The European Insomnia Guideline: An update on the diagnosis and treatment of insomnia 2023
Summary
Progress in the field of insomnia since 2017 necessitated this update of the European Insomnia Guideline. Recommendations for the diagnostic procedure for insomnia and its comorbidities are: clinical interview (encompassing sleep and medical history); the use of sleep questionnaires and diaries (and physical examination and additional measures where indicated) (A). Actigraphy is not recommended for the routine evaluation of insomnia (C), but may be useful for differential-diagnostic purposes (A). Polysomnography should be used to evaluate other sleep disorders if suspected (i.e. periodic limb movement disorder, sleep-related breathing disorders, etc.), treatment-resistant insomnia (A) and for other indications (B). Cognitive-behavioural therapy for insomnia is recommended as the first-line treatment for chronic insomnia in adults of any age (including patients with comorbidities), either applied in-person or digitally (A). When cognitive-behavioural therapy for insomnia is not sufficiently effective, a pharmacological intervention can be offered (A). Benzodiazepines (A), benzodiazepine receptor agonists (A), daridorexant (A) and low-dose sedating antidepressants (B) can be used for the short-term treatment of insomnia (≤ 4 weeks). Longer-term treatment with these substances may be initiated in some cases, considering advantages and disadvantages (B). Orexin receptor antagonists can be used for periods of up to 3 months or longer in some cases (A). Prolonged-release melatonin can be used for up to 3 months in patients ≥ 55 years (B). Antihistaminergic drugs, antipsychotics, fast-release melatonin, ramelteon and phytotherapeutics are not recommended for insomnia treatment (A). Light therapy and exercise interventions may be useful as adjunct therapies to cognitive-behavioural therapy for insomnia (B).
1 SUMMARY FOR PATIENTS
1.1 What is insomnia?
Insomnia is a sleep disorder where people struggle to get off to sleep or to stay asleep. Some individuals have both issues, and others may also have early-morning awakenings, where they are unable to get back to sleep after awakening earlier than desired. Importantly, these night-time sleep difficulties are coupled with significant daytime problems that affect the person's ability to function at their best. Daytime fatigue, low mood or irritability, and problems with attention or concentration are usually experienced. To be diagnosed with an “insomnia disorder”, these difficulties have to occur at least several times a week over a period of 3 months. Insomnia is a very common disorder (up to 10% of the adult population in Europe) and, in addition to a great deal of personal suffering, it also results in increased costs to healthcare services and to society at large (e.g. reduced productivity at work).
1.2 How can insomnia be treated?
Currently, there are two ways to treat insomnia. According to scientific evidence the first, and the most effective, approach is cognitive-behavioural therapy for insomnia (CBT-I). As the name suggests, CBT-I addresses the mental or cognitive aspects of insomnia (e.g. the racing mind), and the behavioural aspect reestablishes a healthy sleep pattern. CBT-I can be offered as single or group therapy (face-to-face [F2F]) or as a digital therapy, where it is delivered as a web-based intervention or on a treatment-based app. The second approach to treat insomnia is pharmacological (i.e. pill-based). There is a variety of sleep medications available, but it is recommended that these are only taken for a short period (no longer than 4 weeks) to avoid the body getting used to them or becoming dependent upon them. In some cases, after weighing the advantages and disadvantages, some medications may be given for longer periods of time.
1.3 Who developed this guideline?
This European Insomnia Guideline 2023 was developed by a group of researchers and clinicians in the European Sleep Research Society (ESRS), and the European Insomnia Network (EIN).
1.4 Which treatment is recommended by this guideline?
It is recommended that all patients with insomnia, whether they have other medical conditions or mental health problems, or not, are offered CBT-I as their initial treatment. CBT-I may be delivered by a clinician or therapist (F2F), or (preferably guided) digitally using a scientifically demonstrated web or mobile treatment platform. If this approach is not sufficiently effective, it is recommended that patients and their treating physicians should come to a shared decision about whether or not medication might be initiated. At present, the evidence suggests that drug treatments in general should be limited to, at most, 4 weeks in duration, and even then with care: tolerance develops within days to weeks. Dose increases are not advised, and may accelerate the development of dependence. In some cases, longer treatment periods may be indicated, carefully weighing the advantages and disadvantages.
