Effect of Body Position, Exercise, and Sedation on Estimation of Pulmonary Artery Pressure in Dogs with Degenerative Atrioventricular Valve Disease
This work was completed at The Ohio State University Veterinary Medical Center, Columbus, OH.
The ACVIM Cardiology Resident Research Grant and the Josie Joy Memorial Fund provided financial support for this study.
This research was presented in poster format at the 2016 ACVIM Forum, Denver, CO.
This article was published online on 2 September 2017. An error was subsequently identified. This notice is included in the online version to indicate that it has been corrected on 8 September 2017.
Abstract
Background
Severity of pulmonary hypertension (PH) in dogs is related to clinical signs and prognosis.
Hypothesis/Objectives
We hypothesized that Doppler echocardiographic (DE) indices of pulmonary artery pressure (PAP) and pulmonary vascular resistance (PVR) are influenced by independent factors that create clinically important variability of DE-based estimates of PH in dogs.
Animals
Thirty-eight client owned dogs with naturally acquired degenerative atrioventricular valve disease and tricuspid regurgitation (TR).
Methods
Dogs were prospectively enrolled, and target variables were acquired during 4 echocardiographic study periods (lateral recumbency, standing, lateral recumbency after a 6-minute walk test [6MWT], and lateral recumbency after sedation with butorphanol 0.25 mg/kg IM). Statistical methods included repeated measures ANOVA, mixed model analysis, and Chi-squared test of association.
Results
There was a significant increase in peak TR flow velocity (TRFV; P < 0.01) after sedation in 78% of dogs, with TRFV increasing by >0.4 m/s in 42% of dogs, independent of stroke volume. A significant effect of study period on DE-estimated PVR was not found (P = 0.15). There were negligible effects of sonographer, body position, and 6MWT on echocardiographic variables of PH. Clinically relevant cyclic variation of TRFV was found. There was an association between estimation of right atrial pressure based on subjective assessment and estimation based on cranial vena cava collapsibility (P = 0.03).
Conclusions and Clinical Importance
The increase in TRFV observed with sedation could change assessment of PH severity and impact prognostication and interpretation of treatment response. Further studies with invasive validation are needed.
Abbreviations
-
- 6MWT
-
- 6-minute walk test
-
- ANOVA
-
- analysis of variance
-
- AT
-
- acceleration time
-
- AV
-
- atrioventricular
-
- CI
-
- cardiac index
-
- CO
-
- cardiac output
-
- CV
-
- coefficient of variation
-
- CrVC
-
- cranial vena cava
-
- CSAPA
-
- cross-sectional area of the pulmonary artery at the level of the pulmonary valve
-
- DE
-
- Doppler echocardiography
-
- DiamPA
-
- maximum diameter of the pulmonary artery at the level of the pulmonary valve
-
- ET
-
- ejection time
-
- Etricuspid
-
- early-diastolic transtricuspid flow velocity
-
- HR
-
- heart rate
-
- HRRT
-
- heart rate recovery time
-
- MaxCrVC
-
- maximum cranial vena cava dimension
-
- MaxRAD
-
- maximum right atrial diameter
-
- MinCrVC
-
- minimum cranial vena cava dimension
-
- mPAP
-
- mean pulmonary artery pressure
-
- PA
-
- pulmonary artery
-
- PAP
-
- pulmonary artery pressure
-
- PH
-
- pulmonary hypertension
-
- PVR
-
- pulmonary vascular resistance
-
- RA
-
- right atrium
-
- RV
-
- right ventricle
-
- RVIDs
-
- right ventricular internal dimension measured at end-systole
-
- RVIDd
-
- right ventricular internal dimension measured at end-diastole
-
- RVFS
-
- right ventricular fractional shortening
-
- SV
-
- stroke volume
-
- TR
-
- tricuspid regurgitation
-
- TRFV
-
- peak tricuspid regurgitation flow velocity
-
- VelPA
-
- peak systolic pulmonary outflow velocity
-
- VTIPA
-
- pulmonary outflow velocity time integral
-
- WU
-
- Wood units
Pulmonary hypertension (PH) leads to debilitating clinical signs including exercise intolerance, syncope, and right-sided congestive heart failure in people1-4 and dogs5-7 and can negatively impact prognosis. Humans and dogs with PH secondary to left heart disease have shorter survival times,7 with a more than 2.5 times increased risk of death in people with heart failure.3 Similarly, in a recent study of dogs with degenerative mitral valve disease, dogs with a tricuspid regurgitant (TR) systolic pressure gradient >55 mmHg (equivalent to a peak TR flow velocity >3.7 m/s) were 2.3 times more likely to die during the study period, as evaluated by multivariate analysis.7 Therefore, appropriate and prompt diagnosis, assessment of severity, and identification of the cause of PH are important for therapeutic intervention and prognostication in both animals and humans.
The exact prevalence of PH in dogs is unknown, but it has been estimated that most dogs with advanced left heart disease and dogs with moderate and severe respiratory disease ultimately develop PH complicating the underlying condition.6, 8-12 Since early reports on the use of Doppler echocardiography (DE) in the noninvasive estimation of pulmonary artery pressure (PAP) in people,13 this method has become an integral part of the comprehensive echocardiographic exam. However, despite its common use and the fact that therapeutic decisions are frequently based on interpretation of DE variables in dogs, validation and quality control studies aimed at documentation of method accuracy and reproducibility have, to our knowledge, not been performed. Information on method reproducibility is becoming particularly important as novel, but also expensive, therapies of PH become increasingly available. Without such knowledge, diagnostic conclusions may be erroneous, therapeutic decisions inappropriate, prognostication misleading, and assessment of treatment success impossible.
The objective of this study was to test a variety of independent factors that might influence DE estimation of PAP and pulmonary vascular resistance (PVR) in dogs. We hypothesized that (1) body position, sedation, and respiration influence DE estimates of PAP and PVR; (2) exercise leads to predictable changes in DE estimates, and the response pattern to exercise is different between dogs with mildly elevated PAP compared to dogs with moderately or severely increased PAP; (3) there is clinically relevant observer imaging and measurement variability of DE estimates of PAP and PVR.
Materials and Methods
The Institutional Animal Care and Use Committee and The Ohio State University Clinical Research Advisory Committee approved all procedures in this study. Written consent authorizing participation of the dogs in the study was obtained from all dog owners.
