Temporal map of the pig polytrauma plasma proteome with fluid resuscitation and intravenous vitamin C treatment
Abstract
Background
Fluid resuscitation plays a prominent role in stabilizing trauma patients with hemorrhagic shock yet there remains uncertainty with regard to optimal administration time, volume, and fluid composition (e.g., whole blood, component, colloids) leading to complications such as trauma-induced coagulopathies (TIC), acidosis, and poor oxygen transport. Synthetic fluids in combination with antioxidants (e.g., vitamin C) may resolve some of these problems.
Objectives
We applied quantitative mass spectrometry-based proteomics [liquid chromatography-mass spectrometry (LC-MS/MS)] to map the effects of fluid resuscitation and intravenous vitamin C (VitC) in a pig model of polytrauma (hemorrhagic shock, tissue injury, liver reperfusion, hypothermia, and comminuted bone fracture). The goal was to determine the effects of VitC on plasma protein expression, with respect to changes associated with coagulation and trauma-induced coagulopathy (TIC).
Methods
Longitudinal blood samples were drawn from nine male Sinclair pigs at baseline, 2 h post trauma, and 0.25, 2, and 4 h post fluid resuscitation with 500 mL hydroxyethyl starch. Pigs were treated intravenously (N = 3/treatment group) with saline, 50 mg VitC/kg (Lo-VitC), or 200 mg VitC/kg (Hi-VitC) during fluid resuscitation.
Results
A total of 436 plasma proteins were quantified of which 136 changed following trauma and resuscitation; 34 were associated with coagulation, complement cascade, and glycolysis. Unexpectedly, Lo-VitC and Hi-VitC treatments stabilized ADAMTS13 levels by ~4-fold (P = .056) relative to saline and enhanced ADAMTS13/von Willebrand factor (VWF) cleavage efficiency based on LC-MS/MS evidence for the semitryptic VWF cleavage product (VWF1275-1286).
Conclusions
This study provides the first comprehensive map of trauma-induced changes to the plasma proteome, especially with respect to proteins driving the development of TIC.
Essentials
- The effects of fluid resuscitation with high-dose vitamin C in polytrauma are ill defined.
- The pig plasma proteome was analyzed to better understand polytrauma dynamics.
- 436 plasma proteins were quantified in the study, of which 136 changed over time.
- High-dose vitamin C most strongly affected ADAMTS13 levels and its VWF cleavage activity.
1 INTRODUCTION
Trauma involving severe tissue injury and hemorrhagic shock is a leading cause of death among both the civilian population1, 2 and military personnel.3 Improving trauma-related and hemorrhage-related survival remains a significant challenge for civilian and military urgent care facilities. Fluid resuscitation and hemorrhage control remain the cornerstone of care for severe trauma-related hemorrhagic shock, with blood and blood-derived components (e.g., platelets, plasma, red blood cells) being preferred to crystalloids or colloids.4-8 Fluid resuscitation is used to stabilize severe trauma-related hemorrhage by restoring blood pressure, enhancing oxygen transport, and normalizing core temperature and coagulation function. During this process, however, the increased fluid volume can exacerbate the body's coagulation and tissue repair response by initiating TIC, an endogenous hypocoagulable condition that can lead to higher mortality and morbidity.9 Trauma-induced coagulopathy is a complex disorder originally thought to result from the depletion and/or dysfunction of procoagulant factors. However, coagulation derangements can occur before major resuscitation.10, 11 Thus, TIC likely arises from a complex imbalance among procoagulant activation, inhibition of anticoagulation pathways, increased fibrinolysis, and impaired platelet function.12-15
Ascorbic acid [vitamin C (VitC)] is a multifunctional nutrient that cannot be biochemically synthesized in humans.16 Vitamin C is an essential cofactor for many enzymatic reactions including HIF1-alpha and collagen synthesis, and constitutes a primary line of defense against reactive oxygen species and lipid peroxidation. Circulating VitC levels drop by more than an order of magnitude in critically injured patients relative to healthy individuals17, 18 because of free radical scavenging and subsequent destruction of the oxidized form of VitC.19, 20, 17 Exogenous intravenous high-dose VitC replacement has been shown to reduce proinflammatory cytokines and coagulation dysfunction in animal models21, 22 via inhibition of the proinflammatory transcription factor nuclear factor kappa B. Despite the benefits of circulating VitC, detailed mechanistic insights into the onset and progression of polytrauma with or without VitC supplementation have been limited.
