Association of Hepatitis B Surface Antigen Levels With Long-Term Complications in Chronic Hepatitis B Virus Infection: A Systematic Literature Review
Funding: This study was funded by GlaxoSmithKline (study 217135). The funders of the study had a role in study design, data analysis, data interpretation and writing of the report. The corresponding author had full access to all the data and the final responsibility to submit it for publication. Literature review and data analysis were performed by Bridge Medical Consulting Ltd funded by GlaxoSmithKline. No funding was provided to Bridge Medical Consulting Ltd for manuscript development.
ABSTRACT
Chronic hepatitis B virus (HBV) infection is a global issue and can lead to cirrhosis and hepatocellular carcinoma (HCC). Hepatitis B surface antigen (HBsAg) is an important marker of HBV infection and HBsAg quantification could be a useful tool in clinical practice. This systematic literature review aimed to explore the association between HBsAg titres and long-term disease outcomes and evaluate the relationship between HBsAg titres, or changes in HBsAg titres, and clinical and treatment characteristics in patients with chronic HBV infection. Structured searches were performed in MEDLINE and Embase (January 2000 to 31 March 2023). Eighty-two studies were included, comprising 51% retrospective cohort studies, mostly conducted in Asia (85%). HBsAg levels were shown to predict the long-term development of cirrhosis and HCC in patients who were untreated prior to and during follow-up; however, these data were inconclusive in mixed and treated populations. HBsAg titres were significantly associated with various virological markers including serum HBV DNA, HBcrAg, HBeAg, HBV RNA levels, intrahepatic covalently closed circular DNA (cccDNA) and intrahepatic HBsAg expression. HBsAg titres generally declined over time; this decline was more pronounced in early (HBeAg-positive) than later disease phases (HBeAg-negative). Higher decline in HBsAg levels was consistently associated with subsequent HBsAg seroclearance and a greater decline in total intrahepatic HBV DNA and cccDNA levels. In conclusion, this review showed that HBsAg levels and rates of decline could inform assessment, management and prediction of outcomes in chronic HBV infection. Further studies in broader, more diverse populations and treated patients are needed.
Abbreviations
-
- ALT
-
- alanine aminotransferase
-
- HBeAg
-
- hepatitis B e-antigen
-
- HBsAg
-
- hepatitis B surface antigen
-
- HBV
-
- hepatitis B virus
-
- HCC
-
- hepatocellular carcinoma
1 Introduction
Chronic hepatitis B virus (HBV) infection is a global issue affecting an estimated 296 million people in 2019, with 1.5 million new infections each year [1]. Chronic HBV infection can lead to life-threatening complications such as cirrhosis and hepatocellular carcinoma (HCC), which account for 96% of the deaths due to viral hepatitis [2]. Chronic HBV infection accounts for ≥50% of HCC, a leading cause of cancer deaths [3, 4].
To inform patient management decisions in clinical practice, HBV treatment guidelines classify patients into disease phases, based on the natural history of chronic HBV infection in the absence of any treatment [5, 6]. Patients are categorized according to hepatitis B e-antigen (HBeAg) status, and HBV DNA and alanine aminotransferase (ALT) levels [5]. These disease phases (HBeAg-positive chronic HBV infection, HBeAg-positive chronic hepatitis B, HBeAg-negative chronic HBV infection and HBeAg-negative chronic hepatitis B) reflect the relationship between viral status and the patient's immune response [5, 7]. High HBV DNA and ALT levels are seen as risk factors for adverse outcomes in patients with chronic HBV infection [8-10].
