High serum creatinine concentration is associated with metabolic perturbations in dogs
Funding information: Academy of Finland, Grant/Award Number: 308887; PetMeta Labs Ltd.
Abstract
Background
The kidneys have many essential metabolic functions, and metabolic disturbances during decreased renal function have not been studied extensively.
Objectives
To identify metabolic changes in blood samples with increased serum creatinine concentration, indicating decreased glomerular filtration.
Animals
Clinical samples analyzed using a nuclear magnetic resonance (NMR) based metabolomics platform. The case group consisted of 23 samples with serum creatinine concentration >125 μmol/L, and the control group of 873 samples with serum creatinine concentration within the reference interval.
Methods
Biomarker association with increased serum creatinine concentration was evaluated utilizing 3 statistical approaches: Wilcoxon rank-sum test, logistic regression analysis (false discovery rate (FDR)-corrected P-values), and random forest classification. Medians of the biomarkers were compared to reference intervals. A heatmap and box plots were used to represent the differences.
Results
All 3 statistical approaches identified similar analytes associated with increased serum creatinine concentrations. The percentages of citrate, tyrosine, branched-chain amino acids, valine, leucine, albumin, linoleic acid and the ratio of phenylalanine to tyrosine differed significantly using all statistical approaches, acetate differed using the Wilcoxon test and random forest, docosapentaenoic acid percentage only using logistic regression (P < .05), and alanine only using random forest.
Conclusions and Clinical Importance
We identified several metabolic changes associated with increased serum creatinine concentrations, including prospective diagnostic markers and therapeutic targets. Further research is needed to verify the association of these changes with the clinical state of the dog. The NMR metabolomics test is a promising tool for improving diagnostic testing and management of renal diseases in dogs.
Abbreviations
-
- AIC
-
- Akaike information criterion
-
- AKI
-
- acute kidney injury
-
- AUC
-
- area under the curve
-
- BCAA
-
- branched-chain amino acid
-
- BCKA
-
- branched-chain ketoacid
-
- CI
-
- confidence interval
-
- CKD
-
- chronic kidney disease
-
- FDR
-
- false discovery rate
-
- IQR
-
- interquartile range
-
- IRIS
-
- International Renal Interest Society
-
- NMR
-
- nuclear magnetic resonance
-
- SCFA
-
- short-chain fatty acid
1 INTRODUCTION
Azotemia is a frequent finding in veterinary medicine.1 Chronic kidney disease (CKD) is considered the most common renal disease in dogs, with an estimated prevalence of 0.37%. Serum creatinine concentration is a clinical biochemistry measurement commonly used as an indicator of glomerular filtration rate. Glomerular filtration rate evaluated using either serum creatinine or symmetric dimethylarginine concentration is a cornerstone for renal disease staging using the International Renal Interest Society (IRIS) guidelines.2-4
The kidneys have several important metabolic functions in addition to their excretory function. Multiple metabolic derangements, such as impaired renal enzymatic activity,5 have been found to occur in both humans and animals with renal disease. Some of these, such as changes in citrate and amino acid metabolism, have even been proposed as therapeutic targets.6-9 However, the occurrence and clinical relevance of changes in systemic metabolism during impaired renal function have not yet been extensively studied in dogs. Moreover, traditional diagnostic approaches do not detect the majority of these metabolic changes, limiting their use in clinical practice. We recently have developed and validated a clinically usable nuclear magnetic resonance (NMR) metabolomics testing platform for dogs,10 a platform providing a broad approach to both veterinary research and clinical practice.
Our objective was to evaluate the systemic metabolic changes that occur in dogs with increased serum creatinine concentrations, and to discuss the possible clinical relevance of these changes as diagnostic biomarkers and therapeutic targets.
2 MATERIALS AND METHODS
2.1 Samples
The workflow for the study is summarized and presented in Figure 1. The study was performed as a retrospective review of clinical blood samples. The samples originated from 2 sources.