To address the shortcomings in our systems-level understanding of polytrauma preresuscitation and post resuscitation, we used high-performance mass spectrometry-based proteomics (LC-MS/MS) to quantitatively study the plasma proteome in a pig model of polytrauma.23 The major goal of this study was to determine the effect of intravenous VitC on modifying plasma protein expression, particularly regarding coagulation and TIC. To this end, we used untargeted LC-MS/MS-based proteomics to map quantitative changes to the plasma proteome in a pig model of hemorrhagic shock/trauma and resuscitation. We predicted that disruptions/aberrancies in proteome expression following acute polytrauma could be reduced or minimized when colloid resuscitation was supplemented by VitC. This study represents the first comprehensive map of the plasma proteome in the first 6 h of polytrauma while identifying novel effects of high-dose vitamin C in the coagulation pathway. This analysis provides vital preliminary data for developing future interventional studies to target aberrant coagulation and inflammatory protein changes following trauma.
2 MATERIALS AND METHODS
2.1 Ethical oversight
This study was approved by Virginia Commonwealth University IACUC (VCU #AD10001189) and Department of Defense Animal Care and Use Office (ACURO: USAMRMC Proposal Number 13011003). Animal use and welfare procedures were conducted according to the Guide for the Care and Use of Laboratory Animals.24
2.2 Animal model
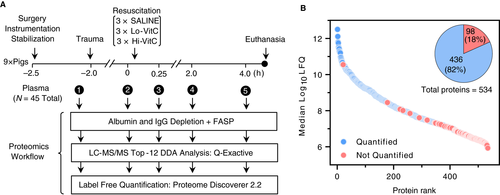
Data for this study were obtained as part of a larger prospective, randomized, blinded swine trial; details of animals, animal husbandry and care, and surgical procedures have been described previously.23 Briefly, male Sinclair swine (mean weight 27 kg on day of experiment) were sedated, anesthetized, intubated, mechanically ventilated, and instrumented for physiological monitoring (Figure 1A). Following ~30-min stabilization, animals were subjected to hemorrhagic hypotension and polytrauma (hypothermia, liver ischemia and reperfusion, comminuted femur fracture). At the conclusion of the 2-h injury phase, the swine were resuscitated with 500 mL colloid (6% hydroxyethyl starch 130/0.4 in 0.9% sodium chloride; VetStarch; Abbott Laboratories, North Chicago, IL, average MW 700 kDa, molar substitution 0.7, C2/C6 ratio 5) via intravenous push, and randomized to receive either intravenous normal saline (Saline 25 mL), low-dose VitC (50 mg/kg in 25 mL normal saline; Lo-VitC), or high-dose VitC (200 mg/kg in 25 mL normal saline; Hi-VitC). Arterial blood samples (15 mL) were drawn into citrated- tubes after initial stabilization before injury (Baseline), 2 h post trauma and immediately prior to fluid resuscitation (0 h) and then at 15 min, 2 h, and 4 h post fluid resuscitation. Blood samples were centrifuged at 2000 g for 10 min and the plasma transferred to cryotubes for storage at −80 °C until retrieved for proteomics processing. Following the experiments, all animals were euthanized under deep anesthesia.
2.3 Sample preparation
Plasma samples from nine animals (three animals/treatment; five longitudinal samples/animal) were defrosted in a 37 °C water bath for 10 min and then depleted of albumin and immunoglobulin G using the MilliporeSigma (Burlington, MA, USA) ProteoExtract® Albumin/IgG Removal Kit according to the manufacturer's instructions. Briefly, 50 μL of plasma was diluted with 450 μL of Binding Buffer, loaded onto a prewashed resin bed, and gravity filtered into a 1.5-mL centrifuge tube. The resin bed cartridge was washed with 2 × 500 μL aliquots of Binding Buffer to a final depleted plasma protein solution volume of 1.5 mL. Depleted plasma samples were prepared for proteomic analysis using a modified filter-assisted sample preparation protocol,25 then 250 μL of depleted plasma from each sample was transferred to a Millipore Amicon Ultra-0.5 mL 10-kDa- molecular-weight cutoff filter, concentrated in a centrifuge for 10 min at 15 000 g, and washed with 400 μL Tris-HCl pH 8.1 in a centrifuge for 10 min at 15 000 g. The concentrated sample was then reduced with 50 μL of 50 mmol/L dithiothriotol (Sigma-Aldrich, St Louis, MO, USA) at 56 °C for 1 h, alkylated with 100 μL 80 mmol/L iodoacetamide (Sigma-Aldrich) in the dark for 30 min, concentrated in a centrifuge for 10 min at 15 000 g, and then digested with 5 μg of sequencing grade trypsin (Promega, Madison, WI, USA) in 350 μL of Tris-HCl pH 8.1 overnight at 37 °C. Samples were eluted through the molecular weight cutoff filter via centrifugation and stored at −80 °C until removed for LC-MS/MS analysis.
