Long-Term Follow-Up of Neuropsychiatric Symptoms After Sustained Virological Response to Interferon-Free and Interferon-Based Hepatitis C Virus Treatment
Funding: This study was funded by the Ellen-Schmidt-Scholarship of Hannover Medical School.
ABSTRACT
Chronic hepatitis C virus (HCV) infection can be associated with neuropsychiatric symptoms like fatigue and cognitive impairment, independent of the liver status. The present study aims to assess changes in the pattern and extent of neuropsychological symptoms after successful treatment with interferon (IFN)-based and IFN-free therapy. HCV-infected patients who underwent neuropsychological assessment in previous studies were invited to a follow-up examination. Patients were grouped according to the treatment status: Sustained virological response (SVR) after IFN treatment (IFN SVR, n = 14) or after therapy with direct acting antivirals (DAA SVR, n = 28) or ongoing HCV infection (HCV RNA+, n = 11). A group of 33 healthy controls served as reference. Patients completed self-report questionnaires addressing health-related quality of life (HRQoL), mood and sleep quality and a neuropsychological test battery including tests of memory and attention (Luria's list of words, PSE test, cancelling “d” test, Word–Figure–Memory Test and computer-based test battery for the assessment of attention [TAP]). At baseline, all three patient groups had worse fatigue, depression, anxiety and HRQoL scores compared to healthy controls. Longitudinal analysis revealed that fatigue and mood slightly improved in all patient groups over time, while HRQoL improved in SVR patients but not in HCV RNA+ patients. Memory test results improved significantly in all patient groups, irrespective of their virological status. In contrast, the attention test results showed no clear change from baseline to follow-up. Our data can be considered as a hint that HCV eradication—independent of therapy regimen—does not substantially ameliorate neuropsychiatric symptoms in HCV-afflicted patients.
1 Introduction
Chronic fatigue and cognitive deficits are frequent extrahepatic manifestations of hepatitis C virus (HCV) infection leading to low health-related quality of life (HRQoL), irrespective of the grade of liver disease [1-5].
Several studies documented that HCV infection can affect the central nervous system (CNS) and may induce neuroinflammation: Negative-stranded HCV ribonucleic acid (RNA) was found in autopsy brain tissues [6] and cerebrospinal fluid [7, 8], and imaging studies showed altered metabolism in distinct brain areas [9, 10], microglial activation [11, 12] and macro- as well as microstructural brain alterations [13-15].
Interferon (IFN)-based HCV therapy showed low sustained virological response (SVR) rates but high rates of side effects including depression and cognitive impairment [16]. In addition, neuropsychiatric impairment remained even after HCV eradication in about half of the formerly affected patients [17]. The treatment of chronic HCV infection profoundly changed in the last decade due to the introduction of direct acting antivirals (DAA). Now, the treatment achieved high SVR rates, and the incidence of side effects decreased considerably [18].
If HCV eradication after therapy with IFN-free treatment ameliorates the neuropsychiatric symptoms in these patients, however, is discussed controversially. Several studies show improvement of fatigue and HRQoL after virus eradication [19, 20]. Studies addressing the effect of a successful therapy on cognitive functions are rare. Vaghi et al. [21] showed a partial improvement of the median test results in different domains of cognition 6 months after DAA treatment in 23 HCV-infected patients. Kleefeld et al. [22] studied 8 HCV- and 14 HCV/human immunodeficiency virus (HIV)-co-infected patients before and 3 months after DAA therapy and observed improvement in fatigue and mental health as well as better performance in five out of eight cognitive domains after treatment.
Data about the course of neuropsychiatric symptoms in the long term after virus eradication are missing, as well as data of HCV-infected patients without therapy or with relapse. In a former cross-sectional study, we compared the features and extent of neuropsychiatric symptoms of HCV PCR-positive and -negative patients with and without interferon therapy and did not find a fundamental difference in the neuropsychiatric symptom profile [23].
The aim of this study was to describe the pattern and extent of neuropsychological symptoms in HCV patients with SVR after IFN-based therapy compared to patients with SVR after IFN-free therapy and HCV RNA-positive patients in a long-term follow-up (FU).
2 Methods
2.1 Patients and Healthy Controls
Ninety-eight chronic HCV-infected patients who had taken part in previous studies about HCV-induced encephalopathy between 2008 and 2010 [23, 24] were invited by letter to take part in the FU examination in 2019/2020.
