Statin use and 30-day mortality in patients with acute symptomatic pulmonary embolism
Manuscript handled by: Zhi-Cheng Jing
Final decision: Zhi-Cheng Jing, 18 April 2022
Abstract
Background
Statins possess antithrombotic and profibrinolytic properties. The association between statin use and short-term outcomes in patients with acute pulmonary embolism (PE) remains unknown.
Methods
We used the data from the Registro Informatizado de Pacientes con Enfermedad TromboEmbólica registry to compare the 30-day all-cause mortality in patients with acute PE according to the use of statins. Secondary outcome was fatal PE. We used cancer-related mortality as a falsification endpoint.
Results
From January 2009 to April 2021, 31 169 patients with PE were recruited. Of these, 5520 (18%) were using statins at baseline: low intensity: 829, moderate: 3636, high intensity: 1055. Statin users were older and had a higher frequency of diabetes, hypertension, or atherosclerotic disease than non-users (P <0.001 for all comparisons). During the first 30 days, 1475 patients died (fatal PE, 255). On multivariable analysis, statin users had a lower risk of all-cause death (odds ratio [OR]: 0.65; 95% confidence interval [CI]: 0.56–0.76) and fatal PE (OR: 0.42; 95% CI: 0.28–0.62) than non-users. The risk for death was lower in patients using either low- (OR: 0.51; 95% CI: 0.34–0.77), moderate- (OR: 0.68; 95% CI: 0.57–0.81), or high-intensity statins (OR: 0.68; 95% CI: 0.51–0.92). Results did not change in mixed effects logistic regression models with hospitals as a random effect. Statins were not associated with a significant chance in cancer mortality (falsification endpoint).
Conclusions
PE patients using statins at baseline had a significantly lower risk of dying within the first 30 days than non-users. Randomized trials are needed to confirm these data.
Essentials
- Statins possess antithrombotic and profibrinolytic properties.
- We used the data from the Registro Informatizado de Pacientes con Enfermedad TromboEmbólica registry to compare the 30-day all-cause mortality in patients with acute pulmonary embolism (PE) according to the use of statins.
- PE patients using statins at baseline had a significantly lower risk of dying within the first 30 days than non-users.
- Randomized trials are needed to confirm these data.
1 INTRODUCTION
The influence of statins on survival in patients with coronary, cerebrovascular, or peripheral artery disease has been largely reported.1-5 In addition to lowering the low-density lipoprotein cholesterol levels, statins have pleiotropic effects, including anti-inflammatory, antithrombotic, and profibrinolytic properties. Statins reduce tissue factor expression and thrombin generation, and attenuate fibrinogen cleavage.6-12
These factors make it plausible that statins may help in the treatment of patients with venous thromboembolism (VTE). In patients with VTE, some authors have found that statin use was associated with a decreased risk of VTE recurrences.13, 14 Moreover, in a population-based registry of patients with acute pulmonary embolism (PE), those using statins during follow-up (median, 199 days) had half the risk of dying than non-users.15 In two studies using the Registro Informatizado de Pacientes con Enfermedad TromboEmbólica (RIETE) registry, we also found that statin users at baseline had a significant decrease in all-cause mortality during follow-up (median, 177 and 192 days, respectively) compared to non-statin users.16, 17 Whether such an association is driven by short-term mortality in patients with acute symptomatic PE has not been thoroughly investigated, in part due to small sample size in prior cohorts.
We used the data from RIETE, a large multicenter, ongoing, international registry of patients with objectively confirmed, acute VTE (ClinicalTrials.gov identifier: NCT02832245). In the current analysis, we aimed to evaluate the association between statin therapy and short-term mortality, compared to no statin therapy. To account for biological plausibility of the potential findings, we decided for the current study to: (1) only include patients with acute symptomatic PE (we excluded patients with isolated deep vein thromboses [DVT]), (2) choose 30-day all-cause mortality as the primary outcome, (3) assess the strength of associations across the strata of intensities of statin therapy on mortality, (4) perform the analyses in multiple ways (multivariable, sensitivity, and propensity-score–matched analysis), and (5) use falsification endpoints to reduce the risk from findings being driven by residual confounding.
2 METHODS
2.1 Data source
Details about the methodology of RIETE have been discussed elsewhere. Briefly, RIETE is a multicenter prospective registry of consecutive patients with objectively confirmed acute DVT or PE with 205 collaborating centers from 27 countries.18 The protocol for patient enrollment was approved by the ethics committees at the participating sites and all patients (or their health-care proxies) provided informed consent. Consecutive patients with acute PE confirmed by objective tests (helical computed tomography [CT] scan of the chest, ventilation-perfusion lung scintigraphy, or angiography) were considered. Patients were excluded if they were currently participating in a blinded/double-blind therapeutic clinical trial. All patients (or their relatives) provided informed consent for participation in the registry, in accordance with local ethics committee requirements. Patients were not involved in the design of the current study, or review of the submitted manuscript.