2.4 Mass spectrometry
The LC-MS/MS system consisted of an Eksigent Ekspert nanoLC 415 (Sciex Concord, ON, Canada) coupled to a Q-Exactive Orbitrap (Thermo Fisher Scientific, Waltham, MA, USA). The reverse nanoflow high-performance liquid chromatography HPLC setup26 consisted of a self-packed 100 μm × 5 cm Integrafrit trap and 100 μm × 20 cm Picofrit analytical column (New Objective, Woburn, MA, USA) containing 5 μm Magic AQ C18, 200 Å stationary phase (Michrom Bioresources, Auburn, CA, USA). Tryptic peptides were eluted at 350 nL/min with the following gradient: 5% B (0-4 min), 35% B (95 min), 75% B (105-110 min), 5% B (115-120 min). Mobile phase compositions of high-performance liquid chromatography -grade water, acetonitrile (Burdick & Jackson, Muskegon, MI, USA), and formic acid (Sigma-Aldrich) were as follows: mobile phase A = water:acetonitrile (98:2; vol:vol), 0.1% formic acid and mobile phase B = water:acetonitrile (2:98; vol:vol), 0.1% formic acid. The electrospray emitter voltage was set to 1.5 kV in positive ion mode and the Q-Exactive inlet temperature and S-lens setting were maintained at 250 °C and 50 V, respectively. The instrument was operated in a top-12 data-dependent acquisition mode with a 30-s dynamic exclusion and a normalized collision energy = 27 for all MS/MS events. Full-scan (400-1600 m/z) resolving power was set to 70 000 full-width at half-max at 200 m/z, automatic gain control target of 3 × 106, and a maximum ionization time (ITmax) of 30 milliseconds (ms). The MS/MS scans were set to a resolving power of 17 500 full-width at half-max at 200 m/z, an automatic gain control automatic gain control target of 2 × 104, and an ITmax of 120 ms.
2.5 Data analysis
Proteomic datasets were processed in Proteome Discoverer 2.2 and searched with SEQUEST HT against the UniProt Sus scrofa (pig) proteome FASTA database (download: 29 August 2018). The LC-MS/MS data were searched using the following conditions: protease = trypsin; full-scan MS mass accuracy = 5 ppm; MS/MS = 0.02 Da; fixed modifications = carbamidomethyl (Cys); variable modifications = acetyl (N-term) and oxidation (Met); and false discovery rate of 1%. Label free quantitative (LFQ) analysis in Proteome Discoverer was restricted to proteins that were identified with high confidence (precursor mass spectrum + tandem mass spectrum of precursor: MS + MS/MS) in at least 5 of 45 LC-MS/MS raw files. The LFQ values in Proteome Discoverer were normalized to the total peptide amount, missing values imputed using the “low abundance resampling” feature, and then log2 transformed for quantitative analysis. A two-tailed paired t test of differential protein levels in Baseline versus 0 h was conducted using the Benjamini-Hochberg procedure to control the false discovery rate. Repeated measures analysis of variance was performed using SAS 9.4 (SAS Institute) to assess the effects of time, treatment, and interactions (i.e., whether longitudinal changes in LFQ data are significantly different between treatment groups). Protein levels that changed with time were analyzed in STRING 10.027 to identify high-confidence interactions (interaction score >0.700) using information from experimental, database, and text mining followed by node reformatting in Cytoscape 3.7.28
2.5.1 Data sharing
The LC-MS/MS raw files, sample identifiers, FASTA file, and search results have been deposited in ProteomeXchange Consortium via the PRIDE29 partner repository with identifier PXD012454.
3 RESULTS
3.1 Quantitative plasma proteome analysis of polytrauma
The temporal effects of fluid resuscitation and high-dose VitC during polytrauma in the pig plasma proteome were investigated with LFQ proteomics (Figure 1A). The proteomics analysis resulted in the identification of 534 proteins (FDR < 0.01; Figure 1B; Table S1) and 436 quantified proteins spanning more than six orders of magnitude of LFQ abundance (Figure 1B; Table S2). The 436 quantified proteins included tandem MS/MS evidence in at least five or more (~10%) LC-MS/MS raw files, which balanced proteome coverage with sufficient MS/MS data to improve downstream inference of statistical significance.