2 GUIDELINE REPORT
This guideline is an update of the European Insomnia Guideline that was published in 2017 (Riemann, Baum, et al., 2017), and developed by a task force of the ESRS and the EIN. The European Insomnia Guideline was based on the German Insomnia Guideline (Riemann, Baglioni, et al., 2017) and was endorsed by the World Sleep Society (Morin et al., 2021). A revision of the German Insomnia Guideline is underway (Spiegelhalder et al., 2023). This first update of the European Insomnia Guideline is inspired by and draws upon this revision of the German Insomnia Guideline.
This guideline focuses on the target population of adult patients suffering from chronic insomnia as defined by the International Classification of Diseases (ICD-10/ICD-11). This includes all subtypes of insomnia, for example, non-organic insomnia/chronic insomnia and insomnia comorbid with somatic (formerly named “organic” insomnia) or mental disorders. The guideline addresses adult patients over the age of 18 years. The literature on insomnia in children and adolescents was not reviewed.
The guideline is based on a review of all relevant international literature and has a particular salience to the provision of clinical services across Europe. It will be of interest to health professionals who are involved in the diagnosis and treatment of insomnia on either an out- or in-patient basis. Most insomnia is managed by general practitioners in primary care settings, and by clinicians who are not specialists in sleep medicine. The guideline should also be helpful to such individuals (i.e. who treat insomnia in routine clinical care without access to advanced expertise or facilities). The guideline should also be useful to specialists in psychiatry, clinical psychology/psychotherapy, psychosomatic medicine, neurology, occupational medicine, pharmacy/pharmacology and other medical specialties who commonly see patients with insomnia in the context of other comorbid physical and mental health conditions. Finally, the guideline will be especially relevant to professionals trained in sleep medicine and who are members of, or are credentialled by, the ESRS.
The revised guideline highlights aspects of clinical management that reflect advances in knowledge and practice that can be delineated from the updated evidence. Accordingly, less emphasis is placed upon detailed reproduction of extant information that is already outlined in the 2017 version (Riemann, Baglioni, et al., 2017).
2.1 Literature search
The 2023 update was designed to build upon scientific knowledge and clinical recommendations from the first guideline, which covered evidence up to June 2016 (Riemann, Baglioni, et al., 2017; Riemann, Baum, et al., 2017). This strategy therefore aimed to both complement and extend previous literature searches, while applying a consistent methodology.
Therefore, to identify relevant studies on the topic of insomnia, a systematic literature search (English language articles only) was conducted using the databases Pubmed and Cochrane Library (www.cochranelibrary.com) for the period from June 2016 until October 2022 (with a further update till May 2023 added). For this update, primarily meta-analyses were identified as the basis for grading recommendations. If there were several meta-analyses on a given topic, the most recent and qualitatively better meta-analyses were chosen to be presented in the first instance. The quality of meta-analyses was judged by methodological rigour, like low risk of bias, number of included studies or sample sizes. For topics without published meta-analyses, systematic reviews or qualitatively adequate randomised controlled studies were used.
The following keywords were used for literature search:
For the area of non-pharmacological treatments, the keyword “insomnia” was searched in connection with other keywords: “sleep hygiene”, “relaxation”, “mindfulness”, “behavior therapy”, “cognitive therapy”, “cognitive behavioral therapy”, “stimulus control”, “sleep restriction”, “psychotherapy”, “light therapy”, “exercise”, “music”, “non-invasive brain stimulation”.
For the area of pharmacological treatments, the keyword “insomnia” was searched in connection with other keywords: “benzodiazepine”, “benzodiazepine receptor agonist”, “sedating antidepressant”, “antipsychotic”, “neuroleptic”, “orexin”, “antihistaminic”, “herbal”, “phytotherapy”, “melatonin”.
