Utility of repeat testing in the evaluation for von Willebrand disease in pediatric patients
Abstract
Background
Von Willebrand disease (VWD) is the most common inherited bleeding disorder and is caused by quantitative and qualitative defects in von Willebrand factor (VWF). The laboratory diagnosis of VWD in pediatric patients is complicated by VWF interassay and intra-assay variability, stress-induced elevations in VWF levels, and a lack of significant bleeding history with which to correlate test results.
Objective
Guidelines recommend repeat testing in patients with a high suspicion of VWD and unclear laboratory assay results; however, no studies have evaluated the utility of repeat VWF testing in pediatric patients.
Methods
This retrospective single-center cohort study aimed to determine clinical variables associated with requiring more than one test to diagnose VWD and to establish a cutoff VWF value above which further testing is not informative.
Results
Of 811 patients evaluated for a suspected bleeding disorder, 22.2% were diagnosed with VWD, with ~70% diagnosed on the first test. Patients with VWD were younger (5.8 vs. 8.5 years, P = .002) and more likely to have a family history of VWD (38% vs. 22%, P < .001) than those without VWD. Univariate analysis failed to identify any clinical variables that correlated with needing multiple tests for a VWD diagnosis. A cutoff of 100 IU/dL for VWF antigen or activity on the first test yielded negative predictive values >95%.
Conclusions
We demonstrate that the majority of pediatric patients had diagnostic VWF values on the first set of testing. Pediatric patients without a family history of VWD and VWF levels >100 IU/dL may not need further testing to rule out the diagnosis of VWD.
Essentials
- Assay variability mitigates diagnostic certainty in pediatric VWD leading to repeat testing.
- Variables associated with a need for repeat testing were tested in a retrospective cohort.
- The first test result is consistent with diagnosis in ~70% of pediatric patients with VWD.
- Initial levels >100 IU/dL have a high negative predictive value (>95%) to rule out VWD.
1 INTRODUCTION
Von Willebrand disease (VWD) is the most common inherited bleeding disorder and affects approximately 0.1% to 1% of the population.1 It is caused by quantitative (type 1 and 3) or qualitative (type 2) defects in VWF.1 Von Willebrand factor plays a role in primary and secondary hemostasis through platelet aggregation and factor VIII (FVIII) carrier protein functions, respectively. Depending on the degree of VWF deficiency, patients may have varying amounts of bleeding ranging from mild, trauma-induced bleeding to spontaneous mucocutaneous bleeds.2 Thus, prompt and accurate diagnosis of VWD is necessary to prevent perioperative bleeding and to optimize outcomes during bleeding or trauma.
The diagnosis of VWD is based on three cardinal features: (a) a personal history of bleeding, (b) laboratory assays, and (c) a family history of VWD.1 The laboratory assays classically used to diagnose VWD include3 (a) VWF antigen, which measures the amount of VWF present; (b) VWF activity assays, such as the VWF ristocetin assay, which measure VWF function; and (c) FVIII activity to assess carrier function.2 The National Heart, Lung, and Blood Institute (NHLBI) cutoff for low VWF is 30 IU/dL for antigen or activity assays; however, levels of 30 to 50 IU/dL have been shown to correlate with increased bleeding.4, 5 Further, the laboratory diagnosis of VWD is complicated by interassay and intra-assay variability in VWF activity and by individual variables that temporarily increase VWF levels from baseline.1 For example, there have been documented variations in VWF levels during the different phases of menstruation and/or with high-dose estrogen therapy used to treat menorrhagia.6 Physiologic stressors can also increase VWF concentration 2-fold to 5-fold. One study showed that phlebotomy-associated fainting increased VWF levels above the normal level in adult patients with previously documented VWD.7 Extrapolating from this, the stress of an intravenous blood draw is thought to be enough to increase VWF levels from baseline, especially in pediatric patients. In type 1 VWD, this complicates the ability to diagnose patients with borderline assay levels.8 Additionally complicating the diagnosis is the occurrence of mild bleeding symptoms even in hemostatically normal people. As such, bleeding scores have been developed to assist with characterizing bleeding severity in an effort to help with VWD diagnosis.9-13 However, these scores are often unreliable in pediatric patients, who may not have experienced hemostatic challenges such as menstruation, surgery, or childbirth.14 The most widely utilized scoring system is the International Society on Thrombosis and Haemostasis (ISTH) Bleeding Assessment Tool (BAT), where a score >2 is deemed positive in pediatric patients.10
Given the difficulties in obtaining a steady-state VWF level, our institutional practice has been to repeat assays at least three times in pediatric patients who have normal VWF levels but a clinical suspicion of VWD. Several guidelines and review articles also recommend repeat testing in patients with mildly low or normal levels and suspicion of VWD.1, 4 It should be noted that this is not a universal practice and that there is some heterogeneity with regard to the assays and numbers of tests needed to make an accurate diagnosis with some suggestion of not repeating testing unless supported by other clinical data.15 In our survey of 16 hemophilia treatment centers within the Mid-Atlantic region, half of the respondents reported having an institutional guideline for repeat testing in the evaluation of VWD (unpublished data). Of these, the majority recommended three or more tests (87.5%). Similarly, 87.5% of providers routinely conducted more than one test in the evaluation of patients for VWD and most sent three or more tests (62.5%). Providers identified age (43.8%), bleeding severity (93.8%), family history (75%), upcoming procedures (18.8%), and test variability or values of first test (18.8%) as factors likely to increase the number of VWF assays sent. However, no studies to date have evaluated the utility of repeat testing in pediatric patients.
