Relationship of microsatellite instability to mismatch repair deficiency in malignant tumors of dogs
Funding information: Japan Society for the Promotion of Science, Grant/Award Number: KAKENHI Grant Number 21H04754.
Abstract
Background
Microsatellite instability (MSI) is a type of genomic instability caused by mismatch repair deficiency (dMMR) in tumors. Studies on dMMR/MSI are limited, and the relationship between dMMR and MSI is unknown in tumors of dogs.
Objectives
We aimed to identify the frequency of dMMR/MSI by tumor type and evaluate the relationship between dMMR and MSI in tumors of dogs.
Animals
In total, 101 dogs with 11 types of malignant tumors were included.
Methods
We extracted DNA from fresh normal and tumor tissues. Twelve microsatellite loci from both normal and tumor DNA were amplified by PCR and detected by capillary electrophoresis. Each microsatellite (MS) was defined as MSI if a difference in product size between the tumor and normal DNA was detected. The dMMR was evaluated by immunohistochemistry with formalin-fixed paraffin-embedded tumor tissues. Next, we confirmed whether dMMR induces MSI by serial passaging of MMR gene knockout cell lines for 3 months.
Results
Microsatellite instability was detected frequently in oral malignant melanoma. The number of MSI-positive markers was higher in cases with dMMR than in those with proficient MMR (P < .0001). Statistical analysis indicated that the occurrence of MSI in FH2305 might have relevance to dMMR. Furthermore, MSI occurred in dMMR cell lines 3 months after passaging.
Conclusions and Clinical Importance
Microsatellite instability and dMMR more frequently were found in oral malignant melanoma than in other tumors, and dMMR has relevance to MSI in both clinical cases and cell lines.
Abbreviations
-
- CFA
-
- chromosome of Canis lupus familiaris
-
- cMLH1
-
- canine MLH1
-
- cMSH2
-
- canine MSH2
-
- cMSH6
-
- canine MSH6
-
- CN-LOH
-
- copy neutral loss of heterozygosity
-
- CRISPR
-
- clustered regularly interspaced short palindromic repeats
-
- DMEM
-
- Dulbecco's modified eagle medium
-
- dMMR
-
- mismatch repair deficiency
-
- DNA
-
- deoxyribonucleic acid
-
- EDTA
-
- ethylenediaminetetraacetic acid
-
- IMAST
-
- increased microsatellite alterations at selected tetra-nucleotide repeats
-
- FFPE
-
- formalin-fixed paraffin-embedded
-
- HEK293T
-
- human embryonic kidney (HEK) 293T
-
- HRP
-
- horseradish peroxidase
-
- ICI
-
- immune checkpoint inhibitor
-
- IHC
-
- immunohistochemistry
-
- LASSO
-
- least absolute shrinkage and selection operator
-
- LOH
-
- loss of heterozygosity
-
- MMR
-
- mismatch repair
-
- MS
-
- microsatellite
-
- MSI
-
- microsatellite instability
-
- MSI-H
-
- microsatellite instability-high
-
- MSI-L
-
- microsatellite instability-low
-
- MSS
-
- microsatellite stable
-
- NP40
-
- nonidet P 40
-
- pMMR
-
- mismatch repair proficiency
-
- RNA
-
- ribonucleic acid
-
- sgRNAs
-
- small guide ribonucleic acids
-
- SDS-PAGE
-
- sodium dodecyl sulfate-polyacrylamide gel electrophoresis
-
- SV40
-
- simian virus 40
1 INTRODUCTION
Microsatellites (MS) are short repetitive sequences composed of 1 to 6 base pairs dispersed throughout noncoding regions in the genome.1 Microsatellite instability (MSI) is a condition in which replication errors, such as insertions and deletions, accumulate in MS and cause differences in MS repeats between normal and tumor tissues. In humans, MSI is detected in some patients with various tumors, particularly Lynch syndrome,2 which is a hereditary disease caused by a congenital dysfunction of DNA mismatch repair (MMR) system, referred to as mismatch repair deficiency (dMMR). Patients with this disease tend to develop tumors. Hence, it was suspected that MSI is related to carcinogenesis, regardless of tumor type. In a previous study, MSI was detected in 4/35 (11%) of dogs with mammary gland tumors,3 but the MMR system was not analyzed in that study. Previously, it was determined that dMMR tended to occur in oral malignant melanoma and hepatocellular carcinoma in dogs.4 To the best of our knowledge, no reports have comprehensively analyzed MSI in various tumors of dogs and evaluated its relationship with dMMR.