Inclusion criteria were chronic HCV infection (detection of HCV RNA) at baseline (BL) and SVR after IFN-based or IFN-free DAA therapy in the meantime or an ongoing chronic HCV infection. Exclusion criteria were liver cirrhosis, coinfection with hepatitis B virus or HIV, regular intake of medication affecting CNS, concomitant diseases with a significant impact on cognitive performance (e.g., stroke, neurodegenerative diseases, malignant diseases and psychoses) and current or past drug or alcohol abuse. Forty-nine of the 98 invited patients could finally be included into the study. Seven had died in the meantime, 14 had moved house and could not be contacted, another 14 patients declined the invitation and the remaining 14 patients met the exclusion criteria (see flowchart in Figure S1). Data of four chronic HCV-infected patients who were treated in the hepatitis outpatient clinic 2016/2017 with DAA were added to the analysis. Thus, finally, data of 53 patients could be analysed. The results of a psychometric assessment from 33 healthy controls from hospital stuff, friends and patients' relatives were used for reference at baseline [23].
The study was approved by the local ethics committee (Nr. 8301_BO_S_2019). From each participant, written informed consent was obtained.
2.2 Study Assessments
2.2.1 Self-Report Questionnaires
All patients were asked to fill in three self-report questionnaires: The Fatigue Impact Scale (FIS) [25], a questionnaire to measure the impact of fatigue upon patients' daily living activities, the Hospital Anxiety and Depression Scale (HADS) [26] for the assessment of depression and anxiety and the short-form questionnaire 36 (SF-36) [27] for the assessment of HRQoL.
2.2.2 Psychometric Tests
The cancelling ‘d’ test [28] and the subtests ‘go/no go’, ‘alertness’, ‘divided attention’, ‘crossmodal integration’, ‘flexibility’ and ‘working memory’ from the computer-based test battery for the assessment of attention (TAP) [29], the portosystemic encephalopathy (PSE) syndromet est [30], Luria's list of words (LLW) [30] and the Word–Figure–Memory Test (WFMT) [31] were used for the assessment of cognitive function.
The test results of the cancelling “d” test are displayed as errors (in %) and processed items—errors. The raw data are transferred into age-corrected percentiles (PRs). For each subtest of the TAP battery, the individual reaction time (RT) and the number of errors and omissions are transferred into PRs as well. PR ≤ 10 is considered abnormal.
The relation between the maximal achievable number of abnormal results and a patient's number of abnormal attention test results—denoted as ‘attention test (ATT) sum score’—represents each patient's individual attention ability. The score covers 15 parameters. If either the parameter ‘errors’ or ‘omissions’ is pathological, this is considered as one pathological parameter. The parameters are: Cancelling ‘d’ test—errors%, items-errors; TAP battery: ‘alertness’ RT with and without warning, ‘go/no go’ RT and errors, ‘divided attention’ RT auditive and visual and errors/omissions, ‘crossmodal integration’ RT and errors, ‘flexibility’ RT and errors and ‘working memory’ RT and errors/omissions. The lower the score, the better is the performance. An ATT sum score > 0.35 is considered abnormal (tantamount to values worse than the mean plus two standard deviations of the controls).
LLW is a short-term memory and learning test. The Luria sum score represents the learning capacity of each patient and is calculated as the sum of recalled words in five consecutive runs. After a 10 min interval, the patient is asked to recall the words learned before. The quotient of this recall (= run 6) and run 5 represents the subject's free recall performance. The WFMT assesses the recognition of words and figures, separately. The results are expressed as z-scores considering norm values adjusted for age and education.
The PSE syndrome test was performed to evaluate attention, concentration and psychomotor ability [32]. This paper–pencil-based test battery comprises the number connection tests A and B, digit symbol test, serial dotting test and line tracing test. The results of the single tests are transferred into age- and—where applicable—sex- and education-corrected scores and finally sum up to the portosystemic hepatic encephalopathy score (PHES), which is considered abnormal if < −4.
2.3 Statistical Analysis
Data were analysed for normal distribution using the Kolmogorov–Smirnov test. Not normally distributed values were expressed as median (and 25th–75th percentile) and normally distributed values as mean (with standard deviation).
One-way ANOVA was performed to test for differences between groups in normally distributed data, while Kruskal–Wallis test was used for not normally distributed data. If significant, the Mann–Whitney U test as a post hoc test was done. Categorial variables were expressed as frequencies (% of total), and group differences were explored by Pearson's Chi-square test and Fisher's exact test, as appropriate.
Comparison between BL and FU data was done by paired t test (for normally distributed data) or Wilcoxon's matched-pairs test (for not normally distributed data). In addition, the average change (FU-BL) for the test scores was calculated.
Statistical analysis was performed with IBM SPSS (Version 28; SPSS Inc., Chicago, Illinois, USA) and GraphPad Prism (Version 5 for Windows; GraphPad Software, Boston, Massachusetts, USA).
3 Results
3.1 Patients and Healthy Controls
Fifty-three patients were included into the study. The patients were subdivided according to their treatment status at FU. All had been HCV PCR-positive at baseline.
