Effects of pimobendan in cats with hypertrophic cardiomyopathy and recent congestive heart failure: Results of a prospective, double-blind, randomized, nonpivotal, exploratory field study
Funding information: Boehringer Ingelheim Vetmedica GmbH
Abstract
Background
The benefits of pimobendan in the treatment of congestive heart failure (CHF) in cats with hypertrophic cardiomyopathy (HCM) have not been evaluated prospectively.
Hypothesis/Objectives
To investigate the effects of pimobendan in cats with HCM and recent CHF and to identify possible endpoints for a pivotal study. We hypothesized that pimobendan would be well-tolerated and associated with improved outcome.
Animals
Eighty-three cats with HCM and recently controlled CHF: 30 with and 53 without left ventricular outflow tract obstruction.
Methods
Prospective randomized placebo-controlled double-blind multicenter nonpivotal field study. Cats received either pimobendan (0.30 mg/kg q12h, n = 43), placebo (n = 39), or no medication (n = 1) together with furosemide (<10 mg/kg/d) with or without clopidogrel. The primary endpoint was a successful outcome (ie, completing the 180-day study period without a dose escalation of furosemide).
Results
The proportion of cats in the full analysis set population with a successful outcome was not different between treatment groups (P = .75). For nonobstructive cats, the success rate was 32% in pimobendan-treated cats versus 18.2% in the placebo group (odds ratio [OR], 2.12; 95% confidence interval [CI], 0.54-8.34). For obstructive cats, the success rate was 28.6% and 60% in the pimobendan and placebo groups, respectively (OR, 0.27; 95% CI, 0.06-1.26). No difference was found between treatments for the secondary endpoints of time to furosemide dose escalation or death (P = .89). Results were similar in the per-protocol sets. Adverse events in both treatment groups were similar.
Conclusions and Clinical Importance
In this study of cats with HCM and recent CHF, no benefit of pimobendan on 180-day outcome was identified.
Abbreviations
-
- D
-
- study day
-
- DCM
-
- dilated cardiomyopathy
-
- CHF
-
- congestive heart failure
-
- FAS
-
- full analysis set
-
- HCM
-
- hypertrophic cardiomyopathy
-
- HR
-
- hazard ratio
-
- LV
-
- left ventricle
-
- LVOTO
-
- left ventricular outflow tract obstruction
-
- SAF
-
- safety data set
-
- PG
-
- pressure gradient
-
- PPS1
-
- per-protocol set 1
-
- PPS2
-
- per-protocol set 2
1 INTRODUCTION
Cardiomyopathy is common in cats and encompasses several morphological variants, the most prevalent of which is hypertrophic cardiomyopathy (HCM).1 Although a long asymptomatic stage is a characteristic of HCM2 in cats, acute decompensation with signs of congestive heart failure (CHF) or thromboembolic disease or both require immediate veterinary intervention.3, 4 However, long-term prognosis in cats with HCM and CHF remains poor,2 in particular in cats with CHF because of end-stage HCM. Additional treatments are needed to improve morbidity and long-term survival in cats with HCM and CHF.
Pimobendan is a calcium-sensitizing and load-reducing agent (inodilator) approved in the United States and Europe for use in small breed dogs with myxomatous valve disease.5, 6 It also has been determined to delay the onset of CHF in Doberman pinschers with dilated cardiomyopathy (DCM).7 In these prospective, controlled trials,5-7 quality of life, delayed onset of cardiac decompensation, and survival time after development of CHF all have been identified after treatment with pimobendan. Although not licensed for cats, pimobendan is used with increasing frequency off-label for cats with cardiomyopathy and heart failure.8-13 Whether or not cats with HCM benefit from pimobendan is currently unknown. Pimobendan might be beneficial in cats with HCM based on several mechanisms.13-17 However, based on hemodynamic assumptions,18-20 positive inotropic agents have been considered contraindicated in HCM associated with diastolic heart failure and, in particular, HCM with dynamic left ventricular outflow tract obstruction (LVOTO).21-23 Data on the use of pimobendan in cats are limited,8, 9, 12, 24-26 and the efficacy of pimobendan in cats with HCM and CHF has not been prospectively evaluated.
Given the paucity of information on the use of pimobendan in cats, evidence-based, prospective data are needed. Our objective was to investigate the short- and intermediate-term effects of pimobendan in cats with HCM and recent CHF, with and without LVOTO. This exploratory study was intended to assist in gathering data on tolerability, morbidity, and mortality and to identify possible efficacy variables and clinical endpoints for a larger pivotal field trial on the use of pimobendan in cats with HCM.
2 MATERIALS AND METHODS
2.1 Study design
This was a prospective, multicenter, double-blind, randomized, placebo-controlled, nonpivotal, exploratory field study.
2.2 Cats
Client-owned cats with before (within the past 60 days) CHF secondary to HCM but without signs of congestion and heart failure at the time of enrollment were recruited at 10 centers (6 in the United States and 4 in Europe) between 2011 and 2013. Because of the nonpivotal (exploratory) nature of the study and absence of any published data in cats with HCM using a dose increase of furosemide as a component of the primary endpoint, and considering feasibility (anticipated length of enrollment period of <18 months), a sample size of approximately 40 cats per treatment group was chosen. All clients of cats enrolled gave written informed consent for participation.