Hepatitis B surface antigen (HBsAg) is another important marker of HBV infection. HBsAg plays a pivotal role in viral persistence and disruption of the immune response underlying the development and progression of chronic HBV infection [11-14]. Total HBsAg includes a large number of subviral particles which act as immunological decoys and drive immune exhaustion by promoting T cell deletion and anergy [11-14]. Current treatments focus on HBV DNA suppression, which reduces the risk of cirrhosis but does not effectively reduce the risk of HCC [5, 6, 15, 16]. A HBsAg-directed goal might potentially achieve greater clinical benefit than HBV DNA suppression alone. Functional cure, defined as HBsAg loss, with or without HBsAg seroconversion, together with sustained undetectable HBV DNA after cessation of therapy [17], may further reduce the risk of cirrhosis and HCC and is now the elevated endpoint for many clinical trials in chronic HBV infection [18-20].
While qualitative HBsAg measurements, such as the lateral flow immunoassay-based rapid strip test, are commonly used in clinical practice for the diagnosis of HBV infection [21], there has been increased interest in the clinical utility of quantitative HBsAg measurements. HBsAg quantification can predict disease activity and allow distinction of disease phases, for example, levels are higher in patients with HBeAg-positive status than in those with HBeAg-negative status [6]. It could also be a valuable tool to monitor treatment response and predict sustained response after treatment cessation [22, 23]. Furthermore, studies have proposed a link between HBsAg levels and long-term outcomes such as cirrhosis and HCC [6].
A systematic literature review was conducted to gather epidemiological data on quantitative HBsAg levels in chronic HBV infection. This review aimed to describe the association between HBsAg titres and long-term disease outcomes and evaluate the cross-sectional relationship between HBsAg titres, or changes in HBsAg titres, and various clinical and treatment characteristics.
2 Materials and Methods
2.1 Identification of Relevant Studies
Structured searches were performed in MEDLINE and Embase from 1 January 2000 to 31 March 2023 (Table S1). Supplementary searches were conducted from the following sources to identify relevant published literature: conference searches (American Association for the Study of Liver Diseases [AASLD], European Association for the Study of the Liver [EASL], Asian Pacific Association for the Study of the Liver [APASL] annual conferences from 2021 to 2023); bibliography searches on recent primary studies, systematic reviews and general reviews to ensure that all relevant evidence was captured; and grey literature searches (keyword-based pragmatic searches were conducted in PubMed, Google and Google Scholar to identify any additional relevant publications).
2.2 Study Selection
Publications were selected in two steps using the predefined inclusion/exclusion criteria described in Table S2. Of note, patients with chronic HBV infection were selected from representative populations and assessed in real-world observational studies. First, the title and abstract of each record identified during the structured and supplementary searches were assessed for relevance as per the screening flowchart (Figure S1). The full-text versions of the selected citations were then examined in more detail to determine the final list for inclusion based on pre-defined eligibility (inclusion/exclusion) criteria. Screening of titles/abstracts and full-text publications was conducted by two independent reviewers and any discrepancies were resolved by a third reviewer.
2.3 Data Collection and Extraction
Data from selected publications were extracted into a Microsoft Excel data extraction sheet by one reviewer and checked by a second reviewer. Quality assessments for all included studies were completed using the Newcastle–Ottawa Scale for cohort studies and cross-sectional studies, as applicable.
2.4 Research Objectives
The objectives of the review were as follows: Objective 1 was to assess the association between HBsAg titres (as measured at identifiable timepoints such as baseline, at treatment initiation and disease stage) and long-term disease outcomes (compensated/decompensated cirrhosis, HCC and mortality); Objective 2 was to assess HBsAg titres in various phases of chronic HBV infection and their cross-sectional relationship with clinical, and treatment characteristics, including patient age, HBV DNA, HBV RNA, hepatitis B core-related antigen (HBcrAg) and intrahepatic cccDNA; Objective 3 will describe the change in HBsAg titres over time and its relationship with the clinical and treatment characteristics listed in Objective 2. The review searches were designed to capture a range of specific clinical and treatment characteristics along with virological biomarkers of interest (Table 1) and disease outcomes.