In this large retrospective single-center cohort study, we aimed (a) to identify clinical variables associated with the need for more than one set of VWF assays to diagnose VWD in pediatric patients and (b) to establish a cutoff value with the first set of VWF assays above which further testing is not informative.
2 PATIENTS AND METHODS
2.1 Study design and setting
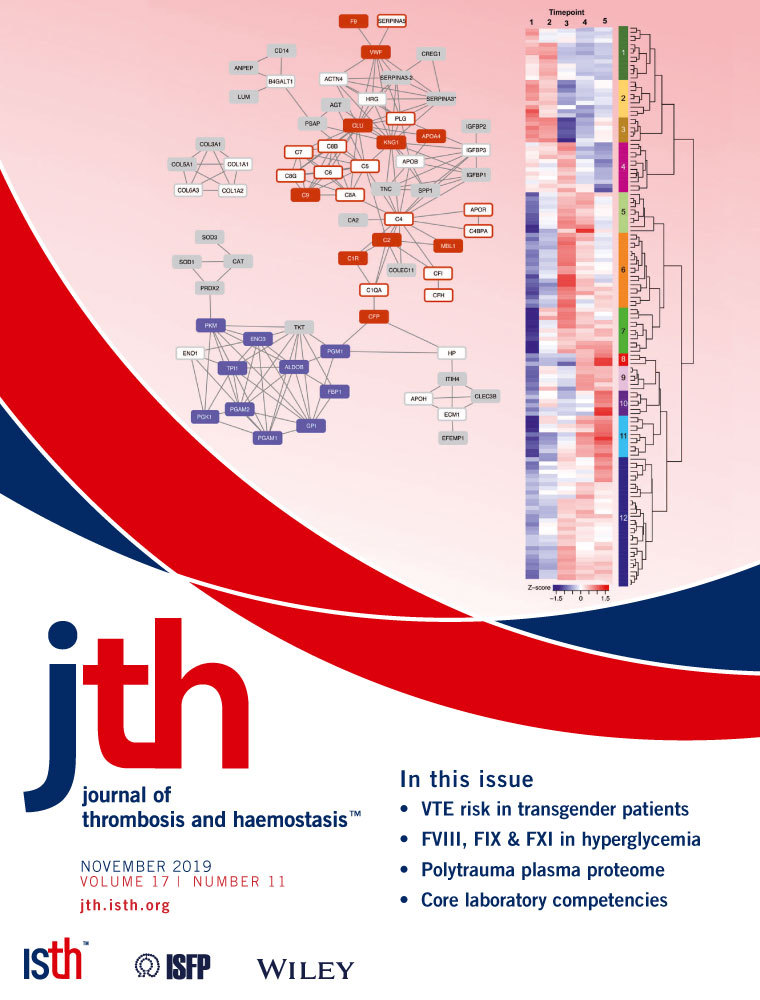
We queried the hospital coagulation laboratory database to identify patients who had VWF activity or antigen testing completed between 1 January 2012 and 31 July 2017. Patients were included in this study if they were 0 to 18 years of age at the time of their initial evaluation and had a documented evaluation by a pediatric hematologist. Those patients who had an incomplete evaluation, who were partially or fully diagnosed at another institution, or who carried a diagnosis of any other bleeding disorder were excluded. On the basis of our statistical modeling, evaluation of 1000 patients would be adequate to address the study objectives. Thus, the first 1000 medical charts were abstracted for demographic information, medications, reason for testing, family history of VWD, results of VWF assays, other illness at time of evaluation, and, if diagnosed, the type of VWD. Each patient had a retrospective ISTH BAT score completed by the abstracter based upon the initial hematology visit. Any missing bleeding data were scored as zero on the BAT form. To minimize variation in scoring between chart abstracters, a percentage of each abstracter's charts were verified by a second abstracter, who was blind to VWD diagnosis, until consensus was reached between abstracters. Although bleeding scores are only validated to be used prospectively, a retrospective BAT score was used in this study as a way to capture clinically documented bleeding data in a standardized manner.
