Proteinuria in dogs with gallbladder mucocele formation: A retrospective case control study
Funding information: American Kennel Club Canine Health Foundation, Grant/Award Number: 01986; American Shetland Sheepdog Association; Collie Health Foundation
Abstract
Background
Proteinuria is an independent risk factor for morbidity and mortality in dogs. An association between proteinuria and gallbladder mucocele formation in dogs is unknown.
Objective
Determine if gallbladder mucocele formation or clinicopathologic comorbidities are associated with proteinuria.
Animals
Twenty-five dogs with mucocele formation and 25 breed and age-matched control dogs from a prior study.
Methods
Retrospective case control study. Proteinuria defined by calculated urine dipstick protein concentration (mg/mL) to urine specific gravity (USG) ratio. Clinicopathologic findings, postcosyntropin cortisol concentration, thyroid function profile, and illness severity score were recorded.
Results
Median urine dipstick protein concentration to USG ratio and number of dogs having a ratio ≥1.5 were significantly higher for dogs with mucocele formation compared to control dogs. Proteinuria was not significantly associated with CBC or serum biochemistry profile abnormalities but increased in relation to severity of illness.
Conclusions and Clinical Importance
Gallbladder mucocele formation is significantly associated with proteinuria in dogs. Diagnosis and treatment of proteinuria in dogs with mucocele formation might minimize long term kidney morbidity in these patients.
Abbreviations
-
- ACVIM
-
- American College of Veterinary Internal Medicine
-
- ALP
-
- alkaline phosphatase
-
- IRIS
-
- International Renal Interest Society
-
- UPC
-
- urine protein to creatinine ratio
-
- USG
-
- urine specific gravity
1 INTRODUCTION
Proteinuria can be an important clinical sign of kidney disease in dogs. Persistent proteinuria promotes progression of kidney disease, and control of proteinuria may prolong maintenance of patient kidney function.1 International Renal Interest Society (IRIS) and American College of Veterinary Internal Medicine (ACVIM) guidelines suggest that proteinuria measured as a urine protein-to-creatinine ratio (UPC) of ≥0.5 in nonazotemic dogs warrants diagnostic investigation for underlying causes of kidney injury.1 Factors identified to predispose to kidney injury and proteinuria include acute extrarenal factors that affect perfusion and oxygenation, chronic processes including inflammatory conditions, remodeling and fibrosis, and systemic endocrine status, any of which may have breed associations.2
Gallbladder disease currently is not recognized as a predisposing cause of proteinuria in dogs. Several studies describe associations between hyperadrenocorticism,3 hyperlipidemia,4, 5 and pancreatitis6 with proteinuria in populations of dogs. Other studies separately have associated these diseases with gallbladder mucocele formation.7-12 A direct association between gallbladder mucocele formation and proteinuria has not been investigated previously. Gallbladder mucocele formation is characterized by excessive secretion of abnormal mucus by the gallbladder epithelium that may lead to gallbladder rupture or bile duct obstruction.13-18 The disease affects older dogs and is observed in some breeds with genetic or metabolic predisposition to proteinuria such as the Shetland Sheepdog,11 Miniature Schnauzer,19 and Cocker Spaniel.20
Our primary aim was to determine, while controlling for the influence of age and breed, if gallbladder mucocele formation or concurrent clinicopathologic comorbidities are associated with increased incidence of proteinuria in dogs.
2 MATERIALS AND METHODS
Medical records of dogs that were included in a prior case-control study of gallbladder mucocele formation12 were retrospectively reviewed. Case and control dogs were selected for inclusion in the present study if both dogs had results of CBC, serum biochemical profile, and urinalysis reported within 1 month of gallbladder ultrasound examination. With regard to recruitment for the prior study, client-owned dogs with diagnosis of gallbladder mucocele formation at the North Carolina State University Veterinary Hospital (NCSU-VH) were prospectively identified over the time period from February 2014 to January 2017. In each case, diagnosis was based on ultrasound findings of an enlarged gallbladder containing nongravity dependent, immobile bile having hypoechoic extensions of mucus into the lumen, resulting in a stellate or finely striated bile pattern.21 In the event that the dog was euthanized or underwent surgery for removal of the gallbladder, the gross pathology and histopathology reports were reviewed to confirm the diagnosis of mucocele formation. For inclusion as matched controls, apparently healthy, age- (to within 2 years) and breed-matched client-owned dogs were recruited by the Clinical Studies Core facility at North Carolina State University over the same time period. Control dogs were determined to be healthy based on history, CBC, serum biochemistry profile, urinalysis and focal hepatobiliary ultrasound examination showing a normal-appearing gallbladder with normal wall structure and thickness. Sludge, if present, was gravity-dependent, occupied <50% of the gallbladder lumen, and was not attached to the wall.