Twenty-eight patients had successfully been treated with DAA with SVR (DAA SVR group). Fourteen patients became HCV PCR-negative after treatment with IFN-based therapy (IFN SVR group). Eleven patients had not been treated between baseline and FU and were still HCV PCR-positive (HCV RNA+) (Figure S1). Data of 33 healthy controls (22 women) served as reference for baseline. The three patient groups and the healthy controls did not differ concerning age and education. The time span between baseline and FU ranged between 11 and 12 years in median and did not differ significantly between the patient groups. Liver cirrhosis was excluded based on clinical findings and liver biopsy or transient elastography (Fibro Scan) (Table 1).
| DAA SVR (1) | IFN SVR (2) | HCV RNA+ [3] | Healthy controls (4) | p | |
|---|---|---|---|---|---|
| N = 28 | N = 14 | N = 11 | N = 33 | ||
| Age, baseline | 54.1 ± 10.6 | 51.1 ± 7.2 | 58.5 ± 8.1 | 51.2 ± 7.7 | 0.089 |
| Age, FU | 64.1 ± 9.7 | 62.6 ± 7.3 | 66.8 ± 6.6 | — | 0.481 |
| Sex (m/f) | 8/20 | 7/7 | 3/8 | 11/22 | 0.532 |
| Years of education | 10.0 (9.0–11.8) | 10.0 (10.0–12.3) | 10.0 (9.0–11.0) | 10.0 (9.5–13.0) | 0.221 |
| Time span baseline–FU | 11.0 (11.0–12.0) | 12.0 (11.0–12.0) | 11.0 (5.0–12.0) | — | 0.400 |
| Time span SVR-FU | 4.0 (1.0–5.0) | 8.5 (8.0–9.0) | — | — | < 0.001 |
| Liver status at baseline | |||||
| F0 | 7 | 3 | 4 | ||
| F1 | 9 | 8 | 4 | ||
| F2 | 9 | 1 | 3 | ||
| F3 | 3 | 2 | — | ||
| Liver status at follow-up | |||||
| F0 | 6 | 2 | 1 | ||
| F1 | 13 | 8 | 5 | ||
| F2 | 6 | 2 | 4 | ||
| F3 | 3 | 2 | 1 | ||
- Note: Results expressed as mean ± SD or median and 25th–75th percentiles. ANOVA p for age, Pearson Chi-square test p for sex, Kruskal Wallis test p for years of education and time span baseline—FU, Mann–Whitney U test p for time span SVR-FU; liver status F0–F1 according to liver biopsy or elastography. p-values in bold are statistically significant.
- Abbreviations: DAA, direct acting antivirals; FU, follow-up; HCV, hepatitis C virus; IFN, interferon; RNA, ribonucleic acid; SVR, sustained virological response.
The DAA SVR group (n = 28, 20 women) had been infected with HCV genotype 1 (genotype 1a [n = 4], 1b [n = 24]). Treatment consisted of Sofosbuvir (SOF) + Ledipasvir (n = 10, with n = 1: +Ribavirin [RBV]), Ombitasvir/Paritaprevir/Ritonavir + Dasabuvir (n = 10, with n = 2: +RBV for 24 weeks), SOF + Daclatasvir (n = 2), Deleobuvir + Faldaprevir + RBV (n = 2), SOF/Velpatasvir (n = 1), SOF/Velpatasvir/Voxilaprevir (n = 1), Elbasvir, Grazoprevir (n = 1) or Glecaprevir/Pibrentasvir (n = 1) for 12 weeks. The time span between SVR and FU was 4.0 (1.0; 5.0) years in median.
The patients treated with IFN-based therapy (± DAA, n = 14, 7 women) were infected with HCV genotype 1a (n = 2), 1b (n = 11) or 3a (n = 1). The treatment regimen consisted of IFN + RBV (n = 5) or IFN in combination with DAA: +Vaniprevir (n = 1), +Boceprevir (n = 3), +Telaprevir (n = 2), +Tegobuvir, +GS-9256 (n = 1), +Simeprevir (n = 1) or +Telaprevir (n = 1). The time span between SVR and FU was about 8.5 (8.0; 9.0) years.
The HCV RNA+ group (n = 11, 8 women) consisted of 10 patients with genotype 1b and 1 with genotype 1a. Five patients were treatment-naïve, six patients were treated before BL and had relapsed after IFN treatment or discontinued therapy because of side effects.