2.2 Von Willebrand factor antigen and activity assays
The VWF antigen level was measured in the clinical laboratory using the STA-Liatest VWF Kit (Stago, Mount Olive, NJ, USA), which determines VWF antigen concentration by measuring the change in turbidity (and thus absorbance) caused by microparticles coated with VWF antibody binding to VWF protein in the plasma. The VWF activity level was measured by two assay systems during the course of the study. Samples obtained before June 2015 were tested using a ristocetin cofactor activity assay (BIO/DATA Corporation, Horsham, PA, USA) by determining the ability of the test plasma and ristocetin to induce agglutination of a standardized platelet suspension. Samples obtained after this date were tested for VWF activity by a latex particle enhanced immunoturbidimetric assay (Hemosil, Bedford, MA, USA), which measures the agglutination generated by a latex reagent coated with an antibody directed against the platelet-binding site of VWF.
2.3 Definitions
The diagnosis of VWD in our patient population was based upon the clinician's assessment/diagnosis of VWD in the patient. Patients with VWD were subdivided into type 1, type 2, or type 3 on the basis of review of patients’ VWF levels. Patients with a diagnosis of type 1 VWD were further separated into: probable type 1 VWD, where the lowest VWF antigen or activity in the study period was >30 and <50 IU/dL, and definite type 1 VWD, where the lowest VWF antigen or activity was <30 IU/dL. Because of small numbers, type 2 VWD patients were not stratified by subtype for this analysis. Type 2 patient diagnosis was confirmed by review of VWF multimer and/or genetic analysis, when available. The diagnostic test was defined as the first test result consistent with the patient's eventual VWD outcome.
2.4 Study objectives
The primary objective of this study was to determine clinical variables associated with requiring more than one VWF assay to diagnose VWD. The secondary objective was to determine, with the first set of VWF assays, whether there is a cutoff value above which further testing is not informative or necessary.
2.5 Statistical methods
Statistical analyses were performed using SAS version 9.4 (SAS Institute). Descriptive statistics were used to summarize patient demographic characteristics, reported as median with interquartile range (IQR) or number values with percentages. Chi-square and Wilcoxon rank sum tests were used to compare patient characteristics by outcome. The odds of VWD diagnosis by reason for VWF evaluation were tested using Fisher's exact method and are reported as odds ratio with 95% confidence interval. Univariate logistic regression models were performed to observe the odds of requiring more than one diagnostic test to diagnose VWD. Odds ratios with 95% confidence interval were used to summarize these relationships. The clinical factors assessed for association with requiring more than one diagnostic test to diagnose VWD included age, race, sex, family history of VWD, ISTH BAT score, anxiety with blood draws (based upon chart documentation, if available), concurrent medications, and clinical status at the time of the blood draw. We hypothesized that the need for additional tests would be higher in young, anxious, or ill patients as they would have a heightened stress response, and thus a physiologic increase in VWF levels from baseline. Family history and high ISTH BAT score were included in the analysis as these could increase probability of diagnosis. Multivariable logistic regression analysis was conducted for factors associated with the need for more than one test for a VWD diagnosis in univariate analysis (P < .1). For determination of VWF antigen and activity cutoffs, a receiver operator characteristic (ROC) curve analysis was generated to measure sensitivity, specificity, and negative predictive value of the first VWF antigen and activity. As there were only two patients with type 3 VWD, these patients were excluded from the final analysis.
2.6 Human subject oversight
This study was approved by the Children's Hospital of Philadelphia Institutional Review Board with a waiver of informed consent and assent.