The following parameters were recorded in RIETE: patient’s baseline characteristics; clinical status including chronic heart or lung disease, recent major bleeding, anemia, or renal insufficiency; risk factors for VTE; the treatment received upon VTE diagnosis; concomitant drugs; and the outcomes during the course of therapy. Immobilized patients were defined as non-surgical patients who had been immobilized (i.e., total bed rest with or without bathroom privileges) for ≥4 days in the 2-month period prior to VTE diagnosis. Surgical patients were defined as those who had undergone an operation in the 2 months prior to VTE. Active cancer was defined as newly diagnosed cancer (<3 months before) or when receiving anti-neoplastic treatment of any type (i.e., surgery, chemotherapy, radiotherapy, hormonal, support therapy, or combined therapies). Recent bleeding was defined as major bleeding <30 days prior to VTE. Anemia was defined as hemoglobin levels <13 g/dl for men and <12 g/dl for women. Creatinine clearance (CrCl) levels were measured according to the Cockcroft & Gault formula.
2.2 Study design
For this study, only patients with acute symptomatic PE and available information on the use of statins at baseline were considered. Thus, we included all patients with acute PE recruited from January 2009 to April 2021. The prescribed statins and doses have been entered by the treating clinicians. Patients were categorized as being on no statins or on low-intensity, moderate-intensity, or high-intensity statin therapy as defined by the 2013 American College of Cardiology/American Heart Association Guidelines (Table S1 in supporting information). The major study outcome was 30-day mortality. Secondary outcome was fatal PE. Fatal PE, in the absence of autopsy, was defined as any death appearing <10 days after PE diagnosis, in the absence of any alternative cause of death. Bleeding events were classified as major if they were overt and required a transfusion of two units of blood or more, or were retroperitoneal, spinal, or intracranial. Fatal bleeding was defined as any death occurring <10 days after a major bleeding episode, in the absence of any alternative cause of death.
2.3 Treatment and follow-up
Patients were managed according to the clinical practice of each participating hospital (i.e., there was no standardization of treatment). The type, dose, and duration of anticoagulant therapy were recorded. After PE diagnosis, all patients were followed-up in the outpatient clinic for at least 3 months. During each visit, any signs or symptoms suggesting VTE recurrences or bleeding complications were noted. Each episode of clinically suspected recurrent VTE was investigated by repeat compression ultrasonography, lung scanning, helical CT scan, or pulmonary angiography as appropriate. Most outcomes were classified as reported by the clinical centers. However, if staff at the coordinating center were uncertain how to classify a reported outcome, the event was reviewed by a central adjudicating committee (less than 10% of events).
2.4 Statistical analysis
Categorical variables were compared using the Chi-square test (two-sided) and Fisher’s exact test (two-sided). Continuous variables with normal distribution were compared using Student’s t-test. Thirty-day mortality rates were compared between patients using versus those not using statins, using odds ratio (OR) as the effect measure, along with and the corresponding 95% confidence interval (CI). We compared the mortality rates between statin users and non-users, and also according to the different intensities of statins. For multivariable analysis, covariates included in the adjusted model were those considered clinically significant or those for which a significant difference (a threshold P-value of <0.1 was set to assess significance of differences) was found. Additionally, a mixed logistic regression model was used to consider the possible cluster effect of participating hospitals. Subgroup analyses were performed by systolic blood pressure levels <90 mm, active cancer, and thrombolytic therapy. Interactions were tested by multivariable mixed logistic regression models. Cancer-related death was considered a falsification endpoint. Statistical analyses were conducted with SPSS for Windows Release (version 20, SPSS Inc.) and Stata 17.0 (Stata Corp.).
3 RESULTS
From January 2009 to April 2021, 31 169 patients with acute symptomatic PE were recruited. Of these, 5520 (18%) were using statins: at low intensity: 829, intermediate intensity: 3636, at high intensity: 1055 (Figure 1). Compared to non-users, statin users were 10 years older and more likely to have hypertension, diabetes, chronic heart failure, prior myocardial infarction, prior stroke, peripheral artery disease, or CrCl levels <60 mL/min (Table 1). They also were more likely to be using antiplatelet drugs and had lower serum levels of total- and low density lipoprotein (LDL) cholesterol than non-users. Compared to patients using low-intensity statins, those on high-intensity statins were the same age but more likely to have chronic heart failure, diabetes, prior myocardial infarction, stroke, peripheral artery disease, or to be using antiplatelet drugs than those using low-intensity statins, and had the lowest levels of total- and LDL cholesterol in serum (Table 1).
