) | |
| II | 2 (4.1%) |
| III | 25 (51.0%) |
| IV | 4 (8.2%) |
| V | 18 (36.7%) |
| Substage (n = 49) | |
| A | 38 (77.6%) |
| B | 11 (22.4%) |
| Anemia (n = 45) | |
| No | 30 (66.7%) |
| Yes | 15 (33.3%) |
| Thrombocytopenia (n = 45) | |
| No | 32 (71.1%) |
| Yes | 13 (28.9%) |
| Lymphocytosis (n = 45) | |
| No | 31 (68.9%) |
| Yes | 14 (31.1%) |
| Neutrophilia (n = 41) | |
| No | 31 (75.6%) |
| Yes | 10 (24.4%) |
| Hypercalcemia (n = 38) | |
| No | 38 (100%) |
| Yes | 0 |
| Hyperglobulinemia (n = 41) | |
| No | 35 (85.4%) |
| Yes | 6 (14.6%) |
- a n, number of cases with available data.
- b n, number of cases affected; %, percentage of cases affected among those cases with available data.
- c Breeds represented by ≥2 cases are included.
3.3 Flow cytometry
Flow cytometry data were compared between lymphoma cases and control lymphocytes from lymph nodes of dogs without lymphoproliferative disease (Figure 1). The mean B-cell size in lymphoma cases, as determined by FS, was 407 (range, 314-474), which is 1.1× the mean size of control B-cells from healthy dog lymph nodes (mean FS 362; range, 301-458 SD 36). The percentage of CD25-expressing B-cells was significantly higher in lymphoma cases compared to control lymph nodes from healthy dogs (P < .001). B-cell class II MHC and CD21 expression were not significantly different between cases and controls. Control samples from healthy dog lymph nodes (n = 7) and blood (n = 5) had 7%-26% Ki67-expressing B-cells (median, 8.5%), with 11/12 controls having Ki67% <20%, which was the threshold used to define low Ki67 expression in 2 previous studies.11, 12 B-cell lymphoma cases had significantly higher Ki67% than controls (P < .001), though there was a wide range of Ki67% values among lymphoma cases (median, 41%; range, 3%-97%).

3.4 Cytology and histology
Thirty-seven cases had lymph node cytology interpreted by a board-certified pathologist and a cytology report available. Most cases (31/37) had cytology performed within 2 days of flow cytometry (range, 0-21 days). Cytology report data from clinical pathologists at different institutions are summarized in Table 3. Most cases (65%) were diagnosed with lymphoma, 27% of cases were diagnosed as possible or probable lymphoma, and 8% were diagnosed with lymphoid hyperplasia. Among cases diagnosed as possible, probable or definitive lymphoma, 2 cases were interpreted as large cell lymphoma cytologically; these cases had a median B-cell FS by flow cytometry of 365 and 460. The criteria used to categorize cell size were variable. Chromatin patterns were highly variable and included the terms finely stippled, finely granular, finely or slightly clumped, stippled, coarse, fine, and smooth. Nucleoli or nucleolar rings were mentioned in 85% of cases, with most cases having indistinct, faint or variably prominent nucleoli.
| Cytologic feature | Number (%) of cases |
|---|---|
| Cytology diagnosis (n = 37) | |
| Lymphoid hyperplasia | 3 (8%) |
| Possible lymphoma | 4 (11%) |
| Probable lymphoma | 6 (16%) |
| Lymphoma | 24 (65%) |
| Cell size (n = 34) | |
| Small to intermediate | 6 (18%) |
| Intermediate | 11 (32%) |
| Intermediate to large | 13 (38%) |
| Large | 2 (6%) |
| Not provided | 2 (6%) |
| Method to determine cell size (n = 34) | |
| Overall cell size | 6 (18%) |
| Nucleus size | 11 (32%) |
| Not provided | 17 (50%) |
| Reference used for size comparison (n = 34) | |
| Red blood cell diameter | 9 (26%) |
| Neutrophil diameter | 5 (14%) |
| Small lymph nuclear diameter | 1 (3%) |
| Micrometer measurement | 2 (6%) |
| Not provided | 17 (50%) |
| Nucleoli description (n = 34) | |
| Indistinct nucleoli | 5 (15%) |
| Nucleolar rings | 2 (6%) |
| Rare faint nucleoli | 8 (24%) |
| Variably prominent nucleoli | 7 (21%) |
| Single prominent nucleolus | 2 (6%) |
| Prominent nucleoli | 5 (15%) |
| Nucleoli not mentioned | 5 (15%) |
- Note: Cellular features are summarized for 34 cases diagnosed as possible, probable or definitive lymphoma.