2.2.1 Inclusion criteria
Cats with body weight ≥2 kg, aged ≥12 months, with HCM and a recent (but not current) diagnosis of CHF, clinical euvolemia, and hematocrit and total plasma protein concentration within the laboratory reference range were eligible. Presence of HCM was confirmed in cats with increased left ventricular (LV) end-diastolic wall thickness ≥6 mm of unknown cause as determined by echocardiography.1, 27 Cats with and without dynamic LVOTO were included. Obstructive HCM was defined by the presence of dynamic LVOTO with a peak systolic pressure gradient (PG) across the obstruction ≥30 mm Hg as assessed by continuous wave Doppler and a late-peaking Doppler flow profile.21, 22, 28 Nonobstructive HCM was defined by a peak systolic PG across the LVOT <30 mm Hg. Cats had to be clinically asymptomatic at enrollment without evidence of pulmonary edema and pleural effusion but with a history of clinical and radiographic evidence of CHF within the last 2 months (≤60 days). One of the following diagnostic criteria had to be met for a cat to be considered to have CHF: (a) medical record documentation of thoracic radiographs from the investigator's site to support the diagnosis of CHF (cardiogenic pulmonary edema or pleural effusion or both), (b) historical diagnosis of CHF made by a board-certified cardiologist, (c) CHF based on thoracic radiographs provided by referring veterinarians and confirmed by the investigator, and (d) in situations where radiography could not be performed before treatment because of instability of the cat but with clinical evidence of tachypnea, open mouth or labored breathing, response to treatment with furosemide, thoracocentesis, or some combination of these. After stabilization, evidence of HCM on echocardiography severe enough to be compatible with CHF was a general requirement for enrollment.
2.2.2 Exclusion criteria
Conditions other than HCM capable of causing LV wall thickening; cardiac arrhythmias judged clinically relevant; CHF precipitated by known noncardiac events such as parenteral fluid administration, treatment with corticosteroids, anesthesia, and before surgery; concurrent primary respiratory disease; thromboembolism; intracardiac thrombi; systemic hypertension (systolic blood pressure ≥160 mm Hg); endocrinopathies; and azotemia (BUN >60 mg/dL [>21 mmol/L] and creatinine concentration >2.5 mg/dL [>221 μmol/L]) were reasons for exclusion. Cats receiving ≥1 of the below treatments were not enrolled: sedation with ketamine and dexmedetomidine; nitroglycerin (within the past 12 hours); angiotensin converting enzyme inhibitors (within the past 24 hours), antiarrhythmics, diuretics other than furosemide, antiplatelet medications other than clopidogrel, anticoagulants, pimobendan (within the past 7 days), and beta blockers (within the past 10 days).
Postinclusion removal criteria included withdrawal of owner consent, development or worsening of dynamic LVOTO 2 to 5 hours postmedication after the first dose on Day (D) 0 (increase in systolic LVOT PG of >25 mm Hg in a previously obstructive cat or development of a systolic LVOT PG >50 mm Hg in a previously nonobstructive cat), total daily furosemide dose >10 mg/kg; development of adverse events necessitating unblinding; concomitant cardiovascular medications deemed necessary by the investigator; thromboembolism; removal deemed necessary for animal welfare reasons; and discovery postenrollment that the animal did not meet inclusion criteria.
2.3 Study medication
Pimobendan tablets (Vetmedin Flavour tablets 1.25 mg in the Europe, Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany, and Vetmedin Chewable tablets 1.25 mg in the United States, Boehringer Ingelheim Vetmedica Inc, Saint Joseph, Missouri) and placebo were identical in terms of appearance, smell, and taste at all study sites. Both pimobendan and placebo were administered PO q12h, depending on body weight: 2 to 3.1 kg (0.5 tablet), >3.1 to 5.2 kg (1 tablet), >5.2 to 7.2 kg (1.5 tablets), and >7.2 kg (2 tablets). The target dose of pimobendan was 0.3 mg/kg q12h.
2.4 Concomitant treatments
At the discretion of the individual investigators, concomitant administration of furosemide (Furozenol 10 mg tablets in the Europe and Salix 12.5 mg tablets in the United States) and clopidogrel (Plavix 75 mg tablets) was allowed.
2.5 Population analyzed
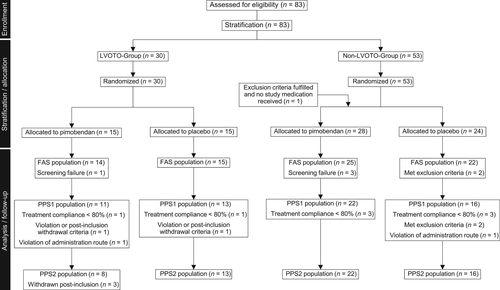
For the purpose of statistical analysis, 4 cat populations were defined: the safety set (SAF), the full analysis set (FAS), the per-protocol set 1 (PPS1), and the per-protocol set 2 (PPS2; Figure 1). The SAF set consisted of all cats that were randomized and received at least 1 dose of the study medication. The FAS set was a subset of SAF with any cats violating inclusion criteria removed. The PPS1 population consisted of all cats of the FAS that reasonably complied with the protocol. Minor deviations from the ideal still may have occurred, but major protocol deviations affecting ability to assess treatment success led to exclusion from this protocol set. Finally, the PPS2 population consisted of all cats of the PPS1 population, but with removal of cats that fulfilled the post-inclusion withdrawal criterion “development or worsening of dynamic LVOTO on D0.”