Microsatellite instability mostly is induced by dMMR. The MMR system preserves genomic stability and is mainly regulated by 4 genes: msh2, msh6, mlh1, and pms2.5 Mismatch repair deficiency is caused by a germline mutation (ie, Lynch syndrome), somatic mutation, or hypermethylation of these MMR genes in various tumors.6-10 It is known that dMMR increases tumor mutation burden and MSI, leading to carcinogenesis.11-14 In humans, dMMR and MSI are simultaneously examined by immunohistochemistry (IHC) and PCR to understand genetic mechanisms in carcinogenesis.15 In addition, numerous reports showed that tumors with dMMR and MSI are highly responsive to immune checkpoint inhibitors (ICIs).16-18 Therefore, dMMR/MSI serves as a predictive biomarker of the likely efficacy of ICIs.19 Furthermore, PCR for MS also provides information about loss of heterozygosity (LOH). Loss of heterozygosity is another type of genomic instability and is caused by various mechanism such as somatic mutations, errors of meiosis, and mitotic recombination. It is known to be associated with tumors and developmental disorders.20-22 Thus, LOH is considered a prognostic biomarker in patients with tumors, independent of MSI.23
We evaluated MSI status in several types of tumors to identify tumor types that frequently have MSI, including its relationship with dMMR in clinical cases and cell lines. Based on these data, MS loci suitable for MSI analysis were narrowed down to make the analysis more productive and accurate. Furthermore, LOH was analyzed simultaneously with MSI because only few reports have examined LOH at MS loci in tumors of dogs.
2 MATERIALS AND METHODS
2.1 Clinical samples
Clinical samples were obtained from tumor-bearing dogs that came to Yamaguchi University Animal Medical Center and Kyoto Animal Medical Center for diagnosis or treatment from May 2020 to March 2021. All dogs underwent biopsy or surgical resection and were diagnosed with malignant tumors by veterinary pathologists at Yamaguchi University or IDEXX Laboratories (Tokyo, Japan), except for some cases with lymphoma that were diagnosed only by cytology. Blood and fresh tumor tissues were collected from each dog to evaluate MSI, and an oral mucosal swab was taken instead of blood to extract normal tissue DNA when tumor cells were observed on a blood smear. Formalin-fixed paraffin-embedded (FFPE) tumor tissues were collected from each dog to evaluate dMMR. The study was approved by the Ethics Committee of Yamaguchi University.
2.2 PCR for microsatellite sequences
Genomic DNA was extracted from blood (or oral mucosal swab) and fresh tumor tissues with a QIAamp DNA Blood Mini Kit (QIAGEN Tokyo, Tokyo, Japan) or QIAamp DNA Mini Kit (QIAGEN Tokyo), respectively, and was stored at 4°C until further use.
For MSI analysis, 12 MS loci were selected (Table 1)24, 25 because of their high instability rates in a previous report3 and our preliminary experiments. Microsatellite sequences were amplified from tumor and normal DNA by PCR in 15 μL of reaction volume, including 12 ng of DNA and 2.5 μM of each primer labeled with fluorescent dye using BIOTAQ DNA Polymerase (Meridian Life Science, Inc, Memphis, Tennessee). The PCR amplicons were detected by capillary electrophoresis (3500xL Genetic Analyzer, Thermo Fisher Scientific K.K., Tokyo, Japan) and analyzed by a software application (Peak Scanner, Thermo Fisher Scientific K.K.). In each MS sequence, electropherograms of tumor and normal DNA were compared and defined as MSI-positive if a difference in product size was detected. In addition, LOH was defined as a ≥35% reduction in the peak of 1 allele in tumor DNA compared to that in normal DNA.26 The electropherograms of each sample were evaluated independently by 3 observers.
