Effect of feline characteristics on plasma N-terminal-prohormone B-type natriuretic peptide concentration and comparison of a point-of-care test and an ELISA test
Funding information: Agria and Swedish Kennel Club´s Research Fund; Foundation Strömsholms Djursjukvård; Michael Forsgren Foundation; Research bursary fund within IVC and Evidensia; Sveland Research Fund; The SLU Companion Animals Research Fund
Abstract
Background
Increased plasma concentration of N-terminal-prohormone B-type natriuretic peptide (NT-proBNP) can be detected in cats with cardiac disease. Potential effects of feline characteristics on NT-proBNP concentration may influence clinical usefulness.
Objectives
To evaluate potential effects of feline characteristics on NT-proBNP plasma concentration and to compare NT-proBNP plasma concentrations in healthy cats with results in hypertrophic cardiomyopathy (HCM) cats with or without left atrial enlargement (LAE) using an ELISA and a point-of-care test (POCT), and assess if POCT results reflect ELISA results.
Animals
One hundred healthy cats of 3 breeds and 39 HCM cats were included.
Methods
Diseases other than HCM were excluded by physical examination, blood pressure measurement, echocardiography, hematology, and serum biochemistry.
Results
Higher NT-proBNP concentrations were found in males than in females in healthy (P = .005) and in HCM cats (P = .0021), but breed had no effect on NT-proBNP concentrations. Using ≥100 pmol/L as a cutoff for abnormal samples, ELISA and POCT had similar sensitivity (SE; 72 and 74%) and specificity (SP; 97 and 98%) for detecting cats with HCM, cats with HCM and LAE (SE, both 100%; SP, 97 versus 98%), and cats with HCM without LAE (SE, both 69%; SP, 97 versus 98%), respectively, when compared to healthy cats.
Conclusions and Clinical Importance
Breed had no effect on plasma NT-proBNP concentrations, but higher concentrations were found in male than in female cats. The ELISA and POCT had similar SE and SP for detecting HCM. Both tests could identify all HCM cats with LAE but not all HCM cats without LAE.
Abbreviations
-
- 2D
-
- 2-dimension
-
- ALT
-
- alanine aminotransferase
-
- Ao
-
- aorta
-
- BCS
-
- body condition score
-
- BNP
-
- B-type natriuretic peptide
-
- BW
-
- body weight
-
- CHF
-
- congestive heart failure
-
- CV
-
- coefficient of variation
-
- DSH
-
- Domestic Shorthair
-
- FS
-
- fractional shortening
-
- HCM
-
- hypertrophic cardiomyopathy
-
- HDO
-
- high-definition oscillometric device
-
- HR
-
- heart rate auscultation
-
- IQR
-
- interquartile range
-
- IVSd
-
- interventricular septum diastole
-
- IVSdinc%
-
- percentage increase interventricular septal diameter in end diastole
-
- LA
-
- left atrium
-
- LA/Ao
-
- left atrial-to-aortic root diameter ratio
-
- LV
-
- left ventricle
-
- LVFWd
-
- left ventricular free wall diameter in end diastole
-
- LVFWdinc%
-
- percentage increase of left ventricular free wall diameter in end diastole
-
- LVIDd
-
- left ventricular internal diameter in end diastole
-
- LVIDdinc%
-
- percentage increase of left ventricular internal diameter in end diastole
-
- LVIDs
-
- left ventricular internal diameter in systole
-
- NF
-
- Norwegian Forest
-
- NP
-
- natriuretic peptide
-
- NT-proBNP
-
- N-terminal-prohormone-B-type natriuretic peptide
-
- proBNP
-
- prohormone-BNP
-
- POCT
-
- point-of-care test
-
- ROC
-
- receiver operator characteristic curve
-
- SAM
-
- systolic anterior motion
-
- SBP
-
- systolic blood pressure
-
- SE
-
- sensitivity
-
- SP
-
- specificity
-
- T4
-
- thyroxine
1 INTRODUCTION
A high prevalence of cardiac disease has been reported in cats, and affected individuals may develop severe dyspnea because of congestive heart failure (CHF), as well as arterial thromboembolism and sudden death.1, 2 Hypertrophic cardiomyopathy (HCM) is the most common cardiac disease in cats,3, 4 and this form of cardiomyopathy is characterized by hypertrophy of the left ventricle (LV).5 Cardiac disease remains preclinical in many affected cats, and suspicion of cardiac disease may occur when the clinician incidentally discovers a murmur, gallop sound, or arrhythmia.3, 4, 6
Cardiac biomarkers in blood samples may be measured as part of clinical assessment, especially when echocardiography is not available. B-type natriuretic peptide (BNP) is produced as prohormone-BNP (proBNP) by cardiomyocytes in response to wall stretch.7 Inactive proBNP is cleaved intracellularly before release from the cardiomyocyte into biologically active BNP, which has a short half-life, and inactive more stable N-terminal-proBNP (NT-proBNP), which usually is measured in clinical settings.8
A quantitative ELISA that measures NT-proBNP concentration is available for cats, as is a semiquantitative point-of-care test (POCT).9, 10 The ELISA test is offered by a commercial laboratory (IDEXX Laboratories Inc, Westbrook, Maine), whereas the POCT offers a quick assessment of NT-proBNP concentration in the clinic. The POCT assay results are normal or abnormal based on a cutoff NT-proBNP concentration of approximately 100 pmol/L according to the manufacturer (SNAP Feline proBNP, IDEXX Laboratories Inc). The transition interval when the POCT becomes positive has been reported to range between 108 and 200 pmol/L.9, 11 Abnormal ELISA or POCT NT-proBNP results in cats have been shown to be associated with cardiac disease, implying that further diagnostic evaluation by echocardiography is indicated.5, 9-14 Previous studies have reported that both quantitative and semiquantitative NT-proBNP concentrations can discriminate cats with cardiac disease from healthy cats. Sensitivity (SE) has been described to vary between 65.4 and 93.9%, and specificity (SP) between 87.8 and 100%.9-12, 14, 15 These studies included cats with various cardiac diseases.