3.2 Proteome response to polytrauma prefluid resuscitation
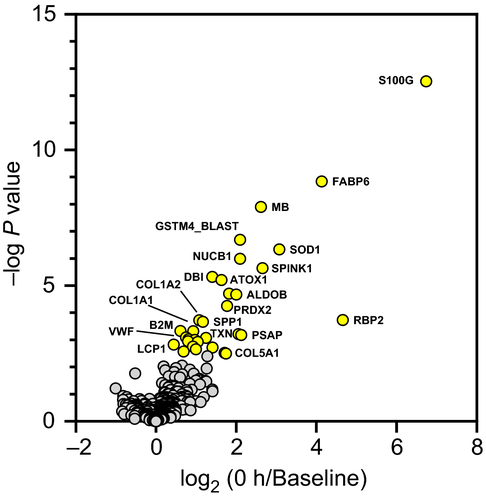
The relative changes to the 436 proteins between Baseline and 0 h were assessed to understand the proteome response prior to fluid resuscitation. The log2 transformed LFQ levels for 0 h/Baseline are plotted in Figure 2 (Table S3) showing 32 proteins that were significantly up-regulated (P < .05). Up-regulated proteins are involved in a number of polytrauma-related processes including platelet activation and degranulation (complement factor D and Saposin-B-Val), coagulation (von Willebrand factor), oxygen binding/transport (myoglobin, hemoglobin A, and hemoglobin subunit alpha, and hemoglobin subunit beta), integrity of soft tissue (collagen chains COL1A1, COL1A2, and COL5A1) and bone (carbonic anhydrase and osteopontin), free radical and toxicant scavenging. (superoxide dismutase [Cu-Zn], betaine homocysteine methyltransferase, peroxiredoxin-2, copper transport protein AT, thioredoxin, and glutathione S-transferase mu 2), lipid binding (gastrotropin and acyl-CoA-binding protein), antimicrobial/immune response (protegrin-3, complement factor D, and beta-2-microglobulin), calcium regulation (protein S100, nucleobindin-1 precursor, and serine protease inhibitor Kazal-type 1), and glycolysis (fructose-bisphosphate aldolase) .
3.3 Plasma protein dynamics
We analyzed the full longitudinal dataset and identified 136 proteins that changed significantly with time (P < .05). Hierarchical analysis of these 136 statistically significant proteins identified 12 clusters (Figure 3A; Table S4) of which two primary groups emerged (Figure 3B): protein levels that either decreased (clusters 1-4) or increased (clusters 5-12) with time. STRING analysis of the 136 proteins (Figure 3C) revealed a highly connected protein interaction network (Table S5) from which 33 KEGG Pathways were enriched (FDR < 0.05). The complement and coagulation cascades pathway was the most dominant enriched pathway, including 26 proteins with 14 down-regulated (clusters 1-4; red-open) and 12 up-regulated (clusters 5-12; red-solid) with time (Figure 3C).
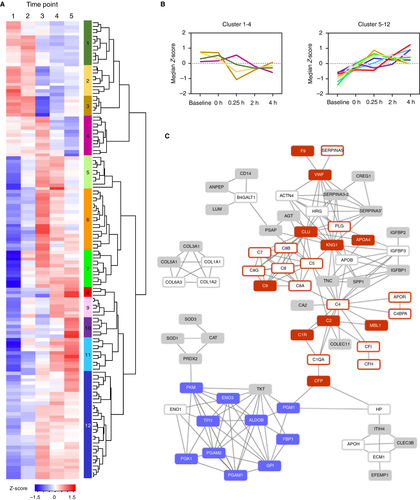
Considering first the complement cascade proteins, the decreased levels of complement factor I and complement factor H are indicative of increased C3 activation via the alternative complement cascade pathway. C5, C6, C7, and C8 decrease with time and are involved in the formation of the membrane attack complex and cell lysis whereas C9, also a part of the membrane attack complex, increased with time. The classical pathway is activated by the antigen-antibody/C1qrs complex, of which C1R and C1QA were enriched in Figure 3C. C1R, the initial receptor for the antibody-antigen complex, increased from Baseline to fluid resuscitation but then leveled off at 2-h and 4-h time points. C1QA, a part of the C1qrs complex that cleaves C2 and C4, decreased immediately after fluid resuscitation. Mannose binding lectin 1, one of the proteins involved with initiating the lectin complement pathway in response to pathogenic carbohydrates, successively increased following polytrauma and then fluid resuscitation. Like the C1qrs complex, the lectin pathway results in the cleavage of C2 (C2b and C2a) and C4 (C4a and C4b), constituting the convergence of both pathways to cleave C3 or C5 to form the membrane attack complex. Remaining dysregulated complement cascade proteins in our dataset included increased levels of clusterin, which blocks membrane attack complex formation, and decreased levels of C4BP, which blocks the C3 activation complex C4b2a.