| Microsatellite marker | Repeat type | Chromosome | Forward primer | Reverse primer | Fluorescent label |
|---|---|---|---|---|---|
| C13.900 | Di | CFA 13 | TTGGACTTCTAATTTTTCATT | CAACTGACTAAATCTCCTAATG | FAM |
| PEZ11 | Tetra | CFA 8 | ATTCTCTGCCTCTCCCTTTG | TGTGGATAATCTCTTCTGTC | FAM |
| FH2054 | Tetra | CFA 12 | GCCTTATTCATTGCAGTTAGGG | ATGCTGAGTTTTGAACTTTCCC | PET |
| C22.763 | Di | CFA 22 | CAGCCCACTTCCTGGAAATA | GACCAGTGTGCATTAAGCC | HEX |
| FH2305 | Tetra | CFA 30 | TCATTGTCTCCCTTTCCCAG | AAGCAGGACATTCATAGCAGTG | FAM |
| PEZ8 | Tetra | CFA 17 | TATCGACTTTATCACTGTGG | ATGGAGCCTCATGTCTCATC | PET |
| FH2175 | Tetra | CFA 16 | TTCATTGATTTCTCCATTGGC | AGGACTCTAAAAACTTGCCTCC | HEX |
| FH3837 | Tetra | CFA 5 | GGCCTCGTAGAATACATTTGG | AGCAAGGAAGGCATCTGG | FAM |
| FH2594 | Tetra | CFA 5 | TTTAAGGAGCTGCTCATGCA | CTGAAATTCCTGGCCCAGTA | FAM |
| FH3113 | Tetra | CFA 5 | CTGAATTATGGGAAAACATGG | CAGGGAAGGAAGAAAACAGC | HEX |
| CPH14 | Di | CFA 5 | GAAAGACAATCCCTGAAATGC | ACCCCATTTATGAGAATCATGT | FAM |
| AHT137 | Di | CFA 11 | TACAGAGCTCTTAACTGGGTCC | CCTTGCAAAGTGTCATTGCT | PET |
2.3 Immunohistochemistry for dMMR analysis
As described in a previous report,4 each FFPE tissue was incubated with mouse monoclonal anti-human MLH1 antibody (clone G168-15, GeneTex, Inc, Irvine, California, diluted at 1:50), rabbit monoclonal anti-human MSH2 antibody (clone SP46, Abcam, Cambridge, UK, diluted at 1:5100), or mouse monoclonal anti-human MSH6 antibody (clone 44/MSH6, BD Transduction Laboratories, San Diego, California, diluted at 1:250). The isotype antibodies, rabbit IgG antibody (clone DA1E, Cell Signaling Technology, Inc, Danvers, Massachusetts) and mouse IgG1 antibody (clone P3.6.2.8.1, eBioscience, Inc, San Diego, California), were used as negative immunostaining controls. A peroxidase stain DAB Kit (Nacalai Tesque, Inc, Kyoto, Japan) was used to visualize the immunoreaction. The sections were counterstained with Mayer's hematoxylin. To bleach the melanocytic granules of melanoma tissues, the sections were incubated in 10% hydrogen peroxide before antigen retrieval. A Simple Stain AEC Solution (Nichirei Bioscience, Inc, Tokyo, Japan) was used to visualize the immunoreaction instead of DAB.
Immunoreactivity was evaluated if the nuclei of the tumor cells were immunostained with each antibody as compared with those in isotype controls. The immunostaining of the normal tissue areas of each sample was used as a control and simultaneously evaluated to confirm positive immunostaining. Negative immunostaining was confirmed when the nuclei of tumor cells showed negative immunostaining. The immunoreactivity of each sample was independently evaluated by 4 observers. Based on the results of IHC, the MMR status of each case was determined; MMR proficiency (pMMR) was confirmed if all 3 MMR proteins showed positive immunostaining, or dMMR was confirmed if ≥1 proteins showed negative immunostaining based on criteria used in studies of human tissues.15
2.4 Cell culture
Human embryonic kidney (HEK) 293 T-cell line and SV40-immortalizated canine gallbladder epithelial cells (kindly provided by Dr. Kenji Baba) were cultured in a Dulbecco's modified Eagle medium (DMEM)-based complete medium (DMEM mixed with 10% fetal bovine serum and 100 units/mL penicillin-streptomycin and 55 μM 2-mercaptoethanol). All cell lines were maintained at 37°C in a humidified CO2 gas incubator.
2.5 Establishment of dMMR/MSI cell lines
According to previous reports,27 CRISPR-Cas9 small guide RNAs (sgRNAs), which target 1 or more of the MMR genes (mlh1, msh2, and msh6), were designed by an online CRISPR design tool (http://zlab.bio/guide-design-resources). The sequences of sgRNAs of canine MLH1, canine MSH2, and canine MSH6 were 5′-GTGGTGAACCGCATCGCGGC, GCGCTTCTTCCAGGCCATGC-3′, and 5′-GAAGTAAGGCCTAAGGTCCA-3′, respectively. These oligonucleotides were synthesized and inserted into a lentiCRISPR v2 plasmid, which was donated by Dr. Feng Zhang (Addgene, Cambridge, Massachusetts, #52961; http://n2t.net/addgene:52961; RRID: Addgene 52961). To produce lentivirus-expressing sgRNAs that target each gene, HEK293T cells were seeded at 3.0 × 105 cells/well on a 6-well plate a day before transfection. On the day of transfection, 1.25 μg of each plasmid was mixed with PEI Max (Polysciences, Inc, Warrington, Pennsylvania) in an OPTI-MEM buffer (Thermo Fisher Scientific K.K.), incubated for 15 minutes at room temperature, and added onto HEK293T cells. After 24 hours, the supernatant of HEK293T cells was replaced with a DMEM-based complete medium without antibiotics. After another 24 hours, the supernatant containing lentiviruses was collected and transferred onto gallbladder epithelial cells. After 12 hours of infection, the supernatant was replaced with a DMEM-based complete medium, followed by the addition of 0.2 mg/mL hygromycin onto the gallbladder epithelial cells to select stably transduced cells. Individual single clones were isolated by limited dilution method and further analyzed by Western blotting to confirm the knockout of each protein. Based on the results of Western blotting, clones with complete knockout of each MMR gene were selected and passaged >30 times over 3 months after single clone isolation.