Dogs were excluded from the prior study if they had a recent (within 2 months) history of treatment with ursodeoxycholic acid or drugs recognized or suspected to interfere with endocrine function testing (eg, topical or systemic corticosteroids, nonsteroidal anti-inflammatory drugs, anticonvulsants, furosemide, sulfa-containing drugs, fatty acid supplements), had history or physical examination findings suggestive of endocrinopathy, or were reproductively intact. Owners of the dogs signed informed consent forms for participation in the study. All study protocols were approved by the Institutional Animal Care and Use Committee of North Carolina State University (ID#14-049-O).
2.1 Clinical pathology and endocrine testing
Each dog underwent a complete physical examination by the attending clinician. Blood was collected by venipuncture and urine by ultrasound-guided cystocentesis after a minimum fasting period of 12 hours. For diagnosis of hyperadrenocorticism, blood was drawn 1-hour after IV injection of synthetic cosyntropin for measurement of serum cortisol concentration as previously described.12 Postcosyntropin serum cortisol concentrations ≥200 ng/mL were considered consistent with a presumptive diagnosis of hyperadrenocorticism.
For diagnosis of hypothyroidism, a serum sample from each dog was stored at −80°C and collectively submitted on dry ice to the Michigan State University Veterinary Diagnostic Laboratory for measurement of serum total thyroxine (T4), free thyroxine by equilibrium dialysis (FT4), and thyrotropin (TSH) concentrations, and for antibodies against thyroxine (T4AA), triiodothyronine (T3AA), and thyroglobulin (TgAA). A laboratory-based diagnosis of hypothyroidism was defined by low serum total T4 and increased serum TSH concentrations or low serum FT4 concentration as previously proposed.9, 22, 23
2.2 Illness severity scoring
To evaluate the impact of overall systemic illness on proteinuria, all dogs were stratified by disease severity into 4 groups based on a previously described scoring system24 as follows: absent (0) for patients that had no clinical signs of systemic illness, mild (1) for patients with signs of clinical disease but suitable for outpatient care, moderate (2) for patients sick enough to require hospitalization and aggressive treatment, and severe (3) for patients with severe illness requiring intensive care and advanced treatment (including all dogs requiring emergency cholecystectomy).
2.3 Assessment of proteinuria
Urinalyses were performed in the North Carolina State University Clinical Pathology Laboratory by an experienced laboratory technician. Urine specific gravity was measured using a digital refractometer (Palm Abbe Digital Refractometer #PA202, MISCO Cleveland, Ohio). Urine chemical analysis was performed using commercially-available dipsticks (Chemstrip 10, Roche Diagnostics, Mannheim Germany) that were read using a calibrated digital analyzer (Chemstrip Criterion II Urine Analyzer, Roche Diagnostics, Mannheim Germany). For sediment examination, 5 mL of urine was centrifuged for 5 minutes at 1600 rpm. After removal of the supernatant, the sediment was suspended, and a drop examined unstained including an average of 10-15 microscopic fields at both 50× and 400× magnification. When requested by the attending clinician, urine protein and urine creatinine concentrations were measured using a clinical chemistry analyzer (Roche Cobas c501, Roche Diagnostics, Mannheim Germany).