Furthermore, the journal Sleep Medicine Reviews was hand searched for meta-analyses on the diagnosis and treatment of insomnia. All issues of this journal until October 2022 were incorporated, furthermore articles were incorporated that were “in press”.
2.2 Writing and consensus
The first draft of this update was formulated and written by Dieter Riemann and Kai Spiegelhalder, following partly, and where adequate, the update of the German insomnia guideline (Spiegelhalder et al., 2023). In the next step, all involved authors received the first draft of the guideline (15 April 2023) and were asked to provide feedback within a period of 4 weeks (15 May 2023). After receiving feedback and incorporating suggested changes/improvements in the second draft of the guideline, two online meetings were held on 21 July and 1 August 2023 to discuss this version of the guideline and to reach consensus. A third draft of the guideline was then sent out asking for final consent from all authors by 7 August 2023. Finally, the guideline was approved by the guideline committee of the ESRS and by the ESRS board, before submission to Journal of Sleep Research (JSR).
2.3 Grading of the evidence/recommendations
In order to grade the evidence of included studies/meta-analyses to update the recommendations in the guideline, a procedure similar to that already outlined in 2017 (Lorenz et al., 2001; Riemann, Baglioni, et al., 2017; Riemann, Baum, et al., 2017) was followed (Table 1).
| Type of study | Level of evidence | Grade of recommendation |
|---|---|---|
| Systematic review/meta-analysis of RCTs | 1a | A |
| One high-quality RCT | 1b | A |
| “All or nothing principle” | 1c | B |
| Systematic review of high-quality cohort studies | 2a | B |
| One cohort study/RCT with adequate design but moderate data quality | 2b | B |
| Outcome research studies | 2c | B |
| One systematic review of case control studies | 3a | B |
| One case control study | 3b | B |
| Case series/cohort or case control studies of moderate quality | 4 | C |
| Expert opinion, etc. | 5 | D |
- Abbreviation: RCT, randomised–controlled study.
The transformation of grades of evidence into grades of recommendations was performed according to this scheme and through consensus decision between all involved authors. For more details, see supplemental material in Riemann, Baum, et al. (2017). Instead of only using two types of recommendations (strong versus weak; Riemann, Baum, et al., 2017), we used four steps of recommendations for this update, ranging from A (very strong recommendation), B (strong), C (weak) to D (very weak recommendation). Please note; levels of evidence do not always directly translate into grades of recommendation, as suggested in Table 1; in some cases, a consensus decision became the decisive factor for the grading of the recommendation.
Reported effect sizes from the meta-analyses were graded as follows: effect sizes (Cohen's D) ≥ 0.2– < 0.5: small effect; effect sizes ≥ 0.5– < 0.8: medium effect; effect sizes ≥ 0.8: large effect.
3 DIAGNOSIS OF INSOMNIA
The 2017 version of this guideline reflected upon marked advances in understanding insomnia as a disorder in its own right (insomnia disorder), that had been recommended by the DSM-5 (Diagnostic and Statistical Manual of The American Psychiatric Association; APA, 2013) and the third edition of ICSD-3 (International Classification of Sleep Disorders; AASM, 2014). Practicing clinicians in most European countries, however, generally have to adhere to the International Classification of Diseases (ICD: World Health Organisation). It is noteworthy, therefore, that the latest revision (ICD-11) was endorsed by the World Health Assembly at its 72nd meeting in 2019 and came into effect globally on 1 January 2022 (WHO, 2022). Whereas the previous version (ICD-10; WHO, 1994) differentiated “non-organic” versus “organic insomnia”, this distinction was abandoned as not evidence based in ICD-11 in favour of the comprehensive category “chronic insomnia” (Code 7A00; and “short-term insomnia” for “transient insomnia”). Criteria for insomnia disorder according to DSM-5, ICSD-3 and ICD-11 are now broadly aligned, and are depicted in Tables 2 and 3.