2.6 Western blotting analysis
Each cell line was collected and lysed with an NP-40 lysis buffer (1% NP40, 10 mM Tris HCl [pH 7.5], 150 mM NaCl, 1 mM EDTA). Equal amounts of protein samples (30 μg) were electrophoresed in 7% acrylamide gel before transferring them onto membranes. The membranes were incubated with each primary antibody (same as the antibodies used in IHC) diluted at 1:1000 in 0.5% skimmed milk in Tris Buffered Saline with Tween20 overnight at 4°C. Horseradish peroxidase (HRP)-conjugated anti-mouse IgG antibody (Merck Millipore, Darmstadt, Germany) or HRP-conjugated anti-rabbit IgG antibody (Jackson ImmunoResearch, West Grove, Pennsylvania) was used as a secondary antibody. The membranes were soaked in Western Lightning Plus-ECL (PerkinElmer, Tokyo, Japan) to visualize the immunoreaction. Immunoreactive bands were quantified and analyzed using AMERSHAM Image Quant 800 (Global Life Sciences Technologies Japan, Tokyo, Japan). The membranes were reused with mouse monoclonal anti-beta actin antibody (Sigma-Aldrich Japan K.K., Tokyo, Japan) to verify equal loading of proteins in each lane.
2.7 Statistical analysis
Statistical analysis was performed by JMP Pro 15 software (JMP Japan, Tokyo, Japan). A Wilcoxon rank-sum test was conducted to determine the association between the number of MSI-positive markers and dMMR/pMMR status. Statistical significance was set at P < .05.
A least absolute shrinkage and selection operator (LASSO) regression model and decision tree model were fitted to further select important variables for the association between dMMR/pMMR status (dependent variable) and MSI-positive markers (independent variables). The LASSO regression model shrank all regression coefficients toward zero and set the coefficients of irrelevant variables to zero according to the regulation weight λ. The optimal value of λ was determined by a 5-fold cross validation. The decision tree analysis used the same variables as LASSO (dMMR/pMMR status as dependent variable and MSI-positive markers as independent variable). The tuning parameters were determined by a 5-fold cross validation. Ten cases with other tumors (Table 2) were excluded in the LASSO regression and the decision tree model analyses.
| Tumor type | Number of cases |
|---|---|
| Oral malignant melanoma | 25 |
| Melanotic | 18 |
| Amelanotic | 7 |
| Lymphoma | 13 |
| T-cell | 3 |
| B-cell | 8 |
| non-T non-B | 2 |
| Mast cell tumor | 3 |
| Low grade | 3 |
| Mammary gland carcinoma | 4 |
| Simple carcinoma | 4 |
| Urothelial carcinoma | 5 |
| Urinary bladder to urethra | 5 |
| Hepatocellular carcinoma | 9 |
| Poorly differentiated | 1 |
| Moderately differentiated | 1 |
| Well-differentiated | 7 |
| Squamous cell carcinoma | 9 |
| Adenocarcinoma | 7 |
| Apocrine gland anal sac adenocarcinoma | 2 |
| Lung adenocarcinoma | 3 |
| Ceruminous adenocarcinoma | 1 |
| Thyroid adenocarcinoma | 1 |
| Hemangiosarcoma | 3 |
| Soft tissue sarcoma | 11 |
| Fibrosarcoma | 4 |
| Perivascular wall tumor | 3 |
| Leiomyosarcoma | 1 |
| Liposarcoma | 1 |
| Myxosarcoma | 1 |
| Undifferentiated pleomorphic sarcoma | 1 |
| Others | 12 |
| Osteosarcoma | 1 |
| Gastrointestinal stromal tumor | 1 |
| Spleen interstitial tumor | 1 |
| Histiocytic sarcoma | 1 |
| Carcinosarcoma | 1 |
| Undifferentiated malignant tumor | 2 |
| Intranasal carcinoma | 1 |
| Carcinoma nos | 3 |
| Sarcoma nos | 2 |
| Total | 101 |
3 RESULTS
3.1 Study population
In our study, 101 dogs with tumors were included. The characteristics of these dogs are shown in Table S1, Supporting Information. The population consisted of 51 males (27 castrated) and 50 females (38 spayed). Median age at diagnosis was 11 years and 11 months (range, 5-16 years and 10 months). The cases were classified into 25 cases of oral malignant melanoma, 13 cases of lymphoma, 3 cases of mast cell tumor, 4 cases of mammary gland carcinoma, 5 cases of urothelial carcinoma, 9 cases of hepatocellular carcinoma, 9 cases of squamous cell carcinoma, 7 cases of adenocarcinoma, 3 cases of hemangiosarcoma, 11 cases of soft tissue sarcoma, and 12 cases of other tumors (Table 2). Four lymphoma cases (cases 27, 29, 37, and 38 in Table S1) and 1 urothelial carcinoma case (case 46 in Table S1) had been treated before the tissues were collected for the study.