2.6 Endpoints
The primary endpoint of the study was successful outcome at D180, defined as remaining in the study without an increase in furosemide dose. Withdrawal from the study for any reason before D180 or an increase in furosemide dose therefore would indicate failure to meet the primary endpoint. The secondary endpoints were time to: withdrawal from the study, morbidity or mortality, first furosemide escalation, furosemide dose >10 mg/kg/d, hospitalization for CHF, requiring precluded medications, aortic thromboembolism (ATE), and increase of severity of LVOTO (as defined previously). Decisions regarding dose escalation of furosemide and initiation of precluded medications were made at the discretion of the individual investigator.3
2.7 Diagnostic methods
The sequence of applied study methods is summarized in Table S1. Physical examination, Doppler blood pressure measurements,29 thoracic radiography,30, 31 transthoracic echocardiography,32 and electrocardiography33 were performed and analyzed (Supplement 1).
2.8 Statistical analysis
Statistical analysis was performed using SAS System Version 9.2, SAS Institute (Cary, North Carolina). Normality of data was assessed by visual inspection, the Kolmogorov-Smirnov test, and the D'Agostino & Pearson test. The null hypothesis “success rate of cats treated with pimobendan is equal to success rate of cats treated with placebo” was tested against its alternative hypothesis by means of a 2-sided Cochran-Mantel-Haenszel test34, 35 controlled for LVOTO as stratification variable. Adjusted Mantel-Haenszel type odds ratios (ORs) with 95% confidence intervals (CIs) were provided to quantify the treatment effect. The Breslow-Day test36 was used to assess homogeneity among LVOTO subgroups. To evaluate the sensitivity of the primary analysis, logistic regression analysis was performed with treatment and LVOTO as fixed effects and the continuous covariate furosemide dose (mg/kg) as baseline. Additional sensitivity analysis was done using a logistic regression model encompassing “center” as a random effect. The “center” effect also was evaluated visually using graphical display methods. Group differences for time-to-event were evaluated using proportional hazard regression with main effects of treatment and LVOTO. Cats that had experienced no events were censored on D180 or on the day of study removal. Effects of treatment, LVOTO, sex, age, body weight, diastolic class, and furosemide dose on D0 on outcome were analyzed using various logistic regression models. A P-value ≤.05 and OR with 95% CI not including 1.0 were considered significant.
(Please see additional information on randomization, allocation, blinding, schedule of events [also in Table S1], and safety monitoring in Supplement 2, Complete Materials and Methods, for review of supporting information not appearing in the parent article.)
3 RESULTS
Because this study was designed as a nonpivotal, explorative field study and in accordance with guidelines of the European Medicine Agency (EMA) on statistical principles for clinical trials for veterinary medical products,37 we primarily present results of the FAS population. Most results on the SAF and PPS2 cohorts are presented in the supplemental files.
Data on demographics and strata of cats included in the FAS population are documented in Table 1. Thirteen different breeds were represented of which most (42, 55%) were domestic shorthair cats. Treatment groups were similar with regard to breed, sex, age, and presence of LVOTO (all P > .05). The number of cats enrolled per center was variable (17, 15, 10, 9, 7, 6, 6, 6, 6, and 1).
| Pimobendan (n = 39) | Placebo (n = 37) | |
|---|---|---|
| Sexa | ||
| Female | 8 (20%) | 10 (27%) |
| Male | 31 (80%) | 27 (73%) |
| BW (kg) | 5.30 ± 2.23 | 5.20 ± 1.54 |
| Age (year) | 6.50 ± 4.68 | 6.40 ± 4.37 |
| LVOTO – yes | 3.7 ± 2.67 | 4.4 ± 3.42 |
| LVOTO – no | 8.1 ± 4.87 | 7.7 ± 4.52 |
| Time since 1st diagnosisb (days) | 12.1 ± 19.5 | 12.8 ± 20.2 |
- Note: Group LVOTO – yes and pimobendan (n = 14), group LVOTO – yes and placebo (n = 15), Group LVOTO – no and pimobendan (n = 25), and group LVOTO – no and placebo (n = 22).
- Abbreviations: BW, body weight; FAS, full analysis set; LVOTO, left ventricular outflow tract obstruction.
- a 97% of cats were neutered or spayed. Absolute and relative frequencies as well as means and standard deviations are presented.
- b Mean (±SD) time between identification and stabilization of CHF and enrollment.
A flow diagram depicting the study populations is presented in Figure 1. Of the 83 cats that were randomized, 1 cat did not receive the study medication because of presence of systemic hypertension. Thus, 82 cats comprised the SAF population. Six cats were considered screening failures (1 each because of abnormal renal function test results, intracardiac thrombi, arrhythmia, not amenable to treatment, diabetes mellitus, and surgery <7 days before enrollment). Therefore, 76 cats remained in the FAS population. Because of minor protocol deviations (eg, treatment noncompliance, meeting postinclusion withdrawal criteria violation of administration route) 62 cats remained in the PPS1 population. Finally, 3 additional cats were removed because of worsening of LVOTO on D0, resulting in 59 cats in the PPS2 population.