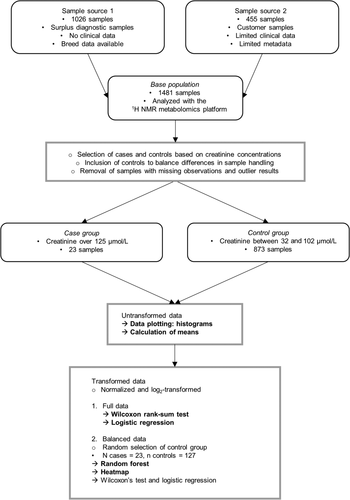
First, diagnostic samples (n = 1026) were collected by Finnish veterinarians and submitted by mail to a single laboratory provider (Movet Ltd, Kuopio, Finland). Altogether, 999 of these samples were collected and sent between February and May 2018. After arrival, the samples were frozen and stored at −20°C for a maximum of 4 weeks before shipment on cold packs for NMR analysis. These samples previously were used in a method comparison study during validation of the NMR metabolomics platform for dogs.10 Data analysis for the current study was started after method validation was completed and the integrity of the generated data confirmed. Twenty-seven samples were collected and sent between October and February 2018-2019, together with the samples from the second source as described below. No clinical data were available for these samples. Signalment data were either completely missing or limited to breed. Both heparinized plasma and serum samples were included, and the individual sample type of each sample was unknown.
The second source included clinical samples (n = 455) collected at 2 veterinary clinics (Kuopion Eläinlääkärikeskus Ltd, Kuopio, Finland and Pieneläinvastaanotto Punaturkki Ltd, Kuopio, Finland) during the NMR metabolomics clinic pilot study. Samples were collected during routine veterinary appointments between October and February 2018-2019, the samples were stored and sent at refrigerated temperature for NMR analysis, with storage times <1 week. Limited disease and signalment data were available for these samples. All samples were heparinized plasma samples.
The samples were analyzed using a validated NMR metabolomics platform for dogs10 quantifying 123 analytes.
Case and control groups were created according to NMR-measured serum or plasma creatinine concentrations. To limit possible confounding factors, clinical data was reviewed for samples for which it was available. No samples were excluded based on clinical data. Although the cause for increased serum creatinine concentrations in the case group samples was unknown, because CKD is the most common renal disease in dogs,11 we used the cutoff serum creatinine concentration for IRIS stage 2 CKD or higher (serum creatinine concentration > 125 μmol/L) as an inclusion criterion for the case group.4
The control group's first inclusion criterion was the NMR-measured serum or plasma creatinine concentration being within the reference interval (32-103 μmol/L) of the NMR method.10 To minimize the confounding effect of preanalytical variability caused by sample handling, our second inclusion criterion was that control group samples must originate in equal proportions from the same sample sets as case group samples.
2.2 Statistical analysis
Data were evaluated for missing observations. We removed biomarkers with multiple missing observations and samples with missing observations. Two samples in the case group were markedly lipemic with measured triglyceride concentration >7 mmol/L. To remove skewness caused by these lipemic samples, we removed them from further analyses as outliers. The resulting case group consisted of 23 samples and control group of 873 samples.
- Wilcoxon rank-sum test with false discovery rate (FDR) corrected P-values to evaluate the significance of differences between case and control group analyte concentrations;
- Separate logistic regression models for each biomarker to evaluate the metabolite's association with increased creatinine concentrations. The case-control status served as the response variable and the individual metabolite as the independent variable. To control for type I errors in multiple testing, we used FDR P-value correction. Linearity between the metabolites and log odds was tested. The models' goodness-of-fit and predictive value were assessed by Akaike information criterion (AIC) and area under the curve (AUC) values. A lower AIC indicates better fit and a higher AUC better predictive value; and
- Random forest classification to identify the biomarkers that best predicted the increased creatinine concentrations. The control group size was decreased by random undersampling to 127 samples to balance the number of cases and controls. Biomarker association with increased creatinine concentrations was assessed by variable importance.
To check whether metabolite association with increased creatinine concentrations was the same using the balanced and full control groups, we also performed a Wilcoxon rank-sum test and logistic regression analysis with the balanced control group.