Animals
Client owned dogs examined between September of 2014 and January of 2016 with degenerative atrioventricular (AV) valve disease and TR, diagnosed by color DE, were screened for possible inclusion into this study. Comprehensive baseline echocardiographic studies were performed and canine and client criteria were evaluated for inclusion in the study. Excluded were dogs with congenital heart disease, dogs with acquired heart disease other than degenerative AV valve disease, and dogs with dynamic right ventricular (RV) outflow tract obstruction. Other exclusion criteria included a poorly identifiable TR velocity profile on spectral DE, clinical instability of the patient to undergo multiple echocardiographic studies, inability of the patient to stand or walk, behavioral disposition preventing acquisition of a complete study, dogs with atrial fibrillation or other hemodynamically relevant arrhythmias, and dogs in which changes of cardiac medications had been made in the prior week.
Dogs were divided into 3 groups determined by their peak TR flow velocity (TRFV).14-18 Group 1 consisted of dogs with TRFV <3.1 m/s (systolic pressure gradient <38 mmHg), suggesting normal or mildly elevated systolic PAP; Group 2 included dogs estimated to have mild-to-moderate PH (TRFV 3.1–4.0 m/s; systolic pressure gradient 38–64 mmHg); Group 3 consisted of dogs estimated to have severe PH (TRFV >4.0 m/s; systolic pressure gradient >64 mmHg).
Study Design
This study consisted of 4 study periods. During Period: Baseline, echocardiography was performed with the dogs in right and left lateral recumbency. During Period: Standing, echocardiography was performed with the dogs in a standing position from the left lateral chest only. During Period: Post-6MWT, echocardiography was performed with the dogs in right and left lateral recumbency immediately after a standardized 6-minute walk test (6MWT).19, 20 During Period: Sedated, echocardiography was performed with the dogs in right and left lateral recumbency approximately 10 minutes after administration of sedation (butorphanol 0.25 mg/kg IM). For all study periods, 2 separate echocardiograms were performed by 2 different sonographers (JDR & KES) in random order, with the exception of Period: Post-6MWT, in which only one echocardiogram was performed by JDR. All echocardiographic variables listed below were recorded during each study, with the exception of cranial vena cava (CrVC) dimensions that were not acquired during Period: Standing.
Echocardiography
Transthoracic 2-D, M-mode, spectral, and color-flow Doppler images were obtained using a digital ultrasound unit1 with either a 4, 5, or 7 MHz nominal frequency transducer, selected based on patient size. Efforts were made to maintain consistent echocardiographic system settings (ie, same probe and gain settings) between sonographers and patients. All dogs were conscious throughout the study and unsedated, aside from Period: Sedated. Multiple cardiac cycles were stored. For all echocardiograms, a simultaneous 1-lead electrocardiogram was recorded. All data were stored on a remote proprietary workstation (GE EchoPac® Version 113.1.3) for offline analysis.
6-Minute Walk Test
A standardized 6MWT was performed in all dogs, as previously described.19, 20 The test was performed by one investigator (JDR) in the same 97 m-long hallway in the basement of the Veterinary Medical Center. Exercise was started with brisk walking; however, the dogs were allowed to set their own pace and to stop or rest if desired. The total walk distance was recorded. Echocardiography was performed immediately after the 6MWT. The time to first obtain an acceptable TR spectral Doppler signal after exercise and the time for heart rate (HR) to return to pre-exercise HR (heart rate recovery time, HRRT) were recorded.
Measurements and Calculations
All measurements and calculations were made by a single observer (JDR), with the exception of one additional observer (KES) involved in assessment of interobserver measurement variability. Depending on signal quality, the mean of 3–5 measurements for each variable was calculated. Therefore, if signals/images were consistent and clearly identifiable, 3 measurements were made to calculate the mean. If the signals/images were variable with less optimal image quality, 5 measurements were made to calculate the mean. Heart rate (including HR used for cardiac output calculations, as well as HRRT) was measured from preceding RR intervals recorded from the ECG associated with the echocardiographic system. Maximum right atrial (RA) diameter (MaxRAD) was acquired from an end-systolic 2-D image from a cranial left apical 2-chamber imaging view measuring from the middle of the atrial septum (oval fossa) to the lateral RA wall at the blood-tissue interface with the measurement line parallel to the tricuspid valve annulus. The maximum diameter of the inflow portion of the RV in end-systole (RVIDs) and end-diastole (RVIDd) was determined using 2-D images from a left apical cranial view, measured immediately below the tricuspid valve annulus in the septal to free wall direction, as previously reported.10 Right ventricular fractional shortening percentage (RVFS), as an index of global RV systolic function, was calculated using the equation: ([RVIDd − RVIDs])/RVIDd) × 100%.
Pulmonary outflow was evaluated by measuring the peak velocity of the pulsed Doppler signal (VelPA), and the RV systolic time intervals were measured as previously described.10, 21, 22 Pulmonary outflow acceleration time (AT) and ejection time (ET) were measured, and the AT : ET ratio was determined. Pulmonary artery (PA) outflow profiles were subjectively evaluated and described as being normal (type I), accelerated (type II), or having midsystolic notching (type III), based on reported standard descriptions.8, 10, 23
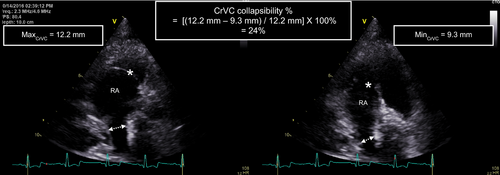
Peak systolic TRFV was measured and used to estimate systolic PAP derived from the simplified Bernoulli equation: systolic PAP = (4 × TRFV2) + RA pressure.13 Right atrial pressure was estimated to be normal (6 mmHg) or increased (10 mmHg) based on subjective 2-D echocardiographic assessment of RA size (normal or mildly increased RA dimension versus moderately to severely increased RA size, respectively).24, 25 The maximum diameter and minimum diameter of the venous entry of the CrVC into the RA (MaxCrVC and MinCrVC, respectively) (Fig 1) were measured during all periods with the exception of Period: Standing. The CrVC collapsibility was calculated using the equation: CrVC collapsibility = ([MaxCrVC − MinCrVC]/MaxCrVC) × 100%. Collapsibility of ≥50% was considered indicative of normal RA pressure and <50% consistent with elevated RA pressure.26 As CrVC collapsibility has not been validated in dogs, this was extrapolated from inferior vena cava collapsibility cutoffs used in human medicine.26
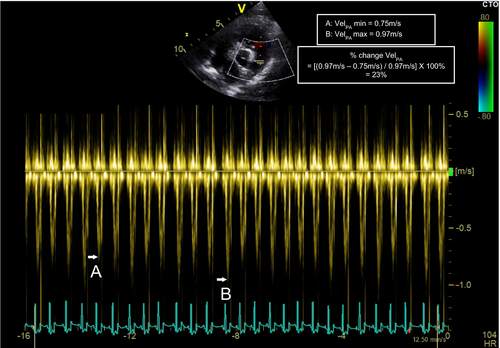
Cyclic variations, likely associated with respiration based on visual observation of breathing during echocardiography of the early-diastolic transtricuspid flow velocity (Etricuspid), TRFV, and VelPA were recorded (Fig 2). The sweep speed was decreased to 12.5 mm/s, and the maximum and minimum velocities were recorded. Percent change was calculated for these measurements using the equation: ([maximum velocity − minimum velocity]/maximum velocity) × 100%.