3.2 Fatigue, Mood and HRQoL at Baseline
At BL, all three patient groups had significantly higher fatigue scores than the healthy control group. The median scores were above 60 in the two groups who later on underwent successful treatment and even above 90 in the HCV RNA+ group (Table 2). This group also showed significantly higher anxiety and depression scores in the HADS compared to the other patient groups (HCV RNA+ vs. DAA SVR group: HADS depression: 11.0 (6.0–12.0) vs. 5.0 (3.2–7.8), p = 0.003; HADS anxiety: 11.0 (7.0–14.0) vs. 7.5 (5.0–10.0), p = 0.037; HCV RNA+ vs. IFN SVR: HADS anxiety: 11.0 (7.0–14.0) vs. 7.0 (4.0–9.3), p = 0.029) and controls (HADS depression and anxiety: 11.0 (6.0–12.0) vs. 2.0 (1.0–4.0), p < 0.001 and 11.0 (7.0–14.0) vs. 3.0 (1.5–5.5), p < 0.001) (Table S1). HRQoL (SF-36) scores did not differ significantly between patient groups at BL, but the HCV RNA+ group was obviously more severely affected than the two other groups. Of note, for all patient groups, the achieved scores were significantly worse than those of healthy controls (Table S1, for all p < 0.001).
| N | DAA SVR | p | N | IFN SVR | p | N | HCV RNA+ | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |||||||
| FIS | 28 | 63.0 (26.3–82.5) | 43.5 (15.5–70.8) | 0.037 | 14 | 62.5 (20.3–85.8) | 19.0 (7.0–40.3) | 0.025 | 11 | 93.0 (45.0–108.0) | 63.0 (48.0–90.0) | 0.169 |
| HADS-depression | 28 | 5.0 (3.3–7.8) | 5.0 (1.0–7.0) | 0.346 | 14 | 7.0 (3.0–10.3) | 3.0 (1.8–5.0) | 0.041 | 11 | 11.0 (6.0–12.0) | 7.0 (5.0–11.0) | 0.113 |
| HADS-anxiety | 28 | 7.5 (5.0–10.0) | 6.0 (3.3–8.8) | 0.033 | 14 | 7.0 (4.0–9.3) | 2.0 (1.0–7.5) | 0.023 | 11 | 11.0 (7.0–14.0) | 9.0 (7.0–11.0) | 0.076 |
| SF36 sumscore | 28 | 443.5 (336.3–637.3) | 547.5 (426.9–669.0) | 0.059 | 14 | 432.0 (317.8–501.8) | 590.5 (537.0–705.3) | 0.002 | 11 | 310.0 (216.5–554.0) | 310.0 (275.5–398.0) | 0.799 |
| SF36 physical | 28 | 256.0 (154.5–315.8) | 298.0 (221.5–350.8) | 0.206 | 14 | 188.5 (148.5–246.8) | 281.5 (246.8–340.8) | 0.004 | 11 | 159.0 (131.0–299.0) | 161.0 (142.0–272.0) | 0.722 |
| SF36 mental | 28 | 221.9 (159.5–314.6) | 281.3 (243.5–329.3) | 0.076 | 14 | 243.1 (173.3–281.6) | 314.5 (270.7–350.1) | 0.004 | 11 | 143.0 (113.0–262.0) | 159.5 (109.0–190.8) | 0.929 |
| WFMT words z | 27 | 0.050 (−1.200–0.870) | 0.953 (−0.059–1.431) | 0.010 | 12 | −12230.505 (−1.823–0.315) | 0.898 (0.207–1.762) | 0.008 | 11 | −1.200 (−1.690- -0.270) | −0.228 (−0.509–0.320) | 0.010 |
| WFMT figures z | 27 | 0.005 (−1.100–0.810) | 0.830 (0.393–1.595) | 0.001 | 12 | −0.854 (−1.553–0.173) | 1.049 (0.088–1.745) | 0.002 | 11 | −1.085 (−1.990–0.850) | 0.651 (−0.066–0.942) | 0.041 |
| Luria sumscore | 23 | 37.0 (34.0–39.0) | 39.0 (35.0–42.0) | 0.042 | 12 | 37.5 (34.3–40.0) | 41.0 (33.3–43.8) | 0.222 | 11 | 35.0 (31.0–41.0) | 37.0 (36.0–40.0) | 0.263 |
| Luria run 6/run 5 | 23 | 0.78 (0.67–0.90) | 0.89 (0.75–1.00) | 0.118 | 12 | 0.86 (0.72–0.98) | 0.89 (0.82–0.98) | 0.314 | 11 | 0.78 (0.63–0.83) | 0.78 (0.75–0.89) | 0.236 |
| PHES | 23 | 0.0 (−1.0–1.3) | 1.5 (−1.0–2.3) | 0.110 | 13 | 0.0 (−1.5–1.0) | 0.0 (−1.5–1.0) | 0.968 | 11 | 0.0 (−2.0–1.0) | 1.0 (−2–0- 2.0) | 0.673 |
| Cancelling “d” test items-errors | 28 | 372.5 (291.0–397.0) | 358.5 (319.0–424.8) | 0.244 | 13 | 354.0 (302.0–401.0) | 325.0 (259.0–398.5) | 0.249 | 11 | 262.0 (174.0–399.0) | 316.0 (245.0–398.0) | 0.026 |
| Cancelling “d” test errors (%) | 28 | 4.8 (2.6–9.3) | 3.5 (2.2–7.3) | 0.124 | 13 | 7.2 (3.0–11.6) | 5.7 (4.3–8.6) | 0.249 | 11 | 7.2 (6.0–13.9) | 5.2 (4.3–9.4) | 0.050 |
| ATT sumscore | 23 | 0.200 (0.133–0.333) | 0.133 (0.067–0.200) | 0.227 | 10 | 0.167 (0.000–0.333) | 0.133 (0.067–0.267) | 0.735 | 11 | 0.200 (0.067–0.667) | 0.267 (0.067–0.400) | 0.075 |
- Note: Results expressed as median and 25th–75th percentiles. p within the group with Wilcoxon's test for paired samples. p-values in bold are statistically significant.