| Disease/virological markers | Liver-structure related factors | Treatment-related factors | Demographic factors |
|---|---|---|---|
| Serum HBsAg | Fibrosis | NA therapy | Age |
| Serum HBcrAg | Cirrhosis | NA treatment duration | Gender |
| Serum HBeAg | HCC | NA consolidation duration | |
| Intrahepatic HBsAg expression | HBsAg seroclearance | ||
| Serum HBV DNA | HBsAg seroconversion | ||
| Intrahepatic HBV DNA (total) | Sustained virological response | ||
| Intrahepatic cccDNA | Clinical relapse | ||
| Serum HBV RNA | Retreatment | ||
| Anti-HBc antibody | |||
| HBV genotype |
- Abbreviations: cccDNA, covalently closed circular DNA; DNA, deoxyribonucleic acid; HBc, hepatitis B core; HBcrAg, hepatitis B core-related antigen; HBeAg, hepatitis B e-antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; NA, nucleos(t)ide analogue.
3 Results
3.1 Literature Search
The PRISMA flow diagram of included citations and studies is presented in Figure S2. The citations arising from the search (4856 unique references from the literature database searches and 28 from the supplementary and conference searches) were reviewed and 82 relevant publications were identified for final data extraction and reporting.
Study characteristics are described in Table S3. Most (51%) were retrospective cohort studies (n = 42/82 [51%]), had a sample size of 100–500 patients (n = 41/82 [50%]) and were conducted in Asia (n = 70/82 [85%]). The data collection period ranged from 1980 through 2020, and most studies reported the follow-up duration as total follow-up (range: 12–1040 weeks; n = 25 [30%]).
The included studies were assessed for quality, and the results of the assessment are shown in Table S4 for cohort studies and Table S5 for cross-sectional studies. Of the 61 (74%) studies reporting details of prior treatment, 33 (54%) included treatment-naïve patients only, 12 (20%) included patients with prior treatments only and 16 (26%) included a mixed population. Of the 77 (94%) studies reporting details of on-study treatment, 26 (34%) included patients receiving no treatment, 33 (43%) included patients who were receiving treatment and 18 (23%) studies included a mixed population. The mean age of patients ranged from 27.3 to 57.2 years and the proportion of female patients was from 0% to 71.9% (Table S6).
3.2 HBsAg Titres and Long-Term Outcomes
Data on the association between quantitative HBsAg titres and long-term disease outcomes were reported in 16 studies (Table S7): HCC (15 studies), cirrhosis (4 studies) and mortality (no studies).
3.2.1 HBsAg Titres and HCC
Data were reported as multivariable hazard ratio (HR; 6 studies [24-29]) and univariable HR (12 studies [24-35]) using Cox's proportional hazards regression analyses (Figure 1). One study reported odds ratios (OR) using logistic regression analysis [36].
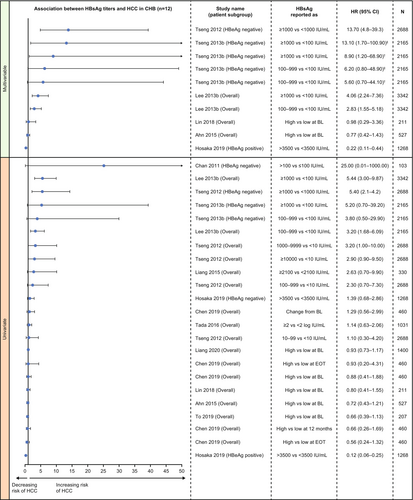
In multivariable analysis, there was evidence for the association of quantitative serum HBsAg with long-term development of HCC, particularly in treatment-naïve populations (Figure 1). In treatment-naïve patients, three studies in Taiwan reported that higher baseline HBsAg levels significantly and independently predicted the development of HCC, including in the overall chronic HBV infection population [26], and in HBeAg-negative patients [28, 29]. In one of the studies in HBeAg-negative patients, no difference was seen between HBsAg levels of 100–999 IU/mL versus <100 IU/mL [28]. In contrast, in HBeAg-positive patients treated with nucleos(t)ide analogues (NA), one study in Japan reported that lower HBsAg levels (<3500 IU/mL vs. >3500 IU/mL) were associated with a significantly higher risk of HCC development [25]. In the overall chronic HBV infection population, two studies reported no significant association between HBsAg levels and HCC development [24, 27].