In dogs, considerable interbreed variation exists in concentration of NT-proBNP.16-19 Studies specifically designed to investigate breed variation in NT-proBNP concentration in cats have not been performed previously. In 1 study designed to investigate NT-proBNP concentration in cats with HCM of different severity, breed had no influence on NT-proBNP concentration.14 In that study, several breeds were included, often comprising only a few cats per breed.14 Furthermore, little information is available concerning other characteristics such as sex, age and body condition score (BCS) on plasma NT-proBNP concentration.12, 14, 20
We hypothesized that plasma concentrations of NT-proBNP (1) vary with characteristics such as breed, sex, age, body weight (BW) and BCS; (2) may differentiate cats with HCM with or without left atrial enlargement (LAE) from healthy cats; and (3) POCT results reflect ELISA results. Our aims were to assess potential effects of feline characteristics on NT-proBNP plasma concentration, to compare NT-proBNP concentrations in healthy cats with concentrations in HCM cats with or without LAE using ELISA and a POCT, and to assess if POCT results reflect ELISA results.
2 MATERIALS AND METHODS
2.1 Recruitment
The study was approved by the Uppsala Animal Experiment Ethics Board. Cats were recruited prospectively to participate in the study through information for cat owners provided on webpages, at seminars or at the recruiting clinic. Client-owned cats were examined at Evidensia Animal Clinic in Västerås between September 2014 and June 2017. Clinically healthy cats and cats with murmurs or previously diagnosed HCM were examined for possible inclusion in the study. Informed written consent was obtained from the owner of each cat.
2.2 Inclusion criteria
Healthy cats of nonpurebred Domestic Shorthair (DSH), purebred Birman and Norwegian Forest (NF), and HCM cats of any breed were allowed into the study. Cats with preclinical or clinical HCM, stabilized as a consequence of CHF treatment, were allowed into the study. Cats aged between 1 and 14 years were eligible for the study. All healthy cats must have had normal echocardiogram. Diagnosis of HCM was based on characteristic findings on an echocardiogram as outlined below.21-23
2.3 Exclusion criteria
Cats with increased mean systolic blood pressure (SBP) >160 mmHg, increased serum concentrations of thyroxine (TT4), creatinine, or fructosamine, increased alanine aminotransferase (ALT) activity, or some combination of these were excluded from the study. Cats with decompensated CHF, thromboembolism, congenital cardiac disease, other acquired cardiovascular disorders, equivocal findings concerning the presence of hypertrophy of the LV, severe dental disease or clinically relevant organ-related or systemic diseases were not included in the study. All cats receiving medical treatment other than those in need of standard CHF treatment, standard antithrombotic treatment, medroxyprogesterone acetate, or deslorelin acetate were excluded from the study.
2.4 Clinical examination and blood pressure measurement
All examinations were performed according to a standardized protocol in a quiet examination room by a single experienced veterinarian (S.H.). The healthy control cats were examined between 0900 and 1300 hours. The cats were allowed to adapt to the environment for 10-15 minutes together with their owners before SBP measurement.24 An appropriate cuff was applied to the base of the tail and the cats then were allowed to rest in their carrier before SBP was indirectly measured using an automated high-definition oscillometric (HDO) device (VET Memodiagnostic HDO monitor, S+B medVET, Babenhausen, Germany).25 Once consistent consecutive readings were obtained, 6 recordings were performed. Mean values for SBP were determined for each cat, after discarding recordings deviating more than 20%.24
After the SBP measurement, all cats underwent general physical examination. The BCS was recorded on a 9-point scale.26
2.5 Echocardiography
Transthoracic 2-dimensional (2D), M-mode, color flow, and spectral Doppler echocardiographic evaluations were performed by an experienced echocardiographer, trained by a board-certified veterinary cardiologist, in unsedated cats placed first in right and then in left lateral recumbency using an ultrasound system (IE33, Philips Ultrasound, Bothell, Washington) equipped with a 4-12 MHz phased-array probe. Continuous electrocardiography monitoring was performed during echocardiographic examination. Images and loops from standardized imaging planes21 were stored digitally. The left atrial-to-aortic root diameter ratio (LA/Ao) was measured in 2D from the right 2D short-axis view.22 Diastolic and systolic LV dimensions (interventricular septum diastole [IVSd], LV internal diameter in end diastole [LVIDd], LV free wall diameter in end diastole [LVFWd], LV internal diameter in systole [LVIDs], and fractional shortening [FS]) were measured from M-mode and 2D using right parasternal short- and long-axis images.21, 23 Mitral, tricuspid, aortic, and pulmonic valves were interrogated using spectral and color-flow Doppler according to published recommendations.21, 23 Two-dimensional images of the LV outflow tract were used to identify the presence of systolic anterior motion (SAM) of the mitral valve.