3 RESULTS
3.1 Patient demographics and characteristics
Within the study period, there were a total of to 2521 VWF antigen and activity sets completed in the hospital coagulation laboratory in 1033 individual patients, of which the first 1000 were evaluated (Figure 1). A total of 811 patients were included in the final analysis, of which 180 (22.2%) were diagnosed with VWD and 631 (77.8%) did not have VWD. Patient baseline characteristics and comparisons by VWD status are listed in Table 1. Of note, patients with VWD underwent testing at a younger age than those without VWD (median 5.8 vs. 8.5 years, P = .002). There was a bimodal peak in age distribution with infants and adolescents constituting the majority of the VWD cohort. There was no difference in sex or race between cohorts. As expected, patients with VWD were more likely to have a family history of VWD (38.1% vs. 21.5%, P < .001). Patients with or without VWD had similar median BAT scores of 2 but with wider IQR in the VWD cohort (IQR 1, 4) versus the non-VWD cohort (IQR 1, 3, P = 0.022). When subdivided by age and sex, only female VWD patients >12 years had significantly higher BAT scores than their non-VWD counterparts with a median (IQR) of 4 (2, 5) versus 2 (1.75, 4), respectively (P < .001, Figure S1B). The VWF activity and antigen levels were lower in the VWD cohort compared to the non-VWD cohort with median VWF activity of 34 (IQR 25, 40) versus 78 IU/dL (IQR 58, 100) and antigen of 45 (IQR 35, 54) versus 80 IU/dL (IQR 64, 106), respectively (Table 1, Figure S2). Of note, the VWF activity assay changed during the course of the study from a ristocetin-based assay to an immunoturbidimetric assay with 409 patients with repeat samples sent with the ristocetin-based assay compared to 178 with repeat samples with the immunoturbidimetric assay. There was not a difference in the number of repeat samples sent between the assays. The coefficient of variation for all ristocetin-based assays is historically reported as ~30% compared to ~20% for immunofunctional assays.16 We do not have data available for the ristocetin-based assay from our laboratory, but our coefficient of variation for the immunoturbidimetric assay is ~10%.
| VWD | No VWD | P value | |
|---|---|---|---|
| n | 180 (22) | 631 (78) | - |
| Age (years) | 5.8 (1.1, 13.9) | 8.5 (3.7, 14.1) | .002 |
| Race | |||
| Caucasian | 120 (67) | 374 (59) | .131 |
| African American | 22 (12) | 114 (18) | |
| Asian/Pacific Islander | 2 (1) | 23 (4) | |
| Hispanic | 11 (6) | 41 (6) | |
| Other | 25 (14) | 79 (13) | |
| Sex | |||
| Male | 73 (41) | 270 (43) | .669 |
| Female | 107 (59) | 361 (57) | |
| Family history of VWD | 69 (38) | 136 (22) | <.001 |
| BAT score | 2 (1,4) | 2 (1,3) | .022 |
| Number of tests for diagnosis | 1 (1,2) | 2 (1,3) | <.001 |
| Laboratory assays | |||
| VWF activity (IU/dL) | 34 (25,40) | 78 (58, 100) | <.001 |
| VWF antigen (IU/dL) | 44 (35, 54) | 80 (64, 106) | <.001 |
| FVIII activity (IU/dL) | 57 (46, 71) | 100 (80, 125) | <.001 |
Note
- Data presented as n (%) or median (interquartile range); P values by Wilcoxon rank sum test (continuous variables) or X2 (categorical variables).
- Abbreviations: BAT, Bleeding Assessment Tool; FVIII, factor VIII; VWD, von Willebrand disease; VWF, von Willebrand factor.
Characteristics of patients with VWD are included in Table 2. Type 1 VWD was most common (30% definite, 61% probable) followed by type 2 (8%); there were only two patients with type 3 VWD (1%). Among patients with VWD, there was no difference in age, race, sex, BAT score, or number of tests for diagnosis between different types. More patients with type 2 and definite type 1 VWD had a positive family history for VWD than those with probable type 1 and type 3 VWD.