The majority of coagulation factors did not change significantly with time, with a few exceptions. Coagulation factor IX (F9), which activates factor X as part of the intrinsic coagulation pathway, increased from Baseline up to 2 h, after which it decreased slightly. Von Willebrand factor increased following polytrauma relative to Baseline and remained elevated. Plasminogen, the precursor for plasmin that degrades fibrin clots, decreased following resuscitation. Plasma serine protease inhibitor, a protease inhibitor that inhibits the activation of several coagulation factors including protein C (PROC),30 decreased following polytrauma and fluid resuscitation. Finally, kininogen-1, which inhibits thrombocyte formation and releases the potent vasodilator bradykinin,31 demonstrated subsequent increased levels particularly immediately following fluid resuscitation.
3.4 Effect of vitamin C on plasma protein dynamics
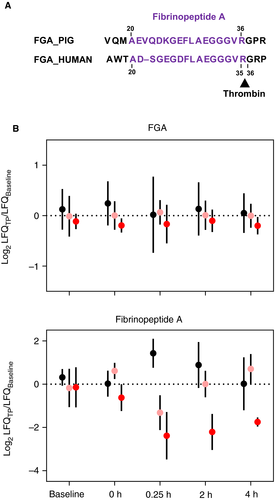
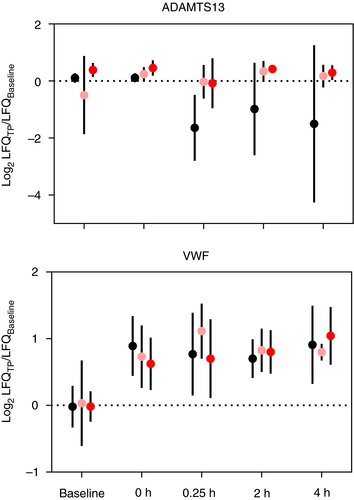
The benefits of high-dose VitC in improving patient outcomes in severe hemorrhagic trauma, shock, and sepsis have been increasingly recognized but the mechanism(s) of action remain largely unknown.18, 21-23, 32 We identified 21 proteins that were significantly different (P < .10) with both time and intravenous VitC treatment (Tables 1 and S6; Figure S1). Several proteins involved with complement activation were modified by Vit C treatment, including three complement C1q subcomponents (C1QA, C1QB, and C1QC). Each subunit was relatively stable through all five time points in the Lo-VitC and Hi-VitC animals, whereas the saline-treated animals showed reduced levels post resuscitation (Figure S1). The pig complement factor 4B homologue (C4_BLAST) showed ~2-fold and ~4-fold increases relative to Baseline at 4 h post fluid resuscitation for the Lo-VitC and Hi-VitC treatments, respectively, whereas the saline-treated animals showed limited change (Figure S1). Lectin complement pathway activation factors mannose-associated serine protease 1/complement component MASP (MASP1) (P = .096) and mannose-binding protein A (MBL1) (P = .089) both showed modest time and treatment effects. Among the remaining proteins in Table 1, superoxide dismutase, fibrinopeptide A (FibA), and a disintegrin and metalloprotease with thrombospondin type 13 showed time and treatment effects with important links to polytrauma phenotypes. First, superoxide dismutase is an important extracellular antioxidant and anti-inflammatory agent that showed no substantial difference in saline-treated animals relative to Baseline, yet it was ~1.5-fold and ~2-fold higher in the Lo-VitC-treated and Hi-VitC-treated animals, respectively (Figure S1). Fibrinopeptide A plasma levels, which are an indirect measure of clot formation via the thrombin-induced cleavage of the fibrinogen alpha chain (Figure 4A), were significantly lower in Lo-VitC and Hi-VitC animals and higher in saline-treated animals relative to Baseline post fluid resuscitation (Figure 4B). Finally, circulating levels of ADAMTS13 are maintained with Lo-VitC and Hi-VitC treatments relative to Baseline whereas the saline-treated animals experienced decreases of ~4-fold post fluid resuscitation (Figure 5).