|
| 1. Difficulty initiating sleep |
| 2. Difficulty maintaining sleep |
| 3. Waking up earlier than desired |
| 4. Resistance to going to bed on appropriate schedule |
| 5. Difficulty sleeping without parent or caregiver intervention |
|
| 1. Fatigue/malaise |
| 2. Attention, concentration or memory impairment |
| 3. Impaired social, family, occupational or academic performance |
| 4. Mood disturbance/irritability |
| 5. Daytime sleepiness |
| 6. Behavioural problems (e.g. hyperactivity, impulsivity, aggression) |
| 7. Reduced motivation/energy/initiative |
| 8. Proneness for errors/accidents |
| 9. Concerns about or dissatisfaction with sleep |
|
|
|
| F.The sleep/wake difficulty is not better explained by another sleep disorder |
| (https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/323148092) |
| Chronic insomnia is a frequent and persistent difficulty initiating or maintaining sleep that occurs despite adequate opportunity and circumstances for sleep, and that results in general sleep dissatisfaction and some form of daytime impairment. Daytime symptoms typically include fatigue, depressed mood or irritability, general malaise, and cognitive impairment. The sleep disturbance and associated daytime symptoms occur at least several times per week for at least 3 months. Some individuals with chronic insomnia may show a more episodic course, with recurrent episodes of sleep/wake difficulties lasting several weeks at a time over several years. Individuals who report sleep-related symptoms in the absence of daytime impairment are not regarded as having an insomnia disorder. If the insomnia is due to another sleep–wake disorder, a mental disorder, another medical condition, or a substance or medication, chronic insomnia should only be diagnosed if the insomnia is an independent focus of clinical attention. |
In this update we will not comment on aetiological and/or pathophysiological concepts of insomnia, as this would go far beyond the remit of this guideline. The interested reader is referred to recent work, much of which is part of the special insomnia issue of JSR (Dressle & Riemann, 2023; Espie, 2023; Fernandez & Perlis, 2023; Palagini et al., 2023; Reffi et al., 2023; Riemann et al., 2022; Tang et al., 2023; van Someren, 2021).
An overview of the relevant diagnostic procedures is given in Table 4.
| General anamnesis and examination (A) |
|
|
|
|
|
|
| Sleep history (A) |
|
|
|
|
| Actigraphy |
|
|
|
| PSG |
|
|
|
- Abbreviation: PSG, polysomnography.
3.1 Insomnia complaint
The diagnosis of chronic insomnia disorder according to DSM 5, ICSD-3 and ICD-11 continues to be solely based on taking a clinical history (anamnesis) and the patient's self-reports of sleep-onset or sleep maintenance difficulties, or early-morning awakening. Likewise, clinical consideration of whether or not these sleep symptoms are coupled with dissatisfaction with sleep and any attributed daytime impairment is mandatory, as well as that symptoms occur despite adequate time allotted for sleep and the opportunity to sleep in a comfortable environment. Quantitative criteria related to sleep-onset latency, sleep duration or the frequency of nocturnal awakenings do not have to be fulfilled in order to diagnose insomnia disorder. The complaint of insomnia occurring at least three nights per week for 3 months with associated daytime sequelae represents minimal criteria for this clinical evaluation of chronic insomnia disorder.