A heat map was created to visualize the results of the biomarkers associated with increased creatinine concentrations based on the Wilcoxon rank-sum test, logistic regression and random forest classification, using the balanced data set of 127 samples.
To determine the direction of the observed changes and to evaluate whether the changes would be detected using clinical diagnostic testing based on reference intervals, we calculated the median concentrations and interquartile ranges (IQR) of the case and control groups using the untransformed data. The medians of both groups were compared to each other and to reference intervals of the NMR method and their 90% confidence intervals (CI).10
To visualize the analyte concentrations in the case and control groups compared to reference intervals of the NMR method, we plotted box plots of the analytes using the untransformed data. Four outliers were removed from the acetate control group results to improve box plot scaling. These changes were hypothesized to be caused by diseases in the control group that consisted of clinical laboratory samples. Three of the outliers had extremely high acetate concentrations and 1 had a very low acetate concentration.
Statistical software used included SAS version 9.4 (SAS Institute Inc, Cary, North Carolina), RStudio Team 2019 (RStudio: Integrated Development for R. RStudio, Inc, Boston, Massachusetts, URL http://www.rstudio.com/), Microsoft Office Excel (Microsoft Corp., Redmond, Washington), and IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, New York).
3 RESULTS
3.1 Sample characteristics
We used a nontargeted NMR metabolomics approach to compare metabolite profiles of clinical samples with increased serum creatinine concentrations (n = 23) to clinical samples with normal serum creatinine concentrations (n = 873). The median creatinine concentration in the case group was 196 μmol/L, with a range of 131-646 μmol/L. The control group's median creatinine concentration was 60 μmol/L with a range of 32-102 μmol/L. Because most of the used samples were surplus clinical laboratory samples, limited signalment and clinical data were available. Breed was unknown for 10 (43.5%) of the case group and 186 (21.3%) of the control group samples (Table 1). The remaining 13 samples in the case group were taken from dogs of 12 different breeds. The control group samples were taken from dogs of 155 different breeds, including 8% mixed breed dogs.
| Cases | Controls | ||
|---|---|---|---|
| Breed | % | Breed | % |
| Unknown | 43.5 | Unknown | 21.3 |
| Lagotto Romagnolo | 8.7 | Mixed breed | 8.0 |
| Poodle | 4.3 | Labrador Retriever | 3.8 |
| Nova Scotia Duck Tolling Retriever | 4.3 | German Shepherd Dog | 2.7 |
| Bichon Frise | 4.3 | Golden Retriever | 2.4 |
| Bernese Mountain Dog | 4.3 | Shetland Sheepdog | 2.2 |
| Schapendoes | 4.3 | Finnish Lapphund | 2.2 |
| Collie Rough | 4.3 | Poodle | 1.9 |
| Miniature Pinscher | 4.3 | Spanish Water Dog | 1.8 |
| Cavalier King Charles Spaniel | 4.3 | Jack Russell Terrier | 1.6 |
| American Staffordshire Terrier | 4.3 | Nova Scotia Duck Tolling Retriever | 1.5 |
| Dogue de Bordeaux | 4.3 | Swedish Elkhound | 1.3 |
| Bouvier des Flandres | 4.3 | Finnish Hound | 1.3 |
| Miniature Schnauzer | 1.1 | ||
| Bichon Frise | 1.1 | ||
- Note: The table shows the 15 most common breeds in the case and control groups, including samples with unknown breed and mixed breeds. The total number of case group samples was 23, and control group samples was 873. The total number of breeds in the control group was 155, including mixed breed dogs, and 45.8% of the control group samples were taken from breeds not included in the 15 most common breeds.
The NMR metabolomics platform used quantitates 123 analytes. Analytes with multiple missing observations were excluded from further analyses, resulting in 97 analyzed analytes (Supporting Information Table 1).