Because of the retrospective nature of the study, direct quantitation of urine protein and creatinine concentrations was not performed for the majority of dogs unless originally requested by the attending clinician. Accordingly, proteinuria was determined based on results of urine dipstick analysis. Dogs were categorized as nonproteinuric if the dipstick results indicated no or trace protein and proteinuric if the dipstick results indicated protein concentration ≥30 mg/dL (≥1+). Dogs with substantial blood contamination (gross hematuria) were excluded as possibly having postrenal causes of proteinuria.25
To provide a quantitative estimate of proteinuria, a ratio of urine dipstick protein concentration (mg/dL)-to-urine specific gravity (USG) was calculated for each dog as previously described.26 A cutoff for assuming abnormal protein concentrations in urine was defined as a dipstick protein-to-USG ratio of ≥1.5, which represents 1+ proteinuria (30 mg/mL) in urine with a USG of 1.020 as the upper limit of normal.27
2.4 Statistical analysis
A Fisher exact test was used to identify significant differences in the percentage of control dogs and dogs with gallbladder mucocele formation that had clinical pathology test results out of reference range limits. A Mann-Whitney rank sum test was used to identify significant differences in the median values of clinical pathology test results between control dogs and dogs with gallbladder mucocele formation. Among dogs with gallbladder mucocele formation, associations between abnormal clinical pathology test results and presence or absence of proteinuria (defined by urine dipstick protein concentration to USG ratio ≥1.5 vs <1.5) were determined using a Fisher exact test. Associations between median values of clinical pathology test results and presence or absence of proteinuria (defined by urine dipstick protein concentration to USG ratio ≥1.5 vs <1.5) were determined using a Mann-Whitney rank sum test. Statistical analysis was performed using commercially available software (SigmaPlot 12.0, Systat Software, Inc, San Jose, California). To correct for the impact of multiple testing, Benjamini-Hochberg28 corrected P-values were calculated using a false discovery rate of 0.05. All results reported as statistically significant had a P-value of <.05 and Benjamini-Hochberg corrected P-value <.05. The relationship between grade of illness severity and urine dipstick protein concentration to USG ratio among dogs with gallbladder mucocele formation was evaluated by fitting of a simple logistic regression model with protein ratio (≥1.5 vs <1.5) as the response and grade of illness severity as the predictor. This analysis was performing using R version 4.0.2.
3 RESULTS
3.1 Case-control study population
A total of 30 case-control pairs of dogs from the previously published study12 had medical records screened for inclusion in the present study. Five case-control pairs were excluded because of lack of urinalysis in 1 or both dogs. Therefore, 25 case-control pairs of dogs met the criteria for inclusion in the study. Dogs were represented by 15 different breeds including 16 Shetland Sheepdogs, 4 American Cocker Spaniels, 4 Bichon Frise, 4 Chihuahuas, and 2 each of the following breeds: American Staffordshire Terrier, Beagle, Border Collie, Cavalier King Charles Spaniel, Cockapoo, Kerry Blue Terrier, Labrador Retriever, Miniature Poodle, Miniature Schnauzer, Pug, and Shih Tzu. There were 27 castrated males and 23 spayed females. The median age was 10 years (range, 6-16 years) and median body weight 9.4 kg (range, 2.9-35.6 kg). There was no statistically significant difference in sex (P = 1), age (P = .17), or body weight (P = .59) between control dogs and dogs with mucocele formation. Illness severity scores of dogs at the time of participation in the study were: 0 (absent) in 12 (48%) dogs, 1 (mild) in 5 (20%) dogs, 2 (moderate) in 6 (24%) dogs, and 3 (severe) in 2 (8%) dogs with mucocele formation. All control dogs had an illness severity score of 0 (absent). In addition to ultrasonographic diagnosis, gallbladder mucocele formation was confirmed by gross examination and histopathology of gallbladder tissue in 8 (32%) dogs, obtained at the time of surgery (5 dogs) or at necropsy (3 dogs).