The diagnosis HCM was made when a subjective impression of hypertrophy (regional or global) was supported by increased M-mode and 2D diastolic LV wall dimensions of the interventricular septum (IVSd), left ventricular free wall (LVFWd), or both defined as outlined below. Left atrial size was assessed using the LA/Ao as previously described.22 Expected BW-dependent values for IVSd, LVIDd, and LVFWd were calculated according to previously generated formulas for cats.27 Percentage deviation from these expected values was calculated according to the formula: Xinc(%) = ([observed value-expected value]/expected value) × 100, where X represents the variable in question. These calculated percentage deviations from expected BW-dependent values,27 and the LA/Ao ratio were used to classify the cats into 3 different groups: healthy controls, HCM without LAE, and HCM with LAE. Cats with subjectively normal LV morphology, LA/Ao <1.5,27, 28 and <30% increase in BW-based predicted values for IVSd and LVFWd27 were classified as healthy cats. Cats with subjective impression of LV hypertrophy and ≥30% increase in BW-based predicted values for IVSd or LVFWd were classified as having HCM without LAE if LA/Ao <1.5, and with LAE if LA/Ao ≥1.5. Cats that could not be classified according to the criteria specified above were excluded from the study.
2.6 Blood collection and analysis
Blood was collected in ethylenediaminetetraacetic acid (EDTA) and serum tubes by venipuncture of the cephalic vein in unsedated minimally restrained cats. Hematology and serum biochemistry profiles, including ALT activity, creatinine, glucose, and total protein concentrations, were performed at Evidensia Animal Clinic in Västerås immediately after sampling using the ProCyte (IDEXX ProCyte Dx, IDEXX Laboratories, Inc) and Catalyst (Catalyst Dx Chemistry Analyzer, IDEXX Laboratories, Inc) systems. Total thyroxine and fructosamine concentrations were analyzed within a few days after each sampling at the Clinical Pathology Laboratory of the University Animal Hospital, Swedish University of Agricultural Sciences using the Immulite (IMMULITE 2000, Siemens Healthcare GmbH, Erlangen, Germany) and Abbott Architect (Abbott Architect c4000, Abbott Park, IL, USA) systems.
For later analysis of NT-proBNP, EDTA blood was centrifuged and plasma frozen at −80°C within 30 minutes of collection for storage. All samples were transported frozen (−80°C) to a commercial laboratory (Vet Med Labor GmbH, Division of IDEXX Laboratories, Ludwigsburg, Germany) for batch analysis in duplicate using a validated second-generation ELISA for cats (CardioPet NT-proBNP assay, IDEXX Laboratories, Inc).29 The reported assay range for NT-proBNP concentration at the commercial laboratory was 24-1500 pmol/L. For statistical analyses, concentrations of NT-proBNP less than the lowest reported concentration were assigned a result of 24 pmol/L. Concentrations of NT-proBNP >1500 pmol/L were assigned a result of 1500 pmol/L.
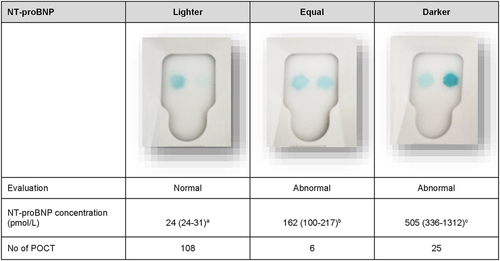
All samples also were analyzed using the POCT feline NT-proBNP (SNAP Feline proBNP, IDEXX Laboratories Inc) according to the instructions of the manufacturer. The samples were randomized and the examiner (S.H.) performing the evaluation of the POCT was blinded to the identity of the cats. Plasma was thawed and kept at room temperature for <30 minutes before analysis. Normal results were recorded when the density of the sample spot appeared lighter than the reference spot, according to the manufacturer's instructions (IDEXX Reference Laboratories Inc). Abnormal results were recorded when the density of the sample spot appeared equal to or darker than the reference spot, which, according to the manufacturer, occurs when concentrations are >100 pmol/L (IDEXX Reference Laboratories Inc). Evaluation of the POCT was performed on the same occasion by visual inspection and using an automated evaluation (SNAP Pro Analyzer, IDEXX Laboratories Inc). The examiner performed the visual inspection before checking and documenting the results from the automated evaluation. Visual inspection of the POCT results was classified into 3 groups (lighter, equal, and darker), based on subjective evaluation of the sample spot color. After all samples had been analyzed, concentrations of NT-proBNP measured by ELISA were revealed.