| Statin therapy (N =5520) | No statins | |||
|---|---|---|---|---|
| Low intensity | Moderate intensity | High intensity | ||
| Patients, N | 829 | 3636 | 1055 | 25,649 |
| Clinical characteristics | ||||
| Male gender | 348 (42%) | 1688 (46%) | 600 (57%) | 12,316 (48%) |
| Age (mean years±SD) | 75±11 | 74±11 | 74±11 | 64±18 |
| Body mass index (mean±SD) | 29±5.3 | 29±5.5 | 29±5.8 | 28±5.9 |
| Risk factors for VTE | ||||
| Recent surgery | 67 (8.1%) | 372 (10%) | 119 (11%) | 2738 (11%) |
| Recent immobility ≥4 days | 173 (21%) | 869 (24%) | 299 (28%) | 5320 (21%) |
| Active cancer | 122 (15%) | 581 (16%) | 147 (14%) | 4337 (17%) |
| Pregnancy or postpartum | 1 (0.1%) | 2 (0.1%) | 1 (0.1%) | 272 (1.1%) |
| Estrogen use | 31 (3.9%) | 73 (2.1%) | 18 (1.7%) | 1906 (7.7%) |
| None of the above (unprovoked) | 489 (59%) | 1977 (54%) | 534 (51%) | 13,141 (51%) |
| Prior VTE | 135 (16%) | 475 (13%) | 140 (13%) | 3572 (14%) |
| Underlying disorders | ||||
| Chronic lung disease | 128 (15%) | 665 (18%) | 218 (21%) | 3327 (13%) |
| Chronic heart failure | 74 (8.9%) | 509 (14%) | 209 (20%) | 1786 (7.0%) |
| Recent major bleeding | 16 (1.9%) | 93 (2.6%) | 35 (3.3%) | 633 (2.5%) |
| Arterial hypertension (N = 29,730) | 615 (75%) | 2740 (76%) | 840 (80%) | 10,619 (43%) |
| Diabetes (N = 29,730) | 211 (26%) | 1119 (31%) | 401 (38%) | 2835 (12%) |
| Current smoking (N = 29,730) | 43 (5.3%) | 338 (9.6%) | 137 (13%) | 3582 (15%) |
| Prior artery disease (N = 29,730) | 151 (18%) | 999 (28%) | 615 (59%) | 2540 (11%) |
| Prior myocardial infarction | 67 (8.2%) | 540 (15%) | 392 (37%) | 950 (3.9%) |
| Prior ischemic stroke | 63 (7.7%) | 390 (11%) | 246 (24%) | 1290 (5.3%) |
| Peripheral artery disease | 37 (4.5%) | 235 (6.6%) | 140 (14%) | 622 (2.6%) |
| Laboratory tests | ||||
| Anemia | 285 (34%) | 1246 (34%) | 381 (36%) | 8206 (32%) |
| Platelet count <100,000/µl | 26 (3.1%) | 73 (2.0%) | 26 (2.5%) | 610 (2.4%) |
| CrCl levels 30–60 ml/min | 334 (40%) | 1256 (35%) | 354 (34%) | 6219 (24%) |
| CrCl levels <30 ml/min | 41 (4.9%) | 242 (6.7%) | 82 (7.8%) | 1212 (4.7%) |
| Total cholesterol (mg/dl) | 172±39 | 167±42 | 154±43 | 179±64 |
| LDL-cholesterol (mg/dl) | 101±37 | 100±88 | 88±37 | 122±51 |
| Concomitant drugs (N = 28,332) | ||||
| Antiplatelets | 245 (32%) | 1308 (38%) | 640 (64%) | 3076 (13%) |
| Corticosteroids | 78 (10%) | 456 (14%) | 116 (12%) | 2365 (10%) |
- Abbreviations: CrCl, creatinine clearance; SD, standard deviation; VTE, venous thromboembolism.
The proportion of patients initially presenting with PE and hypotension (systolic blood pressure levels <90 mm Hg) was similar in statin users and non-users (Table 2). Statin users were slightly less likely to initially present with tachycardia, but more likely to have hypoxemia or raised troponin levels than non-users. The proportion of patients presenting with abnormalities on the echocardiogram (right ventricle dysfunction, raised pulmonary artery pressure levels or low tricuspid annular plane systolic excursion) was similar, but statin users scored worse on PESI (pulmonary embolism severity score) and simplified PESI than non-users. Most patients in all subgroups were initially treated with low molecular weight heparin (LMWH). Then, the proportion of patients receiving vitamin K antagonists, LMWH, or direct oral anticoagulants was similar in all subgroups (Table 2).
| Statin therapy | No statins | |||
|---|---|---|---|---|
| Low intensity | Moderate intensity | High intensity | ||
| Patients, N | 829 | 3636 | 1055 | 25,649 |
| Signs | ||||
| SBP levels (mean mm Hg±SD) | 135 ± 24 | 131 ± 24 | 130 ± 24 | 129 ±23 |