3.2 Microsatellite analysis for clinical cases
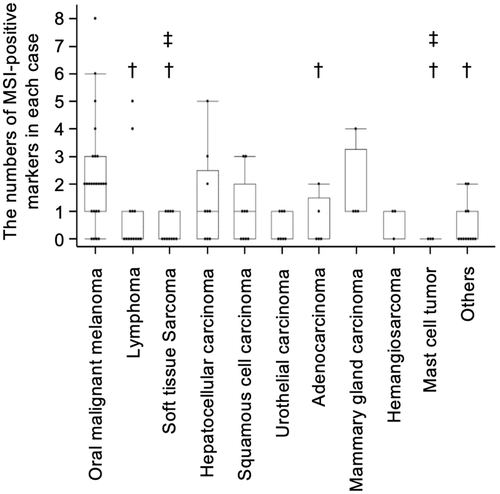
The results of MSI and LOH are shown in Table S1. Microsatellite instability was defined as an alteration in the peak size of MS amplicons in tumor DNA compared to that in normal DNA (Figure 1). At least 1 MSI-positive marker was detected in 20 cases of oral malignant melanoma, 5 cases of lymphoma, 4 cases of soft tissue sarcoma, 6 cases of hepatocellular carcinoma, 5 cases of squamous cell carcinoma, 3 cases of adenocarcinoma, 2 cases of urothelial carcinoma, 4 cases of mammary gland carcinoma, 2 cases of hemangiosarcoma, and 4 cases of others, whereas no MSI-positive marker was detected in mast cell tumors (Figure 2). Oral malignant melanomas tended to have MSI markers more frequently.
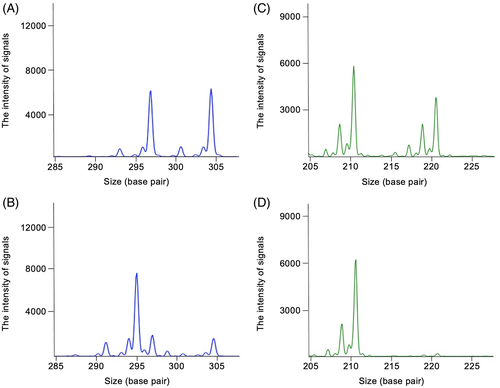
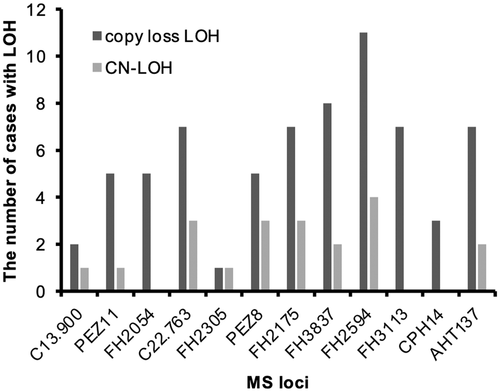
Loss of heterozygosity was defined as a reduction in the peak of 1 allele in tumor DNA compared to that in normal DNA (Figure 1) and was evaluated for all MS loci. In addition, some cases showed increased amplitude of the other allele, which could indicate copy neutral LOH (CN-LOH). Copy loss LOH (no difference in amplitude of the other allele) was observed frequently in markers located in chromosome of Canis lupus familiaris (CFA) 5 (FH3837, FH2594, and FH3113), CFA 16 (FH2175), and CFA 22 (C22.763) across tumor types (Figure 3 and Table S1). Multiple MS markers on CFA 5 showed copy loss LOH in 4 cases (cases 22, 78, 89, and 93 in Table S1).