2.7 Statistical analysis
Statistical analyses were performed using a commercially available software program (JMP, version 12.2.0, SAS Institute Inc, Cary, North Carolina). Group data are presented as medians and interquartile range (IQR). A value of P < .05 was considered significant, unless otherwise indicated.
Univariable analyses were performed separately in 3 groups of cats: all cats, healthy cats and HCM cats. Fisher's exact test was used for comparing proportions. The nonparametric Kruskal-Wallis test was used to evaluate the effects of nominal and ordinal variables (sex, neutered or intact, BCS, presence or absence of SAM) on NT-proBNP concentrations. Furthermore, the Kruskal-Wallis test was used to evaluate the overall association between the POCT results (intensity of color in the POCT) and the plasma NT-proBNP concentrations in all cats, and for evaluating the effect of breed in the healthy cat group. The Kruskal-Wallis test was used followed by Bonferroni correction for post hoc comparison between groups. These statistical analyses were repeated in the HCM cats after excluding 6 cats treated with cardiac medications (furosemide, enalapril, clopidogrel).
Spearman's ρ was used to evaluate potential associations between plasma NT-proBNP concentration and continuous variables (age, SBP, heart rate auscultation [HR] obtained from clinical examination, echocardiographic measurements, and storage time of the plasma samples) for any of the cat groups with a high rate of nondetectable NT-proBNP plasma concentrations.
After logarithmic transformation of the concentration of NT-proBNP, linear regression was used to evaluate potential associations between plasma NT-proBNP concentration and continuous variables (age, BW, SBP, echocardiographic measurements, HR obtained from clinical examination, and storage time of the plasma samples) in any of the cat groups with a high rate of detectable NT-proBNP plasma concentrations. Variables with P < .2 in the univariable regression analysis were included in a multiple regression analysis as were BCS and sex. Multivariable analyses were performed in a backward stepwise manner, starting with all variables included in the model and then removing the variable with the highest P value until all of the remaining variables had a P value <.05. All variables were assessed as main effects; no interaction terms were considered in the model. The distribution of the residuals was assessed for normality by inspection of normal quantile plots. The adjusted R 2 is defined as the percentage of the total sum of squares that can be explained by the regression and it also considers the degrees of freedom for variables added.
The optimal cutoff (combination of highest SE and SP) in NT-proBNP concentration for identifying an abnormal POCT was investigated using receiver operator characteristic (ROC) curves.
3 RESULTS
3.1 Study population
Of 118 examined apparently healthy cats, 18 cats were excluded. Five of these cats had kidney disease, 5 had mild heart disease (4 cats had cardiac disease other than HCM and 1 cat had equivocal findings concerning presence of hypertrophy of the LV), 2 had severe dental disease, 2 had congenital defects (diaphragmatic hernia and peritoneal-pericardial diaphragmatic hernia), 2 had ALT activity above the reference range, and 1 had hyperthyroidism. Of 48 examined cats with murmurs or previously diagnosed HCM, 9 were excluded: 2 of these cats were normal, but did not meet the breed criteria for the healthy controls, 2 had congenital defects (ventricular septal defect and bicuspid Ao), 1 had equivocal findings concerning presence of hypertrophy of the LV, 1 was in decompensated CHF, 1 had kidney disease, 1 had hyperthyroidism, and 1 cat was treated with 4 times the normal dose of clopidogrel.
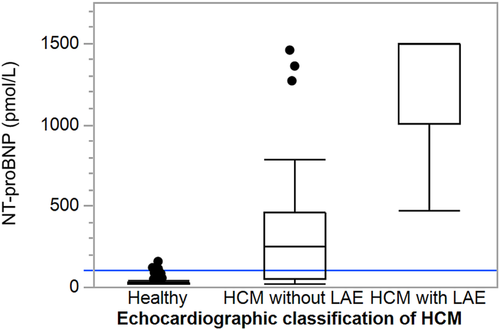
A total of 139 cats (69 males and 70 females) were included in the study: 100 healthy controls, 32 cats with HCM without LAE and 7 HCM cats with LAE. Three of the cats with HCM had experienced CHF and were stabilized by medical treatment. The healthy cats consisted of 35 NF, 33 Birman, and 32 DSH cats. The HCM cats comprised 13 breeds: 18 nonpurebred DSH, 5 Persian, 4 NF, 2 Bengal, 2 Maine Coon, 2 Ragdoll, and 1 each of the following breeds: British Shorthair, Cornish Rex, Exotic, Siberian, and Sphynx. Two healthy male cats were treated with deslorelin, and 1 of the female cats with HCM without LAE was treated with medroxyprogesterone acetate. Three of the cats with HCM without LAE were treated with enalapril, and 3 of the stabilized CHF cats were treated with furosemide and enalapril, of which 1 cat also received clopidogrel. Feline characteristics, concentration of NT-proBNP, and echocardiographic measurements of the 3 cat groups (healthy, HCM without LAE, and HCM with LAE) are presented in Table 1 and Figure 1. Feline characteristics, echocardiographic measurements, concentration of NT-proBNP, and POCT results of the healthy control cats of the 3 different breeds are presented in Table 2.