Four cases had lymph node biopsy. Tissue was not available for evaluation, but histopathology reports were reviewed. None of the cases were diagnosed as large cell lymphoma, but rather all had small to intermediate-sized cells primarily with condensed chromatin and more rarely with small nucleoli. The histology reports were most suggestive of a variety of small to intermediate-sized mature B-cell lymphoma subtypes including nodular and diffuse forms. None of the cases received a definitive WHO classification but 1 case had differentials of MCL or CLL/SLL and 1 case had differentials of marked follicular hyperplasia or possibly nodular lymphoma and subsequent clonality testing showed clonal immunoglobulin gene rearrangements. For 3 cases with a mitotic rate reported, mitotic activity by microscopy was low to intermediate and the B-cell Ki67% ranged from 6% to 23% by flow cytometry.
3.5 Clinical outcome and survival analysis
The median PFS was 119 days across all 49 cases and 15 cases were censored (Figure 2A). The median OS was 222 days and 8 cases were censored at the time of data collection (Figure 2B). Dogs received a variety of first-line therapies including CHOP-based protocol (n = 32), single agent treatment with either chlorambucil (n = 5), lomustine (n = 2), doxorubicin (n = 2) or rabacfosadine (n = 1) with L-asparaginase and prednisone variably included in these protocols, or prednisone +/− L-asparaginase (n = 6). One dog did not receive any lymphoma specific treatment. Among CHOP-treated cases, 16 dogs (50%) achieved a CR, 10 dogs (31%) achieved a PR, and 5 dogs (16%) had stable disease. Best response to therapy was not available for 1 case. Among CHOP-treated cases, the median PFS was 70 days (5 censored) and the median OS was 267 days. Twenty-two dogs initially treated with a CHOP-based protocol were treated with ≥1 rescue protocol; lomustine +/− L-asparaginase (n = 19) and rabacfosadine (n = 10) were the most pursued rescue therapies. There was no significant difference in PFS (P = .27) or OS (P = .38) between CHOP-treated cases and non-CHOP treated cases (receiving no treatment or other treatment).
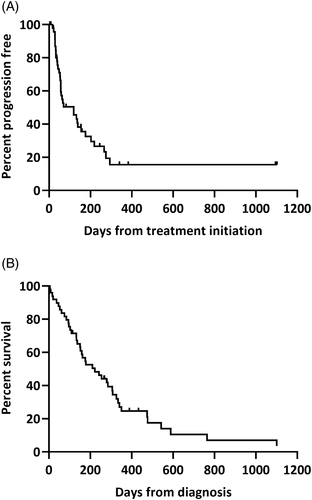
Five dogs had concurrent co-morbidities including splenic histiocytic sarcoma, pulmonary carcinoma, suspected thyroid tumor, poorly controlled diabetes mellitus and IRIS grade IV acute kidney injury (relationship to lymphoma diagnosis unclear). The dog with concurrent histiocytic sarcoma was treated with CCNU, the dog with the AKI was treated with L-asparaginase and prednisone, and the remaining 3 dogs were treated with CHOP chemotherapy. The median OS for these 5 dogs was 37 days (range, 5-434 days) which was not significantly different from OS of the other dogs (n = 44) in the study without significant comorbidities (222 days, P = .09).