3.2 Results of the statistical association tests
The Wilcoxon rank-sum test, logistic regression analysis and classification using random forest, all conducted using the normalized and log2-transformed data, identified similar biomarkers (Table 2, Figure 2).
| Group | Analyte | Wilcoxon rank-sum test Pa | Logistic regression | ||
|---|---|---|---|---|---|
| Pa | AIC | AUC | |||
| Citrate | Citrate | <.001 | <.001 | 97 | 0.922 |
Amino acids |
Phenylalanine/tyrosine | <.001 | <.001 | 178 | 0.861 |
| Tyrosine | <.001 | <.001 | 194 | 0.769 | |
| BCAA | <.001 | .007 | 205 | 0.724 | |
| Valine | <.001 | .007 | 205 | 0.731 | |
| Leucine | .005 | .003 | 203 | 0.717 | |
| Albumin | Albumin | .01 | .003 | 207 | 0.703 |
| Fatty acids | Linoleic acid % | <.001 | <.001 | 195 | 0.729 |
| Docosapentaenoic acid % | .176 | .005 | 207 | 0.642 | |
| Acetate | .004 | .583 | 216 | 0.720 | |
- Note: All P-values are FDR-corrected. The full, log2-transformed data with 23 cases and 873 controls were used in both the Wilcoxon rank-sum test and logistic regression analysis. The control group represents a routine laboratory sample population.
- Abbreviations: AIC, Akaike information criterion; AUC, area under the curve; BCAA, branched-chain amino acids.
- a Significant difference, P < .05.
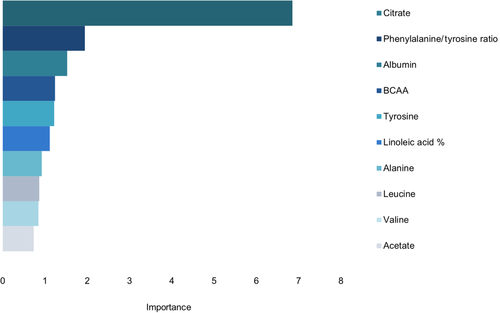
The concentrations of citrate, tyrosine, branched-chain amino acids (BCAA), valine, leucine, albumin, and acetate, linoleic acid percentages, and the ratio of phenylalanine to tyrosine showed significant differences between cases and controls in the Wilcoxon rank-sum test (Table 2). The same analytes, excluding acetate, and including docosapentaenoic acid percentage were associated with increased serum creatinine concentrations in logistic regression analysis. The best model fit and predictive values (AIC and AUC, respectively) were achieved for citrate, followed by phenylalanine to tyrosine ratio, and tyrosine.
In classification using random forest, the 10 biomarkers with the highest variable importance were the same biomarkers that reached significance in the Wilcoxon rank-sum test: citrate, tyrosine, BCAA, valine, leucine, albumin, acetate, linoleic acid percentage and the ratio of phenylalanine to tyrosine, as well as the amino acid alanine (Figure 2).
Because we used different sample sets for different statistical approaches (the full data set for logistic regression analysis and Wilcoxon rank-sum test and a balanced data set with randomly selected samples for random forest classification) we checked whether results of logistic regression and the Wilcoxon rank-sum test were the same in the full and balanced data sets. Both data sets gave similar results.
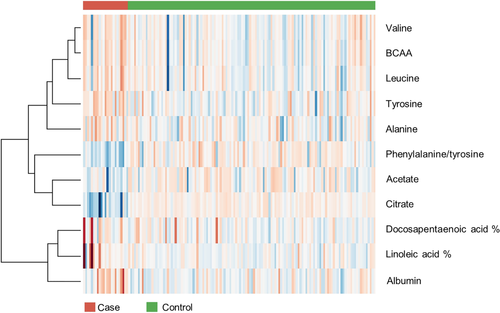
The heat map visualized the aforementioned differences in metabolite concentrations in case and control samples, but it also showed much variability within both the case and control group samples (Figure 3).