Only during Period: Baseline, the maximum PA diameter (DiamPA) at the level of the pulmonary valve was measured from a 2-D image from either the right parasternal short-axis inflow-outflow view or the left apical cranial inflow-outflow view at early systole in the first 1–2 frames after valve opening. The view and frame used to measure the DiamPA were chosen based on optimal image quality. The mean of 5 measurements was used for all computations in the same dog to reduce measurement error intrinsic to this variable. The mean value was then used as a single final estimate of DiamPA. This value was used for all calculations of stroke volume (SV) during all study periods for both observers to further reduce error related to volumetric calculations. Pulmonary artery velocity time integral (VTIPA) was quantified using the built-in calculation software in the ultrasound unit by tracing the outer border of the flow envelope. Pulmonary artery cross-sectional area (CSAPA) was calculated as CSAPA = π[DiamPA/2]2, SV as SV = CSAPAxVTIPA, cardiac output (CO) as CO = SV × HR, and cardiac index (CI) as CI = CO/body surface area. Pulmonary vascular resistance in Wood units (WU) was calculated based on previous reports in people that showed excellent correlation with catheterization-derived estimates of PVR (PVR = [systolic PAP/VTIPA] with 3 units added if notched PA flow signal).25, 27
Intra-observer measurement variability was determined by having one independent observer (JDR) measure key variables in 6 dogs (2 dogs randomly selected from each group) on 3 separate occasions. Interobserver measurement variability was determined by having 2 independent observers (JDR & KES) measure key variables from the same 6 dogs on 3 separate occasions, blinded to each other's measurements.
Statistical Analysis
All data were analyzed using statistical software.2 Normality was assessed after visual inspection of the data and with the Shapiro–Wilk normality test. Logarithmic transformation was applied for some of the variables when the normality assumption was violated. The Winsorization method28 was used to assess and handle outliers.
Analysis of variance (ANOVA) or the Kruskal–Wallis test was used to evaluate differences among groups for continuous demographic variables, disease summary variables, and 6MWT data for normally and non-normally distributed data, respectively. A Chi-square test was used to assess sex differences between groups and association between subjective RA pressure estimates and CrVC collapsibility.
A linear mixed model using an autoregressive (1) covariance structure3 was used to analyze repeated measures outcomes. Period: Baseline was the period to which all subsequent period comparisons were made (ie, Period: Baseline versus Period: Standing; Period: Baseline versus Period: post-6MWT; Period: Baseline versus Period: Sedated). In this model, the study period was considered as a repeated factor, and subject was considered as a random effect. Observations from the same subjects were expected to correlate over time (nonzero correlation). The autoregressive (1) covariance structure was superior to others for modeling this correlation based on the Akaike information criterion. If there was an overall difference observed, then a Bonferroni post hoc test with multiple comparison adjustments was performed to reveal which study period was different from Period: Baseline. This analysis was carried out first for the echocardiographic variables of all dogs, and the same analysis was repeated separately for each group. The group and period interaction terms were included in the model. For the latter analysis, if the interaction test for any group was significant then pairwise comparisons (Period: Baseline versus other periods) were performed. As a significant period effect was found for a key variable (TRFV), multivariate regression analysis was subsequently used to test for an association among independent factors TRFV, HR, RVFS, and SV. We attempted to validate the equation used to calculate PVR by performing a similar analysis was used to detect a difference in baseline PVR between groups. Significance was defined as a P-value of <0.05.
Inter and intra-observer measurement variabilities were calculated using the formula: mean coefficient of variation (CV) = difference between measurements/mean of measurements × 100, expressed in percent and as an absolute parameter value. Based on previously published diagnostic cutoffs variability was interpreted as: CV < 5% very low variability; CV = 5–15% low variability; CV > 15–25% moderate variability; CV > 25% high variability.29
Results
Thirty-eight client owned dogs (Table 1) with a mean (± SD) body weight of 9.3 ± 3.6 kg (minimum to maximum, 2.8–24.3 kg) and a mean age of 10.0 ± 2.2 years (minimum to maximum, 6–14 years) were enrolled. Sixteen dogs had estimated normal to mildly elevated systolic PAP (Group 1), 11 had estimated mild-to-moderate PH (Group 2), and 11 had estimated severe PH (Group 3). There was no significant difference in body weight (P = 0.38), or sex (P = 0.31) between groups. Thirteen dog breeds were represented in the study population (Table 1). Concurrent cardiac medications included (Table 2): furosemide (n = 8), enalapril (n = 16), pimobendan (n = 5), spironolactone (n = 5), and sildenafil (n = 1). Eight dogs were taking additional medications including cough suppressants, analgesics, antibiotics, and gastric protectants.