| Healthy | HCM without LAE | HCM with LAE | |
|---|---|---|---|
| Number | 100 | 32 | 7 |
| Sex (F/M) | 57/43 | 12/20 | 1/6 |
| Sex NF/F/NM/M | 35/22/33/10 | 9/3/16/4 | 0/1/6/0 |
| Age (years) | 4.6 (2.1-8.7)a | 5.3 (3.6-9.1)a | 6.6 (3.0-9.0)a |
| Weight (kg) | 4.3 (3.6-5.5)a | 5.0 (4.1-5.9)a | 5.1 (3.8-5.9)a |
| BCS normal 4-5/overweight 6-7) | 49/501 | 6/224 | 5/2 |
| HR (bpm) | 160 (140-180)a | 160 (143-200)a | 180 (150-200)a |
| Murmur (yes/no) | 4/96 | 30/2 | 7/0 |
| SBP (mmHg) | 135 (123-143)a | 140 (131-148)a | 130 (128-134)a |
| LA/Ao | 1.1 (1.0-1.2)a | 1.1 (1.0-1.2)a | 1.6 (1.6-1.7)b |
| IVSd (mm) | 3.8 (3.5-4.1)a | 6.1 (5.3-6.4)b | 6.8 (6.5-8.5)b |
| IVSd inc (%) | −0.6 (−6.3 to 5.8)a | 52.7 (44.4-68.7)b | 83.0 (63.5-114.6)c |
| LVIDd (mm) | 15.9 (14.9-17.4)a | 15.0 (13.2-16.5)b | 14.7 (14.0-17.6)ab |
| LVIDdinc (%) | 1.1 (−4.8 to 8.3)a | −5.6 (−18.4 to −2.1)b | −10.2 (−14.5 to 15.7)ab |
| LVFWd (mm) | 3.7 (3.5-4.0)a | 5.3 (4.4-6.2)b | 7.7 (7.1-8.1)c |
| LVFWdinc (%) | −0.9 (−6.3 to 4.6)a | 31.7 (14.8-58.1)b | 105.3 (89.6-114.0)c |
| FS (%) | 50 (46-56)a | 60 (52-64)b | 47 (41-59)ab |
| SAM (yes/no) | 0/100 | 24/8 | 5/2 |
- Note: Within each row, values with different letter superscripts are statistically different (P < .017) after Bonferroni correction, and values with the same superscript letter did not differ significantly. For description of the grading of hypertrophic cardiomyopathy, see the main text. Superscript numbers for specific comment for number of missing values: 1 = one missing value, 3 = three missing values, 4 = four missing values. The median and interquartile ranges are shown for continuous variables.
- Abbreviations: BCS, body condition score; F, female; FS, fractional shortening; HCM, hypertrophic cardiomyopathy; IVSd, interventricular septum diastole; IVSdinc%, percentage increase in end-diastolic interventricular septal dimension; LA/Ao, left atrial-to-aortic root diameter ratio; LAE, left atrial enlargement; LVFWd, left ventricular free wall diameter in end diastole; LVFWdinc%, percentage increase in end-diastolic left ventricular free wall dimension; LVIDd, left ventricular internal diameter in end diastole; LVIDdinc%, percentage increase in end-diastolic left ventricular internal dimension; M, male; N, neutered; HR, heart rate auscultation; SAM, systolic anterior motion of the mitral valve; SBP, systolic blood pressure, basic echocardiographic data.
| Group | Birman | Domestic Shorthair | Norwegian Forest |
|---|---|---|---|
| Number | 33 | 32 | 35 |
| Sex (F/M) | 21/12 | 14/18 | 22/13 |
| Sex NF/F/NM/M | 9/12/7/5 | 14/0/18/0 | 12/10/8/5 |
| Age (years) | 3.2 (1.5-7.4)a | 7.1 (3.0-10.8)a | 4.1 (2.1-7.2)a |
| Weight (kg) | 3.4 (2.9-4.0)a | 4.5 (4.0-5.5)b | 5.4 (4.1-6.4)b |
| BCS normal 4-5/overweight 6-7) | 21/111 | 14/18 | 14/21 |
| HR (bpm) | 160 (140-164)a | 140 (132-179)a | 160 (140-180)a |
| Murmur (yes/no) | 2/31 | 1/31 | 1/34 |
| SBP (mmHg) | 124 (116-131)a | 140 (136-147)b | 138 (124-145)b |
| LA/Ao | 1.1 (1.0-1.2)a | 1.1 (1.0-1.2)a | 1.1 (1.0-1.2)a |
| IVSd (mm) | 3.6 (3.4-3.8)a | 3.9 (3.6-4.1)b | 4.0 (3.8-4.3)b |
| IVSd inc (%) | −3.1 (−5.8 to 2.9)a | 0.8 (−7.9 to 7.7)a | −0.3 (−6.4 to 5.9)a |
| LVIDd (mm) | 15.3 (13.9-15.8)a | 16.3 (15.0-18.3)b | 16.4 (15.7-18.0)b |
| LVIDdinc (%) | 0.1 (−4.6 to 7.7)a | 2.5 (−5.6 to 10.5)a | 1.7 (−4.0 to 7.2)a |
| LVFWd (mm) | 3.5 (3.3-3.7)a | 3.8 (3.5–4.0)b | 4.0 (3.6-4.2)b |
| LVFWdinc (%) | −0.9 (−11.0 to 4.5)a | −1.4 (−5.7 to 6.8)a | −0.3 (−5.4 to 3.7)a |
| FS (%) | 48 (45-56)a | 51 (48-57)a | 50 (46-56)a |
| SAM (yes/no) | 0/33 | 0/32 | 0/35 |
| NT-proBNP pmol/L (ELISA) | <24 (<24-39)a | <24 (<24-38)a | <24 (<24-29)a |
POC test Normal/abnormal visual evaluation |
33/0 | 31/1 | 34/1 |
POC test Normal/abnormal automated evaluation |
30/3 | 30/2 | 35/0 |
- Note: Within each row, values with different superscripts are statistically different (P < .017) after Bonferroni correction, and values with the same superscript letter did not differ significantly. Superscript numbers for specific comment for number of missing values; 1 = one missing value. The median and interquartile ranges are shown for continuous variables.