The following cats experienced premature termination of the study before reaching D180: 7 cats because of progression of cardiac disease leading to euthanasia and 3 cats because of sudden death (4 receiving pimobendan and 6 receiving placebo); euthanasia because of a noncardiac cause in 1 cat (pimobendan group); and 1 cat was removed because of owner withdrawal (placebo group). Twenty-three cats met postinclusion removal criteria: worsening of LVOTO in 3 (all receiving pimobendan, removed on D0, D0, and D3); thromboembolic disease in 2 (1 receiving pimobendan and 1 placebo, removed on D6 and D35); daily dose of furosemide >10 mg/kg in 9 (5 receiving pimobendan and 4 placebo, removed on D9, D18, D34, D38, and D109 and D60, D69, D91, and D101, respectively); at owners request in 3 (1 receiving pimobendan and 2 placebo, removed on D6, D23, and D40); and because of investigator withdrawal in 6 (4 receiving pimobendan and 2 placebo, removed on D11, D39, D70, D132, D8, and D105, respectively). Table 2 summarizes the clinical and echocardiographic data at baseline (D0) of the FAS populations separated into strata.
| Pimobendan (n = 39) | Placebo (n = 37) | |
|---|---|---|
| HR (min−1) | 196 ± 28 | 202 ± 24 |
| RR (min−1) | 43 ± 18 | 50 ± 23 |
| LVOTOa | 14 (36%) | 15 (41%) |
| LAD (mm) | 19.50 ± 3.70 | 20.10 ± 4.16 |
| IVSd (mm) | 6.40 ± 1.34 | 6.90 ± 1.10 |
| LVFWd (mm) | 7.30 ± 1.29 | 7.40 ± 1.63 |
| LVIDd (mm) | 14.80 ± 2.67 | 14.20 ± 2.58 |
| LVIDs (mm) | 8.0 ± 2.59 | 7.40 ± 2.03 |
| SF (%) | 47 ± 11 | 47 ± 11 |
| S′ (cm/s) | 4.7 ± 2.0 | 4.9 ± 1.4 |
| IVRT (ms) | 47 ± 11 | 47 ± 17 |
| E (m/s) | 0.90 ± 0.37 | 0.90 ± 0.36 |
| E:A | 1.60 ± 0.78 | 2.10 ± 1.12 |
| E′ (cm/s) | 5.0 ± 2.26 | 6.1 ± 2.65 |
| E:E′ | 19.2 ± 10.4 | 17.5 ± 10.2 |
| LV diastolic function | ||
| Normal | 1 (3%) | 2 (5%) |
| Abnormal | 28 (72%) | 31 (84%) |
| Class 2 and 3 | 10 (26%) | 7 (19%) |
| Class 4 and 5 | 18 (46%) | 24 (65%) |
| Not evaluatedb | 10 (25%) | 4 (11%) |
- Abbreviations: Class 4 and 5, “pseudonormal” and “restrictive” left ventricular filling; d, measured at end-diastole; E, peak velocity of early transmitral flow; E:A, ratio between E and peak velocity of late transmitral flow velocity (A); E:E′, ratio between E and E′; E′, peak velocity of the lateral mitral annulus measured by tissue Doppler imaging in early diastole; HR, heart rate; IVRT, isovolumic relaxation time; IVS, dimension of the interventricular septum; LAD, maximum left atrial cranial-caudal dimension measured parallel to the mitral valve plane; LVFW, dimension of the left ventricular free wall; LVID, left ventricular dimension; LVOT, left ventricular outflow tract obstruction; RR, respiratory rate; s, measured at end-systole; S′, peak velocity of the lateral mitral annulus measured by tissue Doppler imaging in systole; SF, left ventricular shortening fraction.
- a Determined by continuous wave Doppler echocardiography and defined as systolic pressure gradient across the LVOT ≥30 mm Hg.
- b Because of missing data or fusion of filling waves; Absolute and relative frequencies as well as means and standard deviations are presented. There was no difference between treatment groups for any variable (all P > .05).
On D0, 44/76 (58%) cats had systolic anterior (cranial) motion of the mitral valve (SAM), with 22 cats in each treatment group of which dynamic LVOTO with a systolic PG ≥30 mm Hg was present in 29/76 (38%) cats (14 receiving pimobendan and 15 placebo). In Table S3, mean systolic PGs across the LVOT are summarized for each visit during the entire study period including all cats (FAS population). In Tables S4 and S5, data on the systolic PG across the LVOT for each visit according to presence or absence of LVOTO are summarized. The PG across the LV outflow tract in obstructive cats was not different 2 to 5 hours after pimobendan (Figure 2A; P = .55) and placebo (Figure 2B; P = .15) compared to premedication PGs on D0. In 4 obstructive cats, the systolic PG was increased after pimobendan by 60, 45, 35, and 30 mm Hg. More specifically, systolic PG increased from 70 to 135 mm Hg in 1 cat and 46 to 91 mm Hg in another cat after pimobendan, with no clinical signs observed. One cat in which PG increased from 67 to 102 mm Hg after pimobendan vomited and temporarily appeared less active. The cat with an increased PG after pimobendan of 30 mm Hg (from 52 to 82 mm Hg) had a brief episode of open-mouth breathing on the car ride home. In 4 obstructive cats, the systolic PG after pimobendan was decreased by 36, 29, 15, and 14 mm Hg. In the placebo group, the systolic PG was increased in 3 obstructive cats by 24, 15, and 15 mm Hg and decreased in 6 cats by 70, 56, 32, 23, 18, and 16 mm Hg after medication. No clinical signs were reported. Considering treatment groups, neither pimobendan (P = .08) nor placebo (P = .45) induced dynamic LVOTO in nonobstructive cats (Figure 2C,D). In 6 nonobstructive cats, the systolic PG was increased by 31, 14, 14, and 12 mm Hg (after pimobendan) and by 20 and 13 mm Hg (after placebo). No clinical signs were observed. Results on the long-term (180 days) effect of pimobendan (n = 4) and placebo (n = 9) on LVOTO in all cats with a successful outcome are presented in Figure 3. In both groups, the PG was decreased at the end of the study (P = .009 for pimobendan and P = .001 for placebo).