3.3 Magnitudes of the differences in analyte concentrations: medians and box plots
The concentrations of BCAA, leucine, valine, tyrosine, alanine, albumin, docosapentaenoic acid percentage, and linoleic acid percentage were lower in the case than in the control group. In contrast, median acetate, citrate, and phenylalanine/tyrosine ratio were higher in the case group (Table 3). The case group's median citrate concentration was higher than the reference interval of the NMR method, indicating that the median concentration in the case group was a clinically observable change.
| Analyte | Median case (IQR) | Median control (IQR) | RI (CI)10 |
|---|---|---|---|
| Citrate (mmol/L) | 0.149 (0.117–0.180)a | 0.075 (0.067-0.086) | 0.061 (0.059-0.063) to 0.123 (0.120-0.125) |
| Phenylalanine/tyrosine ratio | 0.998 (0.925-1.288) | 0.767 (0.664-0.894) | 0.513 (0.494-0.530) to 1.049 (1.026-1.098) |
| Tyrosine (mmol/L) | 0.050 (0.044-0.058) | 0.065 (0.056-0.074) | 0.041 (0.039-0.043) to 0.089 (0.086-0.091) |
| BCAA (mmol/L) | 0.309 (0.266-0.386) | 0.384 (0.333-0.446) | 0.242 (0.215-0.251) to 0.515 (0.502-0.531) |
| Valine (mmol/L) | 0.149 (0.131-0.183) | 0.189 (0.163-0.224) | 0.113 (0.099-0.119) to 0.251 (0.243-0.261) |
| Leucine (mmol/L) | 0.106 (0.086-0.132) | 0.128 (0.112-0.149) | 0.083 (0.078-0.088) to 0.185 (0.181-0.189) |
| Alanine (mmol/L) | 0.338 (0.283-0.446) | 0.398 (0.342-0.462) | 0.216 (0.199-0.222) to 0.597 (0.580-0.624) |
| Albumin (g/L) | 27.0 (25.3-28.9) | 28.9 (27.5-30.2) | 25.2 (24.2-25.7) to 32.4 (32.1-32.8) |
| Linoleic acid (%) | 26.3 (25.5-26.7) | 27.3 (26.3-27.9) | 24.1 (23.9-24.4) to 28.6 (28.4-28.7) |
| Docosapentaenoic acid (%) | 1.2 (.9-1.4) | 1.3 (1.1-1.5) | 1.1 (1.0-1.1) to 2.0 (1.9-2.0) |
| Acetate (mmol/L) | 0.028 (0.025-0.037) | 0.024 (0.020-0.029) | 0.021 (0.020-0.021) to 0.037 (0.036-0.037) |
- Note: The full, untransformed data with 23 cases and 873 controls was used when calculating case and control group medians and their interquartile ranges (IQR). The control group represents a routine laboratory sample population. Reference intervals (RI) represent the reference intervals of the NMR method, and CI represent the confidence intervals of the reference limits.10
- a Analyte's case group median higher than the analysis reference interval and its 90% CI.
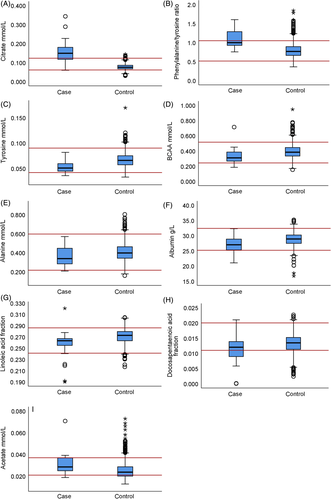
Box plots of the analytes confirmed that citrate concentration and phenylalanine/tyrosine ratio were notably higher in samples with increased serum creatinine concentrations, and often exceeded the reference interval (Figure 4). The dispersion of citrate was notably higher in the case than in the control group. The box plot of acetate suggested that control group results approached the lower analysis reference limit. Although the case group distribution of albumin approached the lower reference limit, certain samples had albumin concentrations near the upper reference limit.