3.2 Sleep diaries and questionnaires
As recognised in the 2017 guideline (Riemann, Baglioni, et al., 2017), the evaluation of insomnia should be supported by the use of a sleep diary for a period of at least 7–14 days (see consensus sleep diary: Carney et al., 2012). The Insomnia Severity Index (ISI; Bastien et al., 2001) is an established tool to gauge the severity of the insomnia. The ISI can range with a score from 0 to 28. Values from 8 to 14 suggest subclinical insomnia, values from 15 to 21 moderately severe insomnia, and values from 22 to 28 severe insomnia. The authors recommend a cut-off score of 10 for caseness in community settings, and a change score of 8.4 as a sign of moderate improvement (Morin et al., 2011). The ISI has a two-item version that can be used for weekly monitoring or other situations where a shorter version may be needed (Kraepelien et al., 2021) The eight-item Sleep Condition Indicator (SCI), based upon DSM-5 criteria for insomnia disorder, is an alternative instrument to the ISI (Espie, Kyle, Hames, et al., 2014). The SCI also has a two-item short-form version (Luik et al., 2019), and has age and sex reference data on a sample of 200,000 adults (Espie et al., 2018), with a Reliable Change Index of 7 scale points. The frequently used Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989) may be helpful to gain a broader picture of sleep-related symptoms that can be relevant for the judgement of insomnia. The PSQI delivers values from 0 to 21, with values above 5 supposedly reflecting clinically relevant sleep impairments. It should be noted, however, that the PSQI is not a measure of insomnia disorder, but it broadly asks about symptoms from a range of other sleep disorders.
In the meantime, a variety of questionnaires has become available, not only to measure insomnia, but for many other aspects of other sleep disorders (the interested reader is referred to Shahid et al., 2012). A single comprehensive and validated questionnaire for sleep and daytime impairment for clinical use has not been developed yet, to our knowledge, and is urgently needed.
3.3 Actigraphy
Although the literature on actigraphy continues to expand, and its availability may become more pervasive, there is limited support for the mandatory application of actigraphy in the routine clinical evaluation and diagnosis of insomnia. Medical grade actigraphic devices may be used to evaluate “free living” bedtimes and sleep times, and their variability, at a rather low cost for longer periods of time, and so have particular application where a circadian rhythm sleep–wake disorder is suspected (Smith et al., 2018). According to two systematic reviews, many devices based on actigraphy underestimate the severity of sleep disorder symptoms and overestimate sleep duration compared with polysomnography (PSG; Kolla et al., 2016; Smith et al., 2018). Rösler et al. (2023) concluded that actigraphy was not able to differentiate patients with insomnia from good sleepers. Actigraphy may be primarily helpful in a differential-diagnosis, to identify irregular bedtime patterns—actigraphy, however, does not deliver a valid estimation of sleep stages like the PSG.
Further, there are numerous lifestyle products available, in the form of watches or similar wearable devices, that incorporate some form of actigraphic and/or heart rate measurement. However, the validity and utility of such devices for the diagnosis of insomnia has not been satisfactorily researched or demonstrated at this point in time (Kolla et al., 2016). It has even been suggested that using these devices might have a negative impact on sleep behaviour.
3.4 Polysomnography (PSG)
It is well established that PSG is indicated when there is clinical suspicion of other sleep disorders like periodic limb movement disorder (PLMD) or any kind of sleep-related breathing disorders (SRBD; Crönlein et al., 2012), such as obstructive sleep apnea (OSA). Because insomnia does not protect against, or exclude, such disorders, PSG is equally indicated if clinical suspicion is present in people with insomnia. However, PSG is not necessary or sufficient for the diagnosis of insomnia per se (Dikeos et al., 2023). A meta-analysis of the PSG literature in insomnia showed that, as compared with controls without sleep complaints, patients with insomnia have a significant reduction in sleep efficiency, a reduction in total sleep time, an increase in nocturnal wake times, and reductions in slow-wave sleep and rapid eye movement (REM) sleep (Baglioni et al., 2014). These differences compared with good sleepers, however, are considerably lower than would be expected from subjective (i.e. sleep diaries/questionnaires) measures of sleep. Moreover, similar deviations can be found in several other sleep disorders and other conditions, resulting in insufficient specificity. Although it has been suggested that feedback about any discrepancy between physiological and objective and subjective measures might be used therapeutically (Tang & Harvey, 2006), CBT-I and all other treatments are substantially effective for insomnia disorder in the absence of such data. We recommend that PSG should also be performed in patients with insomnia who have been refractory to various therapeutic interventions (CBT-I and/or hypnotics) with the aim to detect hitherto occult sleep disorders, in patients at risk for tiredness/fatigue-related accidents (i.e. professional drivers, etc.: to gauge the extent of sleep deprivation) and in patients where a huge mismatch between subjective and objective findings is suspected. Riemann et al. (2022) have outlined that a potential explanation for the discrepancy between subjective and objective findings may be via an altered sleep microstructure in insomnia, including an increased frequency of micro-arousals (especially in REM sleep) and increased amounts of fast-frequency waves (in the sigma and beta-band).