Forty-eight of 76 cats (63.2%) in the FAS population received clopidogrel; 23 (59%) in the pimobendan group and 25 (68%) in the placebo group. The dose of furosemide on D0, the dose of furosemide averaged over time, and the time-adjusted furosemide dose compared to baseline are presented in Table S6. The dose of furosemide was comparable between pimobendan and placebo, and between cats with and without LVOTO at any time. Thirty-three of 76 (43.4%) cats were sedated at least once during the examinations, with no differences among treatment groups.
3.1 Assessment of efficacy
Efficacy of treatment was assessed in the overall FAS population (n = 76) and consisted of 39 cats in the pimobendan group (mean daily dose of 0.6 ± 0.16 mg/kg, 25th to 75th percentile 0.5 to 0.7) and 37 cats receiving placebo. Separate subgroup analyses for cats with LVOTO (n = 29) and without LVOTO (n = 47) also were done. In addition, the primary endpoint was assessed for the PPS1 and PP2 populations (see Table S7 for PPS2). Proportions of cats in the 4 analysis sets are presented in Table S2.
3.2 Assessment of efficacy: Primary endpoint
In total, 53.9% (41/76 cats) completed the study on D180; 51.3% (20/39 cats) that received pimobendan and 56.8% (21/37 cats) that received placebo. Of these cats, 25 cats did not need an increase in furosemide, and therefore 32.9% (25/76 cats) successfully completed the study endpoint in the FAS population. The proportion of cats with a successful outcome in the pimobendan group was 30.8% (12/39 cats) and 35.1% (13/37 cats) in the placebo group (P = .75). The OR was 0.85 with a 95% CI of 0.33 to 2.22. In the LVOTO stratum (n = 29), the success rate for the pimobendan group was 28.6% (4/14 cats) and 60% (9/15 cats) for the placebo group (OR, 0.27; 95% CI, 0.06-1.26; Table 3; Figure 4). In the non-LVOTO group (n = 47), the success rate for the pimobendan group was 32.0% (8/25 cats) and 18.2% (4/22 cats) for the placebo group (OR, 2.118; 95% CI, 0.54-8.34; Figure 4). Because of the low number of observations, additional testing generating P values beyond the 95% CIs of ORs was not done.
| Treatment group | N-1 | N-2 | Odds ratio | 95% CI | |
|---|---|---|---|---|---|
| All | Pimobendan | 39 | 12 | 0.855 | 0.33-2.22 |
| Placebo | 37 | 13 | |||
| LVOTO | Pimobendan | 14 | 4 | 0.267 | 0.06-1.26 |
| Placebo | 15 | 9 | |||
| No LVOTO | Pimobendan | 25 | 8 | 2.118 | 0.54-8.34 |
| Placebo | 22 | 4 |
- Notes: There was no statistical difference between treatments in each group (P > .05).
- Abbreviations: CI, confidence interval; LVOTO, left ventricular outflow tract obstruction; N-1, all cats; N-2, cats that reached the primary endpoint of successful outcome at D180.
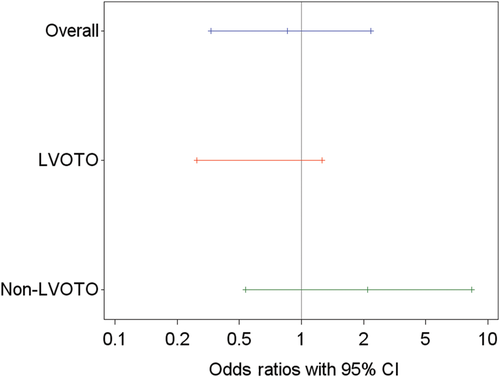
The results for the PPS1 and PPS2 population (Table S7; Figure S1) were similar to those of the FAS population. No significant differences were found between treatment groups for the primary endpoint in PPS1 (P = .64) and PPS2 (P = .97) populations with similar results observed in the 2 strata. Specifically, within the PPS2 population, success rate in the LVOTO stratum was 50% (4/8 cats) in the pimobendan group and 61.5% (8/13 cats) in the placebo group (OR, 0.625; 95% CI, 0.11-3.71); in the non-LVOTO stratum, success rate was 31.8% (7/22 cats) in the pimobendan group and 25% (4/16 cats) in the placebo group (OR, 1.40; 95% CI, 0.33-5.93; Figure S7).
Treatments and LVOTO subgroups were not homogeneously distributed across the study centers. However, the overall treatment effect and the interaction between treatment *LVOTO (y/n) were not affected by “center” as a random effect.