| Type 1 | Type 2 | Type 3 | P value | ||
|---|---|---|---|---|---|
| Definite | Probable | ||||
| n (%) | 53 (29) | 110 (61) | 15 (8) | 2 (1) | |
| Age (years) | 5.5 (1.4, 13.1) | 6.4 (1.4, 15.1) | 7.4 (0.6, 16.4) | 0.2 (0.01, 0.34) | .058 |
| Race | |||||
| Caucasian | 29 (55) | 77 (70) | 12 (80) | 2 (100) | .668 |
| African American | 6 (11) | 15 (14) | 1 (6) | 0 (0) | |
| Asian/Pacific Islander | 1 (2) | 1 (1) | 0 | 0 (0) | |
| Hispanic | 5 (9) | 5 (5) | 1 (6) | 0 (0) | |
| Other | 12 (23) | 12 (11) | 1 (6) | 0 (0) | |
| Sex | |||||
| Male | 19 (36) | 47 (43) | 6 (40) | 0 (0) | .561 |
| Female | 34 (64) | 63 (57) | 9 (60) | 2 (100) | |
| Family history VWD | 24 (45) | 35 (32) | 10 (67) | 0 (0) | .025 |
| BAT score | 2 (0,3) | 2 (1,4) | 2 (1,5) | 6 (4,8) | .057 |
| Number of tests for diagnosis | 1 (1,2) | 1 (1,2) | 1 (1,2) | 1 | .880 |
| Laboratory assays | |||||
| VWF activity (IU/dL) | 24 (21, 26) | 38 (34, 43) | 30 (23, 33) | 15 (10, 20) | <.001 |
| VWF antigen (IU/dL) | 33 (27, 40) | 47 (41, 59) | 54 (33, 84) | 1.5 (1,2) | <.001 |
| FVIII activity (IU/dL) | 50 (38, 61) | 61 (53, 76) | 47 (32, 62) | 1 (1,1) | <.001 |
Note
- Data presented as n (%) or median (interquartile range); P values by Kruskall-Wallis test (continuous variables) or X2 (categorical variables).
- Abbreviations: BAT, Bleeding Assessment Tool; FVIII, factor VIII; VWD, von Willebrand disease; VWF, von Willebrand factor.
3.2 Reason for testing and odds of VWD diagnosis
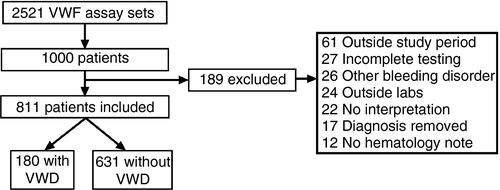
As summarized in Figure 2A, patients were tested for a variety of reasons. The most common reasons for testing were family history, epistaxis, cutaneous bleeding, menorrhagia, and abnormal coagulation test results. Of all reasons for testing, the odds of being diagnosed with VWD were only significantly higher in patients with a family history of VWD (odds ratio 1.78, 95% confidence interval 1.23, 2.54) and those referred for abnormal coagulation tests (odds ratio 1.61, 95% confidence interval 1.07, 2.24) as seen in Figure 2B. Of the 133 patients referred for abnormal coagulation parameters, 49 had abnormal results of VWF or FVIII studies, 74 abnormal prothrombin time or partial thromboplastin time, and 10 had other coagulation laboratory abnormalities. Nearly all of the abnormal VWF study findings were from an outside laboratory and 26 (53%) of these patients were eventually diagnosed with VWD after repeat testing compared to 15% of those with prolonged prothrombin time/partial thromboplastin time and 30% of those with other abnormal coagulation results. Patients tested for anemia were unlikely to be diagnosed with VWD with odds ratio 0.16 (95% confidence interval 0.03, 0.62).
One hundred and forty-seven females were tested for heavy menstrual bleeding, comprising 18% of the population. Of these, 30 were on hormonal therapy at the time of testing and 11 of these patients were diagnosed with VWD at a median of two tests (IQR 1,3). Only one of these patients was concurrently anemic at the time of the initial diagnostic test. As high-dose estrogen may artificially elevate VWF levels, we evaluated whether these patients were on estrogen-containing compounds at the time of testing. Eight of these 11 patients were on low-dose estrogen formulations (<25 mcg ethinyl estradiol) while 3 were on high-dose estrogen. Of the latter, two-thirds were diagnosed at initial testing and the remaining patient diagnosed on test 3 but while still on high-dose estrogen.