| SBP levels <90 mm Hg | 24 (2.9%) | 137 (3.8%) | 31 (2.9%) | 786 (3.1%) |
| Heart rate ≥110 bpm | 131 (16%) | 655 (18%) | 190 (18%) | 4997 (20%) |
| Laboratory data | ||||
| Sat O2 levels <90% (N = 15,264) | 121 (29%) | 581 (31%) | 187 (35%) | 3361 (27%) |
| Raised troponin levels (N = 17,210) | 219 (47%) | 1078 (48%) | 322 (49%) | 5387 (39%) |
| Echocardiogram | ||||
| RV dysfunction (N = 12,691) | 82 (22%) | 345 (22%) | 100 (21%) | 2235 (22%) |
| PAP levels >45 mm Hg (N = 7982) | 86 (37%) | 420 (42%) | 116 (41%) | 2642 (41%) |
| TAPSE <16 mm (N = 7453) | 34 (15%) | 180 (18%) | 60 (20%) | 1045 (18%) |
| Burden of PE on CT scan | ||||
| Subsegmental arteries | 36 (4.3%) | 141 (3.9%) | 57 (5.4%) | 1074 (4.2%) |
| Segmental arteries | 169 (20%) | 773 (21%) | 274 (26%) | 4608 (18%) |
| Lobar arteries | 225 (27%) | 937 (26%) | 280 (27%) | 5647 (22%) |
| Principal or central arteries | 314 (38%) | 1338 (37%) | 337 (32%) | 7481 (29%) |
| No available information | 85 (10%) | 447 (12%) | 107 (10%) | 6839 (27%) |
| Prognostic scores | ||||
| PESI >85 points | 536 (65%) | 2419 (67%) | 723 (69%) | 13,699 (53%) |
| sPESI >0 points | 579 (70%) | 2546 (70%) | 771 (73%) | 15,971 (62%) |
| Initial therapy | ||||
| Low molecular weight heparin | 738 (89%) | 3043 (84%) | 894 (85%) | 21,112 (82%) |
| Unfractionated heparin | 33 (4.0%) | 200 (5.5%) | 66 (6.3%) | 1739 (6.8%) |
| Direct oral anticoagulants | 15 (1.8%) | 139 (3.8%) | 36 (3.4%) | 1069 (4.2%) |
| Thrombolytic drugs | 11 (1.3%) | 102 (2.8%) | 16 (1.5%) | 672 (2.6%) |
| Inferior vena cava filter | 19 (2.3%) | 107 (2.9%) | 36 (3.4%) | 705 (2.7%) |
| Pulmonary embolectomy | 4 (0.5%) | 31 (0.8%) | 10 (0.9%) | 255 (0.1%) |
| Long-term therapy | ||||
| Vitamin K antagonists | 485 (59%) | 1826 (50%) | 521 (49%) | 13,289 (52%) |
| Low-molecular-weight heparin | 208 (25%) | 1036 (28%) | 297 (28%) | 6785 (26%) |
| Direct oral anticoagulants | 109 (13%) | 621 (17%) | 182 (17%) | 4308 (17%) |
- Abbreviations: bpm, beats per minute; CT, computed tomography; PAP, pulmonary artery pressure; PE, pulmonary embolism; PESI, Pulmonary Embolism Severity Index; RV, right ventricle; SBP, systolic blood pressure; sPESI, simplified Pulmonary Embolism Severity Index; TAPSE, tricuspid annular plane systolic excursion.
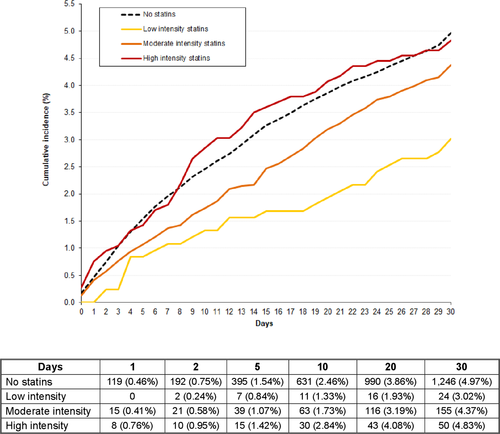
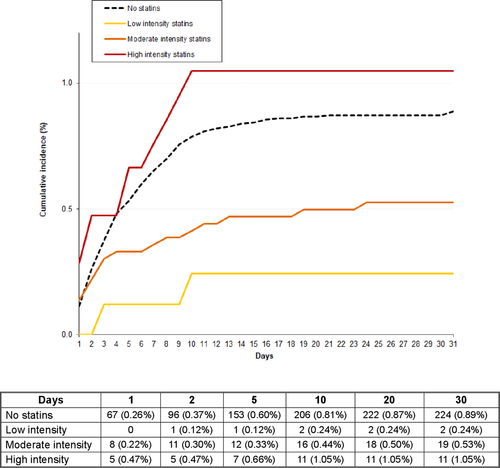
During the first 30 days, 198 patients (0.64%) developed VTE recurrences (recurrent PE: 123, DVT: 75), 544 (1.75%) suffered major bleeding (gastrointestinal: 139, haematoma: 149, intracranial: 75), and 1475 (4.73%) died (fatal PE: 255, fatal bleeding: 75; Table 3). The all-cause mortality rate was slightly lower in statin users than in non-users: 4.15% versus 4.86% (OR: 0.85; 95% CI: 0.73–0.98). The fatal PE rate was: 0.40% versus 0.87%, respectively (OR: 0.45; 95% CI: 0.29–0.69). The all-cause mortality rate according to the intensity of statin therapy was low intensity: 2.90%, moderate intensity: 4.26%, high intensity: 4.74%. Fatal PE rates were: 0.24%, 0.52%, and 1.04%, respectively. Compared to non-users, patients receiving low-intensity statins had a lower all-cause mortality rate (OR: 0.59; 95% CI: 0.39–0.88), those on moderate intensity had a non-significantly lower mortality rate (OR: 0.87; 95% CI: 0.74–1.03), and those on high intensity had a similar rate (OR: 0.98; 95% CI: 0.74–1.30) of all-cause mortality. The differences in all-cause mortality (OR: 0.32; 95% CI: 0.05–1.07) and in fatal PE rates (OR: 0.32; 95% CI: 0.02–1.62) between patients using low-intensity statins and non-users were highest during the first 48 hours (Figures 1 and 2).