- Abbreviations: ATT, attention test sum score; DAA, direct acting antivirals; FIS, Fatigue Impact Scale; FU, follow-up; HADS, Hospital Anxiety and Depression Scale; HCV, hepatitis C virus; IFN, interferon; PHES; portosystemic hepatic encephalopathy score; RNA, ribonucleic acid; SF-36, short form-36 questionnaire; SVR, sustained virological response; WFMT, Word–Figure–Memory Test.
3.3 Cognition at Baseline
The BL results for cognition are displayed in Table 2 and Table S2. Each patient group showed significantly worse results than controls for almost all outcomes (Table S2). The patient groups did worse than the controls in the WFMT, the Luria sum score (p < 0.001) and the PHES. In the cancelling “d” test only, the HCV RNA+ patients processed significantly less items (minus errors) compared to healthy controls (HCV RNA+ vs. controls: 262.0 (174.0–399.0) vs. 401.5 (349.3–459.0), p = 0.002). The percentage of errors showed no significant difference between the four groups. The DAA SVR and the HCV RNA+ patients scored significantly worse than the healthy controls in the ATT sum score (p = 0.008 and p = 0.016, respectively).
All in all, at BL, more than 60% of the patients showed abnormal results in the fatigue score in all three patient groups compared to only 6% in the healthy control group (Table 3). HADS depression and anxiety scores were more often abnormal in the patients than in the controls as well. This holds true, especially for the HCV RNA+ group. In this group also, nearly half of the patients had abnormal results in the ATT sum score compared to 19% in the DAA SVR group and 10% in the IFN SVR group. The WFMT results were abnormal in 45% of the HCV RNA+ group patients compared to 33% in the IFN SVR group and 19% in the DAA SVR group (Table 3).
| DAA SVR | p * | IFN SVR | p * | HCV RNA+ | p * | Healthy controls | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | Baseline | ||||
| FIS (> 45) | 19/28 (68%) | 14/28 (50%) | 0.398 | 9/14 (64%) | 4/14 (29%) | 0.166 | 8/11 (73%) | 9/11 (82%) | 0.879 | 2/33 (6%) |
| HADS depression (> 7) | 7/28 (25%) | 6/28 (21%) | 0.951 | 5/14 (36%) | 1/14 (7%) | 0.258 | 8/11 (73%) | 4/11 (36%) | 0.231 | 1/33 (3%) |
| HADS anxiety (> 7) | 14/28 (50%) | 10/28 (36%) | 0.559 | 5/14 (36%) | 3/14 (21%) | 0.705 | 8/11 (73%) | 8/11 (73%) | 0.9 | 4/33 (12%) |
| ATT sumscore (> 0.35) | 2/23 (9%) | 1/23 (4%) | 0.837 | 1/10 (10%) | 1/10 (10%) | 0.9 | 5/11 (45%) | 3/11 (27%) | 0.675 | 1/33 (3%) |
| WFMT words (z < −1.3) | 5/27 (19%) | 2/27 (7%) | 0.478 | 4/12 (33%) | 0/12 (0%) | 0.091 | 5/11 (45%) | 0/11 (0%) | 0.039 | 3/33 (9%) |
| WFMT figures (z < −1.3) | 5/27 (19%) | 0/27 (0%) | 0.064 | 4/12 (33%) | 0/12 (0%) | 0.091 | 4/11 (36%) | 0/11 (0%) | 0.087 | 2/33 (6%) |
- Note: p-values in bold are statistically significant.
- Abbreviations: ATT, attention test sum score; DAA, direct acting antivirals; FIS, Fatigue Impact Scale; HADS, Hospital Anxiety and Depression Scale; HCV, hepatitis C virus; IFN, interferon; RNA, ribonucleic acid; SVR, sustained virological response; WFMT, Word–Figure–Memory Test.
- * p between baseline and follow-up with Fisher's exact test.