3.3 Assessment of efficacy: Secondary endpoints
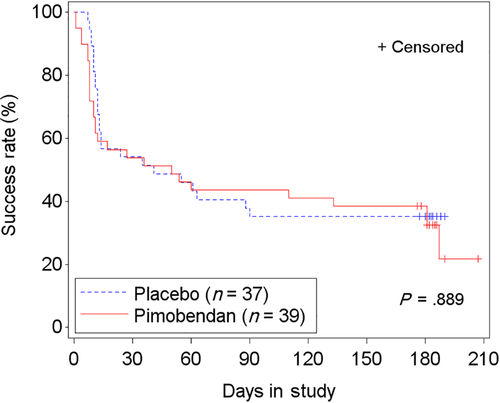
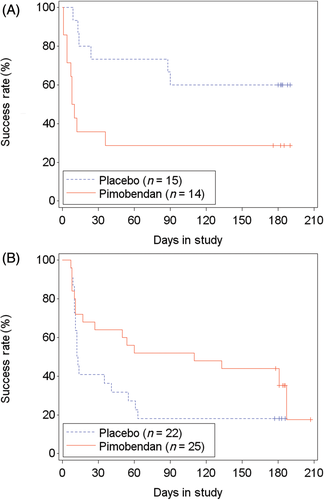
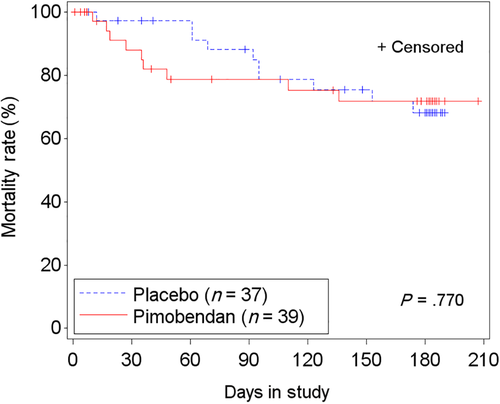
In the FAS population, 67.1% (51/76 cats) failed to successfully complete the study: 69.2% (27/39 cats) in the pimobendan group and 64.9% (24/37 cats) in the placebo group. The time-to-event analysis indicated no differences in time to study failure between groups (P = .89; hazard ratio [HR], 0.96; 95% CI, 0.54-1.71; Figure 5; Table S8). Similar results were found in the PPS2 population (Figure S2; Table S8). In FAS, of 29 cats with LVOTO, 55.2% (16 cats) failed to complete the study. Those receiving pimobendan (14/29 cats) were more likely to be removed from the study before D180 or to have at least 1 increase of the dose of furosemide (P = .03; HR, 3.14; 95% CI, 1.13-8.73; P = .03; Figure 6A) compared to 15 cats receiving placebo. This included 3 cats in the pimobendan-LVOTO group that were removed early because of increased severity of LVOTO by >25 mm Hg for general safety reasons. In the non-LVOTO population, 74.5% (35/47 cats) failed to successfully complete the study: 25 treated with pimobendan and 22 with placebo. No difference was found between treatment groups in time to removal from the study or to have at least 1 increase of the dose of furosemide (P = .09; HR, 0.55; 95% CI, 0.28-1.10; Figure 6B).
Cardiac mortality (death or euthanasia; Table 4) occurred in 10% of cats (4) in the pimobendan group and in 16% of cats (6) in the placebo group, equally distributed between groups in cats with LVOTO (2 in each group) and without LVOTO (4 in each group). Cardiac morbidity, defined as a dose escalation of furosemide to a daily dose >10 mg/kg, was observed in 13% of cats (5) receiving pimobendan and in 11% of cats (4) receiving placebo (Table 4). The time-to-event analysis for the composite endpoint “morbidity/mortality” was not different between treatment groups (P = .77; HR, 0.87; 95% CI, 0.35-2.18) or between cats with (P = .48) and without (P = .5) LVOTO (Figure 7). Analysis of time to first dose escalation of furosemide was not different between treatment groups (P = .45; HR, 0.77; 95% CI, 0.39-1.52) and was similar for cats with LVOTO (P = .35; HR, 1.77; 95% CI, 0.54-5.87) and without LVOTO (P = .14; HR, 0.56; 95% CI, 0.25-1.22). No difference was found between treatment groups in time to hospitalization for recurrence of CHF (P = .25), and this was true both in cats with LVOTO (P = .73; HR, 0.67; 95% CI, 0.07-6.46) and without LVOTO (P = .27; HR, 0.55; 95% CI, 0.19-1.59). Similar results were found in the PPS2 population (Table S9).
| Pimobendan (n = 39) | Placebo (n = 37) | Total (n = 76) | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Euthanasia | 3 | 8 | 4 | 11 | 7 | 9 |
| Death | 1 | 3 | 2 | 5 | 3 | 4 |
| Daily dose of furosemide >10 mg/kg | 5 | 13 | 4 | 11 | 9 | 12 |
A total of 141 adverse events were recorded (Table 5) with no difference between groups. For the pimobendan group, 33 (76.7%) cats with 72 adverse events and for the placebo group, 29 (74.7%) cats with 69 adverse events were reported. Based on the investigator's best assessment as to whether the adverse events might have been related to the study drug or were unrelated to the study medication, 26 adverse events were classified as possibly related to the study medication and 115 were classified as possibly unrelated. Four (9.3%) cats in the pimobendan group had an increase in severity of LVOTO defined as systolic PG increase >25 mm Hg after the first dose of pimobendan, as discussed above. None of the cats required emergency treatment, but all cats were removed from the study in agreement with the study protocol.