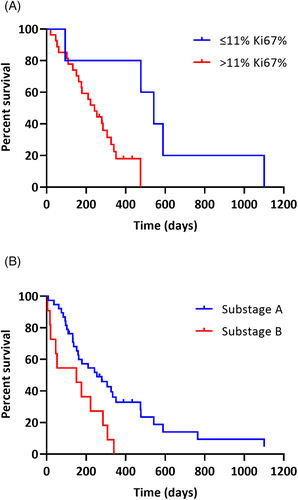
Potential prognostic factors were evaluated for association with OS by univariable analysis (Table 4). Each variable was evaluated across all cases (n = 49) and cases treated with a CHOP-based protocol (n = 32). For continuous variables, the best predicted cutoff value using X-Tile Software was used to divide groups (Table 4).16 B-cell Ki67% was prognostic among CHOP-treated dogs, with very low Ki67 expression cases (≤11% Ki67-expressing B-cells) having significantly longer OS (MST 542 days) than cases with Ki67% >11% (MST 242 days; P = .01; Figure 3A). However, the number of cases with Ki67% ≤11% was small (n = 5). When Ki67% was assessed across all cases of all treatment types, there was a similar difference in MST (542 days vs 176 days) but the association with OS was no longer statistically significant (P = .08). Smaller B-cell size as determined by flow cytometry was associated with shorter survival among CHOP-treated cases, but not across all cases. B-cell class II MHC expression and CD25 expression were associated with survival among all cases, but not CHOP-treated cases only. Across all cases, younger dogs (≤7 years) had significantly longer survival than older dogs. This association was not significant among CHOP-treated cases. There was no significant difference in age between CHOP-treated and non-CHOP-treated cases or between cases that did or did not receive a rescue protocol. Among all cases (Figure 3B) and CHOP-treated cases, dogs assessed as substage B had significantly shorter OS than dogs in substage A. Among CHOP-treated cases, the presence of lymphocytosis was associated with significantly shorter OS. Among CHOP-treated cases, dogs that achieved a CR had significantly longer PFS and OS than dogs that achieved a PR or had stable disease. Among cases with lymphocyte cell size assessed on lymph node cytology (n = 32), there was no difference in OS between cases with lymphocytes described as small to intermediate or intermediate-sized and those with intermediate to large or large-sized lymphocytes (P = .54).
| Variable | Categorya | All cases (n = 49) | CHOP-treated cases (n = 32) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Median PFS (days) (range) | P | Median OS (days) (range) | P | n | Median PFS (days) (range) | P | Median OS (days) (range) | P | ||
| B-cell Ki67%b | ≤11 | 7 | 203 (48-1095) | .17 | 542 (94-1101) | .08 | 5 | 176 (48-1095) | .21 | 542 (94-1101) | .01 |
| >11 | 42 | 63 (4-1102) | 176 (5-1102) | 27 | 58 (16-383) | 242 (20-475) | |||||
| B-cell size (FS)b | ≤410 | 24 | 63 (16-1102) | .12 | 179 (20-1102) | .45 | 16 | 56 (16-383) | .14 | 195 (20-475) | .01 |
| >410 | 25 | 176 (4-1095) | 306 (5-1101) | 16 | 147 (28-1095) | 340 (46-1101) | |||||
| Class II MHC MFIb | ≤290 | 13 | 58 (8-340) | .66 | 133 (8-340) | .01 | 9 | 57 (20-340) | .72 | 222 (20-340) | .10 |
| >290 | 36 | 119 (4-1102) | 279 (5-1102) | 23 | 119 (16-1095) | 285 (46-1101) | |||||
| CD21 MFIb | ≤54 | 31 | 57 (4-1102) | .05 | 151 (5-1102) | .06 | 19 | 56 (20-266) | .009 | 210 (20-589) | .12 |
| >54 | 18 | 203 (16-1095) | 308 (46-1101) | 13 | 219 (16-1095) | 340 (46-1101) | |||||
| CD25%b | ≤90 | 37 | 132 (16-1102) | .35 | 279 (17-1102) | .02 | 26 | 58 (16-1095) | .90 | 279 (20-1101) | .33 |
| >90 | 12 | 63 (4-383) | 117 (5-433) | 6 | 95 (28-383) | 195 (46-433) | |||||