- Abbreviations: BCS, body condition score; F, female; FS, fractional shortening; HR, heart rate auscultation; IVSd, interventricular septum diastole; IVSdinc%, percentage increase in end-diastolic interventricular septal dimension; LA/Ao, left atrial-to-aortic root diameter ratio; LVFWd, left ventricular free wall diameter in end diastole; LVFWdinc%, percentage increase in end-diastolic left ventricular free wall dimension; LVIDd, left ventricular internal diameter in end diastole; LVIDdinc%, percentage increase in end-diastolic left ventricular internal dimension; M, male; N, neutered; NT-proBNP, N-terminal-prohormone B-type natriuretic peptide; SAM, systolic anterior motion of the mitral valve; SBP, systolic blood pressure, basic echocardiographic data.
Blood glucose concentration was mildly increased in 11/139 cats. However, serum fructosamine concentrations were measured and were within normal limits in all cats, as were hematology and other serum biochemistry variables.
3.2 Evaluation of NT-proBNP plasma concentration measured by ELISA
The intra-assay coefficient of variation (CV) for the feline NT-proBNP ELISA was 5.2%-8.1%, and the interassay CVs were 7.2, 3.1, and 4.2%, respectively, for samples with mean concentrations of 57, 229, and 639 pmol/L, respectively.
3.3 All cats
3.3.1 Univariable analysis
The median NT-proBNP plasma concentration was higher in male (49 pmol/L; IQR, <24-300) than in female cats (<24 pmol/L; IQR, <24-40; P < .0001), and higher in cats with SAM (P < .0001) than in those that did not have SAM. The NT-proBNP concentration increased with increasing IVSdinc% (R2 = .22; P < .0001), LVPWdinc% (R2 = .21; P < .0001), and FS (R2 = .07; P = .002).
3.4 Healthy cats
The median NT-proBNP concentration in the healthy cats was <24 pmol/L in all 3 breeds with similar IQR across the breeds (Birman, <24-39; DSH, <24-38; NF, <24-29 pmol/L). Three of the healthy controls had concentrations >100 pmol/L (110, 117, and 155 pmol/L, respectively).
3.4.1 Univariable analysis
Median NT-proBNP concentration was higher (P = .005) in male (25 pmol/L; IQR, <24-49) than in female cats (<24 pmol/L; IQR, <24-26).
3.5 Cats with HCM
Of the 39 cats with HCM, 11 cats had an NT-proBNP concentration <100 pmol/L (range, <24-69 pmol/L).
3.5.1 Univariable regression analysis
N-terminal-proBNP concentrations increased with increasing IVSdinc% (R2 = .17; P = .006), percentage increase of LVFWd (LVFWdinc%; R2 = .36; P < .0001), and LA/Ao (R2 = .17; P = .007), and with decreasing SBP (R2 = .11; P = .02).
3.5.2 Multiple regression analysis
The effect of increasing LVFWdinc% (P < .0001) on NT-proBNP concentration was maintained in the multiple regression analysis. Furthermore, the final model also included sex (males had higher concentrations, P = .002). The final model included the variables LVFWdinc%, sex, and BW had an adjusted R2 of .50.
Only marginal changes of the results presented above were found after excluding the 6 cats treated with any cardiac medication (furosemide, enalapril, clopidogrel) from the data set, except that IVSdinc% was no longer significantly associated with plasma concentration of NT-proBNP.
3.6 Evaluation of POCT NT-proBNP test results
3.6.1 All cats
The ELISA concentration of NT-proBNP was higher in cats when the subjective evaluation of the sample spot was darker than when the sample spot color was equal to the control spot (P < .0001), and higher in cats when the subjective evaluation of the sample spot was equal to the control spot compared to when it was lighter (P < .0001) (Figure 3). The transition interval between normal and abnormal for the POCT was between 69 and 117 pmol/L. A ROC curve showed that the optimal cutoff value (combination of highest SE and SP) for ELISA NT-proBNP concentration to identify an abnormal POCT was 110 pmol/L.