| Type | Pimobendan (n = 43) | Placebo (n = 39) | ||||
|---|---|---|---|---|---|---|
| F | N | % | F | N | % | |
| Cardiovascular | 51 | 25 | 58.1 | 56 | 24 | 61.5 |
| General | 14 | 9 | 20.9 | 12 | 11 | 28.2 |
| Renal | 13 | 6 | 14.0 | 12 | 7 | 17.9 |
| Gastrointestinal | 12 | 8 | 18.6 | 14 | 8 | 20.5 |
| Dermal | 4 | 3 | 7.0 | 3 | 3 | 7.7 |
| Behavioral | 1 | 1 | 2.3 | 2 | 2 | 5.1 |
| Blood | 1 | 1 | 2.3 | 0 | 0 | 0.0 |
| Hepatic | 1 | 1 | 2.3 | 0 | 0 | 0.0 |
| Neurologic | 0 | 0 | 0.0 | 1 | 1 | 2.6 |
| Respiratory | 0 | 0 | 0.0 | 1 | 1 | 2.6 |
- Notes: Because of the coding process multiple adverse events at the same time in the same animal have been split into single adverse events. The adverse event type “General” includes nonspecific clinical signs such as lethargy, intermittent shaking, urinating outside the litter box, inappetence, fluctuating appetite, and other nonorgan specific client observations according to coding principles of the Veterinary Dictionary for Drug Related Affairs (VeDDRa) terminology of the European Medicines Agency.
- Abbreviations: %, percent cats with at least 1 adverse event; F, frequency (number) of adverse events; N, number of cats with at least 1 adverse events.
4 DISCUSSION
This study in cats with HCM and a history of CHF failed to identify an overall benefit of pimobendan when added to furosemide, with or without clopidogrel, over a 6-month trial period. Considering the FAS population, in cats without dynamic LVOTO, treatment with pimobendan was associated with a tendency toward a decreased likelihood of early removal from the study or increase in furosemide dose. In contrast, cats with dynamic LVOTO had an increased likelihood of early removal from the study or an increase of furosemide dose if treated with pimobendan. Considering the PPS2 population, which corrected for the early removal of cats because of subclinical worsening of LVOTO after treatment, no effect of pimobendan on primary and secondary outcomes was found for any subgroup.
The study also indicated that administration of pimobendan is clinically well tolerated in cats with HCM. Pimobendan did not induce LVOTO in cats with nonobstructive HCM or worsen existing obstruction based on mean systolic PG. However, the PG across the LVOT did increase under the influence of pimobendan in individual cats, but without major clinical signs such as weakness, severe hypotension, syncope, or death. Finally, in cats with HCM that reached the primary 6-month study endpoint, severity of dynamic LVOTO consistently decreased over time under the influence of pimobendan or placebo in individual cats, lessening concerns regarding long-term detrimental effects of pimobendan related to dynamic LVOTO.
The survival benefits of pimobendan in dogs5-7 have been identified. In contrast, the pathophysiology of HCM in cats is distinct from heart disease in dogs, and is associated with dysregulation of Ca2+ signaling in the cardiac myocyte with enhanced Ca2+ sensitivity and prolonged Ca2+ binding, leading to increased inotropy, diastolic dysfunction, and arrhythmogenicity.4, 19, 38-40
Short- and long-term effects of pimobendan have been reported in healthy cats24-26 and cats with cardiomyopathy.8-13 In a retrospective open-label study9 of 170 cats, pimobendan was found to be well tolerated and safe, similar to our findings. Adverse effects were rare (3%). However, pimobendan was not administered to most cats with dynamic LVOTO. In 27 cats with systolic dysfunction and CHF,8 the effect of pimobendan on survival time was analyzed retrospectively. Addition of pimobendan to standard CHF treatment did not improve outcome, neither using univariate (P = .25) nor multivariate analyses (P = .1). In a young cat with congenital mitral valve dysplasia and CHF, dynamic LVOTO, systolic dysfunction, and systemic hypotension,8 administration of pimobendan was associated with an additional decrease in systolic blood pressure, requiring discontinuation of pimobendan. Whether or not this effect was caused by worsening of LVOTO, vasodilatory effects of pimobendan, additive effects of either mechanisms, or unrelated events remains unknown. Pimobendan improved mean survival time in 32 cats with DCM in an uncontrolled retrospective study (P = .05), but effect size (mean, 37 days) was small.11 Finally, another open-label, case-control study in 27 cats with HCM and CHF treated with pimobendan reported a large survival benefit (mean, 503 days; P = .02).12 Nevertheless, interpretation of the results is challenging because of the retrospective nonrandomized nature of the study and the fact that groups were not contemporaneous and not well matched regarding concurrent medications (clopidogrel, 63% vs 22% in favor of the pimobendan group and atenolol, 33% vs 11% in favor of the nonpimobendan group). These results are in contrast to our prospective and randomized study where mortality after 6 months was not different between treatments. Despite the larger number of cats enrolled in our study, it was designed as a phase-2 trial with the results (if favorable) intended to inform a pivotal study to define feasible endpoints and enrollment number for a larger pivotal phase-3 trial. Our study did not unequivocally identify such endpoints for a pivotal field study. However, focusing on cats with nonobstructive disease in long-term (>180 days), randomized and placebo-controlled studies seems warranted to better explore the potential therapeutic benefits of pimobendan in cats with HCM.