In univariable analysis, one study in Taiwan reported a significant association between higher HBsAg levels and HCC development in treatment-naïve patients with chronic HBV infection [29]. However, this was only observed in the subgroup of patients who were HBeAg-negative and had a low viral load, with no significant association seen in other subgroups [29]. One study from Japan reported that HBsAg level ≥3.32 log IU/mL (vs. <3.32 log IU/mL) was significantly associated with HCC development based on univariable analysis [38]. Another eight studies reported no significant association between HBsAg levels and HCC development in univariable analysis (Figure 1) [25, 28, 30-35].
The study using logistic regression analysis (univariable only), conducted in the overall chronic HBV infection population, reported no significant association between baseline HBsAg levels (continuous) and HCC development (crude OR 0.79; 95% confidence interval [CI]: 0.56–1.10) [36].
Three studies [28, 37, 38] did not report any quantitative data for HR or CIs (only p values were reported) and these are not covered in Figure 1. Data for all other studies reporting quantitative HR data (univariable and multivariable) are presented in Figure 1.
3.2.2 HBsAg Titres and Cirrhosis
Data were reported as multivariable HR using Cox's proportional hazards regression analyses in two studies [26, 39] and univariable HR in all four studies [24, 26, 35, 39] (Figure 2). There was evidence for an association between serum HBsAg levels and long-term development of cirrhosis, specifically in treatment-naïve patients, although the data were limited.
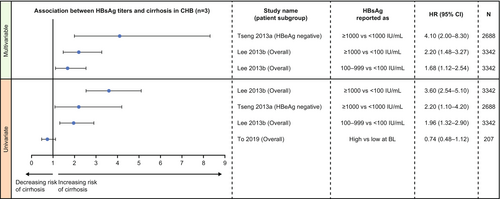
In multivariable analysis, both studies in Taiwan, one in HBeAg-negative patients and one in the overall population, showed that higher baseline HBsAg levels significantly predicted the development of cirrhosis in treatment-naïve patients with chronic HBV infection (Figure 2) [26, 39]. The same results were also seen in these studies in univariable analysis [26, 39]. However, baseline HBsAg levels were not found to be a risk factor for the development of cirrhosis in a mixed cohort of treated and treatment-naïve patients in one study (univariable analysis; Figure 2) [35]. No significant association between baseline HBsAg levels (continuous) and development of cirrhotic complication was found in one retrospective cohort study [24].
3.3 HBsAg Titres by Disease Phase and Their Relationship With Patient Characteristics
Data on quantitative HBsAg level by disease phase were reported in 38 studies (Table S7) [40-77]. In keeping with the natural disease progression, HBsAg titres were generally higher in the initial HBeAg-positive phases than the later HBeAg-negative phases (Table S8).
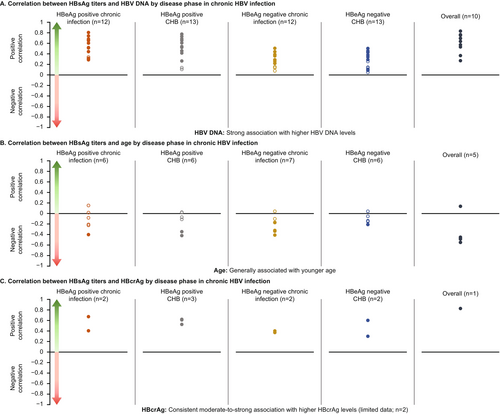
Data on the relationship between HBsAg titres and various patient characteristics by disease phase were available from 34 studies (Table S7). HBV DNA (n = 20) and age (n = 9) were the most commonly assessed factors followed by HBcrAg and HBV RNA (n = 5 studies each). Higher HBV DNA levels, younger age, higher HBcrAg levels and higher HBV RNA levels were associated with higher HBsAg levels across disease phases (Figure 3). Positive associations were noted between high HBsAg titres and high HBeAg level [77], intrahepatic HBsAg expression [58] and retreatment [46] in specific disease phases, although data were very limited (Table 2). There was no (consistent) association between high HBsAg titres and HBsAg clearance (n = 3), gender (n = 3), anti-HBc antibody levels (n = 1), fibrosis (n = 1) or cirrhosis (n = 1) (Table 2).