Baseline results of the single tests of the TAP battery are displayed in Table 4 as the number of patients with abnormal results in relation to the number of patients who performed the test. The patient groups performed worse than the controls in the alertness test, the crossmodal integration test, the working memory test, the flexibility test and the divided attention test with no specific pattern for the different groups.
| DAA SVR | p | IFN SVR | p | HCV RNA+ | p | Healthy controls (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (%) | Follow-up (%) | Baseline (%) | Follow-up (%) | Baseline (%) | Follow-up (%) | |||||
| Go/no go | ||||||||||
| RT, PR | 6/23 (26) | 1/23 (4) | 0.053 | 2/10 (20) | 2/10 (20) | 0.9 | 3/11 (27) | 3/11 (27) | 0.9 | 10/33 (30) |
| Errors PR | 0/23 (0) | 0/23 (0) | 0.9 | 0/10 (0) | 1/10 (10) | 0.9 | 0/11 (0) | 0/11 (0) | 0.9 | 1/33 (3) |
| Alertness | ||||||||||
| RT, without warning PR | 7/23 (30) | 3/23 (13) | 0.284 | 5/10 (50) | 3/10 (30) | 0.410 | 5/11 (45) | 7/11 (64) | 0.670 | 6/33 (18) |
| RT, with warning PR | 8/23 (35) | 7/23 (30) | 0.9 | 4/10 (40) | 4/10 (40) | 0.9 | 5/11 (45) | 6/11 (54) | 0.9 | 5/33 (15) |
| Divided attention | ||||||||||
| RT, auditive PR | 10/23 (43) | 13/23 (57) | 0.556 | 3/10 (30) | 4/10 (40) | 0.9 | 4/11 (36) | 2/11 (18) | 0.635 | 4/33 (12) |
| RT, visual PR | 2/23 (9) | 3/23 (13) | 0.9 | 2/10 (20) | 1/10 (10) | 0.9 | 3/11 (27) | 1/11 (9) | 0.587 | 5/33 (15) |
| Errors or omissions PR | 7/23 (30) | 8/23 (35) | 0.9 | 3/10 (30) | 3/10 (30) | 0.9 | 6/11 (54) | 6/11 (54) | 0.9 | 9/33 (27) |
| Crossmodal integration | ||||||||||
| RT, PR | 7/23 (30) | 10/23 (43) | 0.542 | 2/10 (20) | 3/10 (30) | 0.9 | 5/11 (45) | 5/11 (45) | 0.9 | 5/33 (15) |
| Errors PR | 5/23 (22) | 5/23 (22) | 0.9 | 2/10 (20) | 2/10 (20) | 0.9 | 3/11 (27) | 1/11 (9) | 0.587 | 2/33 (6) |
| Flexibility | ||||||||||
| RT, PR | 1/23 (4) | 0/23 (0) | 0.9 | 1/10 (10) | 0/10 (0) | 0.9 | 2/11 (18) | 2/11 (18) | 0.9 | 2/33 (6) |
| Errors PR | 4/23 (17) | 1/23 (4) | 0.346 | 1/10 (10) | 0/10 (0) | 0.9 | 3/11 (27) | 1/11 (9) | 0.587 | 1/33 (3) |
| Working memory | ||||||||||
| RT, PR | 4/23 (17) | 0/23 (0) | 0.109 | 1/10 (10) | 1/10 (10) | 0.9 | 3/11 (27) | 2/11 (18) | 0.9 | 2/33 (6) |
| Errors or omissions PR | 7/23 (30) | 5/23 (22) | 0.738 | 5/10 (50) | 3/10 (30) | 0.9 | 7/11 (64) | 4/11 (36) | 0.395 | 5/33 (15) |
- Note: p between baseline and follow-up with Fisher's exact test; RT, reaction time expressed as median in ms.
- Abbreviations: DAA, direct acting antivirals; HCV, hepatitis C virus; IFN, interferon; PR, percent range; RNA, ribonucleic acid; RT, reaction time; SVR, sustained virological response.
3.4 Fatigue, Mood and HRQoL at Follow-Up Examination
Both successfully treated patient groups—DAA SVR and IFN SVR—improved in fatigue, HADS and SF-36 scores but remained worse than the healthy controls regarding FIS and SF-36 (Tables 2 and 3). Of interest, the improvement in FIS was more pronounced in the IFN SVR group than in the DAA SVR group. HCV RNA+ showed better median FIS and HADS scores on follow-up than at BL but did still significantly worse than the SVR groups (and controls). Furthermore, the number of patients with abnormal scores did not significantly change. Of interest, the physical as well as the mental HRQoL scores remained virtually unchanged in the patients with ongoing HCV infection (Table 2). Table S3 shows the extent of changes in median for FIS, HADS and SF-36 (Table S3).
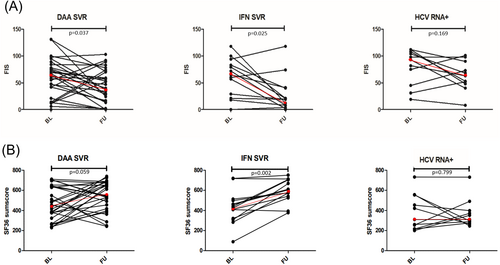
Individual FIS results over time are illustrated in Figure 1A. The graph shows the heterogeneity within each group. While most of the patients improved over time, a few patients also showed an increase in fatigue. Figure 1B shows the corresponding results for the SF36 sum score.