One of the most relevant clinical concerns in the use of pimobendan in cats with HCM is worsening of LVOTO. In humans with HCM, severity of dynamic LVOTO is a strong predictor of disease progression, exercise intolerance, and cardiac death41, 42 with obstruction being an accepted therapeutic target.18 However, data failed to confirm the clinical and prognostic importance of LVOTO in cats with HCM,2, 43, 44 and medical treatment relieving obstruction did not lead to improved survival.45 Our study indicated that pimobendan does not induce or worsen dynamic LVOTO in the majority of cats, a finding of clinical relevance. Moreover, severity of obstruction decreased considerably during chronic pimobendan treatment in cats that completed the study. However, individual cats may have an acute increase of their dynamic PG after initiation of pimobendan with a maximum increase of 60 mm Hg observed. Although not associated with specific clinical signs in our study, vigilance is advised in particular after starting treatment with pimobendan in cats with dynamic LVOTO. Surprisingly, severity of dynamic LVOTO also changed considerably in many placebo-treated cats with a maximum increase of 24 mm Hg and a maximum decrease of 70 mm Hg found. This observation leads to speculation on the accuracy of Doppler methods in the quantification of dynamic LVOTO, the variability in an individual cat's momentary level of excitement from examination to examination influencing LVOTO, or whether progression of disease over time leads to a decrease in LVOTO. Obstruction is a dynamic process41, 42 influenced by flow rate associated with loading conditions, systolic function, heart rate, autonomic tone, and mitral valve and chamber geometry.46 Because of its labile nature with high physiological variability and rather poor reproducibility, it may not be a feasible monitoring variable for drug efficacy studies targeting outflow obstruction in cats.
The effect on the primary endpoint of our study was not statistically different between treatment groups indicating that pimobendan did not worsen 180-day outcome. Although not statistically significant, 32% of nonobstructive cats treated with pimobendan had a successful outcome compared to 18.2% placebo-treated nonobstructive cats. For cats with dynamic LVOTO in the FAS population, only 28.6% of pimobendan-treated cats had a successful outcome compared with 60% placebo-treated cats. In addition, secondary endpoint analysis indicated that nonobstructive cats may benefit from pimobendan (P = .03), whereas obstructive cats may not (P = .09). However, the fact that 3/8 (38%) cats in the LVOTO-pimobendan group that failed to reach the primary study endpoint were excluded early because of worsening of LVOTO by >25 mm Hg, a per-protocol decision addressing initial safety concerns, must be taken into consideration when interpreting our results. These 3 cats, despite being either nonsymptomatic or only mildly symptomatic after the first dose of pimobendan, were not given the opportunity to reach D180. This may have affected our results and biased toward a potentially worse effect of pimobendan in cats with dynamic LVOTO compared to placebo as found in the FAS population. Removing these 3 cats and focusing only on the PPS2 population eliminated the potentially worse effects of pimobendan in cats with dynamic LVOTO with no group differences for primary and secondary endpoints observed (Table S8). Because of the low number of observations, caution is advised when interpreting these results. It remains to be determined using larger populations of cats with HCM, with and without LVOTO, whether or not addition of pimobendan is associated with a survival benefit.
Our study had some limitations. The number of cases enrolled was low, and using a factorial design by splitting of the 2 treatment groups into cats with and without LVOTO further decreased statistical power to detect treatment effects. The long “run-in” period (average, 12-13 days) may have introduced selection bias affecting conclusions. Our study only followed outcome to 6 months. Although it was not a primary objective of the trial, a true survival study with a larger number of cats and a longer follow-up period potentially could have led to different conclusions. Considering the labile nature of LVOTO in cats with HCM, binary classification of cats (LVOT obstruction, yes/no) and elimination of cats from the FAS population using arbitrary diagnostic cutoffs may have led to misclassification of cats, confounding efficacy data. Finally, only treatment with the investigational product, clopidogrel, and furosemide was permitted. Therefore, our study failed to address the potential benefit of other drugs.
In conclusions, addition of pimobendan to furosemide with and without clopidogrel in the treatment of cats with HCM and recent CHF had no effect on 180-day outcome in our study. However, the study suggests that cats with nonobstructive HCM and recent CHF might benefit from pimobendan whereas cats with LVOTO might not. Overall, concerns that the use of pimobendan in cats with HCM with or without LVOTO potentially might worsen outcome seem unfounded. Considering the heterogeneity of cardiomyopathy in cats, it is possible that some, but not all, subpopulations of cats with HCM might benefit from treatment with pimobendan. Given the general lack of approved treatments for cats with HCM, identification of these subgroups and a more tailored approach to treatment are needed.
ACKNOWLEDGMENTS
Funding proved by Boehringer Ingelheim Vetmedica Inc and Boehringer Ingelheim Vetmedica GmbH. Part of this work was presented at the International Cardiology Veterinary Symposium in Dubrovnik, Croatia, 22 and 23 October 2016 and the 2019 ACVIM Forum, Phoenix, Arizona, 6-8 June 2019. The authors thank Nicole Bridger, Kathryn Christmas, Diana Swisher, Balazs Albrecht, Kevin Christiansen, Gregory Moore, Carla Kroh, Sabine Volbon, Sonja Möbus, Richard Hunter, Roland Ludwig, Alexandra Hirsch, Karen Krauel-Göllner, Heidi Francis, Annie Altorfer, Simon Dennis, Thaibinh Nguyenba, Jose Matos, and Marc Hardman. The authors are particularly grateful to the owners of the enrolled cats who made this study possible.
CONFLICT OF INTEREST DECLARATION
All authors have received funding from Boehringer Ingelheim Vetmedica Inc. and Boehringer Ingelheim Vetmedica GmbH within the last 10 years for some or all of the following activities: research, travel, speaking fees, consultancy fees, and preparation of educational materials. A representative of Boehringer Ingelheim Vetmedica GmbH read and approved the final draft of this manuscript before submission. The writing committee (Drs Schober, Rush, and Luis Fuentes) did not receive compensation for their publication activities.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved at each investigator site by the IACUC or equivalent committee if required. The study conduct was approved by the national authorities in the United Kingdom and Germany.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