| Statin therapy | No statins | |||
|---|---|---|---|---|
| Low intensity | Moderate intensity | High intensity | ||
| Patients, N | 829 | 3636 | 1055 | 25,649 |
| Recurrent DVT | 2 (0.2%) | 13 (0.4%) | 2 (0.2%) | 58 (0.2%) |
| Recurrent PE | 2 (0.2%) | 14 (0.4%) | 2 (0.2%) | 105 (0.4%) |
| Recurrent VTE | 4 (0.5%) | 27 (0.7%) | 4 (0.4%) | 163 (0.6%) |
| Major bleeding | 15 (1.8%) | 79 (2.2%)* | 24 (2.3%) | 426 (1.7%) |
| Sites of bleeding | ||||
| Gastrointestinal | 0 | 22 (0.6%) | 6 (0.6%) | 111 (0.4%) |
| Hematoma | 6 (0.7%) | 24 (0.7%) | 10 (0.9%) | 109 (0.4%) |
| Intracranial | 3 (0.4%) | 11 (0.3%) | 2 (0.2%) | 59 (0.2%) |
| Retroperitoneal | 2 (0.2%) | 5 (0.1%) | 1 (0.1%) | 49 (0.2%) |
| Hematuria | 2 (0.2%) | 8 (0.2%)* | 2 (0.2%) | 22 (0.1%) |
| Hemoptysis | 0 | 3 (0.1%) | 1 (0.1%) | 22 (0.1%) |
| Myocardial infarction | 1 (0.1%) | 8 (0.2%) | 1 (0.1%) | 40 (0.2%) |
| Ischemic stroke | 0 | 2 (0.1%) | 2 (0.2%) | 10 (0.04%) |
| Death | 24 (2.9%)* | 155 (4.3%) | 50 (4.7%) | 1246 (4.9%) |
| Causes of death | ||||
| Pulmonary embolism | 2 (0.2%) | 19 (0.5%)* | 11 (1.0%) | 224 (0.9%) |
| Bleeding | 2 (0.2%) | 7 (0.2%) | 1 (0.1%) | 65 (0.2%) |
| Respiratory failure | 1 (0.1%) | 22 (0.6%) | 8 (0.8%) | 166 (0.6%) |
| Sudden, unexpected | 0 | 7 (0.2%) | 1 (0.1%) | 36 (0.1%) |
| Disseminated malignancy | 4 (0.5%) | 39 (1.1%) | 9 (0.9%) | 301 (1.2%) |
| Multiorganic failure | 3 (0.4%) | 16 (0.4%) | 5 (0.5%) | 120 (0.5%) |
| Infection | 7 (0.8%)* | 13 (0.4%) | 5 (0.5%) | 77 (0.3%) |
| Heart failure | 1 (0.1%) | 9 (0.2%) | 2 (0.2%) | 54 (0.2%) |
| Bronchoaspiration | 1 (0.1%) | 3 (0.1%) | 0 | 36 (0.1%) |
- Abbreviations: DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
- Note: Comparisons between statin users (every subgroup) vs. non-users: *P <0.05; †P <0.01.
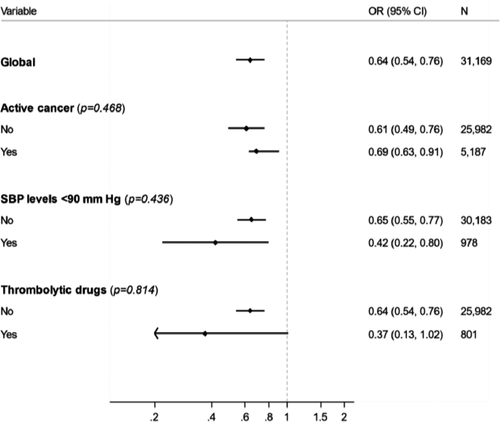
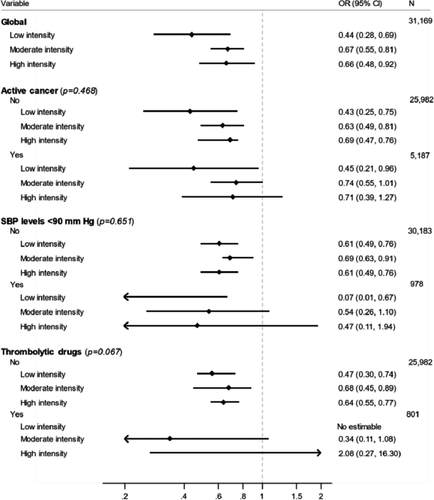
On multivariable analysis, statin users had a significantly lower risk for all-cause death (OR: 0.65; 95% CI: 0.56–0.76) and for fatal PE (OR: 0.42; 95% CI: 0.28–0.62; Table 4). We repeated the multivariable analyses after replacing statin use (yes/no) for the three intensities of statin therapy. Compared to non-users, the risk for all-cause death was significantly lower in patients using low- (OR: 0.51; 95% CI: 0.34–0.77), moderate- (OR: 0.68; 95% CI: 0.57–0.81), or high-intensity statins (OR: 0.68; 95% CI: 0.51–0.92). As for fatal PE, only patients using low- (OR: 0.22; 95% CI: 0.06–0.90) or moderate-intensity (OR: 0.39; 95% CI: 0.24–0.63) statins were at a lower risk than non-users.