3.3 Relationship between dMMR and MSI in clinical cases
The FFPE specimens of tumor tissues were obtained from 81 of 101 dogs. The canine MLH1, MSH2, and MSH6 (referred to cMLH1, cMSH2, and cMSH6) in these specimens were analyzed by IHC. The classifications of the 81 tumor specimens are shown in Table 3.
| Tumor type | Total cases | dMMR cases (%) |
|---|---|---|
| Oral malignant melanoma | 21 | 8 (38) |
| Lymphoma | 1 | 0 |
| Mast cell tumor | 2 | 0 |
| Mammary gland carcinoma | 4 | 1 (25) |
| Urothelial carcinoma | 5 | 1 (20) |
| Hepatocellular carcinoma | 9 | 3 (33) |
| Squamous cell carcinoma | 9 | 0 |
| Adenocarcinoma | 7 | 0 |
| Hemangiosarcoma | 2 | 0 |
| Soft tissue sarcoma | 11 | 0 |
| Others | 10 | 0 |
- Abbreviation: dMMR, mismatch repair deficiency.
Representative results of IHC are shown in Figure 4A,B. Based on criteria for dMMR used in humans, all analyzed cases were classified into pMMR or dMMR. The proportion of tumors with dMMR was 8/21 (38.1%) cases of oral malignant melanoma, 1/4 (25%) cases of mammary gland carcinoma, 1/5 (20%) cases of urothelial carcinoma, and 3/9 (33.3%) cases of hepatocellular carcinoma, whereas the remainder of the specimens were classified into pMMR (Table 3 and Table S1). For 20 cases, including 2 dMMR cases (cases 1 and 46), out of 101 cases, FFPE samples of normal tissues also were available, and expression of MMR proteins was detected in all of them (Figure S1).
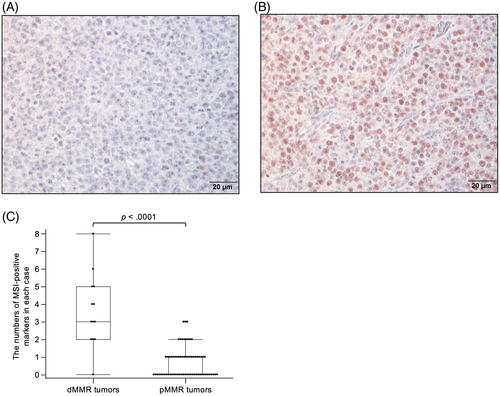
Then, whether or not dMMR was associated with MSI in clinical cases was confirmed. Comparing the number of MSI-positive markers between cases with dMMR or pMMR in entire tumors, these markers were significantly higher in cases with dMMR (Figure 4C, P < .0001). Further analysis of the relationship by tumor type identified significant results in oral malignant melanoma and hepatocellular carcinoma (P < .01 and P < .02, respectively) and entire tumors, but not in urothelial carcinoma and mammary gland carcinoma (P = .11 and P = .08, respectively). On the other hand, there was no relationship between dMMR and number of LOH (data not shown).
3.4 Statistical evaluation to select MS loci suitable for dMMR/pMMR status
To identify which MSIs contributed substantially to dMMR in tumors of dogs, the LASSO regression model and decision tree analysis were used. In LASSO, 6 variables were estimated as nonzero coefficients (but not statistically significant), which might indicate that the appearance of MSI in FH2305 and the total number of MSI-positive markers reflect dMMR (Table 4).
| Variants | Coefficients | Odds ratio | P-value | 95% confidence interval |
|---|---|---|---|---|
| AHT137 | 1.21 | 3.34 | .52 | 0.08-137.0 |
| FH2305 | 1.27 | 3.57 | .14 | 0.67-19.1 |
| CPH14 | 0.87 | 2.38 | .44 | 0.26-21.7 |
| C13.900 | 1.26 | 3.52 | .53 | 0.07-173.2 |
| FH2175 | −0.16 | 0.85 | .86 | 0.14-5.35 |
| Total MSI | 0.33 | 14.2 | .4 | 0.64-3.01 |
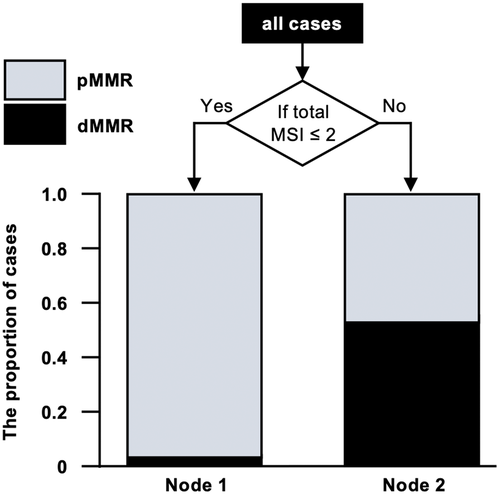
Additionally, the decision tree model indicated that the cases with dMMR or pMMR could be classified with high probability by setting up nodes with ≥2 or ≤1 MSI-positive loci, respectively (Figure 5).