3.2 Proteinuria, urinalysis, and urinary tract ultrasound examination
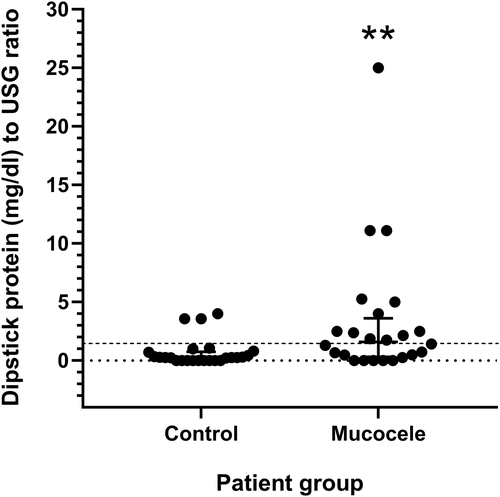
A significantly larger number of dogs with gallbladder mucocele formation had dipstick-measured urine protein concentration ≥30 mg/dL compared to control dogs (P = .003). The median urine dipstick protein concentration to USG ratio (P = .008) and number of dogs having a urine dipstick protein concentration to USG ratio ≥ 1.5 (P = .005) was significantly higher for dogs with gallbladder mucocele formation compared with control dogs (Table 1 and Figure 1). One dog was not included in the analysis because of lack of recorded USG. A quantitative urine protein-to-creatinine (UPC) ratio was measured in 3 dogs with mucocele formation and ranged from 0.82 to 6.7 (concurrent urine dipstick protein concentration to USG ratio range, 1.76-11.11). A UPC was measured in 4 control dogs and ranged from 0.1 to 0.14 (concurrent urine dipstick protein concentration to USG ratio range, 0-0.7).
| Urine specific gravity and protein dipstick | No gallbladder mucocele (25 dogs) | Gallbladder mucocele (25 dogsa) | ||||
|---|---|---|---|---|---|---|
| Median | Range | Number (%) of dogs | Median | Range | Number (%) of dogs | |
| Urine specific gravitya | 1.028 | 1.009-1.045 | 1.018 | 1.003-1.062 | ||
| Dipstick protein (mg/dL) | 10 | 0-100 | 30** | 0-300 | ||
| No or trace proteinuria | 18(72) | 7(28) | ||||
| Proteinuria (≥30) | 7(28) | 18(72)** | ||||
| Dipstick protein to USG ratio ≥ 1.5a | 0.270 | 0–4 | 3(12) | 1.60** | 0-25 | 12(50)** |
| Urine sediment examination | Median | Range | Number (%) of dogs above reference range | Median | Range | Number (%) of dogs above reference range | Reference range |
|---|---|---|---|---|---|---|---|
| Red blood cells/hpf | 0-5 | 0-100 | 1(4) | 0-5 | 0-40 | 6(24) | 0-5 |
| White blood cells/hpf | 0-5 | 0-30 | 1(4) | 0-5 | 0-5 | 0(0) | 0-5 |
| Casts/hpf | 0 | 0-2 | 0(0) | 0 | 0-2 | 0(0) | 0-2 |
| # Dogs with casts observed | 5(25) | 9(36) |
- a USG measurement was unavailable for 1 dog.
- ** P < .01; Fisher exact test (for proportional variables) and Mann-Whitney rank sum test (for continuous variables). Exact P-values reported in text.
Prevalence of microscopic hematuria (P = .10) and presence of casts (P = .34) in the urine was not significantly different between dogs with gallbladder mucocele formation compared to control dogs. No dogs in the study were observed to have gross hematuria or pigmenturia. The number of casts of any given type did not exceed 2 per high power field in any dog (Table 1). Nine dogs (36%) with gallbladder mucocele formation had cylindruria with casts of the following types: hyaline (7 dogs), fine granular (4 dogs), waxy (2 dogs), coarse granular (1 dog), epithelial (1 dog), and fatty (1 dog). Among mucocele dogs with cylindruria, 2 dogs had a single cast type, 6 dogs had 2 cast types, and 1 dog had 3 cast types. Five (25%) of the control group dogs had cylindruria with casts of the following types: hyaline (3 dogs), fine granular (3 dogs), and coarse granular (1 dog). Among control dogs with cylindruria, 3 dogs had a single type and 2 dogs had 2 types of casts. Thirteen dogs had urine pH ≥8 (8 dogs with gallbladder mucocele formation and 5 control dogs). No association was found between presence of urine pH ≥8 and urine dipstick protein concentration to USG ratio ≥ 1.5 among dogs in the study (P = .30).
Quantitative urine cultures were performed in 9 gallbladder mucocele dogs with 1 positive for Escherichia coli on a catheterized sample with an inactive sediment and urine dipstick protein concentration to USG ratio of 0.5. One control dog had an active sediment on a voided sample with urine dipstick protein concentration to USG ratio of 0.8. No urine cultures were performed on control dogs.