3.5 Cognition at Follow-Up Examination
From BL to FU, all three patient groups improved significantly in the performance of the WFMT for both categories (Table 2). The percentage of pathological results also decreased over time in each patient group (Table 3). The long-term change of the results did not differ between the patient groups (Table S4). Accordingly, Figure 2 illustrates the change of the z-score (category words of the WFMT) for each patient group and the individual patient results. Here, especially the values of the DAA SVR group varied in both directions.

The DAA SVR group showed significantly improved performance in the Luria sum score (BL vs. FU 37.0 [34.0–39.0] vs. 39.0 [35.0–42.0], p = 0.042), whereas patients with ongoing HCV infection significantly improved in the cancelling ‘d’ test for processed items—errors (262.0 (174.0–399.0) vs. 316.0 (245.0–398.0), p = 0.026) and errors % (7.2 [6.0–13.9] vs. 5.2 [4.3–9.4], p = 0.050, Table 2).
The percentage of pathological results regarding the ATT sum score did not change over time in all patient groups, nor did most of the results of the single TAP battery tests. Only the DAA SVR group showed a slight, though not significant, improvement at follow-up in the subtests: ‘go/no go’ (RT), ‘flexibility’ (errors) and ‘working memory’ (RT) (Table 4). These data, however, must be interpreted with caution due to the small number of patients studied.
4 Discussion
In this follow-up study, we examined the pattern and extent of neuropsychiatric symptoms in HCV patients before and after successful virus eradication (with IFN or DAA therapy) compared to patients with ongoing HCV infection (HCV RNA+) and a reference group of healthy controls. Mood alterations and reduced HRQoL are common in HCV-infected patients independent of the grade of liver disease. Accordingly, in our study, all patient groups showed signs of fatigue, depression, anxiety and reduced HRQoL at baseline compared to healthy controls. Fatigue and mood alterations slightly improved over time in all groups, but the differences between BL and FU were not striking, and the results were still in the pathological range. The change between FU and BL did not differ significantly between the groups. However, looking at the data, the improvement of FIS, HADS scores, SF-36 scores and WFMT results is clearly more pronounced in the IFN SVR group. Considering the known side effects of IFN therapy (+Ribavirin), such as depression, sleep disturbances, fatiguability and loss of appetite, for example, this finding was unexpected. However, the longer time interval between SVR and follow-up in the IFN group compared to the DAA SVR group may play a role.
Of interest, despite the modest improvement in fatigue and mood, the SF-36 scores in the HCV RNA+ group did not change, contrary to the findings in both SVR groups.
As expected, and in line with previous publications [22, 23] all patient groups showed significantly worse results in the memory tests (WFMT and Luria's list of words) compared to healthy controls before treatment. The percentage of patients with abnormal results ranged between 19% and 45%. The results of the attention tests represented by the ATT sum score differed between the patient groups and the controls as well. However, the percentage of patients with abnormal ATT results differed remarkably from that of controls only in the HCV RNA+ group.
The differences between the SVR groups and the HCV RNA+ group at baseline were unexpected and must be discussed. All patients had been examined for the first time about 11–12 years before follow-up and had undergone the same procedure. The group differences thus could be explained as fortuity. However, one may speculate that this difference could eventually explain why the HCV RNA+ group had not sought medical treatment. Fatigue and perception of a diminished quality of life could have been one reason for turning away from medical treatment. It has also to be highlighted that 6 of the 11 HCV RNA+ patients had been treated before baseline with IFN-based therapy without success or with side effects that led to the stop of the treatment. Their test results might dominate the findings for the whole group. However, they do not differ from those of the five patients who were never treated. Thus, the differences between SVR groups and the HCV RNA+ group at baseline remain unexplained. Finally, they remain secondary for this paper, as this study aimed to detect changes in neuropsychiatric symptoms after successful HCV therapy.
Interestingly, between BL and FU, all patient groups improved in the WFMT and Luria's list of words, while the other test results remained virtually unchanged, except for the cancelling ‘d’ test results of the HCV RNA+ patients, who achieved a significant increase in their median scores. This improvement in all groups begs the question whether it is due to changes in the test application or evaluation. The test procedure remained the same over the years and is defined in the text manual. At least for the WFMT, the ‘improvement’, however, could be due to less strict evaluation of the raw data with increasing age. This would apply to all three groups and explain the similar changes in WFMT results in the three patient groups.