| Age (years) | ≤7.0 | 10 | 129 (22-1102) | .32 | 542 (176-1102) | .003 | 8 | 129 (28-383) | .25 | 477 (176-542) | .13 |
| >7.0 | 39 | 70 (4-1095) | 159 (5-1101) | 24 | 58 (16-1095) | 232 (20-1101) | |||||
| Sex | Male | 28 | 139 (4-1095) | .07 | 254 (5-1101) | .89 | 19 | 136 (16-1095) | .11 | 279 (46-1101) | .84 |
| Female | 21 | 57 (8-1102) | 152 (8-1102) | 13 | 57 (20-294) | 210 (20-589) | |||||
| Stage | ≤IV | 31 | 119 (4-1095) | .30 | 210 (5-1101) | .85 | 22 | 119 (16-1095) | .74 | 282 (20-1101) | .23 |
| V | 18 | 133 (8-1102) | 222 (8-1102) | 10 | 57 (28-340) | 199 (46-434) | |||||
| Substage | A | 38 | 119 (8-1102) | .13 | 254 (8-1102) | .01 | 24 | 126 (28-1095) | .09 | 306 (61-1101) | .01 |
| B | 11 | 43 (4-340) | 151 (5-340) | 8 | 33 (16-340) | 164 (20-340) | |||||
| Anemia | Present | 15 | 119 (4-1095) | .43 | 254 (5-1101) | .72 | 8 | 89 (16-1095) | .51 | 270 (46-1101) | .46 |
| Absent | 30 | 70 (20-1102) | 232 (20-1102) | 22 | 70 (20-340) | 261 (20-589) | |||||
| Lymphocytosis | Present | 14 | 57 (8-1102) | .99 | 133 (8-1102) | .53 | 7 | 54 (28-266) | .12 | 133 (46-266) | .003 |
| Absent | 31 | 132 (0-1095) | 279 (5-1101) | 22 | 134 (28-1095) | 326 (94-1101) | |||||
| Hyperglobulinemia | Present | 6 | 86 (33-136) | .27 | 129 (75-254) | .06 | 4 | 86 (48-136) | .32 | 192 (94-254) | .12 |
| Absent | 35 | 95 (4-1102) | 242 (5-1102) | 23 | 70 (28-1095) | 279 (46-1101) | |||||
| Treatment response | CR | 16 | 147 (33-1095) | .04 | 326 (53-1101) | .01 | |||||
| PR, SD | 15 | 48 (16-340) | 176 (46-475) | ||||||||
- Note: Bold value indicates statistical significance with a P value < .05.
- Abbreviations: CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CR, complete response; FS, forward scatter; MFI, median fluorescent intensity; MHC, major histocompatibility complex; n, number of cases; OS, overall survival; P, P value; PFS, progression free survival; PR partial response; SD, stable disease.
- a For continuous variables, the cutoff was determined by X-Tile Software.
- b Factors determined by flow cytometry.
Multivariable analysis results are provided in Table 5. Though treatment type (CHOP-based protocol vs other or no treatment) did not significantly affect OS by univariable analysis (P = .38), the multivariable analysis was performed with and without treatment type included as a factor. Five variables had a P-value < .25 by univariable analysis for PFS and were included in the PFS multivariable model: B-cell Ki67%, B-cell size, B-cell CD21 expression, sex, and substage. Only substage had a P-value < .1 in the PFS multivariable model (P = .02). Six variables had a P-value < .25 by univariable analysis for OS and were included in the OS multivariable model: B-cell Ki67%, class II MHC expression, CD21 expression, CD25%, age and substage. Though hyperglobulinemia had a P-value < .25 by univariable analysis, this variable was excluded because data were only available for a subset of cases. All variables tested in the OS multivariable model, including treatment type, had a P-value < .1 except B-cell Ki67% (P = .33), which was excluded from the final model. Older age, substage B, low B-cell CD21 expression and high B-cell CD25 expression were independently associated with increased likelihood of death irrespective of whether treatment type was included in the model. Low B-cell class II MHC expression was associated with increased risk of death when treatment type was included in the model.