| UniProt ID | Gene | Protein | P value |
|---|---|---|---|
| I3LKV5 | ADAMTS13 | ADAM metallopeptidase with thrombospondin type 1 motif 13 | .0556 |
| F1S574 | AMY2 | Alpha-amylase | .0155 |
| A0A286ZPG0 | APOC4 | Apolipoprotein C-IV precursor | .0840 |
| A0A287BNF7 | C1QA | Complement C1q subcomponent subunit A | .0240 |
| A0A286ZKA5 | C1QB | Complement C1q subcomponent subunit B precursor | .0526 |
| A0A286ZSJ7 | C1QC | ComplementC1q subcomponent subunit C precursor | .0401 |
| I3LKJ7 | C4Ba | Uncharacterized protein (Complement 4B)a | .0657 |
| P28491 | CALR | Calreticulin | .0992 |
| P14460 | FibA | Fibrinogen alpha chain (Fragment) | .0294 |
| A0A287BKJ2 | FGL1 | Fibrinogen like 1 | .0510 |
| A0A2C9F356 | HP | Haptoglobin | .0228 |
| A0A286ZV86 | IgG-like | Uncharacterized protein | .0413 |
| D5L7X4 | MASP1 | Complement component MASP3 | .0960 |
| Q5U9S1 | MBL1 | Mannose-binding protein A | .0887 |
| F1RRT2 | MYL4 | Myosin light chain 4 | .0242 |
| A0A287AVQ3 | PLCE1 | Phosphoinositide phospholipase C | .0815 |
| A0A287BPP9 | PSMB1 | Proteasome subunit beta type | .0434 |
| A0A287AQB6 | PTPN11 | Tyrosine-protein phosphatase non-receptor type | .0493 |
| P50447 | SERPINA1 | Alpha-1-antitrypsin | .0494 |
| I3LUD1 | SOD3 | Superoxide dismutase [Cu-Zn] | .0988 |
| A0A287AVF2 | TNC | Tenascin | .0001 |
- a Indicates gene name was inferred from BLAST search against UniProt Human Database.
4 DISCUSSION
The global effect of polytrauma followed by fluid resuscitation on the plasma proteome is poorly characterized in humans. This study was an extension of our previous investigation that showed VitC therapy resulted in less end-organ tissue damage, less inflammation related to interleukins 1β and 8, tumor necrosis factor α, and modest correction of coagulation dysfunction in a pig polytrauma model.23 The current study explored the potential mechanism(s) of the protective effect of VitC, as seen through the plasma proteome. One potential limitation is that following completion of the study, new Tactical Combat Casualty Care guidelines were published by the Joint Trauma System of the Department of Defense that now recommend, when possible, blood products be administered for battlefield resuscitation.33 However, hydroxyethyl starch, which was used in our experiments, is still recommended when blood products are not available, or when logistically not possible to administer on the battlefield, which is the clinical scenario we were trying to mimic. Second, this was not a clinical study of fluid resuscitation per se, but rather a study with the stated goal of testing efficacy of our proposed therapeutic (VitC) to reduce inflammation and coagulation dysfunction.
Previous studies in trauma have identified proteins that are indicative of TIC pathophysiology and patient outcomes.9 In human trauma, decreased circulating levels of PROC and increases in activated protein C are associated with acute traumatic coagulopathy.34-36 These perturbations led to increases in fibrinolysis, extended prothrombin time and partial thromboplastin time, and depletion of several coagulation factors. However, PROC levels in our study did not demonstrate statistically significant changes with time or time-treatment interactions. Furthermore, the predicted thrombin cleavage site of pig PROC based on the human form (HUMAN_PROC200-211) occurs at two arginine residues (PIG_PROC98-213) that would be targeted by trypsin in our proteomic workflow. Thus, we would not be able to detect the activated PROC unambiguously in this study. However, the primary inhibitor of PROC activation, plasma serine protease inhibitor,30 decreased with time (Figure 3) indirectly suggesting the conditions for activated PROC are present.