3.3 Number of tests
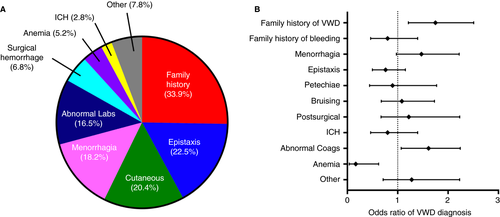
The median number of tests completed prior to the diagnosis in the VWD cohort was one (IQR 1,2) compared to two (IQR 1,3) for the non-VWD cohort (P < .001). As seen in Figure 3A, the first test was diagnostic in 125/180 (69.4%) of patients with VWD, the second test was diagnostic in 18.4%, and the third test was diagnostic in 10.6%. Only 1.6% of patients required more than three tests to establish the diagnosis of VWD. Of note, although the first test was consistent with the diagnostic result in ~70% of VWD patients, the clinical diagnosis was not established until the second test in 25% of these patients. In the non-VWD cohort, the proportion who had three tests completed before the provider excluded the diagnosis of VWD was 42.1% whereas 4.5% of patients had more than three tests completed for the exclusion of VWD. Within the VWD cohort (Figure 3B), the first test was diagnostic in 69% of patients with definite or probable type 1 VWD, in 73% of those with type 2, and in 100% of those with type 3 VWD.
 ) and with (
) and with ( ) VWD. B, Histogram of number of tests before diagnosis by VWD subtype: definite type 1 VWD (
) VWD. B, Histogram of number of tests before diagnosis by VWD subtype: definite type 1 VWD ( ), probable type 1 VWD (
), probable type 1 VWD ( ), type 2 VWD (
), type 2 VWD ( ), and type 3 (
), and type 3 ( ) VWD
) VWD3.4 Clinical variables associated with the need for repeat VWF testing
Univariate and multivariable logistic regression analysis was completed for clinical variables that might be associated with the need for more than one test to establish the diagnosis of VWD. Clinical variables included age, sex, race, family history, BAT score, estrogen use at time of testing, clinical/health status at phlebotomy, and VWD type. In the VWD cohort, only one variable (race) achieved a P value < .1 and thus a multivariable analysis was not performed (Table 3). When the same analysis was conducted in the non-VWD cohort, univariate analysis showed P values < .1 for race, family history, and health status at phlebotomy. A multivariable model of these variables was created (Table 4) using stepwise backward regression to reduce error, and no significant potential interaction effects were found with an area under the curve of 0.70 in ROC curve analysis. In this model, the odds of the clinician's ordering more than one test to rule out VWD were higher in patients with a family history of VWD or with a high BAT score (>2) and were lower in those who were clinically well at the time of phlebotomy.
| OR | 95% CI | P | |
|---|---|---|---|
| Age | 1.00 | 0.95, 1.06 | .914 |
| Sex | 0.78 | 0.40, 1.49 | .448 |
| Race | |||
| Caucasian | 1.00 | - | - |
| African American | 1.23 | 0.46, 3.29 | .679 |
| Hispanic | 0.99 | 0.25, 3.95 | .987 |
| Other | 2.11 | 0.89, 4.98 | .088 |
| Family history of VWD | 0.71 | 0.36, 1.38 | .306 |
| BAT score | 0.99 | 0.84, 1.16 | .891 |
| Estrogen use | 2.06 | 0.12, 136.96 | .213 |
| Anxious with phlebotomy | 4.00 | 0.12, 136.96 | .442 |
| VWD type | |||
| Type 1 definitive | 1.00 | - | - |
| Type 1 probable | 1.12 | 0.55, 2.28 | .748 |
| Type 2 | 0.86 | 0.24, 3.12 | .823 |
- Abbreviations: BAT, Bleeding Assessment Tool; CI, confidence interval; FVIII, factor VIII; VWD, von Willebrand disease; VWF, von Willebrand factor.
| Univariate analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age | 0.99 | 0.97, 1.03 | .931 | - | - | - |
| Sex | 0.90 | 0.64, 1.25 | .527 | - | - | - |
| Race | ||||||
| Caucasian | 1.00 | - | - | - | - | - |
| African American | 0.68 | 0.44, 1.05 | .082 | - | - | - |
| Hispanic | 1.97 | 0.88, 4.40 | .097 | - | - | - |
| Other | 0.92 | 0.58, 1.45 | .709 | - | - | - |
| Family history of VWD | 2.39 | 1.51, 3.78 | <.001 | 4.98 | 2.92, 8.47 | <.001 |
| BAT score | 1.26 | 1.13, 1.41 | <.001 | 1.53 | 1.33, 1.76 | <.001 |
| Estrogen use | 1.61 | 0.68, 3.83 | .282 | - | - | - |
| Clinical status at phlebotomy | 0.43 | 0.27, 0.68 | <.001 | 0.46 | 0.29, 0.74 | .002 |
- Abbreviations: BAT, Bleeding Assessment Tool; CI, confidence interval; FVIII, factor VIII; VWD, von Willebrand disease; VWF, von Willebrand factor.