3.5 Medical evaluation
Table 5 lists a wide range of medical, mental, neurological disorders and substances, which are implicated in this context (please note: probably any kind of medical disease might be involved as a causative or contributive factor for insomnia).
| Mental | Medical | Neurological | Substance use/dependence |
|---|---|---|---|
| Depressive disorders | Cardiovascular disorders | Neurodegenerative diseases | Alcohol |
| Bipolar disorders | Diabetes mellitus | Cerebrovascular diseases | Nicotine |
| Anxiety disorders | Chronic kidney diseases | Traumatic brain injury | Caffeine |
| Borderline personality disorder | Chronic obstructive pulmonary diseases | Multiple sclerosis | Tetrahydrocannabinol /marihuana |
| Posttraumatic stress disorder | Rheumatic disorders | RLS/PLMD | Opioids |
| Schizophrenia | Chronic pain | Fatal familial insomnia | “Designer” drugs |
| Substance use disorders | Any kind of malignant disorder | Cocaine | |
| SRBD/OSA | Amphetamines |
- Abbreviations: OSA, obstructive sleep apnea; PLMD, periodic limb movement disorder; RLS, restless legs syndrome; SRBD, sleep-related breathing disorder.
A thorough physical examination is therefore indicated in patients with insomnia with suspected comorbidities, to be able to exclude specific medical disorders that are in need of specific treatment and for, when necessary, treating insomnia as a comorbid condition. The historical and now outdated clinical perspective, that insomnia is primarily a symptom, was strongly challenged by evidence that chronic insomnia is an independent risk factor for mental health conditions such as depression or anxiety disorders (Baglioni et al., 2011; Hertenstein et al., 2019). Indeed, almost any kind of mental disorder is frequently accompanied by insomnia or can contribute to a worsening of insomnia symptoms (Table 5). Many patients with insomnia suffer comorbidly from another mental disorder, which may be stigmatised and therefore will not be reported spontaneously by patients. Therefore, within the context of the clinical evaluation of insomnia, any kind of mental disorder should be actively explored.
Insomnia is highly prevalent in such neurological disorders as stroke, Parkinson's disease, epilepsy, headache/migraine and traumatic brain injury (Bassetti et al., 2015, 2020). More recently, insomnia has also been shown to represent an independent risk factor for some neurological disorders such as stroke and dementia (Damsgard et al., 2022; Zheng et al., 2019).
Insomnia is highly prevalant also in medical disorders including diabetes (LeBlanc et al., 2018) and cardiovascular disease/hypertension (Li, Gan, et al., 2021; Li, Li, et al., 2021; Sofi et al., 2014). Indeed, it seems there is a truly bi-directional relationship between sleep disturbances and health conditions, such that insomnia should be evaluated and actively treated in comorbid conditions. Moreover, this underscores medical and psychiatric evaluations being part of a thorough insomnia assessment. Accordingly, normal sleep is recognised as an important predictor of brain and mental health (Bassetti et al., 2015).