Two coagulation factors showed statistically significant differences: coagulation factor IX (F9) with time (P = .02) and fibrinopeptide A(FibA) with time × treatment (P = .03; Table 1). Factor IX levels in plasma did not change significantly between Baseline and 0 h (Figure 2; Table S3) despite previous studies in humans that showed decreases in F9 with trauma severity.36 However, F9 levels increased ~1.5-fold immediately following fluid resuscitation (Figure 3A; Cluster 6 and Table S5) before recovering to Baseline levels at 4 h. The levels of other coagulation factors I, II, V, VIII, X, XI, XII, and XIII detected in this study did not change, even with fluid resuscitation. No mechanistic precedent is known to exist for the increase of F9, but it is assumed to be related to a dissociation-based mechanism of existing F9 rather than an increase in biosynthesis, which would occur primarily in the liver.37 Fibrinopeptide A is not a coagulation factor per se but rather a byproduct of fibrinogen clot formation. Activated thrombin (i.e., coagulation factor IIa) cleaves coagulation factors fibrinogen, PROC, V, and XI in the coagulation cascade. In the case of fibrinogen, thrombin cleaves the fibrinogen alpha chain (FGA) at the N-terminus to produce the N-terminal peptide FibA (Figure 4A). Thus, circulating levels of FibA is a useful indicator for fibrin clot formation.38, 39
Pig FibA shares significant sequence homology to humans (Figure 4A) with the thrombin cleavage site to the C-terminal side of Arg36. However, the assignment of FibA as a FGA “fragment” is confounded by the fact that it could originate from endogenous thrombin cleavage or exogenous trypsin cleavage during sample preparation as both enzymes share the Arg36 cleavage site. The relative LFQ values for FibA and FGA are plotted as a function of time in Figure 4B. Despite no statistically significant change for FGA levels over time, FibA levels in the three treatment groups differ significantly (P = .03). The saline-treated animals show >2-fold increase post fluid resuscitation versus >2-fold decrease for Lo-VitC and Hi-VitC. The >2-fold lower levels of FibA persist at 4 h in the Hi-VitC animals, whereas FibA levels return to baseline at 2 h in the Lo-VitC-treated and saline-treated animals. As mentioned, the interpretation of the FibA data is confounded by the shared thrombin and trypsin cleavage site, raising the question of whether the measured peptide is endogenous or is exogenously produced. Second, the sample preparation used a 10-kDa filter in the filter-assisted sample preparation method25 which means in principle any endogenously produced FibA would have been removed prior to trypsin digestion. Thus there could be multiple interpretations of the FibA levels in Figure 4B. One interpretation is that the endogenous FibA was removed and that FibA was generated by tryptic digest of intact FGA. This would suggest more clot formation in VitC-treated versus saline-treated animals. A second interpretation is that sufficient amounts of FibA were retained during the filtering process and that the LC-MS/MS measured endogenous and residual intact FGA exogenous FibA. This would suggest less clot formation in VitC-treated versus saline-treated animals. At present there is insufficient direct evidence in our dataset to support one over the other interpretation, but the importance of clot formation in polytrauma and the clear effect of VitC warrant further studies.
An unexpected finding in this study was the discovery that high-dose VitC affected ADAMTS13 levels post fluid resuscitation (Table 1; Figure 5). Resuscitation with saline resulted in a ~4-fold decrease in ADAMTS13 relative to baseline levels, whereas both Lo-VitC-treated and Hi-VitC-treated animals had relatively stable ADAMTS13 levels over the 6-h study. Several studies in humans and animal models have reported decreases in ADAMTS13 levels and activity as a result of trauma,40 sepsis,41-44 and fluid resuscitation.45 Russel et al40 recently showed in 106 pediatric patients with severe trauma that ADAMTS13 levels were significantly lower upon admission and at 24 h compared to a control group of pediatric patients without trauma. Importantly, those with the lowest ADAMTS13 levels died from their injuries. Bockmeyer et al44 showed using a pig polytrauma/sepsis model with fluid resuscitation (Ringers's and HAES) that ADAMTS13 activity decreased from 2 to 12 h post sepsis induction.
ADAMTS13 is the enzyme responsible for cleaving VWF multimers46-50 in the A2 collagen binding region. Following tissue injury, acute phase stimuli (e.g., inflammatory cytokines, thrombin, and fibrin) trigger the secretion of VWF from Weibel Palade bodies to bind at the site of injury and serve as a binding scaffold for platelets during clot formation and tissue repair. The ADAMTS13-induced trimming of VWF multimers maintains a critical balance between hemostasis and thrombosis that is perturbed in thrombotic thrombocytopenic purpura, thrombotic microangiopathy, and sepsis-induced disseminated intravascular coagulation.51 When the ADAMSTS13/VWF ratio is unbalanced, the unchecked formation of ultralarge VWF multimers leads to blockage of microcapillaries, tissue damage, and potentially to organ failure. Notably, while these differences were noted in this subanalysis of our larger dataset, the multivariate analysis in the primary study23 showed that the VitC groups could be clearly distinguished from the normal saline group.