4 DISCUSSION
Azotemia is a common finding in veterinary medicine, and several important clinical conditions manifest with increased serum creatinine concentrations. Serum creatinine concentration routinely is used as a measure of renal function, with increased creatinine concentration indicating decreased glomerular filtration. Systemic metabolic derangements that occur during impaired renal function recently have gained attention, but have not yet been extensively studied in dogs, and therefore are not yet utilized in clinical practice. Our study aimed to utilize a novel quantitative metabolomics platform to identify circulating metabolites associated with increased serum creatinine concentrations, and evaluate its usefulness in patient management. A broad range of physiologically relevant metabolites, such as citrate, BCAA, albumin, and fatty acids were associated with decreased glomerular filtration. These findings may provide insight into compromised renal function and opportunities for patient management.
Blood citrate concentrations were markedly increased in cases, and were markedly dispersed, suggesting different degrees of altered citrate metabolism in these animals. Increased blood citrate concentrations previously have been associated with impaired renal function in both humans and rats.12, 13 The kidneys are responsible for citrate removal from plasma by both urinary excretion and renal tubular cell citrate metabolism, which contributes to renal energy supply.14 An important determinant of citrate excretion is blood pH, and increased citrate reabsorption can occur because of acid retention and metabolic acidosis.7, 15 Because citrate retention has been associated with the early stages of metabolic acidosis, the use of dietary H+ reduction has been suggested in CKD patients with impaired citrate excretion.7
The aromatic amino acids phenylalanine and tyrosine have been associated with impaired renal function.5, 9, 16 In normal conditions, tyrosine is considered a nonessential amino acid, because the body is capable of producing sufficient amounts from phenylalanine. During impaired renal function, however, phenylalanine hydroxylation to tyrosine can be decreased.5, 16 Insufficient phenylalanine hydroxylation to tyrosine leads to a decrease in plasma tyrosine concentrations, a normal to slightly increased plasma concentration of phenylalanine, and an increase in the plasma ratio of phenylalanine to tyrosine.5, 9, 17 These changes also were observed in our study, with the tyrosine concentration being significantly lower, and the ratio of phenylalanine to tyrosine being significantly higher, in the case as compared with the control group. Because tyrosine is a precursor of several important molecules, such as epinephrine and the thyroid hormones, dietary tyrosine supplementation has been suggested when renal tyrosine formation is insufficient.6, 9 Additional studies are needed to identify the tyrosine concentrations, that would benefit from tyrosine supplementation.
We also observed significantly lower concentrations of total BCAA as well as the individual BCAAs valine and leucine in dogs with increased serum creatinine concentration compared to the routine laboratory population. Concentrations of BCAA are known to be decreased in CKD,9, 18 especially during metabolic acidosis.19, 20 This phenomenon is caused by increased catabolism of muscle and BCAA because of the increased activity of liver and muscle branched-chain keto acid dehydrogenase, with decreased protein intake also contributing to the condition.21 This condition can be treated in humans by supplementing the low-protein diet with BCAA or their keto analogues (BCKA).22, 23 Supplementation with BCAA and BCKA also has been suggested in hypoalbuminemic and hypoaminoacidemic dogs, when adequately controlled by routine treatments including clinical renal diets and angiotensin-converting enzyme inhibitors.8
Both hypo- and hyper-albuminemia were observed in the case group. Hypoalbuminemia frequently occurs in advanced CKD because of urinary loss of albumin.4 However, nonrenal conditions also can cause hypoalbuminemia.24 Because albumin is responsible for maintaining blood oncotic pressure, severe hypoalbuminemia causes fluid leakage from the blood vessels, causing edema or ascites.25 Hyperalbuminemia, on the other hand, frequently occurs as a result of dehydration.24 Correcting fluid imbalance is a crucial treatment goal in these patients, because dehydration can have serious consequences, including ischemic kidney injury.
The acetate concentration was significantly higher in case samples than in control samples. Increased blood acetate concentrations previously have been observed in a rat model of CKD.13 Short-chain fatty acids (SCFA), such as acetate, are organic anions mainly produced by gut microbial fermentation.26 The SCFA have immunomodulatory effects27 and contribute to renal blood pressure regulation.28 Long-term administration of large amounts of SCFA has been associated with development of ureteritis and hydronephrosis.29 However, the control group acetate concentrations were relatively low in our study. Thus, characteristics of the control group consisting of routine clinical laboratory samples also could be the reason for significant differences between the case and control groups. Therefore, additional studies of acetate concentrations in dogs with azotemia and other common clinical disease manifestations are warranted.