Special note must be made of other sleep disorders, like SRBD/OSA, restless legs syndrome (RLS) and PLMD. Only recently has it become evident that there is a considerable percentage of patients with OSA also presenting with insomnia—now termed COMISA (comorbid insomnia and sleep apnea). Recent meta-analyses indicate that 30%–40% of patients with insomnia may have OSA, and 30%–50% of patients with OSA meet insomnia criteria (Zhang, Ren, Zhou, et al., 2019; Sweetman et al., 2021). These data argue for a strict inclusion of an OSA screening tool (e.g. STOP-Bang questionnaire; Chung et al., 2008) in the diagnostic process for insomnia and conversely insomnia screening for each patient with suspected SRBD. If the screening questionnaire is positive for OSA, a home sleep apnea test or PSG is required for a valid diagnosis of OSA. Insomnia is a crucial symptom of many patients with RLS. PLMD extremely frequently accompanies RLS and may contribute independently to the experience of insomnia. To determine the extent to which PLMD/RLS may be contributing to the insomnia, the International Restless Legs Syndrome Study Group Rating Scale (IRLSGR; Abetz et al., 2006) should be used to gauge the occurrence and severity of symptoms. The diagnosis of RLS is mainly a clinical diagnosis, but PLMD requires a PSG.
Given the high comorbidity between insomnia complaints and OSA/SRBD, PLMD and RLS, the diagnostic and differential-diagnostic process should pay utmost attention to delineate the probable bi-directional relationships between insomnia and the “other” sleep disorder—this information should also directly inform the therapeutic process, meaning that in many cases the insomnia and the “other” sleep disorder will be treated simultaneously according to suggested guidelines/articles (Garcia-Borreguero et al., 2016; Sweetman et al., 2023).
Furthermore, a number of substances may lead to the development, maintenance or aggravation of insomnia.
Alcohol consumption may play a prominent role in this context (Perney & Lehert, 2018; Hu et al., 2020; Hertenstein et al., 2019). Frequently, alcohol is also used mal-adaptively as self-medication to self-manage sleep-onset or sleep maintenance problems. Therefore, alcohol consumption and other substances should be specifically probed. Insomnia as a possible side-effect of prescribed medications may occur with a huge variety of substances, including corticosteroids, beta-blockers, beta-sympathomimetics, antibiotics, antidementives, selective serotonin reuptake blockers and probably many more (for an overview, see Riemann & Nissen, 2012; Schierenbeck et al., 2008) impacting on sleep.
It is important to know even in clear cases of the causative/maintaining role of a medical or mental disorder or consumption of a given substance, the typical psychophysiological vicious circle of insomnia may have developed in many patients. This includes anticipatory anxiety of a poor night, nocturnal ruminations, increased psychophysiological arousal and behavioural changes like excessive time in bed. Insofar, these clinical phenomena also may need to be addressed as part of the therapeutic approach towards comorbid insomnias.
Please note that with DSM-5, ICSD-3 and ICD-11, comorbidity of insomnia with any kind of medical disorder is acknowledged—the former hierarchy of primary versus secondary insomnia was abandoned because it lacked sufficient evidence, especially the assumed direct causality between medical disorders and associated insomnia symptoms, which should disappear when the primary disorder is treated, never became evidence-based.
In summary, the diagnostic and differential-diagnostic process for chronic insomnia disorder is complex and may require not only a clinician's direct time, but also the involvement of medical tests and interdisciplinary collaborations between different medical specialties. The process is not made easier due to the intricate bi-directional relationships between insomnia and almost any kind of medical disorder. Comorbidity may be more the rule than the exception, which entails the integration of insomnia treatments under a very broad medical context.
Needless to say, a proper diagnostic and differential diagnostic evaluation has to take place prior to any therapy, be it CBT-I, digital CBT-I (dCBT-I) or any kind of pharmacological treatment.
4 TREATMENT OF INSOMNIA
Up to 2016/2017, published insomnia guidelines tended to favour pharmacotherapy over non-pharmacological approaches. Both fields existed in parallel worlds (i.e. on the one hand medical specialists favouring pharmacotherapy, and on the other hand, mainly clinical psychologists and psychotherapists, favouring CBT-I or other forms of psychotherapy). This antagonism seems to have been reconciled over the last decade (see guidelines/summaries by Quaseem et al., 2016; Wilson et al., 2019; Edinger et al., 2021), and is reflected by the wide authorship of this guideline.