3.5 Establishment of cell lines with dMMR and MSI analysis
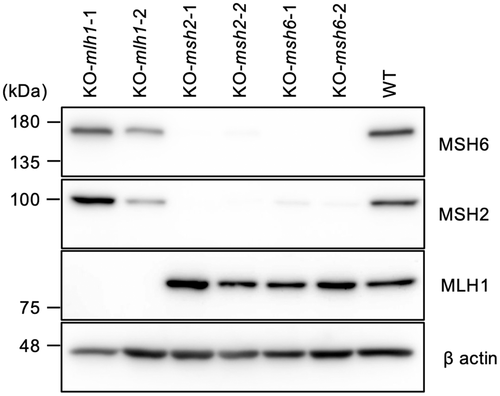
We investigated whether dMMR induces MSI in vitro by canine cell lines after knockout of the MMR gene. A CRISPR/Cas9 system was used to establish the dMMR cell lines, and was followed by confirmation of knockout by Western blotting (Figure 6) and immunocytochemistry (Figure S2). No MLH1 protein was detected in both clones of the MLH1 knockout cell line. The MSH2 and MSH6 knockout cell lines expressed no MSH6 and MSH2, in addition to defective expression of MSH2 and MSH6, respectively, as shown in a previous study.4 The knocked-out cell lines and wild-type cells were serially passaged for 3 months to allow mutations to accumulate in MS loci. Then, the MSI status in these cell lines was analyzed at 2 time points (0 and 3 months). Based on results of the MSI analysis, an MSI-positive marker occurred in dMMR cell lines but not in wild-type cell lines from 0 to 3 months. At 3 months, 2 loci (FH2054 and AHT137) showed MSI in a cMLH1-deficient cell line, 2 loci (PEZ11 and FH3837) in a cMSH2-deficient cell line, and 4 loci (PEZ11, FH2054, FH2594 and AHT137) in a cMSH6-deficient cell line (Table 5).
| Cell lines | Microsatellite markers | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C13.900 | PEZ11 | FH2054 | C22.763 | FH2305 | PEZ8 | FH2175 | FH3837 | FH2594 | FH3113 | CPH14 | AHT137 | |
| KO-mlh1 | MSI | MSI | ||||||||||
| KO-msh2 | MSI | MSI | ||||||||||
| KO-msh6 | MSI | MSI | MSI | MSI | ||||||||
| Wild type | LOH | |||||||||||
- Note: Blank cells indicates no alteration.
- Abbreviations: LOH, loss of heterozygosity; MSI, microsatellite instability.
4 DISCUSSION
In this study, MSI and dMMR frequently were detected, mainly in canine oral malignant melanoma. A previous report by a different cohort indicated that oral malignant melanoma and hepatocellular carcinoma in dogs tended to have a defective MMR system,4 which is consistent with our current study. In addition, we found that dMMR was related to MSI in both clinical cases and cell lines, which was supported by our results in the LASSO regression and decision tree model. However, the number of MSI-positive markers in the cell lines and some clinical cases with dMMR was lower than expected based on a previous study of humans showing that knockout of MMR genes results in high frequencies of MSI.28 There are several possible reasons for this difference: (a) presence of factors other than dMMR involved in the appearance of MSI; (b) other proteins that function as major MMR proteins; and (c) inappropriate MS loci used. Increased MS alterations at selected tetra-nucleotide repeats (IMAST) especially must be considered because 8 of all 12 MS loci used in our study were tetra-nucleotide repeat sequences (Table 1). The IMAST are caused by dysfunction of MSH3,29 which is 1 of the MMR proteins, rather than that of MSH2, MSH6, MLH1, and PMS2. It was reported that IMAST occurring at tetra-nucleotide MS consisted of “AAAG” or “ATAG.” In our study, 6 markers (PEZ11, FH2305, PEZ8, FH2175, FH3837, FH2594) consisted of “AAAG” repeats and 1 marker (FH2054) consisted of “ATAG” repeats. However, the expression of MSH3 proteins was not examined in our current study.