Results of abdominal ultrasound examination that included the urinary tract were available for 12 dogs with gallbladder mucocele formation and 3 control dogs. Regarding the lower urinary tract, 1 control dog had small suspected uroliths and a urine dipstick protein concentration to USG ratio of 0.4. One dog with a gallbladder mucocele had mild irregularity and thickening of the apical urinary bladder, urine dipstick protein concentration to USG ratio of 11.1, an inactive urine sediment and negative urine culture. All remaining dogs had no ultrasonographic evidence of lower urinary tract disease. Abnormal ultrasound findings related to the kidneys were described in 9/12 (75%) dogs with gallbladder mucocele formation and included decreased corticomedullary distinction (6 dogs), peridiverticular mineralization (6 dogs), hyperechoic kidney cortices (3 dogs), chronic kidney infarcts (2 dogs), and cortical cysts (1 dog). Changes also were described in the kidneys of 2/3 (67%) control dogs, including decreased corticomedullary distinction (2 dogs), peridiverticular mineralization (2 dogs), and cortical rim sign (1 dog).
3.3 Clinicopathologic comorbidities
After correction for the impact of multiple testing on false discovery, results of CBC and serum biochemical profiles identified significantly higher results for the number of polymorphonuclear leukocytes (P = .01) and bands (P = .004), serum activities of alkaline phosphatase (ALP; P = .001), alanine aminotransferase (ALT; P = .008), γ-glutamyl transferase (GGT; P = .002) and lipase (P = .003), and concentration of total bilirubin (P = .005) in dogs with gallbladder mucocele formation compared to control dogs. A significantly higher number of dogs with mucocele formation had results out of reference range limits for serum activities of ALP (P < .001), GGT (P < .001), and lipase (P = .004; Table 2).
| Clinical variable | No gallbladder mucocele (25 dogs) | Gallbladder mucocele (25 dogs) | Direction of abnormality | Reference range | Mann-Whitney P-value (comparison of median values) | Fisher exact P-value (comparison of % abnormal values) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | Range | Number (%) of dogs with abnormal value | Median | Range | Number (%) of dogs with abnormal value | |||||
| Complete blood cell count | ||||||||||
| Packed cell volume (%) | 44 | 34-52 | 1(4) | 42 | 25-50 | 8(32) | Low | 39-58 | .14 | .02 |
| Total white blood cells (×103/mL) | 7.47 | 4.490-13.370 | 3(12) | 9.820* | 4.670-34.570 | 9(36) | High | 4.39-11.61 | .02 | .09 |
| Polymorphonuclear leukocytes (×103/mL) | 5.358 | 2.371-10.450 | 3(12) | 7.286* | 3.468-28.926 | 8(32) | High | 2.841-9.112 | .01 | .17 |
| Bands (×103/mL) | 0 | 0.0-0.190 | 8(32) | 0.098** | 0-6.568 | 16(64) | High | 0.0-0.0 | .004 | .05 |
| Platelets (×103/mL) | 359 | 189-616 | 1(4) | 394 | 143-695 | 2(8) | Low | 191-468 | .48 | 1 |
| Serum biochemical analysis | ||||||||||
| Alkaline phosphatase (IU/L) | 52 | 6-251 | 4(16) | 205*** | 21-3188 | 17(68)*** | High | 16-140 | .001 | <.001 |
| ALT (IU/L) | 48 | 11-215 | 8(32) | 108** | 11-5393 | 16(64) | High | 12-54 | .008 | .046 |