The omega-3 fatty acid docosapentaenoic acid percentage and the omega-6 fatty acid linoleic acid percentage were significantly lower in case group samples than in controls. Omega-3 fatty acids are considered renoprotective, whereas omega-6 fatty acids are considered detrimental to renal function.30 Because of their bioactive role, omega-3 fatty acids are supplemented in clinical renal diets.31 Additional studies are needed to confirm the association of fatty acid concentrations with the clinical state of the patient.
Alanine concentrations were associated with increased creatinine concentrations, with the case group having lower alanine concentrations than the control group. Previously, decreased alanine concentrations have been found in human patients with impaired renal function,32, 33 and urinary alanine excretion has been associated with proteinuria.33
Two case group samples exhibited severe hypertriglyceridemia of >7 mmol/L. Although hyperlipidemia can be a potential clinical finding in azotemic conditions, such as nephrotic syndrome,34 these samples were excluded from further data analyses because of extreme outlier results. Severe hypertriglyceridemia is an important clinical condition, associated with development of serious diseases, such as pancreatitis, vacuolar hepatopathy and gallbladder mucocele.35
The major limitation of our study is the relatively small case group size, and sample inclusion based only on serum creatinine concentrations, and not actual clinical diagnoses. This approach was taken because clinical data was available only for a minority of the samples. Because of the inclusion criteria, the case group may include samples from animals with diverse, acute and chronic, prerenal, renal or postrenal conditions. We also could not evaluate the effects of other physiological variables, such as age and sex, because complete demographic information was lacking for most samples. Using routine clinical laboratory samples as the control group enabled us to find metabolites differentiating samples with high serum creatinine concentrations from other conditions. It also allowed us to use samples with similar sample handling procedures. However, general changes associated with multiple disease states are not well visualized by this approach, and variability in metabolite results within the control group was high.
In summary, we identified metabolic changes associated with increased serum creatinine concentrations with possible implications for disease diagnostic testing and management. The quantitative NMR metabolomics approach is a promising tool for identifying the metabolic alterations that occur in renal diseases and monitoring these changes in response to treatment. Additional studies with well-defined clinical cohorts are needed to evaluate how these changes are associated with the specific clinical status of canine patients.
ACKNOWLEDGMENTS
Funding provided by PetBIOMICS Ltd and Academy of Finland (308887). We thank the staff of Movet Ltd for sample acquisition and coordination of sample flow. The staff of Kuopion Eläinlääkärikeskus Ltd, and Pieneläinvastaanotto Punaturkki Ltd are acknowledged for help in sample handling and acquisition. We thank the customers of Movet Ltd for enabling the scientific use of leftover diagnostic sample material. We thank all dog owners, that participated in the metabolomics clinic pilot for enabling the scientific use of their dogs' samples and metadata. We thank Kibble Labs Ltd for NMR analysis of the samples and MediSapiens Ltd for sample data management. The canine genetics research group at the University of Helsinki is thanked for help in sample management. Tuomas Poskiparta and Katja Jauni are acknowledged for advice and help in project conceptualization. Jenni Puurunen is thanked for advice in data visualization.
CONFLICT OF INTEREST DECLARATION
Claudia Ottka is an employee, Katariina Vapalahti is a previous employee, and Hannes Lohi is an owner and the Chairman of the Board of PetBIOMICS Ltd. Ann-Marie Määttä is the CEO, and Nanna Huuskonen is a member of board of Movet Ltd. Sinikka Sarpanen is an owner and CEO of Kuopion Eläinlääkärikeskus Ltd. Liisa Jalkanen is an owner and chairman of board of Pieneläinvastaanotto Punaturkki Ltd.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study was performed as a retrospective evaluation of clinical blood samples and surplus of clinical samples. All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. Committee: Finnish national Animal Experiment Board, permit number: ESAVI/7482/04.10.07/2015. Permission for scientific use was obtained for all samples.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