In humans, MSI is evaluated by analysis of 5 selected MS loci, and a tumor sample is defined as MS stable (MSS) if all 5 sequences are normal, MSI-low (MSI-L) if 1 sequence is mutated, or MSI-high (MSI-H) if ≥2 sequences are mutated. High microsatellite instability is highly consistent with dMMR in various tumors.30, 31 Several studies reported that tumors with dMMR/MSI-H have approximately 25 times more mutations than other tumors,14 suggesting that tumors with dMMR/MSI-H express a number of neoantigens and are more immunogenic compared to tumors with pMMR/MSS or pMMR/MSI-L. Thus, it was expected that tumors with dMMR/MSI-H might be sensitive to ICIs, particularly for PD-1 or PD-L1 inhibitors. In fact, pembrolizumab, a PD-1 inhibitor used in humans was reported in clinical trials to be effective in patients with dMMR/MSI-H solid malignancies that progressed after chemotherapy, regardless of tumor type.32-35 These results made pembrolizumab the first drug authorized for treatment based on MSI status rather than the pathological diagnosis.36
Recently, clinical trials of ICIs by canine PD-1 or PD-L1 inhibitors were reported in spontaneous tumors of dogs including oral malignant melanoma, and showed efficacy in some cases.37-39 In these studies, some dogs with oral malignant melanoma experienced clinical benefits. Hence, in veterinary medicine, tumors with MSI/dMMR possibly could have a good response to PD-1 and PD-L1 inhibitors. Although few reports on ICI treatment for mammary gland carcinoma and hepatocellular carcinoma in dogs exist, studies evaluating its efficacy against these tumors are expected because of the high likelihood of MSI/dMMR.
As mentioned above, only 5 MS loci are used for MSI analysis in human medical practice. This MS panel is composed of mono- or di-nucleotide repeats because tri-nucleotide repeats are less altered than are mono- and di-nucleotide repeats.40 Previous studies analyzed the MSI of multiple MS loci and MMR status and narrowed down the loci suitable for MSI analysis to 10 candidates.40, 41 Based mainly on these reports, the National Cancer Institute recommended an MS panel for MSI analysis and defined the border between MSI-H and MSI-L in 1998.42 In our study, the appearance of MSI in C13.900, FH2054, CPH14, and AHT137 may particularly have relevance to dMMR however none of the results were significant. Lack of significance was probably a consequence of insufficient sample size. Moreover, our MS panel contained only a few di-nucleotide and no mononucleotide repeats. Furthermore, MSI analysis by mono- or di-nucleotide repeats is needed if the influence of IMAST also is considered. The border between MSI-H and MSI-L in tumors of dogs also should be decided.
Furthermore, LOH frequently was identified in the markers located in CFA 5, 16, and 22, suggesting mutations of tumor suppressor genes in the same region. Copy loss LOH in MS loci suggests that inactivation of nearby tumor suppressor genes may contribute to carcinogenesis.22 In fact, tumor suppressor genes TP53, WRN, and RB1 are located in CFA 5, 16, and 22, respectively.43 Thus, it was suspected that there were tumors with pMMR in our study caused by somatic deletion of the wild-type allele of these genes. Another study found that TP53 is mutated in 16.7% of tumors in dogs,44 which is similar to our results showing the detection of LOH at MS loci FH2594 in 15 cases. Tumor suppressor genes located in CFA 16 also may contribute to carcinogenesis. The CN-LOH was also detected sporadically across tumor types. Copy neutral loss of heterozygosity by mitotic recombination also can contribute to oncogenesis. For example, BRCA1/2 and Cyclin D1 are implicated in CN-LOH in tumors of humans.45, 46 In dogs, oncogenes also may be involved in the occurrence of CN-LOH. However, we did not evaluate expression of such tumor suppressor genes and oncogenes in our study, and more detailed studies are needed.
In our study, immunolabeling of PMS2 protein was not done because no monoclonal antibodies were certified to cross-react with canine PMS2 protein. In addition, the number of cases varied markedly depending on the tumor type. Because of the small number of cases of each tumor type, except for oral malignant melanoma, it will be necessary to recruit more tumor cases to evaluate the frequency of dMMR/MSI more accurately.
In conclusion, to our knowledge, ours is the first report in veterinary medicine to identify tumor types with frequent dMMR/MSI using a comprehensive analysis of dMMR and MSI. Our results also suggest a relationship between dMMR and MSI in clinical cases and in vitro. Additional studies are needed to consider other candidates.
ACKNOWLEDGMENT
Funding provided by the Ministry of Education, Culture, Sports, Science and Technology, Japan Society for the Promotion of Science (JSPS), KAKENHI Grant Number 21H04754. We thank Drs Masaru Okuda, Satoshi Kambayashi, Munekazu Nakaichi, and Kenji Tani for providing tumor samples. We are grateful to all YUAMEC clinical staff for helping us in the clinics. We also thank Dr Naoto Tsujimoto (Eli Lilly Japan K.K.) for consulting a statistical analysis.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the Ethics Committee of Yamaguchi University Animal Medical Center.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