In summary, a clear difference or a definitive improvement of cognitive function could not be observed in any patient group between BL and FU. Similar to the findings in the short-term follow-up in the former randomised controlled trials on HCV treatment [20], however, the patients showed a significant increase of HRQoL scores after successful treatment with both DAA and IFN-based therapy, though not up to a normal range. Indeed, literature is inconsistent in this regard: Some studies underline the improvement of HRQoL after therapy with DAA [19] or PEG-IFN + RBV [33], whereas others show that a clinically relevant improvement of HRQoL occurs only in about half of the patients [34]. Several studies indicate that fatigue and impaired cognitive performance persist despite the clearance of the virus after successful therapy with PEG IFN/RBV [33, 35, 36]. Others show an incomplete recovery from HCV-related neuropsychiatric disorders with SVR after DAA therapy [22]. MRI diffusion tensor imaging (DTI) studies showed that patients with better cognitive function after successful treatment presented an improved white matter integrity of the brain in contrast to those who remained cognitively impaired [35]. Also, regional alterations in distinct cerebral metabolites measured by MR spectroscopy (MRS) remained despite viral clearance [33].
Finally, all these studies—including our own—showed no clear evidence that the successful antiviral treatment of HCV patients results in recovery from HCV-associated neuropsychiatric symptoms.
These clinical observations are very much in line with immunological findings that show permanent immunological signatures despite clearance of the virus [37]. HCV patients show a distinct pattern of immune cell activation and soluble inflammatory mediators that is linked to the neuropsychiatric symptoms of HCV encephalopathy and differs, for example, from the respective findings in patients with autoimmune liver disease [38]. The immune cell dysfunction caused by HCV infection persists even after the clearance of the virus [38-40]. Of note, long-lasting imprints of HCV infection were also observed regarding soluble inflammatory mediators not only after the successful antiviral treatment of acute hepatitis C [41] but also chronic hepatitis C [42].
The details of the relationship between neurocognitive symptoms and immune alterations in HCV infection are not yet fully understood. Studies indicate that infected peripheral blood mononuclear cells (PBMCs) could mediate HCV entry in the CNS by the ‘Trojan horse’ mechanism [43]. Postmortem studies found viral replicative forms and viral proteins in brain tissue [6, 44]. Indeed, astrocytes and macrophages/microglia were identified as cells harbouring HCV [44, 45]. In addition, chronic HCV infection induces proinflammatory cytokines in the periphery. These cytokines could cause blood–brain barrier alterations and/or chronic microglia activation which indirectly affect brain function and mood alterations, for example, via induction of cerebral cytokine production with the consecutive alteration of glutaminergic and cholinergic neurotransmission [10, 46]. MRS studies revealed increased myo-inositol and choline levels in the brain of HCV patients which was interpreted as a sign of neuroinflammation [10, 14]. Of note, the metabolite levels increased with decreasing fatigue scores, indicating a neuroprotective effect of microglia activation [14]. In a single photon emission tomography (SPECT) study, HCV patients with abnormal dopamine and/or serotonin transporter binding capacity showed worse psychometric test results than those with normal SPECT results, indicating the alteration in neurotransmission as the possible cause for fatigue and mood alterations [9].
Many viruses, especially flaviviridae, are capable of inducing cognitive decline and mood alterations. Most recently, the effect of severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) infection upon brain function could be observed in millions of patients, and the long-term aftermath of COVID-19 upon brain function, irrespective of the severity of the symptoms during the acute phase of the disease, is one major topic in medical science—though contradictorily discussed. Of note, symptoms and signs are quite similar between HCV encephalopathy and post-COVID syndrome. Like patients with HCV encephalopathy, also patients with post-COVID syndrome, in contrast to those without, show distinct alterations of soluble and cellular immunological mediators [47-49].
Our study has some limitations. We conducted the study with a small sample size, especially in the HCV RNA+ group. In addition, data of healthy controls were only available for baseline. Further, the FU period was about 11–12 years so that new accompanying diseases (like neurodegenerative diseases or malignant diseases) can be expected, on principle, though we tried to control these factors by a detailed anamnesis and neurological examination. Nevertheless, our findings must be interpreted with caution.
Sampling bias should also be taken into account: Patients with persisting symptoms (with or without therapy) are more interested to participate in a study. However, the flowchart of patient recruitment does not indicate sampling bias, as only 14 of 102 patients invited declined participation.
Our study shows long-term results of neuropsychiatric assessments in HCV-infected patients after different treatment regimens. Patients with and also those without SVR improved over time in a few memory and attention tests; however, by and large, the virus eradication in the periphery did not change the neuropsychiatric status of the patients. As the sample size studied is small, the results of our study can only be considered as a hint that virus eradication in the periphery is not equivalent to full recovery from CNS-related extrahepatic manifestations of HCV infection.
Acknowledgements
We would like to thank all those who participated in the study for their time and involvement. Open Access funding enabled and organized by Projekt DEAL.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