| Variable | Category | n | Treatment type included in model | Treatment type excluded from model | ||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratioa | 95% CI | P | Hazard ratioa | 95% CI | P | |||
| OS model (Likelihood of death) | ||||||||
| Class II MHC MFIb | ≤290 | 13 | Reference | Reference | ||||
| >290 | 36 | .37 | .16-.90 | .03 | .44 | .19-1.05 | .06 | |
| CD21 MFIb | ≤54 | 31 | Reference | Reference | ||||
| >54 | 18 | .37 | .17-.80 | .01 | .34 | .16-.74 | .01 | |
| CD25%b | ≤90 | 37 | Reference | Reference | ||||
| >90 | 12 | 4.80 | 1.96-11.73 | <.001 | 4.82 | 1.99-11.67 | <.001 | |
| Age (years) | ≤7.0 | 10 | Reference | Reference | ||||
| >7.0 | 39 | 4.30 | 1.64-11.25 | .003 | 3.78 | 1.51-9.43 | .004 | |
| Substage | A | 38 | Reference | Reference | ||||
| B | 11 | 3.20 | 1.20-8.53 | .02 | 3.29 | 1.24-8.73 | .02 | |
| Treatment typec | Non-CHOP | 17 | Reference | |||||
| CHOP | 32 | .52 | .25-1.07 | .08 | ||||
- Note: Bold value indicates statistical significance with a P value < .05.
- Abbreviations: CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CI, confidence interval; MFI, median fluorescent intensity; MHC, major histocompatibility complex; n, number of cases; OS, overall survival; P, P value.
- a Hazard ratios estimate the risk of death in those patients compared to patients in the reference group.
- b Factors determined by flow cytometry.
- c CHOP, treated with CHOP-based chemotherapy protocol; non-CHOP, received no treatment (n = 1), prednisone only (n = 3), chlorambucil (n = 5), or other chemotherapy (n = 8).
4 DISCUSSION
This study found that most cases of nodal small cell B-cell lymphoma, as defined by >80% small B-cells by flow cytometry, had an aggressive clinical course with a median OS of 222 days. Age, substage, and the levels of CD21, CD25 and class II MHC expression on B-cells were associated with outcome, while the cytologic size description of the cells was not.
Small cell B-cell lymphomas have historically been considered a primarily indolent disease, but these results demonstrate that cases often have shorter survival compared to the most common subtype of B-cell lymphoma, DLBCL. Most cases (65%) of small cell B-cell lymphoma were treated with a CHOP-based protocol, but even these cases treated with standard-of-care therapy for B-cell lymphoma had shorter OS (median OS, 267 days) compared to published cohorts of DLBCL cases treated with CHOP (median OS, 316-341 days).9, 17, 18 Only 50% of CHOP-treated small cell B-cell lymphoma cases achieved a CR, compared to a CR rate >76%-82% reported in DLBCL.17-19 Additionally, CHOP-treated small cell B-cell lymphoma cases had a short median PFS (70 days) compared to the median PFS previously reported for DLBCL (233-252 days),9, 17, 18 suggesting that small cell B-cell lymphoma cases have less durable remissions than DLBCL when treated with multi-agent chemotherapy. Overall, these data suggest that a subset of small cell B-cell lymphoma cases have more aggressive behavior and less favorable responses to chemotherapy compared to DLBCL.
The cases in this study likely represent a range of histologic subtypes. A limitation of this study is that histologic subtyping was not available for these cases and flow cytometry currently cannot differentiate WHO classification subtypes of B-cell lymphoma.5, 8, 9 While a core needle biopsy is often adequate to diagnose DLBCL, a greater section of tissue or whole lymph node allowing assessment of tissue architecture is required to classify non-DLBCL subtypes, which owners might be reluctant to pursue because of perceived increased invasiveness of the procedure. Therefore, while biopsy remains the gold standard to differentiate B-cell lymphoma subtypes, the ability to provide prognostic information for dogs with an expansion of small-sized B-cells using flow cytometry benefits pet owners and veterinarians when lymph node biopsy is declined. Based on a previous study assessing histologic subtypes,5 we hypothesize that cases in this study predominantly include histologic subtypes of DSBCL, nMZL, and FL. It is possible BCLL/SLL cases were also included since 31% of cases had lymphocytosis, however, lymphocytosis is seen in DSBCL (27%), nMZL (13%), and FL (33%) cases as well.5 Studies evaluating outcome in these non-DLBCL subtypes suggest variable clinical courses, with a subset of these cases having aggressive disease. Cases with DSBCL had a median OS of 140 days and only 4/22 cases achieved a CR.5 Historically MZL was considered an indolent lymphoma, though some studies included both splenic and nodal MZL cases and outcomes are variable.3, 20, 21 More recent studies focused on nodal MZL indicate this subtype has a more aggressive clinical course, with a median time to progression of 147 to 149 days and median lymphoma specific survival of 254 to 259 days among cases treated with chemotherapy.22, 23 Though these studies indicate nMZL overall has a poor outcome, a