| Variable | All (n = 38) | Group 1 (n = 16) | Group 2 (n = 11) | Group 3 (n = 11) | P |
|---|---|---|---|---|---|
| Age (years) | 10.0 ± 0.3 | 9.1 ± 2.2 | 10.3 ± 2.2 | 11.1 ± 3.9 | 0.07 |
| Sex (female/male) | 14/24 | 4/12 | 6/5 | 4/7 | 0.31 |
| BW (kg) | 9.3 ± 3.6 | 10.1 ± 3.2 | 9.2 ± 4.0 | 8.2 ± 3.9 | 0.38 |
| Breed (n) | |||||
| CKCS | 13 | 7 | 4 | 2 | |
| Mixed breed | 9 | 4 | 4 | 1 | |
| Boston Terrier | 3 | 0 | 1 | 2 | |
| Miniature Schnauzer | 2 | 1 | 0 | 1 | |
| Miniature Poodle | 1 | 0 | 0 | 1 | |
| Shetland Sheepdog | 1 | 1 | 0 | 0 | |
| Pomeranian | 1 | 1 | 0 | 0 | |
| Chihuahua | 1 | 0 | 0 | 1 | |
| French Bulldog | 1 | 0 | 0 | 1 | |
| Border Collie | 1 | 1 | 0 | 0 | |
| Shih Tzu | 1 | 0 | 1 | 0 | |
| English Toy Spaniel | 1 | 0 | 0 | 1 | |
| Maltese | 1 | 0 | 1 | 0 | |
| Cairn Terrier | 1 | 0 | 1 | 0 | |
| Cocker Spaniel | 1 | 0 | 0 | 1 | |
| ACVIM DAVVD staginga | |||||
| A | 0 | 0 | 0 | 0 | |
| B1 | 17 | 9 | 4 | 4 | |
| B2 | 13 | 6 | 6 | 1 | |
| C | 8 | 1 | 1 | 6 | |
| D | 0 | 0 | 0 | 0 | |
- PAP, pulmonary artery pressure; TRFV, tricuspid regurgitant flow velocity; PH, pulmonary hypertension.
- Age and body weight (BW) are presented as mean ± SD. Group 1: dogs with normal to mildly elevated systolic PAP (TRFV < 3.1 m/s); Group 2: dogs with mild-to-moderate PH (TRFV = 3.1–4.0 m/s); Group 3: dogs with severe PH (TRFV > 4.0 m/s). CKCS, Cavalier King Charles Spaniel. ACVIM, American College Veterinary Internal Medicine. DAAVD, degenerative atrioventricular valve disease.
- a See reference 61.
| Medication | All (n = 38) | Group 1 (n = 16) | Group 2 (n = 11) | Group 3 (n = 11) |
|---|---|---|---|---|
| Furosemide | 8 | 2 | 0 | 6 |
| Enalapril | 16 | 6 | 3 | 7 |
| Pimobendan | 5 | 1 | 1 | 3 |
| Spironolactone | 5 | 1 | 0 | 4 |
| Sildenafil | 1 | 0 | 0 | 1 |
- See Table 1 legend for definition of groups.
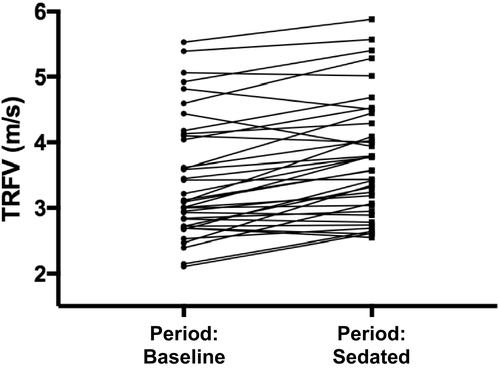
Echocardiographic data are presented in Table 3. A significant effect of study period on TRFV (P < 0.01) was observed. Tricuspid regurgitant flow velocity in Period: Sedated was significantly higher compared to Period: Baseline (mean ± SD; Period: Sedated: 3.75 ± 0.88 m/s versus Period: Baseline: 3.43 ± 0.95 m/s; P < 0.01; Fig 3). This effect of sedation on TRFV was observed in Group 1 dogs (Period: Sedated: 2.99 ± 0.32 m/s versus Period: Baseline: 2.65 ± 0.32 m/s; P < 0.05) and Group 2 dogs (Period: Sedated: 3.77 ± 0.33 m/s versus Period: Baseline: 3.29 ± 0.28 m/s; P < 0.01), but not in Group 3 dogs (P = 0.16). After sedation, TRFV was increased in 78% of dogs (range of increase 0.02–1.2 m/s). Thirty-two percent of dogs had an increase of >0.5 m/s, and 42% of dogs had an increase of >0.4 m/s. Tricuspid regurgitant flow velocity decreased in 7 dogs (range of decrease 0.04–0.5 m/s) and did not change in 1 dog. Multivariate analysis found a correlation between TRFV and HR (r2 = 0.42, P < 0.01).