3.5 Determination of cutoff values with first test
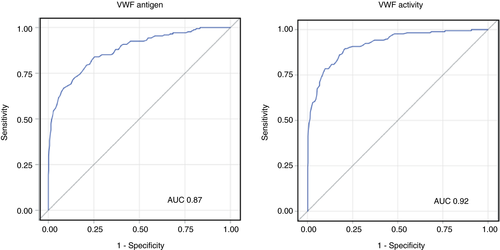
An ROC analysis was performed on the first VW set to determine a cutoff above which repeat testing was not informative in establishing the diagnosis of VWD (Figure 4). The VWF antigen and activity established the diagnosis of VWD with an AUC of 0.87 and 0.92, respectively. Using a cutoff of 100 IU/dL for VWF antigen yielded a sensitivity of 95%, specificity of 38%, and negative predictive value of 96.6%. Similarly, a cutoff of 100 IU/dL for VWF activity yielded a sensitivity of 98%, specificity of 38%, and negative predictive value of 98.6%. We further evaluated whether lower cutoffs would perform similarly. The inflection point on the ROC curve is at ~75 IU/dL providing a target lower cutoff value. Using a cutoff of 75 IU/dL, the negative predictive values were 94.1% and 97.4% for antigen and activity, respectively.
4 DISCUSSION
To our knowledge, this is the first study to evaluate repeat laboratory testing for VWD in a pediatric population. This single-center retrospective cohort study was designed to identify the clinical variables associated with the need for more than one test to establish the diagnosis of VWD in pediatric patients. In this population of 811 patients, we found that 22% were diagnosed with VWD. The first VW assay was diagnostic in nearly 70% of patients. The VWD cohort was more likely to be younger (5.8 vs. 8.5 years, P = .002) and have a family history of VWD (38 vs. 22%, P < .001) than the non-VWD cohort. Although mucocutaneous bleeding (menorrhagia, epistaxis, easy bruising) constituted the vast majority of bleeding symptoms in our patient population, it was not associated with increased odds of VWD diagnosis. Notably, the only two reasons for testing that were associated with an increased odds ratio of VWD diagnosis were family history of VWD and referral for abnormal coagulation study results. On ROC analysis with the first set of VW testing, a cutoff of 100 IU/dL for VWF antigen or activity on the first test yielded a negative predictive value >95% for both VWF antigen and activity. Pediatric patients without a family history of VWD and VWF levels >100 IU/dL on initial test may not need further testing to rule out the diagnosis of VWD.
Unfortunately, none of the evaluated clinical variables including age, sex, race, family history, bleeding severity, estrogen use, health status at phlebotomy, or VWD type showed an association with the need for more than one test to establish the diagnosis of VWD in univariate analysis. It is notable that 70% of patients with VWD had diagnostic initial laboratory results but 25% of these patients did not have the diagnosis made clinically until after a second assay set. The reason for this may be secondary to providers’ wanting to confirm initial laboratory findings because of assay variability, concern for preanalytic variables, or may be a function of documentation delay. Although approximately 18% of our patient population was tested for heavy menstrual bleeding and this population is often treated with estrogen-containing therapy, which is known to affect VWF levels, only 20% of our population was concurrently on estrogen therapy. Of these patients, 36.7% were diagnosed with VWD at a median of two tests and high-dose estrogen therapy in three patients did not change the testing requirements. As these numbers are relatively small, further study is warranted as this is a population that may require repeat testing when off high-dose estrogen. Additionally, patients with blood type O have VWF levels that are ~25% lower than non-O blood group patients.17 Since approximately 2008, guidelines began recommending against using blood-type-specific reference values and instead using either absolute cutoffs or population-based reference ranges in conjunction with the clinical and family history in determining whether a patient carries a diagnosis of VWD.4, 15 Consequently, our practice stopped blood-typing patients after publication of these guidelines in 2009 and, thus, blood group typing was not included in our variable analysis.
Despite our institutional guideline, ~50% of non-VWD patients had their diagnosis excluded before three tests were completed. Within the non-VWD cohort, a multivariable model including family history, positive BAT score, and poor health status at phlebotomy was associated with the need for more than one test to exclude the diagnosis of VWD. In our prior survey of hemophilia treatment center providers, these same clinical variables were identified as reasons to conduct repeat testing (unpublished). It is not surprising that patients with a family history of VWD would have additional tests completed to rule out the diagnosis as these patients are more likely to have inherited a genetic VWF defect. Although the BAT score in the overall VWD cohort was slightly higher than the non-VWD cohort, the difference is only pronounced in female patients >12 years. This is consistent with prior studies and most likely due to the fact that younger patients have not had significant prior hemostatic challenges, such as teeth extractions, menorrhagia, and surgical procedures, which compose a large portion of the BAT score. Thus, a high BAT score could help differentiate bleeding severity in the adolescent VWD cohort from hemostatically normal patients.