| All-cause death | PE-related death | |||
|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |
| Demographics | ||||
| Male sex | 1.03 (0.93–1.14) | – | 1.05 (0.82–1.34) | – |
| Age (per year) | 1.04 (1.03–1.04) | 1.03 (1.03–1.04) | 1.03 (1.02–1.04) | 1.02 (1.01–1.03) |
| Body weight (per kg) | 0.97 (0.97–0.98) | 0.99 (0.99–0.99) | 0.99 (0.98–1.00) | 1.00 (0.99–1.01) |
| Initial PE presentation | ||||
| Heart rate ≥110 bpm | 1.94 (1.73–2.16) | 1.66 (1.48–1.86) | 2.83 (2.20–3.63) | 2.11 (1.62–2.74) |
| SBP levels <90 mm Hg | 3.60 (3.03–4.28) | 2.17 (1.81–2.59) | 8.71 (6.45–11.8) | 5.08 (3.70–6.98) |
| Sat O2 levels <90% | 1.79 (1.58–2.03) | 1.21 (1.06–1.38) | 2.18 (1.64–2.89) | 1.30 (0.96–1.74) |
| Risk factors | ||||
| Unprovoked | Ref. | Ref. | Ref. | Ref. |
| Active cancer | 6.54 (5.72–7.46) | 4.76 (4.14–5.48) | 3.33 (2.41–4.59) | 2.49 (1.77–3.51) |
| Transient risk factors | 2.52 (2.19–2.89) | 2.12 (1.83–2.44) | 2.48 (1.84–3.34) | 1.97 (1.45–2.68) |
| Prior VTE | 0.59 (0.50–0.71) | 0.70 (0.59–0.84)‡ | 0.47 (0.29–0.75) | 0.55 (0.34–0.89)* |
| Concomitant diseases | ||||
| Recent major bleeding | 2.33 (1.86–2.92) | 1.53 (1.21–1.92) | 2.66 (1.60–4.41) | 1.81 (1.08–3.03) |
| Chronic heart failure | 2.22 (1.94–2.55) | 1.34 (1.16–1.56) | 1.78 (1.24–2.54) | 0.91 (0.62–1.33) |
| Chronic lung disease | 1.72 (1.52–1.95) | 1.18 (1.03–1.34) | 2.12 (1.60–2.81) | 1.57 (1.16–2.12) |
| Arterial hypertension | 1.29 (1.16–1.43) | 0.84 (0.75–0.94) | 1.14 (0.89–1.46) | – |
| Diabetes | 1.52 (1.34–1.72) | 1.18 (1.04–1.35) | 1.91 (1.44–2.54) | 1.51 (1.12–2.04) |
| Current smoking | 0.73 (0.61–0.86) | 1.10 (0.92–1.32) | 0.87 (0.60–1.28) | – |
| Prior artery disease | 1.87 (1.65–2.11) | 1.34 (1.17–1.55) | 2.46 (1.87–3.23) | 2.09 (1.52–2.87) |
| Anemia | 2.64 (2.38–2.93) | 1.45 (1.30–1.62) | 2.00 (1.57–2.56) | 1.20 (0.92–1.56) |
| Platelet count <100,000/µl | 4.49 (3.76–5.36) | 2.65 (2.21–3.18) | 4.23 (2.76–6.50) | 2.54 (1.62–3.96) |
| CrCl levels >60 ml/min | Ref. | Ref. | Ref. | Ref. |
| CrCl levels 30–60 ml/min | 2.18 (1.95–2.44) | 1.25 (1.10–1.43) | 2.23 (1.71–2.93) | 1.67 (1.21–2.30) |
| CrCl levels <30 ml/min | 4.56 (3.91–5.31) | 1.90 (1.58–2.28) | 5.33 (3.77–7.55) | 2.75 (1.80–4.22) |
| Concomitant therapies | ||||
| Antiplatelets | 1.55 (1.37–1.75) | 1.01 (0.88–1.17) | 1.51 (1.13–2.02) | 0.87 (0.62–1.22) |
| Corticosteroids | 2.78 (2.46–3.14) | 1.68 (1.48–1.91) | 2.29 (1.68–3.13) | 1.40 (1.01–1.94) |
| Statin use | ||||
| No statins | Ref. | Ref. | Ref. | Ref. |
| Statins | 0.85 (0.74–0.98) | 0.65 (0.56–0.76) | 0.66 (0.46–0.96) | 0.42 (0.28–0.62) |
| Low intensity | 0.59 (0.39–0.88) | 0.51 (0.34–0.77) | 0.27 (0.07–1.11) | 0.22 (0.06–0.90) |
| Moderate intensity | 0.87 (0.74–1.03) | 0.68 (0.57–0.81) | 0.60 (0.37–0.95) | 0.39 (0.24–0.63) |
| High intensity | 0.98 (0.74–1.30) | 0.68 (0.51–0.92) | 1.20 (0.65–2.19) | 0.65 (0.34–1.22) |
| Statin use adjusting by center, mixed models | ||||
| No statins | Ref. | Ref. | Ref. | Ref. |
| Statins | 0.86 (0.74–0.99) | 0.64 (0.54–0.76) | 0.87 (0.69–1.10) | 0.61 (0.47–0.88) |
| Low intensity | 0.60 (0.39–0.88) | 0.44 (0.28–0.69) | 0.25 (0.09–0.68) | 0.18 (0.06–0.49) |
| Moderate intensity | 0.88 (0.74–1.05) | 0.67 (0.55–0.81) | 0.90 (0.68–1.18) | 0.66 (0.49–0.88) |
| High intensity | 0.98 (0.72–1.32) | 0.66 (0.48–0.92) | 1.26 (0.83–1.91) | 0.78 (0.50–1.23) |
- Abbreviations: bpm, beats per minute; CrCl, creatinine clearance; PE, pulmonary embolism; Ref., reference; SBP, systolic blood pressure.
Adjustment used a mixed effects logistic regression model to take into account the possible random effect of the hospitals; the previous results did not have a substantive change (Table 4). Further, there was no association between the use of statins and cancer-related mortality (OR: 0.87; 95% CI: 0.65–1.22).