| Variable | Period | ||||
|---|---|---|---|---|---|
| Baseline | Standing | Post-6MWT | Sedation | P | |
| TRFV (m/s) | |||||
| All | 3.43 ± 0.95 | 3.40 ± 0.94 | 3.47 ± 1.03 | 3.75 ± 0.88c | <0.01 |
| Group 1 | 2.65 ± 0.32 | 2.59 ± 0.33 | 2.67 ± 0.27 | 2.99 ± 0.32b | |
| Group 2 | 3.29 ± 0.28 | 3.44 ± 0.42 | 3.3 ± 0.32 | 3.77 ± 0.33c | |
| Group 3 | 4.68 ± 0.63 | 4.58 ± 0.62 | 4.81 ± 0.83 | 4.82 ± 0.60 | |
| sPAP (mmHg) | |||||
| All | 56.9 ± 30.1 | 53.1 ± 30.8 | 58.8 ± 34.5 | 63.9 ± 30.8c | <0.01 |
| Group 1 | 34.6 ± 6.7 | 31.1 ± 10.3 | 34.8 ± 6.0 | 42.2 ± 7.7 | |
| Group 2 | 49.7 ± 7.4 | 49.0 ± 19.7 | 50.0 ± 8.6 | 57.5 ± 21 | |
| Group 3 | 96.7 ± 25.3 | 92.8 ± 23.6 | 102.6 ± 34.1a | 101.6 ± 24.8a | |
| PVR (WU) | |||||
| All | 7.8 ± 5.9 | 8.1 ± 6.4 | 8.8 ± 7.1 | 9.1 ± 6.6 | 0.15 |
| Group 1 | 3.9 ± 0.8* | 3.8 ± 1.1 | 4.6 ± 1.2 | 4.7 ± 1.2 | |
| Group 2 | 6.8 ± 1.4# | 6.7 ± 1.5 | 6.6 ± 1.8 | 7.4 ± 2.0 | |
| Group 3 | 13.9 ± 7.6*,# | 16.1 ± 6.7 | 17.1 ± 8.3 | 16.9 ± 7.1 | |
| CI (mL/min/m2) | |||||
| All | 3,101 ± 1,065 | 3,402 ± 1,131 | 3,265 ± 1,370 | 2,714 ± 1,035 | 0.02 |
| Group 1 | 2,971 ± 777 | 3,326 ± 1,079 | 3,050 ± 1,139 | 2,580 ± 672 | |
| Group 2 | 2,593 ± 660 | 3,242 ± 1,027 | 2,822 ± 677 | 2,406 ± 737 | |
| Group 3 | 3,799 ± 1,384 | 3,675 ± 1,304 | 4,021 ± 1,902 | 3,189 ± 1,487 | |
| HR (bpm) | |||||
| All | 120 ± 23 | 127 ± 28 | 138 ± 26c | 107 ± 26c | <0.01 |
| Group 1 | 113 ± 22 | 110 ± 22 | 125 ± 29 | 98 ± 22c | |
| Group 2 | 113 ± 14 | 127 ± 26a | 133 ± 16b | 98 ± 22 | |
| Group 3 | 137 ± 24 | 151 ± 22b | 160 ± 13c | 126 ± 24 | |
| MaxRAD (mm) | |||||
| All | 20.6 ± 4.8 | 22.6 ± 5.6b | 20.6 ± 5.0 | 22.1 ± 5.1b | <0.01 |
| Group 1 | 20.3 ± 3.3 | 21.8 ± 3.0b | 19.8 ± 3.0 | 20.8 ± 3.2 | |
| Group 2 | 18.8 ± 3.5 | 20.2 ± 4.6 | 19.1 ± 3.4 | 21.5 ± 1.0b | |
| Group 3 | 22.8 ± 6.8 | 26.7 ± 7.8 | 23.2 ± 7.6 | 24.5 ± 7.3 | |
- Within a row, superscript letters indicate significant differences as compared to Period: Baseline. P < 0.05 was considered significant for overall comparisons. For within-group comparisons, superscript a, b, and c indicate P < 0.1, P < 0.05, P < 0.01, respectively. For between-group comparisons of baseline PVR, superscript * indicates P < 0.01 (between Group 1 and Group 3), # indicated P < 0.01 (between Group 2 and Group 3); Period: Baseline = lateral recumbency, nonsedated; Period: Standing = standing position, nonsedated; Period: Post-6MWT = post-6MWT, lateral recumbency, nonsedated; Period: Sedated = postsedation, lateral recumbency. See Table 1 legend for definitions of groups. sPAP, estimated systolic pulmonary artery pressure; PVR, estimated pulmonary vascular resistance; TRFV, peak tricuspid regurgitant flow velocity; CI, cardiac index, MaxRAD, maximum right atrial diameter.
An effect of study period on DE-estimated PVR (P = 0.15) was not found. A significant difference in baseline PVR between Group 1 and Group 3 (3.9 ± 0.8 WU versus 13.9 ± 7.6 WU; P < 0.01) and between Group 2 and Group 3 (6.8 ± 1.4 WU versus 13.9 ± 7.6 WU; P < 0.01) was found, but not between Group 1 and Group 2 (P = 0.41).
A significant effect of study period on HR (P < 0.01) was found. In Period: Post-6MWT and Period: Sedated, a significant effect on HR compared to Period: Baseline was identified (mean ± SD; Period: Post-6MWT: 138 ± 26 bpm versus Period: Baseline: 120 ± 23 bpm; P < 0.01 and Period: Sedated: 107 ± 26 bpm versus Period: Baseline: 120 ± 23 bpm; P < 0.01). Specifically, in Group 1 dogs HR decreased during Period: Sedated (from 113 ± 22 bpm to 98 ± 22 bpm; P < 0.01). In Group 2 HR increased during Period: Post-6MWT (Period: Post-6MWT: 113 ± 14 bpm versus Period: Baseline: 98 ± 22 bpm; P < 0.01). In Group 3 HR increased during Period: Standing and Period: Post-6MWT compared to Period: Baseline (Period: Standing: 151 ± 22 bpm versus Period: Baseline: 137 ± 24 bpm; P < 0.05 and Period: Post-6MWT: 160 ± 13 bpm versus Period: Baseline: 137 ± 24 bpm; P < 0.01) but did not change during Period: Sedated (P > 0.05).
A significant effect of study period on AT and ET was found (P = 0.01 and P < 0.01, respectively). In Period: Post-6MWT a reduction in AT and ET compared to Period: Baseline was identified (Period: Post-6MWT: 60 ± 16 ms versus Period: Baseline: 67 ± 20 ms; P < 0.05 and Period: Post-6MWT: 171 ± 22 ms versus Period: Baseline: 185 ± 28 ms; P < 0.05, respectively). Multivariate analysis found an association between AT and HR (r2 = 0.55, P < 0.01) and ET and HR (r2 = 0.60, P < 0.01). An effect of study period on VelPA and AT : ET was not observed (P = 0.32 and P = 0.81, respectively). Based on baseline studies, 26 dogs had a type I pulmonary outflow profile, 8 dogs had a type II profile, and 4 dogs had a type III profile.
A significant period effect on VTIPA (P = 0.02) was observed. In Period: Post-6MWT a decrease of VTIPA compared to Period: Baseline was identified (Period: Post-6MWT: 7.7 ± 2.0 cm versus Period: Baseline: 8.4 ± 1.9 cm; P < 0.05). This effect was present in Group 1 dogs (Period: Post-6MWT: 8.1 ± 2.3 cm versus Period: Baseline: 9.2 ± 2.2 cm; P < 0.05), but absent (P > 0.05) in the other 2 study groups.
Although a significant effect of study period on CO and CI was found (P = 0.03 and P = 0.02, respectively), a specific study period or group in which there was a significant change was not identified with the statistical model used. The greatest mean difference in CI was found between Period: Baseline and Period: Sedated (Period: Baseline: 3,101 ± 1,065 mL/min/m2 versus Period: Sedated: 2,714 ± 1,035 mL/min/m2; P = 0.1). A significant effect of study period on SV was not found (P = 0.25).