| Characteristicsa | Disease phase | Association with HBsAg levels |
|---|---|---|
| HBeAg levels [77] | HBeAg-positive phases | Positive strong |
| Intrahepatic HBsAg expression [58] | HBeAg-negative phases | Positive moderate |
| Retreatment [46] | HBeAg-negative phases | Positive association |
| Anti-HBc antibody levels [67] | All phases | No consistent association |
| Fibrosis [64] | HBeAg-negative phases | No association |
| Cirrhosis [69] | All phases | No association |
- Abbreviations: HBc, hepatitis B core; HBeAg, hepatitis B e-antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus.
- a n = 1 each.
Correlation analyses showed a clear positive correlation between HBsAg titres and HBV DNA, which was generally moderate-to-strong in the initial HBeAg-positive phases and weak-to-moderate in the late HBeAg-negative phases and was statistically significant in most cases (Figure 3).
Higher HBsAg levels were generally associated with lower age across individual phases and overall (all phases combined). The statistically significant results were weak-to-moderate in strength and the non-significant results also followed the same trend (Figure 3).
A consistent moderate-to-strong association was noted between higher HBsAg levels and higher HBcrAg levels in individual phases in five studies (Figure 3) [42, 66, 74, 77, 78]. Correlation analyses showed a positive correlation between HBsAg titres and HBV RNA in three studies with moderate-to-strong correlation in HBeAg-positive phases [48, 62, 74], a weak correlation in HBeAg-negative phase [62] and no association reported in one study [71].
Correlation analyses showed a positive correlation between HBsAg titres and intra-hepatic cccDNA levels in two studies with moderate-to-strong positive correlation in HBeAg-positive phases [43, 45] and no association was reported in one study [48].
3.4 Change in HBsAg Titres Over Time and Its Relationship With Patient Characteristics
Data on change in HBsAg titres over time in patients with chronic HBV infection were reported in 41 studies (Table S7) [25, 27, 31, 37, 42, 43, 45, 46, 48, 55, 57, 60, 68, 73, 78-104]. HBsAg titres generally declined over time and this decline was slightly more pronounced in HBeAg-positive patients than HBeAg-negative patients (Table S9).
Data regarding the change in HBsAg titres and its relationship with various patient characteristics were reported in 34 studies (Table S7) [31, 37, 43, 45, 46, 48, 55, 57, 60, 68, 72, 78-93, 98-104]. HBsAg seroclearance (n = 16) and HBV DNA (n = 13) were the most assessed factors followed by intrahepatic HBV DNA (n = 5).
In multivariable analyses, higher decline in HBsAg titres over time was consistently associated with HBsAg seroclearance in the overall chronic HBV infection population, as well as in HBeAg-negative and positive patients (Figure 4). In univariable analysis, four studies in mixed/treatment-experienced HBeAg-negative patients reported no significant association between HBsAg decline and HBsAg seroclearance (Figure 4A) [31, 81, 90, 91]. Seto et al. showed that HBsAg reduction of >0.166 log IU/mL/year was highly predictive of HBsAg seroclearance [98].