In the subgroup analyses, we did not find differences in the estimates of the effect of statins in subgroups according to systolic blood pressure levels, active cancer, or use of thrombolytic therapy. All interactions were statistically non-significant (Figures 3 and 4).
4 DISCUSSION
Our data, obtained from a large series of consecutive patients with acute symptomatic PE, confirm that statin users were 10 years older and more than 3-fold more likely to have arterial hypertension, diabetes, prior artery disease, or to be using antiplatelet drugs than non-users. The severity of the PE (according to PESI or simplified PESI) scored worse in statin users, and their treatments were similar. However, the 30-day rates of all-cause death (crude OR: 0.85; 95% CI: 0.73–0.98) or fatal PE (crude OR: 0.45; 95% CI: 0.29–0.69) were significantly lower in statin users than in non-users. Compared to non-users, the older age, the higher proportion of patients with comorbidities, the worse severity of the initial PE event, and the similar therapy were common in all three subgroups of patients using different statin intensities. Multivariable adjusted analyses confirmed that the lower risk for all-cause death or fatal PE persisted after adjusting for several potential confounders. The lower risk for all-cause death consistently appeared in patients using low- (51%), moderate- (68%), or high-(68%) intensity statins. The 33% reduction in the adjusted risk for 30-day mortality in our study was consistent with the 47% reduction found in a Dutch study in which PE patients were followed-up for a median of 199 days,19 and our two prior studies in VTE patients followed-up for more than 100 days.16, 17 Of note, although we noted a significant reduction in PE-related mortality, there was no significant change in the falsification endpoint, that is, cancer-related mortality.
This is the third study on the association between statin use and mortality in patients with VTE using the RIETE registry. The first study included 32 062 patients with a first episode of VTE (either DVT or PE) followed-up for a median of 177 days.16 During the course of anticoagulant therapy, statin users and non-users had a similar risk for VTE recurrences or major bleeding, but users had a lower risk for death (HR: 0.62; 95% CI: 0.48–0.79). The second study validated our prior findings (HR: 0.68; 95% CI: 0.59–0.79) in a subsequent cohort of 19 557 VTE patients followed-up for a median of 192 days.17 In the current study, we focused only on patients with acute PE, and limited follow-up to the first 30 days because in RIETE we do not gather information on how many patients using statins at baseline kept receiving statins after discharge. Again, statin users had a significantly lower risk to die compared to non-users. These data are fascinating but should be taken with caution. They must be confirmed in specifically designed randomized trials before considering statins an additional therapy for patients with acute PE.
The exact mechanism underlying any protective effects of statins on the risk for death in patients with acute PE is unknown. Statins inhibit the enzyme 3-hydroxy-3-methylglutaril coenzyme and decrease serum levels of cholesterol. In addition to these effects, statins have pleiotropic mechanisms such as platelet inhibition, reduction of inflammation and C-reactive protein, increased production of nitric oxide, or down-regulation of the coagulation cascade.7, 20-24 Other authors have suggested that the beneficial effect of statins beside the cholesterol-lowering effect may be due to the fact that persons who receive treatment with statins have a healthier lifestyle and/or a lower risk profile.25, 26
Data from RIETE reflect routine, unmonitored medical practice involving a broad spectrum of patients with VTE. It can, therefore, provide insights into the natural history of VTE and to be hypothesis generating. However, it has several limitations that need to be addressed. First, this study is an observational study with no randomization to statin versus no statin users. Thus, some non-measured variables, such as the reason for receiving statins, or patient preference or socioeconomic factors impacting the use of statins have not been considered. In this study, we performed extensive adjustment for several variables that may have impacted the use of statins, and the results remained substantively similar. Nevertheless, residual confounding may remain, as certain potential confounding variables may have not been available or may have not had the desired level of granularity. Third, in RIETE we only gather information on the use of statins at baseline, and have no information on adherence afterward. However, the length of follow-up was only 30 days; differences in mortality started to appear during the first few days and anticoagulant therapy is not a contraindication to statin use. Our falsification endpoint analysis did not indicate an association between statin use and short-term cancer-related mortality. This observation, although not conclusive, makes the possibility of residual confounding as the only explanation of our findings less likely. Fourth, uncontrolled healthy-user or healthy-adherer bias cannot be excluded either. However, statin users were disadvantaged for mortality considering that they were, on average, 10 years older and were more likely to have severe comorbidities than non-users. Finally, we failed to find differences among patients receiving different intensities of statin therapy, maybe because patients receiving higher doses could be sicker at baseline.
In conclusion, our findings confirm that PE patients using statins at baseline are at a lower risk for all-cause death and for fatal PE within the first 30 days. This decrease was independent of different intensities of statins. Intervention studies specifically designed to confirm our findings are warranted.
INFORMED CONSENT
On behalf of all the authors of the publication “Statin use and 30-day mortality in patients with acute symptomatic pulmonary embolism,” I declare that all the authors have read, critically reviewed the paper, and agree to its publication.
ACKNOWLEDGMENTS
We express our gratitude to Sanofi Spain, LEO PHARMA, and ROVI for supporting this Registry with an unrestricted educational grant. We also thank the RIETE Registry Coordinating Center, S&H Medical Science Service, for their quality control data, logistic, and administrative support. We thank Salvador Ortiz, Universidad Autónoma Madrid and Silvia Galindo, both Statistical Advisors in S&H Medical Science Service, for the statistical analysis of the data presented in this paper.