A significant period effect on MaxRAD (P < 0.01) was observed. In Period: Standing an increase in MaxRAD compared to Period: Baseline was identified (Period: Standing: 22.6 ± 5.6 mm versus Period: Baseline: 20 ± 4.8 mm; P < 0.05). This effect was most obvious in Group 1 (Period: Standing: 21.8 ± 3.0 mm versus Period: Baseline: 20.3 ± 3.3 mm; P < 0.01). In Period: Sedated an increased in MaxRAD compared to Period: Baseline was identified (Period: Sedated: 22.1 ± 5.1 mm versus Period: Baseline: 20.6 ± 4.8 mm; P < 0.05). This effect was most obvious in Group 2 (Period: Sedated: 21.5 ± 4.1 mm versus Period: Baseline: 18.8 ± 3.5 mm; P < 0.01).
An effect of study period on the CrVC collapsibility was not found (P = 0.34). An association between subjective assessment of RA pressure based on 2-D evaluation of RA size and estimation of RA pressure based on collapsibility of the CrVC during respiration was identified (P = 0.03). There was 94% agreement between subjective assessment of RA pressure by 2-D echocardiography evaluating RA size and estimation of RA pressure based on collapsibility of the CrVC when they were both assessed to be low but only 17% agreement when they were both estimated to be elevated.
Cyclic variation during data recording was observed in all DE variables examined including TRFV (% change mean ± SD: 14 ± 6.8%; minimum to maximum, 2.2–40.7%), Etricuspid (mean ± SD: 23 ± 8.7%; minimum to maximum, 4.4–53.3%), and VelPA (mean ± SD: 18 ± 6.7%; minimum to maximum, 4.1–37.8%). However, the effect was not different among the study periods (P = 0.73, P = 0.49 and P = 0.06, respectively).
Results on intra- and interobserver measurement variabilities are presented in Table 4. Intra-observer measurement variability for all variables was low to very low. Interobserver measurement variability for most variables was also considered low with the exception of MinCrVC (moderate variability) and AT and CrVC collapsibility (high variability).
| Variable | Mean Coefficient of Variation (CV) | |||
|---|---|---|---|---|
| Intra-observer | Interobserver | |||
| Mean Difference | CV (%) | Mean Difference | CV (%) | |
| DiamPA (cm) | 1.49 | 2 | 1.52 | 8 |
| TRFV (m/s) | 3.66 | 1 | 3.69 | 4 |
| sPAP (mmHg) | 63 | 2 | 64 | 6 |
| VTIPA (cm) | 8.67 | 2 | 8.71 | 6 |
| PVR (WU) | 8.6 | 2 | 8.8 | 5 |
| AT (ms) | 77 | 5 | 70 | 27 |
| ET (ms) | 193 | 1 | 183 | 12 |
| AT : ET | 0.40 | 5 | 0.38 | 15 |
| MaxCrVC (mm) | 11.9 | 4 | 11.2 | 15 |
| MinCrVC (mm) | 6.7 | 8 | 6.7 | 18 |
| CrVC collapsibility (%) | 42 | 15 | 40 | 26 |
- DiamPA, maximum diameter of the pulmonary artery at the level of the pulmonary valve; TRFV, peak tricuspid regurgitant flow velocity; systolic PAP, estimated systolic pulmonary artery pressure; VTIPA, pulmonary artery velocity time integral; PVR, estimated pulmonary vascular resistance; AT, acceleration time; ET, ejection time; MaxCrVC, maximum CrVC dimension; MinCrVC, minimum CrVC dimension; CrVC, cranial vena cava.
Descriptive statistics regarding the 6MWT are summarized in Table 5. The average total walk distance was 550 ± 160 m (minimum to maximum, 60–759 m). The average HRRT was 174 ± 63 seconds (minimum to maximum, 60–240 seconds). Heart rate never returned to baseline in 1 dog (from Group 2) within the allotted time period (maximum of 8 minutes after exercise). The average time to obtain a high-quality TR signal after completion of the 6MWT was 65 ± 21 seconds (minimum to maximum, 36–130 seconds). There was no difference between groups with regard to the total walk distance (P = 0.6), HRRT (P = 0.29), and time to obtain TR the signal (P = 0.51).
| Variable | All (n = 38) | Group 1 (n = 16) | Group 2 (n = 11) | Group 3 (n = 11) | P |
|---|---|---|---|---|---|
| Distance walked (m) | 550 ± 160 | 598 ± 136 | 576 ± 111 | 455 ± 202 | 0.06 |
| HRRT (s) | 174 ± 63 | 174 ± 53 | 148 ± 75 | 200 ± 58 | 0.29 |
| Time to TRFV (s)a | 65 ± 21 | 61 ± 17 | 66 ± 23 | 71 ± 26 | 0.51 |
- 6MWT, 6-minute walk test; HRRT, heart rate recovery time (defined as the time for heart rate to return to baseline after cessation of the 6MWT); TRFV, tricuspid regurgitant flow velocity; See Table 1 legend for definitions of groups.
- a Time to first recording TRFV after cessation of the 6MWT.
Discussion
The results of this study revealed a significant effect on the DE estimates of PAP in the setting of mild sedation that has not previously been demonstrated in veterinary medicine. Considerable variation associated with respiration and technical factors (recording and measurement variability) was also identified. However, the majority of these effects were mild during most study periods and, with the exception of sedation, not likely to be clinically relevant. Given DE is the most common modality used to diagnose and quantify PH in dogs,5, 6, 9 the latter observation deserves clinical attention and consideration when interpreting echocardiographic findings.