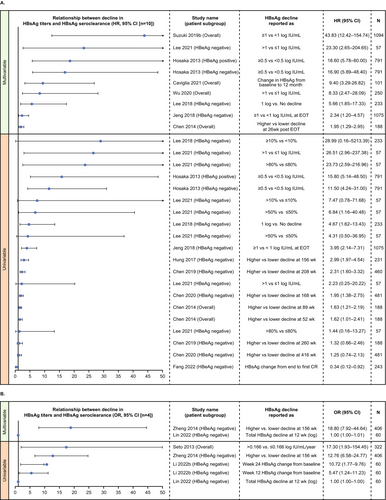
Higher decline in HBsAg titres over time was also consistently associated with lower HBV DNA (Figure 5). However, the association was not significant for all studies at all timepoints. In multivariable analysis, association between decline in HBsAg titres and lower HBV DNA was mostly seen in treatment-experienced HBeAg-negative patients [90, 91] and in the overall population with chronic HBV infection in three studies in Taiwan [82, 90, 91]. Results of univariable analyses were mixed, with significance depending upon the definition of HBsAg decline or the timepoint of interest (Figure 5).
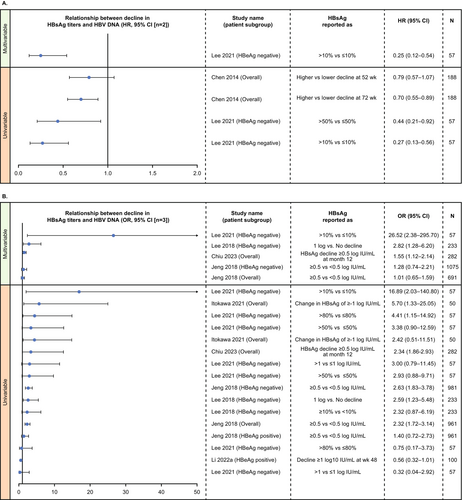
A higher decline in HBsAg titres was consistently associated with a higher decline in intrahepatic HBV DNA in five studies. Using Pearson correlation analysis, decline in HBsAg levels had a moderate positive correlation with reduction in (1) total intrahepatic HBV DNA levels in the overall population, (2) HBeAg-positive and HBeAg-negative subgroups in both treatment-experienced and treatment-naïve patients [78, 99], (3) with intrahepatic cccDNA levels in both HBeAg-positive chronic HBV infection [45, 48, 78] and (4) HBeAg-negative chronic HBV infection [78], treatment-experienced patients. Using Spearman correlation analysis, one study reported a significant positive correlation between a decline in HBsAg titres and intrahepatic cccDNA in a mixed population of patients with HBeAg-positive chronic HBV infection [43]. There were limited data for association of decline in HBsAg titres with HBV genotype (n = 3 studies) Inconsistent associations were noted with HBV genotype B based on the timepoint of interest and definition of HBsAg decline in the overall chronic HBV infection population in two studies [82, 85] and no association was noted in the overall population, patients with HBeAg-positive and HBeAg-negative chronic HBV infection [87].
There were also limited and inconsistent data for associations between decline in HBsAg titres with retreatment, HBeAg level, HBcrAg level, NA therapy, age and gender (n = 2 studies each). In two NA cessation studies, an increase in HBsAg level was associated with a higher risk of retreatment after clinical relapse in patients with HBeAg-negative chronic HBV infection [46], whereas HBsAg decline was not significantly associated with retreatment in both patients with HBeAg-negative and HBeAg-positive chronic HBV infection [93]. There was no consistent association between HBsAg decline and changes in HBeAg and HBcrAg levels [45, 57, 85, 102], NA therapy (tenofovir disoproxil fumarate vs. entecavir) [82, 87], or age and gender [82, 85].
The relationship between the decline in HBsAg titres and other patient characteristics were reported in only one study each (Table S10); there was no association between HBsAg decline and treatment duration and consolidation duration [81], HBsAg seroconversion [104] or clinical relapse [91].