| GGT (IU/L) | 3 | 3-6 | 0(0) | 6** | 3-78 | 11(44)*** | High | 0-6 | .002 | .02 |
| Total bilirubin (mg/dL) | 0.1 | 0.1-0.1 | 0(0) | 0.1** | 0.1-11.5 | 6(24) | High | 0-0.2 | .005 | <.001 |
| Cholesterol (mg/dL) | 249 | 165-452 | 4(16) | 301 | 107-754 | 9(36) | High | 124-344 | .03 | .2 |
| Blood urea nitrogen (mg/dL) | 16 | 9-26 | 0(0) | 17 | 5-170 | 6(24) | High | 8-26 | .93 | .02 |
| Creatinine (mg/dL) | 0.8 | 0.5-1.1 | 0(0) | 0.7 | 0.2-4.1 | 2(8) | High | 0.7-1.5 | .31 | .49 |
| Total protein (g/dL) | 6.2 | 5.1-7.0 | 1(4) | 5.8 | 3.2-7.2 | 4(16) | Low | 5.2-7.3 | .18 | .35 |
| Albumin (g/dL) | 3.6 | 2.9-4.6 | 1(4) | 3.4 | 1.6-4.2 | 4(16) | Low | 3-3.9 | .07 | .35 |
| Globulin (g/dL) | 2.4 | 1.9-4.0 | 1(4) | 2.5 | 1.6-3.4 | 0(0) | High | 1.7-3.8 | .78 | 1.00 |
| Lipase (IU/L) | 75 | 24-1032 | 2(8) | 124** | 41-3920 | 12(48)** | High | 12-147 | .003 | .004 |
| Postcosyntropin cortisol (ng/mL) | 92 | 30-135 | 0(0) | 110 | 37-699 | 3(12) | High | <200 | .06 | .23 |
- Notes: Mann-Whitney rank sum test P-values compare the median value of each clinical pathology variable between control dogs and dogs with gallbladder mucocele formation. Fisher exact test P-values compare the % of dogs with an abnormal value for each clinical pathology variable between control dogs and dogs with gallbladder mucocele formation. All asterisked values shown have Benjamini-Hochberg corrected P-values <.05.
- * P < .05.
- ** P < .01.
- *** P < .001.
Among dogs with gallbladder mucocele formation, no significant differences were found between median results of any CBC or serum biochemistry variables between dogs having a urine dipstick protein concentration to USG ratio ≥1.5 compared to those having a ratio <1.5. As categorized based on whether or not results were outside of reference range limits, no CBC or serum biochemistry variables were significantly associated with urine dipstick protein concentration to USG ratio ≥1.5 vs <1.5 in dogs with gallbladder mucocele formation (Table 3).
| Clinical variable | Urine dipstick protein to USG ratio < 1.5 (n = 12) | Urine dipstick protein to USG ratio ≥ 1.5 (n = 12) | Direction of abnormality | Reference range | Mann-Whitney P-value (comparison of median values) | Fisher exact P-value (comparison of % abnormal values) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | Range | Number (%) of dogs with abnormal value | Median | Range | Number (%) of dogs with abnormal value | |||||
| Urine dipstick protein to USG ratio | 0.37 | 0-1.30 | 0(0) | 3.25*** | 1.76-25 | 12(100)*** | High | <1.5 | <.001 | <.001 |
| Complete blood cell count | ||||||||||
| Packed cell volume (%) | 43 | 38-50 | 2(17) | 40 | 25-46 | 5(42) | Low | 39-58 | .05 | .37 |
| Total white blood cells (×103/mL) | 8.130 | 4.670-34.570 | 3(25) | 10.460 | 6.390-34.030 | 5(42) | High | 4.39-11.61 | .12 | .67 |
| Polymorphonuclear leukocytes (×103/mL) | 5.585 | 3.468-24.545 | 2(17) | 8.573 | 4.556-28.926 | 5(42) | High | 2.841-9.112 | .09 | .37 |
| Bands (×103/mL) | 0.025 | 0.0-6.568 | 6(50) | 0.203 | 0-1.475 | 9(75) | High | 0.0-0.0 | .3 | .4 |