The most clinically important finding in this study was the significant and nearly consistent increase in TRFV with sedation, seen in the majority of dogs. This observation was most obvious in dogs with normal to mildly elevated PAP and dogs estimated to have mild-to-moderate PH (Groups 1 and 2). There was not only a statistically significant increase of TRFV after sedation, but the increase was clinically relevant in a number of dogs. This could change the assessment of the severity of PH in dogs potentially leading to misclassification and inappropriate decisions regarding treatment, disease staging, and prognostication. For example, in 1 dog, the TRFV increased from 3.1 to 4.1 m/s corresponding with an estimated systolic PAP of 44 and 73 mmHg, respectively. Doppler signal quality was considered excellent in both examinations. This would change the estimated classification from mild-to-moderate PH to severe PH in this dog. A TR pressure gradient of >55 mmHg is a negative prognostic indicator in dogs with degenerative mitral valve disease.7 As such, the difference in this case could alter prognostication. Also, the latter estimated systolic PAP might trigger treatment by some clinicians.30, 31 Importantly, upon recheck DE, if that patient was unsedated, a decrease in the TRFV could be misinterpreted as a response to treatment. It is important to mention that while in this example, there was an obvious relevant effect of TRFV, only about 1/3 of dogs had an increase that would be considered clinically significant, whereas 7 of the dogs actually had a decrease in TRFV after sedation, the range of decrease was much less than the range of increase seen in the majority of dogs.
There are potential reasons why sedation increased TRFV in these dogs, including direct and indirect effects on HR, SV, RV systolic function, and PVR. With sedation, there was an increase in TRFV in some dogs, as well as a mild decrease in HR (mean reduction of 14 bpm). Stroke volume is largely determined by diastolic fiber length (preload) and inotropy.32 By reducing HR and increasing cycle length, diastolic filling could increase with related augmentation of SV. One may hypothesize that the increase in TRFV was related to an expected increase in total SV secondary to the decrease in HR or increase in RV systolic function. However, estimated SV did not significantly change between the periods. Similar to SV, RV systolic function, as assessed by RVFS, did not change significantly between the periods. Given the complex structure and peristaltic-like contraction of the RV,33-35 additional assessment of other indices of RV function (tricuspid annular plane systolic excursion, fractional area change, lateral tricuspid annular S', RV free wall strain, and strain rate)36, 37 might have found differences between the periods, better explaining the effect of sedation.
Butorphanol tartrate is a centrally acting mixed kappa agonist/μ antagonist opioid drug that provides sedation and analgesia.38 There is little published evidence that butorphanol significantly affects PAP or PVR in humans and dogs. Popio et al found a mild increase in mean PAP (mPAP) by 2.9 mmHg and PVR by 23 dynes*sec/cm5 10 minutes after butorphanol administration in a study of people with angina pectoris undergoing cardiac catheterization.39 In a study of 6 anesthetized healthy laboratory dogs undergoing cardiac catheterization, butorphanol significantly reduced HR by 21 bpm, but there was no effect on mPAP.40 In a more recent report of 20 anesthetized healthy dogs undergoing right heart catheterization, there was a decrease in HR by 20 bpm and mPAP by 2 mmHg and no change in PVR after butorphanol administration.41 Butorphanol is often the drug of choice for sedation in animals with cardiopulmonary disease given the minimal adverse cardiocirculatory effects that include mild decreases in HR and systemic blood pressure;42 however, a centrally depressive effect on respiration is often seen which could conceivably lead to mild alveolar hypoxia and reflex pulmonary vasoconstriction resulting in an increase in PAP. If that hypothesis were true, one would also expect PVR to increase, but this was not observed in this study. Whether or not findings from studies in anesthetized healthy dogs or humans regarding the effect of butorphanol on PAP and PVR can be extrapolated to dogs with naturally acquired AV valve disease is unknown.
Another consideration for the variability of TRFV after sedation includes the potential inaccuracies associated with DE estimates of PAP including poor DE envelope definition, malalignment between TR and the DE signal, and impaired RV systolic function.43, 44 As demonstrated by Soydan et al, only a moderate correlation was found between invasive measurements of PAP and DE estimates in anesthetized dogs with experimentally-induced PH (r = 0.78).18 The tranquilization effects of butorphanol alone could have allowed better image acquisition and Doppler alignment to create a more accurate assessment of the TRFV,45 although this is unlikely because image quality was considered good in all dogs for all study periods.
A significant period effect on PVR, as determined by a simplified DE method previously validated in humans,25 was not found in this study. While this could indicate that PVR was truly unaffected by the study period, the equation used in this study to estimate PVR has not been validated in dogs. Moreover, although inter and intra-observer variabilities were considered low in this study for DE-derived VTIPA and estimated systolic PAP, there is inherent risk of inaccurate measurements when estimating PVR echocardiographically. Estimated systolic PAP is calculated from squaring TRFV; an inaccurate spectral Doppler measurement can exponentially increase error. Pulmonary artery VTI, a measurement of volumetric flow, can change significantly depending on position and size of the sample volume as well as the alignment to flow, further instilling error into the calculation of PVR.46 The equation used in this study also involves assessment of the presence of a midsystolic notch in the PA flow signal, which can be affected by respiration and suboptimal sample volume placement,25 therefore, creating another, albeit minor, source of error. In an attempt to validate the equation used,25 a difference was found in baseline PVR between Group 3 compared with Group 1 and Group 2, but not between Group 1 and Group 2. The explanation for not finding a difference between Group 1 and Group 2 is likely related to inherent error in the equation, as mentioned above, and small sample size. Given the increasing availability of novel drugs targeting PH47-50 and the challenges associated with right heart catheterization in pets, a noninvasive evaluation of PVR in dogs is very desirable.
Pulmonary artery AT and ET were both significantly decreased after exercise but were not influenced by sedation. Given the lack of change in the AT : ET ratio and the negative correlation with HR, the shortening of AT and ET is most likely related to changes in HR associated with exercise. The lack of significant change with sedation (when HR was lower) could be explained by small sample size failing to detect a difference. Also, panting induced by exercise could have affected imaging windows causing the measurements of AT and ET to falsely appear different. Others have found that AT and AT : ET ratio can predict the presence of increased TRFV10 in dogs with interstitial lung disease. There was variation in AT and ET with regard to exercise in this study, whereas AT : ET ratio remained static after exercise. This may indicate that the latter is a more reliable measurement of PH and PVR and less affected by changes in HR.
Maximum RA diameter was also affected by study period, specifically when standing and after sedation, with MaxRAD being significantly increased with both. The increase in MaxRAD after sedation was greater in dogs estimated to have mild-to-moderate PH. Interestingly, this is also the group of dogs where TRFV increased after sedation, whereas this could indicate a true increase in RA size and pressure as would be expected with an increase in PAP, this increase in RA diameter was relatively small (~2 mm change). The increase in MaxRAD seen with the dogs standing during echocardiography was most obvious in dogs with normal to mildly elevated estimated systolic PAP. Although this very minimal and likely clinically insignificant increase (<2 mm) could be real, it could also be related to acquiring images from a slightly different imaging plane.