In ROC analysis of the first VWF antigen or activity assay and VWD diagnosis, a cutoff value of 100 IU/dL yielded a negative predictive value of 96.6% and 98.6%, respectively. Interestingly, a cutoff of 75 IU/dL on the first assay still yielded negative predictive values of 94.1% and 97.4% for VWF antigen and activity, respectively. The number of patients in the non-VWD cohort who underwent more than one test after an initial result >100 IU/dL was 163 (26%) and after an initial result >75 IU/dL was 328 (51%). Given the cost of VWF assays and the health care costs associated with laboratory and clinical visits, ~$150 000 to $300 000 could have been saved if additional testing was not completed in these patients.
We recognize that our study has several limitations. First, as it is a retrospective cohort study there are possible biases with respect to incomplete information and abstracter bias. We attempted to limit these biases by omitting analysis of variables with limited data and using standardized abstraction forms to limit abstracter bias. The BAT score was completed retrospectively and thus was limited by the data entered by the clinician at the time of the visit and may be prone to variability in questions asked during the visit. In an effort to account for this, any areas with missing data were scored as 0 and we attempted to limit classification bias with cross-validation of BAT scores with blinded abstracters. Further, the BAT score is only validated to be used in a prospective manner and, as such, the use of the scoring system in a retrospective manner may not yield an accurate representation of the correlation between this score and the patient's bleeding severity and/or diagnosis. Although several pediatric bleeding questionnaires have been evaluated in different cohorts,13, 18, 19 there are no guidelines that recommend consistent use of a single bleeding questionnaire in pediatric patients. Given the retrospective nature of this study and the fact that our institution does not routinely administer a bleeding questionnaire in a prospective manner, a retrospective BAT score was chosen as the most consistent way to allow a surrogate measure of bleeding severity to account for this important clinical variable in the need for repeat testing.
A further limitation of our study is that the study population was mostly composed of type 1 VWD patients. Thus, these results may not be generalizable to patients with type 2 or type 3 disease. Moreover, although our institutional standard is to obtain three independent laboratory assays for VWD evaluation, ~50% of patients without VWD were classified as such before the third set and the reasons behind this are unknown because of the retrospective nature of our study. The ROC cutoffs from our study may not be universal to other laboratories because of interlaboratory assay differences. As we excluded laboratory studies done at outside facilities, we cannot comment on the interlaboratory variation within our population, and extrapolation of cutoff values from this study should be cautiously approached. Finally, whether repeat testing is truly necessary remains an open question and may represent an inherent confirmation bias in our institutional practice. Given the variability in VWF assays, it is possible that repeat testing may bias our population toward overdiagnosis of VWD, specifically in patients with low VWF levels of 30 to 50 IU/dL. Overall, our population has a lower rate of VWD compared to the large prospective Zimmerman VWD study, but a higher rate of probable/low VWF than in that population.20
Our findings, in a large cohort of 811 pediatric patients, suggest that the majority of patients with VWD will have levels consistent with their diagnosis on the first test and repeat testing is not indicated. Unfortunately, for the minority of patients diagnosed with repeat VW testing, no clinical factors were identified to aid clinicians in decision making. The negative predictive value of the initial VWF antigen or activity being >75 IU/dL approaches 95% and at 100 IU/dL is >95%. Thus, in patients with a low clinical suspicion of VWD, it may be reasonable to avoid further testing if levels are above 75 to 100 IU/dL on the initial test, which could result in significant cost and health care utilization savings.
ACKNOWLEDGMENTS
The authors acknowledge Dr. Michele Lambert and Dr. Leslie Raffini at Children's Hospital of Philadelphia for their insight and guidance. This work was supported by a research grant from the HRSA 340b program (H30MC24050).
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
B. S. D., R. B. B., and C. M. W. designed the study; R. S. R. conducted statistical analysis; A. O. provided laboratory database; B. S. D., H. B. W., E.S., and J. B. abstracted data. All authors reviewed and approved the manuscript.