3.6.2 Healthy cats
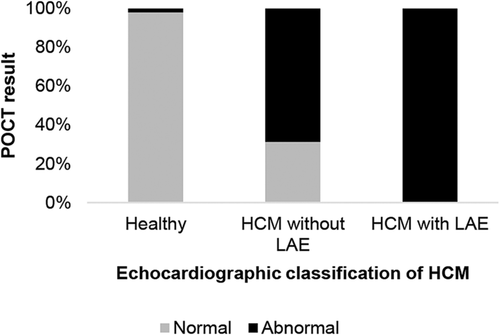
By visual inspection of the POCT, 98/100 healthy cats were classified as normal and 2/100 as abnormal (ELISA NT-proBNP concentrations in these cats were 110 and 155 pmol/L, respectively; Figures 2 and 3).
By automated evaluation of the POCT, 95/100 healthy cats were evaluated as normal and 5/100 healthy cats as abnormal (corresponding ELISA NT-proBNP concentrations in these cats were 49, 61, 78, 88, and 155 pmol/L, respectively).
3.6.3 Cats with HCM
By visual inspection of the POCT, 29/39 (74%) HCM cats were classified as abnormal using the POCT (corresponding plasma NT-proBNP concentrations using the ELISA were 69 pmol/L in 1 cat and >100 pmol/L in the other 28 cats), and 10 HCM (26%) cats were classified as normal (all had ELISA concentrations <100 pmol/L; Figures 2 and 3).
By the automated evaluation, 29/39 (74%) HCM cats were classified as abnormal using the POCT (corresponding ELISA NT-proBNP concentrations were >100 pmol/L in 27 cats, and 61 and 69 pmol/L, respectively, in 2 cats), and 10/39 (26%) HCM cats were classified as normal (corresponding ELISA NT-proBNP concentrations were <100 pmol/L in 9 cats and 168 pmol/L in 1 cat).
3.6.4 SE and SP for detecting HCM
Using ≥100 pmol/L as a cutoff value for identifying abnormal samples, the ELISA and visual inspection and automated evaluation of the POCT had SE of 72 and 74% (both visual inspection and automated evaluation) and SP of 97, 98, and 95%, respectively, for detecting HCM when healthy cats were compared to all cats with HCM. The ELISA and the visual inspection and automated evaluation of the POCT had a SE of 100% and SP of 97, 98, and 95%, respectively, for detecting HCM cats with LAE, and a SE of 69%, and SP of 97, 98, and 95% respectively, for detecting HCM cats without LAE (Figure 2) when the specific groups were compared to healthy cats.
4 DISCUSSION
Male cats had higher plasma NT-proBNP concentrations than did female cats in our population of cats. The NT-proBNP concentration in healthy cats was not affected by age, BW, BCS, or breed. In all cats, and in the cats with HCM, the NT-proBNP concentration increased with increasing IVSd and LVFWd. Measuring NT-proBNP using the POCT and the ELISA tests showed similar SE and SP for detecting HCM. The ELISA and the POCT results were abnormal in all HCM cats with LAE, but not in all HCM cats without LAE.
Median concentration of NT-proBNP was higher in healthy male cats than in female cats in our study. This finding contrasts with studies in people in which the NT-proBNP concentration has been reported to be higher in women than in men,30-32 and with studies in dogs, which have shown ambiguous results such as no effect of sex on the NT-proBNP concentration,33 or a higher concentration in female dogs.34, 35 In previous studies in cats, sex has not been reported to significantly influence the NT-proBNP concentration.12, 14 Testosterone also may be a factor. A suppressive effect of testosterone has been suggested to contribute to the difference in BNP concentration between men and women,36 and in rats, testosterone has been shown to suppress the release of NPs.37 In our study, most of the male cats (77%) were neutered, which may be a reason for the different results compared to those in humans. A significant difference was found between sexes in the healthy cat group, and the reason for this difference hopefully will be further explored in future studies.