There is continuous debate whether RA pressure can be estimated by echocardiography with reasonable accuracy.18, 21, 51, 52 For this study, we used the collapsibility of the CrVC during the cardiac cycle, as opposed to the caudal (inferior) vena cava, which is the standard in people.53 Investigators' preference to use CrVC over the caudal vena cava was given mostly due to better image quality for CrVC compared to images obtained from either the RA entrance or transdiaphragmatic views of the caudal vena caval diameter, which based on clinical experience, are markedly affected by ventilation and translational motion. We found an association between estimated RA pressure based on subjective RA size from 2-D echocardiography and CrVC collapsibility, especially when RA pressure was estimated to be low. Due to absence of direct RA pressure measurements, collapsibility of the CrVC was not validated in this study and thus requires further evaluation as to whether there is a clinically relevant correlation between CrVC collapse and right heart catheterization-derived RA pressure.
While there was not a statistical difference in the distance walked between groups, this might be explained by small sample sizes, limiting the statistical power of the analysis. Group 3 dogs numerically walked shorter distances (mean, 455 m in Group 3 versus 576 m in Group 2 and 598 m in Group 1), (P = 0.06). Given the hemodynamic consequence of elevated PAP, one would expect dogs with more advanced PH to walk shorter distances. In people, the “minimal clinically important difference” in walk distance (after treatment of PH) is as little as 24 m;54, 55 therefore, albeit not statistically different, the difference in walk distance between dogs with normal and mildly elevated systolic PAP and dogs with severe PH seems clinically relevant and deserves further study. In addition to small group size, other factors may have played a role in why differences in walk distance were not identified. Although body weight in dogs of all groups was similar, body conformation and body condition score may have been different leading to different stride duration and exercise ability and thus possibly unbalanced group composition. The dogs in Group 3 tended to be older than dogs in Group 1. Although this difference did not achieve statistically significance (P = 0.07), other factors such as temperament and degenerative joint disease could also play a factor. Finally, the tendency of reduced 6-minute walk distance with increased severity of PH may simply relate to increased severity of left heart disease or concurrent pulmonary disease. However, it was not a primary goal of the study to evaluate the exercise capacity of the dogs. Rather, we aimed at evaluating the effect of exercise on DE variables of PAP and PVR in a uniform way, with walk distance only being a tool to report quantity of exercise. Heart rate recovery time was not statistically different in dogs with severe PH. In people, the 6-minute walk distance and HR reduction within 1 minute after exercise are 2 of most important variables derived from the 6MWT.56
Heart rate increased after the 6MWT as expected, most notably in dogs categorized as severe PH. This may represent decreased exercise capacity in these dogs. To meet metabolic needs during exercise, CO is increased.57 When cardiopulmonary disease is present, a more substantial increase in HR compensates for a reduced ability to increase SV.58 Swimmer and Rozanski similarly demonstrated this in a study comparing 6MWT in healthy dogs versus dogs with pulmonary disease. Whereas there was not a significant difference in HR before 6MWT among groups, HR was significantly increased after exercise in dogs with pulmonary disease.20
There are several limitations of this study including a relatively small sample size in each group. Moreover, the study samples were not uniform in disease severity, and current therapies were not controlled. Comprehensive assessment of the underlying cause of PH was not performed in these dogs and different etiologies of disease might respond differently to body positioning, exercise, and sedation. Comorbidities and occult disease state were also not controlled for, likely leading to unbalanced study groups or a different response to interventions. Gold standard right heart catheterization measurements of RA pressure, PAP, PA wedge pressure, SV, and CO were not performed to validate our results. While we chose to use one single measurement of DiamPA throughout all calculations of SV to reduce error, there may have actually been a change in the DiamPA, and this could have affected these calculations. A 6MWT was used in this study to test a response to exercise. For some dogs, especially apparently healthy dogs, this might not represent an adequate exercise test to unmask impaired cardiopulmonary function. Also, interday variability of the 6MWT was not evaluated and, if high, could influence walk distances. As mentioned before, the average time between butorphanol administration and echocardiography was about 10 minutes. The mean time to onset of clinically detectable sedation has been reported to be about 16 minutes in healthy dogs;59 therefore, this could have affected our assessment of the postsedation echocardiograms. Nonetheless, all dogs appeared adequately sedated at the time of their final echocardiogram. Regarding cyclic variation of DE signals, we contributed the variability to changes in intrathoracic pressure and preload related to respiration,60 as respiration was the only variable that consistently changed. Some of the variability may have been related to imaging factors such as changes in Doppler envelope definition during certain parts of respiration (ie, movement of the heart during respiration in relation to the stationary position of the sample volume). Finally, RV systolic function was only assessed by RVFS, an index applicable to the inflow portion of the RV, and thus is not useful in the comprehensive evaluation of RV function.34, 53 As discussed before, other indices of RV function may have more accurately found differences in RV systolic function between study periods affecting the interpretation of our results.
In conclusion, to the authors' knowledge, this is the first report to systematically evaluate the effect of body position, exercise, and sedation on echocardiographic variables of PH in dogs with AV valve disease. The major finding of this study was a clinically relevant increase of TRFV after butorphanol administration in over 1/3 of the dogs studied. In contrast, body position and exercise had minimal effects on DE estimates of PAP. This effect of sedation could impact clinical decisions and prognostication and should be considered when interpreting TRFV. The results of the repeatability study emphasize the importance of colleagues and laboratories to standardize methods for measuring images and signals. The intra-observer variability was always smaller than the difference quantified between examiners, with the magnitude of some differences achieving clinical importance. This study also investigated an estimate of PVR derived from DE and evaluated CrVC collapsibility as an estimate of RA pressure, which are potentially useful variables in the comprehensive evaluation of dogs with PH. While larger studies and catheter-based validations are needed, these results provide further information for clinicians and researchers applying echocardiography to the evaluation of PH in dogs.
Acknowledgment
The authors acknowledge Tammy Muse, Patricia Mueller, Alicia Byrd, and Tim Vojt for their contributions.
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off-label Antimicrobial Declaration
Authors declare no off-label use of antimicrobials.