subset of nMZL cases have a more indolent clinical course, with a 3-year survival rate of 10% in 1 study.22 Older studies including small numbers of FL cases also suggested this subtype might have a more indolent course,20, 21 but a recent study evaluating 6 FL cases treated with chemotherapy found a median time to progression of 168 days and median lymphoma specific survival of 200 days.22 B-cell CLL can have a variable clinical course, with many cases having indolent disease, while Boxer breed cases and cases with high cellular proliferation have poorer prognosis.12, 24 It is possible dogs with BCLL were included in this study as a subset of BCLL cases can have nodal involvement.12, 25 However, dogs with lymphocytosis in this study appeared to have a much more aggressive clinical course with an OS of 133 days, as compared to a MST of 300 to 480 days12, 24 for dogs with BCLL, despite a majority of dogs having a lymphocytosis of <60 000/μL which was associated with an improved prognosis for dogs with BCLL.12 MCL, another subtype comprised of small to intermediate-sized B-cells, primarily affects the spleen in dogs and nodal MCL appears quite uncommon.3, 20, 21
We hypothesized that Ki67 expression, which is a marker of cellular proliferation, would be a useful prognostic factor for small cell B-cell lymphoma. Several studies in veterinary medicine have evaluated Ki67 expression measured by immunohistochemistry (IHC)21, 26-30 or flow cytometry10, 11, 31-33 in lymphoma for diagnostic and prognostic purposes for dogs. A recent study demonstrated correlation in Ki67 activity between flow cytometry and IHC with a small subset of outliers, which could be attributed to flow cytometry evaluating far more cells than microscopy and to pathologists generally assessing Ki67 positivity in the most proliferative areas.32 A different study identified a Ki67 index cutoff of 12.2% by flow cytometry to discriminate high-grade and low-grade lymphomas in dogs,10 which is very similar to the 11% cutoff proposed here to identify cases with different outcomes. This cutoff of 11% was more useful in the current study than cutoffs of 20% or 40% which have been used for other B-cell tumors to discriminate cases with different outcomes.11, 12 The CHOP-treated small B-cell lymphoma cases with low Ki67% (≤11%) had significantly longer survival (median OS, 542 days) than cases with higher Ki67% (median OS, 242 days). Though there was a similarly marked difference in MSTs across all cases regardless of treatment type (542 days, [range, 94-1101 days] vs 176 days [range, 5-1102 days]), this association did not reach statistical significance (P = .08). We had few cases with very low Ki67% in this study which could have limited statistical power to detect significant associations. We hypothesize that the cases in this study are comprised of a heterogeneous group of small cell subtypes, and Ki67% is useful in identifying low-grade forms with a more indolent clinical course.
Cell size as assessed by cytology can be a useful indicator that neoplastic cells are present but should not be used to predict biological behavior. A study assessing the performance of cytology to diagnose and characterize lymphoma in dogs revealed that cytology is useful in accurately diagnosing lymphoma, but accuracy decreases when attempting to predict grade and phenotype, and ancillary diagnostics must be used for further characterization.34 The cases in this study had small cell size defined by flow cytometry FS. Though only 4 cases had histology, all 4 had lymphocytes described as small to intermediate in size. Cytologic descriptions were variable, but descriptions were summarized from cytology reports from different pathologists and not blindly re-evaluated with consistent criteria. Therefore, it is difficult to interpret how much variability is attributed to differences in interpretation rather than variability in cytomorphology across cases. Most cases were interpreted cytologically as lymphoma and often had a predominance of intermediate-sized cells, but cell size assessments were variable. Some discrepancy between flow cytometry and cytology is likely attributed to differences in methodology. Flow cytometry measures the entire cell volume in 3 dimensions while cytology is evaluating 2 dimensions and the cell size determination method is variable across pathologists. A benefit of flow cytometry is that a neoplastic population can be gated based on a homogeneous phenotype and the median FS of that population is determined to objectively assess size across the entire neoplastic population. Additionally, flow cytometry evaluates 25 × 103 cells in a sample while cytology evaluates far fewer cells and microscopy cell size determination can be highly affected by sample quality. Other groups have noted cell size differences between flow cytometry and microscopy, indicating flow cytometry is likely a more objective method.35-37