CONFLICTS OF INTEREST
Dr. Carmine Siniscalchi received a speaker’s fee for congress presentation by MediK. Dr. Behnood Bikdeli reports that he is a consulting expert, on behalf of the plaintiff, for litigation related to two specific brand models of IVC filters. The other authors disclose no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Carmine Siniscalchi and Manuel Monreal conceived and designed the study. Carmine Siniscalchi, Alfonso Muriel, Behnood Bikdeli, José María Surinach, David Jimenez, Josè Luis Lobo, Cristina Amado, Aida Gil-Diaz, Egidio Imbalzano, and Manuel Monreal either performed and/or interpreted the tests and data. All authors revised the manuscript critically for important intellectual content and gave final approval of the manuscript submitted.
APPENDIX
Coordinator of the RIETE Registry: Manuel Monreal.
RIETE Steering Committee Members: Paolo Prandoni, Benjamin Brenner, and Dominique Farge-Bancel.
RIETE National Coordinators: Raquel Barba (Spain), Pierpaolo Di Micco (Italy), Laurent Bertoletti (France), Sebastian Schellong (Germany), Inna Tzoran (Israel), Abilio Reis (Portugal), Marijan Bosevski (R. Macedonia), Henri Bounameaux (Switzerland), Radovan Malý (Czech Republic), Peter Verhamme (Belgium), Joseph ACaprini (USA), Hanh My Bui (Vietnam).
RIETE Registry Coordinating Center: S & H Medical Science Service.
Members of the RIETE Group
SPAIN: Adarraga MD, Agudo P, Aibar J, Aibar MA, Amado C, Arcelus JI, Ballaz A, Barba R, Barbagelata C, Barrón M, Barrón-Andrés B, Blanco-Molina A, Beddar Chaib F, Botella E, Camón AM, Castro J, Chasco L, Criado J, de Ancos C, de Miguel J, del Toro J, Demelo-Rodríguez P, Díaz-Brasero AM, Díaz-Pedroche MC, Díaz-Peromingo JA, Domínguez IM, Dubois-Silva A, Escribano JC, Espósito F, Farfán-Sedano AI, Fernández-Capitán C, Fernández-Reyes JL, Fidalgo MA, Font C, Francisco I, Gabara C, Galeano-Valle F, García MA, García-Bragado F, García de Herreros M, García de la Garza R, García-Díaz C, García-Mullor MM, Gil-Díaz A, Gómez-Cuervo C, Gómez-Mosquera AM, González-Martínez J, Grau E, Guirado L, Gutiérrez J, Hernández-Blasco L, Jara-Palomares L, Jaras MJ, Jiménez D, Jiménez R, Jiménez-Alfaro C, Jou I, Joya MD, Lainez-Justo S, Latorre-Díez A, Lalueza A, Lecumberri R, Lobo JL, López-Brull H, López-De la Fuente M, López-Jiménez L, López-Miguel P, López-Núñez JJ, López-Reyes R, López-Sáez JB, Lorenzo A, Lumbierres M, Madridano O, Maestre A, Marchena PJ, Marcos M, Martín-Martos F, Martínez-Urbistondo D, Mella C, Mellado M, Mercado MI, Monreal M, Muñoz-Blanco A, Muñoz-Gamito G, Morales MV, Nieto JA, Núñez-Fernández MJ, Olid-Velilla M, Otalora S, Otero R, Paredes-Ruiz D, Parra P, Parra V, Pedrajas JM, Pellejero G, Pérez-Jacoiste A, Peris ML, Porras JA, Portillo J, Rivera A, Roca M, Rosa V, Ruiz-Artacho P, Ruiz-Giménez N, Ruiz-Ruiz J, Ruiz-Sada P, Salgueiro G, Sánchez-Muñoz-Torrero JF, Sancho T, Sigüenza P, Soler S, Suárez-Rodríguez B, Suriñach Caralt JM, Torres MI, Trujillo-Santos J, Uresandi F, Usandizaga E, Valle R, Varona JF, Vela L, Vela JR, Villalobos A, Villares P, Zamora C. AUSTRIA: Ay C, Nopp S, Pabinger I, BELGIUM: Engelen MM, Vanassche T, Verhamme P. COLOMBIA: Esguerra G, Montenegro AC, Roa J. CZECH REPUBLIC: Hirmerova J, Malý R, FRANCE: Accassat S, Bertoletti L, Bura-Riviere A, Catella J, Chopard R, Couturaud F, Espitia O, El Harake S, Helfer H, Le Mao R, Mahé I, Moustafa F, Poenou G, Sarlon-Bartoli G, Suchon P. GERMANY: Schellong S. ISRAEL: Braester A, Brenner B, Kenet G, Tzoran I. ITALY: Basaglia M, Bilora F, Bortoluzzi C, Brandolin B, Ciammaichella M, De Angelis A, Di Micco P, Grandone E, Imbalzano E, Mastroiacovo D, Merla S, Pesavento R, Prandoni P, Siniscalchi C, Tufano A, Visonà A, Vo Hong N, Zalunardo B. LATVIA: Kigitovica D, Rusa E, Skride A. PORTUGAL: Fonseca S, Manuel M, Meireles J. REPUBLIC OF MACEDONIA: Bosevski M, Trajkova M, SWITZERLAND: Bounameaux H, Mazzolai L. USA: Bikdeli B, Caprini JA, Weinberg I. VIETNAM: Bui HM.