4 Discussion
This systematic literature review of epidemiological data on quantitative HBsAg levels in chronic HBV infection evaluated three objectives: the association between HBsAg titres and long-term disease outcomes, HBsAg titres in various disease phases and their cross-sectional relationship with clinical and treatment characteristics and change in HBsAg titres over time and their relationship with clinical and treatment characteristics.
In treatment-naive patients, data suggest quantitative HBsAg has a clinical utility in predicting the risk of cirrhosis and HCC, particularly in patients who are HBeAg-negative with low-intermediate viral load (≤20,000 IU/mL) over a long duration of follow-up [26, 29, 30, 39]. This could potentially contribute to decisions around treatment initiation to reduce this risk [25, 28, 30-35].
The difference between treatment-naïve and treated populations could also be attributed to reduced HBsAg levels following NA treatment [105], complicating assessment of between-group differences, though this has not been shown consistently. Additionally, NA treatment affects the risk of cirrhosis and HCC, complicating assessment of any association in this population [15]. Finally, all but one study with mixed or treatment-experienced patients were smaller than studies with treatment-naïve patients, therefore the lack of association shown could be due to study size. It is also important to note that all these studies were in Asia; further high-quality studies in broader populations and in treated patients are needed to assess the clinical utility of HBsAg quantification in predicting cirrhosis and HCC.
The role of HBsAg level in determining outcomes is likely dependent on when HBsAg is measured and the disease phase. In the HBeAg-positive chronic HBV (‘immune-active’) phase, there is an immune response to HBV and high HBV DNA, ALT and HBsAg levels [5, 106]. Immune activity and immune-mediated clearance of infected hepatocytes lead to a decrease in HBsAg levels [107]. Immune activity is also a main cause of liver inflammation and fibrosis [108]. A SONIC-B database analysis found a strong association between lower HBsAg levels and higher rates of fibrosis and cirrhosis in HBeAg-positive patients, indicating that in these patients HBsAg levels can predict a higher risk of liver disease [108]. These patients could be nearing the end of the HBeAg-positive chronic HBV phase, whereby the active immune response may have destroyed the infected HBsAg-producing hepatocytes [106, 109]. No relationship was observed in HBeAg-negative patients [108]. Another study showed HBsAg and HBeAg levels were negatively correlated with liver inflammation and cirrhosis in patients with chronic HBV infection in the HBeAg-positive chronic HBV (‘immunoclearance’) phase, highlighting the role of the immune system and HBV proteins in mediating liver damage [5, 110]. This might help explain why measuring HBsAg levels during initiation of NA therapy will not show a relationship. While a short and effective immune reaction could lead to immunoclearance of serum HBeAg, anti-HBeAg/anti-HBsAg seroconversion and HBsAg loss with complete recovery, a long and ineffective immune response could result in anti-HBeAg seroconversion with ongoing liver inflammation, resulting in cirrhosis and HCC. The reduction in HBsAg and HBeAg levels can therefore be linked to both liver disease progression and complete recovery; further studies are required to elucidate this relationship [110].
HBsAg titres were significantly associated with various virological markers; the most consistent significant association was with HBV DNA. Higher HBsAg levels were associated with higher HBV DNA levels across all disease phases, with a higher concordance in the early disease phases, reflecting the high levels of both markers in these initial phases. This association weakened in later phases, likely reflecting the disconnect between HBV DNA replication (viral load) and HBsAg production in these phases, possibly due to differences in the regulation of viral replication and HBsAg synthesis pathways, resulting in altered ratios of HBV virion to subviral HBsAg particles [51, 53, 63].