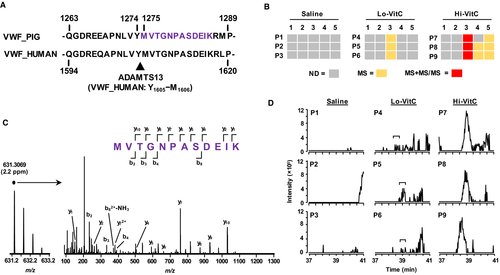
In the current study, VWF increased by ~2-fold following polytrauma injury relative to baseline and then remained at that relative level for the duration of the study (Figure 3A, cluster 7) with no statistically significant treatment effect (P = .72; Table S6 ) or change immediately following volume resuscitation. As previously discussed, the bottom-up proteomic measurements used in this study rely on trypsin-induced proteolysis of the plasma proteomes prior to LC-MS/MS analysis. A consequence of this approach is that the intact form(s) of proteins including activated forms or protein-protein complexes can be lost or confounded (e.g., FGA vs. FibA). However, in humans, ADAMTS13 fortuitously cleaves VWF at a non-tryptic -Tyr1605-Met1606- that resides in the middle of a full tryptic peptide (Figure 6A). Sequence alignment with the pig form of VWF shows a near-identical amino acid alignment including the -Tyr1274-Met1275- cleavage site. A new analysis of the plasma proteomic dataset that included -YM- enzyme cleavage identified the VWF semi-tryptic peptide (VWF1275-1286) in the Hi-VitC treatment group at 0.25 h (Figure 6B). Representative MS and MS/MS evidence for the VWF1275-1286 peptide from one of the Hi-VitC-treated animals at 0.25 h is shown in Figure 6C. The sole presence of MS/MS data in the Hi-VitC-treated animals at 0.25 h is due to the fact that its abundance was sufficiently high to trigger a data-dependent scan. This is confirmed when examining the extracted ion chromatograms (631.305 ± 0.005 m/z) for all 0.25-h plasma samples (Figure 6D). Low levels of VWF1275-1286 without MS/MS evidence were observed in the Lo-VitC animals at 0.25 h (Figure 6B, D) and in the Hi-VitC animals at 2 h and 4 h (Figure 6B). Importantly, the plasma concentrations of VitC in the Hi-VitC-treated animals are approximately the same as those of the Lo-VitC animals at 0.25 h.23 Thus, the LC-MS/MS data provide direct evidence that VitC affects ADAMTS13 cleavage of VWF multimers.
In conclusion, quantitative LC-MS/MS analysis of the pig plasma proteome resulted in the identification of 136 proteins that changed significantly pre and post resuscitation. Furthermore, the coadministration of high-dose VitC during fluid resuscitation was found to affect 21 proteins involved with coagulation, complement cascade, and oxidative stress. The most significant finding in this study with regard to high-dose VitC treatment was the stabilization of ADAMTS13 plasma levels relative to saline while also enhancing ADAMTS13-induced proteolytic cleavage of VWF multimers. These data suggest one of potentially many mechanisms by which high-dose VitC improves patient outcomes in acute care settings.
ACKNOWLEDGEMENTS
This research was supported by United States Department of Defense USAMRMC Contract Number W81XWH-15-2-0064 to R. Natarajan. E. K Cudjoe Jr. was supported in part by a NIH Training Grant (R25HL128639-04). The sponsor had no role in study design, data collection, analysis, and interpretation; writing the manuscript; or the decision to submit the article for publication.
AUTHOR CONTRIBUTION
R. Natarajan, P. S. Reynolds, A. A. Fowler, and A. M. Hawkridge designed the study. R. Natarajan, P. S. Reynolds, B. J. Fisher, J. McCarter, C. Sweeney, P. Middleton, and M. Ellenberg carried out all animal husbandry, surgery, and blood collection. E. K. Cudjoe, Z. H. Hassan, and A. M. Hawkridge performed all proteomic sample preparation, LC-MS/MS analysis, and proteomic data analysis. L. Kang and A. M. Hawkridge performed statistical analysis of the proteomic data. A. M. Hawkridge, E. K. Cudjoe, Z. H. Hassan, and L. Kang prepared the figures and tables. E. K. Cudjoe, Z. H. Hassan, D. F. Brophy, R. Natarajan, and A. M. Hawkridge wrote the manuscript. All authors reviewed and edited the manuscript.
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.