Although the healthy cats were from 3 genetically distant breeds,38 with >30 cats/breed (see Table 2), no significant difference in NT-proBNP concentration was found among the breeds, which is in accordance with a previous study that included several different breeds with variable and often small breed group sizes.14 This finding is in contrast to studies in dogs, in which significant interbreed differences have been found in NT-proBNP concentrations.19, 39
In the cats with HCM, lower SBP was associated with higher concentrations of plasma NT-proBNP. A possible explanation for this finding may be that cats with more prominent HCM might have impaired cardiac output leading to lower, but still within normal variation, SBP.40 Furthermore, NPs regulate several physiological processes, such as arteriolar relaxation and promotion of urinary sodium excretion (natriuresis), which potentially might lower the SBP further.41
Left ventricular hypertrophy, LAE or both were associated with significantly increased NT-proBNP concentrations in cats with HCM in our study. Similar findings have been described previously in cats,12, 14, 15 dogs,34 and people.42-44 The POCT had similar SE and SP as did the ELISA for diagnosing HCM in cats, which is in agreement with previous studies in which SE of the tests ranged between 71%-92% and SP between 94%-100% for detecting cardiac disease.12, 14, 15 The test results obtained by visual inspection of the POCT were similar to the automated evaluation. The POCT in our study had a SE of 74% with visual inspection or automated evaluation for detecting HCM, and the SP was slightly lower when analyzed by automated evaluation (95%) compared to visual inspection (98%) of the NT-proBNP concentration. However, our study design, with a study population consisting of either cats with HCM or healthy cats, and excluding cats with equivocal findings concerning presence of hypertrophy of the LV, other diseases than HCM, and cats with abnormal blood test results, might have overestimated the accuracy of the test and thereby resulting in higher SE and SP for the ELISA and the POCT test than if the test had been used in the general feline population. Differences in SE (65%-84%) and SP (83%-100%) in POCT results in previous studies of cats9, 11 may be explained by different study populations including cats with different cardiac diseases and different disease severities. In our study, the observed echocardiographic measurements were related to BW-dependent expected values both in the healthy cats and in the cats with HCM.27 Evaluations of the accuracy of the POCT for assessing HCM in cats categorized using echocardiographic measurements adjusted for BW have not been published previously. In our study, none of the HCM cats with LAE had a normal POCT result, whereas 31% of the HCM cats without LAE had a normal POCT result (see Figure 2). The POCT showed a higher false-negative rate than true positive rate, which implies that a cat with a normal POCT may need further evaluation by echocardiography to exclude mild HCM. Similar results have been reported previously for both the POCT and the quantitative ELISA in cats with cardiac disease.9, 11, 14
Evaluation of the color of the sample spot at visual inspection of the POCT (see Figure 3) showed that NT-proBNP plasma concentration differed significantly among the 3 POCT groups: lighter (normal), equal (mildly abnormal) and darker (abnormal). This finding may increase the value of the POCT for the assessment of whether cardiac disease is likely or not, because the results are readily available and give an estimate of the NT-proBNP concentration.
In our study, cats with severe dental disease obvious on clinical examination were excluded because periodontitis is an inflammatory disease that causes pain, gingival bleeding, and may impact overall health.45 In people, patients with periodontitis have been shown to have higher serum NT-proBNP concentrations than individuals without periodontitis and the greater the extent of periodontal destruction, the higher the concentration of NT-proBNP in serum.46
4.1 Study Limitations
Blood was collected only once from each cat. Therefore, biological variation in NT-proBNP concentration within an individual cat could not be evaluated. Studies have indicated a high biological variation for NT-proBNP concentrations in cats, dogs, and humans, and this variability may affect the SE and SP.47-49 The healthy control cats were examined in the morning to decrease the effect of diurnal variation among the cats included in the study. Cats with HCM were not examined at a standardized time of day because of the clinical condition of the cat and for practical reasons.
Plasma samples were stored at −80°C up to 4 years before batched analyses were performed, but storage time was not shown to be associated with the NT-proBNP plasma concentration. Furthermore, previous studies have shown that NT-proBNP concentrations are stable at the freezing temperatures used in our study.50-52 It thus is unlikely that storage had any major effect on the results.
The POCT becomes abnormal within a transition interval rather than a set cutoff value, which is a limitation. In our study, the transition interval was found to between 69 and 117 pmol/L, which is slightly lower than a previous study in which the transition occurred between 108 and 122 pmol/L9 but different from another study in which the transition interval was between 150 and 200 pmol/L.11 However, the SE and the SP for the ELISA and the POCT were similar, therefore the POCT results seem reliable.
In our study, the cats with HCM without LAE generally were mildly affected and few cats had HCM with LAE. Furthermore, a few of the cats included were treated with deslorelin, medroxyprogesterone acetate, or cardiac medications. Three of the HCM cats with LAE were on medical treatment for previous CHF, and plasma concentrations of NT-proBNP in cats treated for CHF may decrease after stabilization of CHF.53
The design of our study, using selected inclusion and exclusion criteria, may have influenced the accuracy of the test and thereby overestimated SE and SP. Our study was intended as an explorative study, and further research in a mixed cat population is warranted.
Although no differences in NT-proBNP concentration were found among the 3 genetically distant breeds in the study, differences among other breeds cannot be excluded.
5 CONCLUSIONS
Male cats had higher plasma NT-proBNP concentrations than did female cats, but other characteristics, such as breed, age, BW, and BCS, were not associated with plasma NT-proBNP concentration.
The POCT gave a quick semiquantitative estimate of NT-proBNP concentration, with similar SE and SP to those found using the quantitative ELISA test for detecting HCM in cats. Both tests could identify all HCM cats with LAE but not all HCM cats without LAE.
ACKNOWLEDGMENTS
The study was supported by Agria and the Swedish Kennel Club's research fund, Sveland Research Fund, The SLU Companion Animals Research Fund, Michael Forsgrens Foundation, Research bursary fund within IVC and Evidensia, and the Foundation Strömsholms Djursjukvård. Presented in part at XVIII ISACP congress in Tokyo, 2018 and at the 28th ECVIM congress in Rotterdam, 2018, as short abstracts (oral presentations). The authors thank dedicated veterinary technicians at Evidensia Animal Clinic in Västerås for their important role in completing this study. Special thanks to all participating cats and their owners. We thank Claudia Von Brömssen, for valuable assistance with the statistics.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Uppsala Ethical Committee for Animal Research, Uppsala, Sweden C137/13.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