This study evaluated the prognostic value of clinical and flow cytometry variables. The most useful variables identified were age, substage, and B-cell CD21, CD25, and class II MHC expression. Older age (>7 years) was associated with poorer survival. There was no significant difference in age between cases treated with CHOP and cases that received no treatment or an alternative treatment, so it did not appear that age affected the initial treatment plan. Age also did not appear to affect whether a rescue protocol was pursued. Associations between age and outcomes have been variable across studies of lymphoma in dogs,9, 38-42 but greater age was associated with poorer outcomes in 1 recent study of DLBCL.17 Cases in substage B, defined as having clinical signs,43 had poorer outcomes. Substage is a commonly used prognostic factor for lymphoma in dogs, with several studies demonstrating that substage B is associated with poorer outcomes,38, 41, 44-46 though not all studies have found this association.18, 47, 48 B-cell CD25 (Interleukin-2 receptor alpha chain) expression was significantly higher in lymphoma cases compared to healthy control nodal B-cells and high CD25 expression was associated with shorter survival, corroborating previous studies and suggesting that high CD25-positivity can be useful diagnostically to increase suspicion for lymphoma and identify cases with poorer prognosis.9, 12, 49 Low class II MHC expression on B-cells was associated with poorer outcomes in dogs with small cell B-cell lymphoma and in 2 studies of B-cell lymphoma,40, 50 but was not associated with outcome in a study of DLBCL.9 B-cell CD21 expression was not associated with outcome in a study of B-cell lymphoma40 or DLBCL9 in dogs, though low CD21 expression was associated with shorter survival in BCLL12 in dogs as seen in these small cell B-cell lymphoma cases. Among CHOP-treated cases, cases achieving a CR had better outcomes, which has been shown previously for DLBCL.17
Limitations of this study include incomplete staging in all cases, variability of induction and rescue therapies and the retrospective study design. Dogs with circulating lymphocytosis were classified as stage V in this study, however, presence of changes in the peripheral blood is not always reflective of bone marrow infiltration in dogs with lymphoma51 and without bone marrow evaluation, stage might have been underestimated in this study cohort. The degree of bone marrow infiltration as assessed by flow cytometry has been demonstrated to be predictive of outcome for nMZL52 in dogs so the lack of effect of stage on outcome should be interpreted cautiously for this study. Lymph node measurements were not consistently reported in medical records for assessment using VCOG Nodal Response Criteria,15 which might have affected accuracy of overall response rates and PFS. Additionally, there were several dogs with co-morbidities that could have affected owner treatment decisions as well as decreased survival; lack of statistical significance could be because of type II error. The cases included in this study had a high proportion of B-cells by flow cytometry (>80%). It is possible that these inclusion criteria limited this study to cases with a more diffuse pattern of lymphoma, such as DSBCL, or more advanced disease. Potentially cases with a smaller proportion of neoplastic B-cells would better represent cases with a nodular pattern or indolent clinical course. Cases with a smaller proportion of neoplastic small B-cells are more challenging to diagnose by flow cytometry and often require clonality testing unless the B-cells have an aberrant phenotype. The current study provides useful information for cases with a high proportion of neoplastic B-cells, but this outcome data might not be applicable to cases with more heterogeneous flow cytometry cell populations.
ACKNOWLEDGMENT
No funding was received for this study. The authors thank Dr. Sangeeta Rao for her contributions to statistical design and the veterinarians and clients who provided samples and clinical information for this study.
CONFLICT OF INTEREST DECLARATION
The authors Rout, Yoshimoto, Hughes, and Avery are employed by the Colorado State University-Clinical Hematopathology Laboratory, which offers diagnostic tests, including flow cytometry, on a fee-for-service basis. No other authors have a conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
All dogs with lymphoma included in this retrospective study were privately owned and lymph node samples were collected for diagnostic purposes with informed client consent. According to the Colorado State University Clinical Review Board, a specific approval was not required for this retrospective study. Control samples for flow cytometry were collected from dogs without lymphoproliferative disease used on an unrelated study after euthanasia and maintained at an AAALAC accredited facility.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