Higher HBsAg titres were also associated with higher levels of most other virological factors (HBcrAg, HBeAg, intrahepatic cccDNA, intrahepatic HBsAg expression and HBV RNA); however, evidence was limited. Higher HBsAg titres were associated with higher intrahepatic HBsAg expression in HBeAg-negative phases only, maybe because in HBeAg-negative patients, HBsAg synthesis occurs mainly via HBV cccDNA transcription and lower levels of serum HBsAg parallel lower intrahepatic HBsAg expression [58]. Higher HBsAg levels were generally associated with lower age across individual disease phases (moderate correlation), likely reflecting disease progression and the decrease in HBsAg levels over the disease course [51, 53, 69]. There was no clear association between HBsAg levels and other factors such as gender and fibrosis.
HBsAg titres generally declined over time; higher decline in HBsAg levels was consistently associated with subsequent HBsAg seroclearance, a key therapeutic endpoint [111], both in the overall population and the HBeAg-negative and HBeAg-positive patient subgroups. One study showed that HBsAg reduction of >0.166 log IU/mL/year was highly predictive and likely reflects the substantial restoration of host immune control that precedes HBsAg seroclearance [98]. HBsAg decline from the end of lamivudine treatment until 6 months post-treatment was also independently associated with HBsAg seroclearance [80]. Five studies found a greater decline in HBsAg titres was consistently associated with a greater decline in total intrahepatic HBV DNA and intrahepatic cccDNA levels [43, 45, 48, 78, 99]. In previous cross-sectional studies, relatively lower reductions in intrahepatic HBV DNA paralleled similar lower reductions in serum HBsAg levels during seroconversion, as compared with the far higher decline in serum HBV DNA levels [112]. Decline in HBsAg levels was strongly associated with HBeAg levels in patients with HBeAg seroconversion but not in those without [102].
The correlation of HBsAg levels/decline with demographic factors, virological markers and treatment-related factors, shows that quantitative HBsAg could be a valuable tool in the clinical assessment and management of chronic HBV infection. It can detect changes in HBsAg which can identify patients who might have inactive chronic HBV infection and the possibility of HBsAg seroclearance [5]. Additionally, quantitative HBsAg might help predict response to interventions such as NA cessation, pegylated interferon and novel therapies targeting RNA interference and may therefore provide a useful marker of the need for additional monitoring or treatment decisions in clinical practice [46].
This literature review has several limitations in addition to those already mentioned. Most studies (>90%) were conducted in Asian countries (primarily Taiwan, China and Japan); regional differences in HBV genotype mean the applicability of these results to other populations is unknown. No studies identified an association between HBsAg titres and mortality. Although these data are promising on the value of quantitative HBsAg, this is often not routinely measured in clinical practice and data on certain markers are limited; therefore, more studies with larger cohorts are also needed to better understand the value of quantitative HBsAg measurement as part of clinical assessment, management and treatment of chronic HBV infection.
In conclusion, this systematic literature review showed that HBsAg levels and rates of decline could potentially have value in informing the assessment, management and prediction of various outcomes in chronic HBV infection; although more data is needed in broader populations including more diverse race and ethnicity and for treated patients.
Author Contributions
The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors, take responsibility for the integrity of the work as a whole, contributed to the writing and reviewing of the manuscript, and have given final approval for the version to be published. Saifuddin Kharawala, Anadi Mahajan, Stuart Kendrick and Vera Gielen contributed to the conception and design of the study and the acquisition of data. Supriya Desai contributed to the acquisition of data. All authors contributed to data analysis and interpretation.
Acknowledgements
Embase is a trademark owned by or licensed to Elsevier BV and MEDLINE is a trademark owned by or licensed to Medline Industries, LP. Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors' comments for each draft, assembling tables and figures, grammatical editing and referencing) was provided by Sheekha Amin, MPharmacol, of Fishawack Indicia Ltd, UK, part of Avalere Health, and was funded by GSK.
Conflicts of Interest
S. Kendrick, V. Gielen and J. Das are employees of GSK and hold stock/shares in the company. S. Kharawala, A. Mahajan and S. Desai are employees of Bridge Medical Consulting Ltd, which received funding from GSK to conduct the literature review.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