| Platelets (×103/mL) | 393 | 193-695 | 0(0) | 409 | 143-564 | 2(17) | Low | 191-468 | .83 | .48 |
| Serum biochemical analysis | ||||||||||
| Alkaline phosphatase (IU/L) | 140 | 21-2817 | 6(50) | 261 | 66-3188 | 10(83) | High | 16-140 | .1 | .19 |
| ALT (IU/L) | 45 | 11-5393 | 5(42) | 172 | 30-3877 | 10(83) | High | 12-54 | .09 | .09 |
| GGT (IU/L) | 4 | 3-78 | 4(33) | 12 | 3-77 | 6(50) | High | 0-6 | .69 | .68 |
| Total bilirubin (mg/dL) | 0.1 | 0.1-11.5 | 1(8) | 0.1 | 0.1-3.5 | 4(33) | High | 0-0.2 | .38 | .32 |
| Cholesterol (mg/dL) | 342 | 107-711 | 6(50) | 283 | 206-754 | 2(17) | High | 124-344 | .54 | .19 |
| Blood urea nitrogen (mg/dL) | 16 | 6-32 | 2(17) | 15 | 5-170 | 3(25) | High | 8-26 | .98 | 1 |
| Creatinine (mg/dL) | 0.8 | 0.2-1.2 | 0(0) | 0.6 | 0.4-4.1 | 2(17) | High | 0.7-1.5 | .88 | .48 |
| Total protein (g/dL) | 6.0 | 3.2-6.8 | 2(17) | 5.8 | 4.9-7.2 | 2(17) | Low | 5.2-7.3 | .66 | 1 |
| Albumin (g/dL) | 3.5 | 1.6-4.2 | 2(17) | 3.2 | 2.3-4.2 | 2(17) | Low | 3-3.9 | .31 | 1 |
| Globulin (g/dL) | 2.3 | 1.6-3.2 | 0(0) | 2.6 | 2.0-3.4 | 0(0) | High | 1.7-3.8 | .52 | – |
| Lipase (IU/L) | 111 | 41-3920 | 4(33) | 167 | 43-1558 | 7(58) | High | 12-147 | .37 | .41 |
| Postcosyntropin cortisol (ng/mL) | 108 | 37.8-143 | 0(0) | 113 | 67-699 | 2(17) | High | <200 | .51 | .48 |
| Diagnosis of hypothyroidism | 2(17) | 3(25) | 1 | |||||||
- Notes: Mann-Whitney rank sum test P-values compare the median value of each clinical pathology variable between control dogs and dogs with gallbladder mucocele formation. Fisher exact test P-values compare the % of dogs with an abnormal value for each clinical pathology variable between control dogs and dogs with gallbladder mucocele formation. One dog excluded from analysis due to unavailable USG measurement.
- *** P < .001. All asterisked values shown have Benjamini-Hochberg corrected P-values < .05.
3.4 Endocrinopathy
Among dogs with gallbladder mucocele formation, test results consistent with diagnoses of hyperadrenocorticism or hypothyroidism were obtained for 3 and 5 dogs, respectively. No control dogs met criteria for diagnosis of either endocrinopathy. Postcosyntropin serum concentrations of cortisol in dogs with gallbladder mucocele formation (median, 110 ng/mL; interquartile range, 93-140 ng/mL) did not differ significantly from concentrations measured in control dogs (median, 92 ng/mL; interquartile range, 80-116 ng/mL; P = .06; Table 2). Among dogs with gallbladder mucocele formation, no significant association was found between urine dipstick protein concentration to USG ratio ≥1.5 vs <1.5 and postcosyntropin serum cortisol concentration (P = .51), diagnosis of hyperadrenocorticism (P = .48), or diagnosis of hypothyroidism (P = 1; Table 3).
3.5 Illness severity
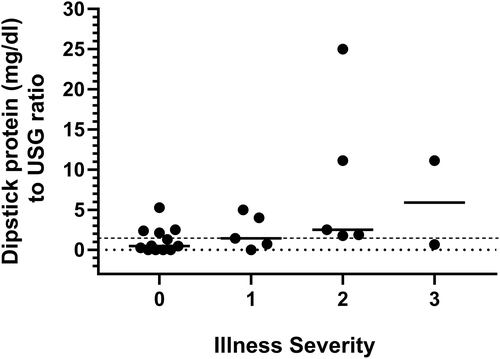
Illness severity score was significantly associated with urine dipstick protein concentration to USG ratio. Each unit increase in the severity of illness was associated with a 173% increase in odds of a urine dipstick protein concentration to USG ratio ≥1.5 (P = .04; Figure 2).
4 DISCUSSION
Our case-control study documents a significant association between ultrasonographic diagnosis of gallbladder mucocele formation and incidence of proteinuria in dogs. Important features of the study were breed and age-matched design, ultrasound documentation of absent gallbladder mucocele formation in control dogs, and concurrent serum biochemistry and endocrine testing of all dogs. This design enabled the examination of potentially confounding influences of concurrent disease on presence of proteinuria in this population. As defined by a urine dipstick protein concentration to USG ratio ≥1.5, proteinuria in dogs with gallbladder mucocele formation was not significantly associated with any CBC or serum biochemistry profile abnormalities in our study. Proteinuria significantly increased in relation to severity of systemic illness. Although a causal relationship between illness severity and proteinuria in dogs with mucocele formation could not be further examined by our retrospective study, this finding suggests that systemic mechanisms such as underling metabolic, vascular, inflammatory, or xenobiotic exposures should be considered.29
No association was observed between proteinuria and postcosyntropin serum cortisol concentration or diagnosis of hyperadrenocorticism among dogs with gallbladder mucocele formation. Hyperadrenocorticism is known to be associated with proteinuria in dogs4, 5, 17 and is a common comorbidity in dogs with mucocele formation.8, 9, 12 Lack of association between proteinuria and hyperadrenocorticism in our study likely is related to selection criteria that decreased the number of dogs ultimately diagnosed with hyperadrenocorticism. However, hypercortisolemia was not responsible for proteinuria in these dogs.
Dogs with gallbladder mucocele formation are at increased risk for concurrent diagnoses of hypertriglyceridemia, hypercholesterolemia, and hypothyroidism.7, 9, 12, 30 Dyslipidemia also is associated with proteinuria in people and dogs.4, 5, 31, 32 We did not identify an association between proteinuria and median serum cholesterol concentration or number of dogs having high serum cholesterol concentrations. Dogs in our study did not have serum triglyceride concentrations measured. Also, no association was found between proteinuria and diagnosis of hypothyroidism.
Important limitations of our study include the retrospective nature of the case-control cohort, in which the UPC ratio was not systematically measured. This resulted in the need to approximate proteinuria using a ratio between the urine dipstick-measured protein concentration and USG.33 Accordingly, these results should be validated with future prospective studies. Our study was not designed to comprehensively investigate underlying causes of proteinuria in these dogs. Only a subset of dogs had urinary tract imaging or urine culture performed, but explanatory reasons for proteinuria were not identified in those dogs that did. Dogs did not have fasting triglyceride concentrations or systolic blood pressure measurements recorded, and these should be included in future studies. Dogs did not undergo an exhaustive search for underlying immune-mediated, neoplastic or infectious diseases that could have contributed to proteinuria, but these conditions were not reported in accessions and considered systematically unlikely within the study population. Although an association between serum lipase activity and proteinuria was not observed in our study, pancreatitis can be associated with proteinuria in dogs6 and is a common comorbidity in dogs with mucocele formation.11 Accordingly, future studies utilizing specific tests for pancreatitis in dogs with gallbladder mucocele formation and proteinuria may be warranted. Finally, we used a generic scoring system to stratify the severity of systemic illness among dogs. The system initially was described for examining the influence of systemic illness on thyroid function.24 Its use in our study served a similar purpose in capturing levels of illness severity without introducing biasing variables with respect to proteinuria.
In conclusion, assessment of proteinuria is warranted in dogs diagnosed with gallbladder mucocele formation. Moreover, occult gallbladder mucocele formation should be considered as a possible cause for proteinuria in dogs of predisposed age and breed. Prospective studies are warranted to more closely examine contributing factors to proteinuria in dogs with gallbladder mucocele formation with suggested emphasis on gaps left by our study such as measurement of systemic blood pressure, serum fasting triglyceride concentrations and markers of systemic inflammation, more specific testing for pancreatitis, and characterization of associated kidney pathology. Whether proteinuria can be ameliorated by surgical removal of the gallbladder in dogs with mucocele formation also is worthy of additional study.
ACKNOWLEDGMENT
Funding provided by the American Kennel Club Canine Health Foundation, American Shetland Sheepdog Association, and Collie Health Foundation (grant #01986; http://www.akcchf.org/).
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the IACUC of North Carolina State University (ID#14-049-O).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