One crucial question when discussing insomnia therapy is the question who and how many patients with insomnia seek help from professional healthcare. Data from Germany (Marschall et al., 2017; data from health insurance) indicate that probably not more than 30% of those afflicted with insomnia have ever sought medical help. Considering over the last 12 months, only 15% indicated having seen a doctor specifically because of their insomnia. These sobering data need to be borne in mind when considering the treatment recommendations of this guideline. Given the bi-directional relationships between insomnia and all medical disorders, a strong case can be made to raise not only public awareness but also of the medical community concerning insomnia.
Concerning the choice of treatment option, we strongly suggest to follow a shared decision-making approach (Bomhof-Roordink et al., 2019). Diagnostic and treatment options should be outlined by the clinician and discussed together with the patient.
4.1 Non-pharmacological treatments
4.1.1 Cognitive-behavioural therapy for insomnia (CBT-I)
The CBT-I is typically provided as a multicomponent treatment comprising psychoeducation (including sleep hygiene), relaxation therapy, sleep-restriction therapy (SRT), stimulus control therapy (SCT) and several cognitive therapeutic strategies. These are administered over four–eight therapy sessions in single or group format by certified health professionals (mainly clinical psychologists/psychological or medical psychotherapists). Baglioni et al. (2022) summarised these treatments in a comprehensive manual/textbook aimed at health professionals involved in the treatment of insomnia. Chapter 2 in this book (pp. 19–41) describes the CBT-I “standard protocol”, and provides detailed information on the scientific basis, therapy rationale and treatment instructions for each CBT-I component (Espie, 2022a). Baglioni, Altena, et al. (2020), have summarised CBT-I training strategies and the dissemination of CBT-I to health professionals. The website of the ESRS lists presently available teaching courses for health professionals in Europe (www.esrs.eu).
The multicomponent delivery approach is typical of the pattern of CBT provision in other common disorders, such as anxiety and depression. That said, substantial early evidence was gathered on single component treatments for insomnia (e.g. progressive relaxation, stimulus control, paradoxical intention, sleep restriction), and there has been a return to evaluating separate components in recent years. This is in keeping with broader moves to “deconstruct” multimodal approaches, generate evidence on specific therapeutics, understand mechanisms of action, and the growth in personalised medicine.
Psychoeducation/sleep hygiene
Psychoeducation: This typically comprises foundational information on the roles and functions of sleep, age-related changes, and how sleep–wake (circadian) patterns are regulated (e.g. the two-process model of sleep regulation; Borbély, 1982). Importantly, psychoeducation is usually integrated into elements of CBT-I rather than being standalone information. For example, the two-process model can be used to explain to patients that “bad nights” are often followed by “good nights” due to sleep homeostasis, serving to reinforce the rationale for sleep restriction and SCT.
Sleep hygiene: These recommendations (first presented by Hauri, 1991) are also reinforced in CBT-I, and comprise advice on bedroom factors (e.g. temperature, light and noise levels, bed comfort) and habits (e.g. caffeine, alcohol, establishing a regular routine, stop clock-watching) that can impact on sleep. It should be noted, however, that sleep hygiene on its own does not constitute an evidence-based treatment for insomnia, and sleep hygiene behaviours sometimes have a function of “safety behaviours” in patients with insomnia, heightening the perceived threat of not being able to sleep.
Recently the “five principles of good sleep health” have been proposed, and these may form a useful introduction to the cognitive-behavioural approach (Espie, 2022b).
Relaxation therapy
Many of the earliest insomnia studies included some form of relaxation intervention to facilitate de-arousal (Baglioni et al., 2022). This was consistent with the widespread adoption, in behavioural psychotherapy, of relaxation as a re-conditioning agent. Empirical research on insomnia suggests that abbreviated progressive muscle relaxation, autogenic training and imagery exercises are most frequently used for the treatment of insomnia. At present there is no evidence that one or the other method is superior, but the range of possibilities provides a multitude of treatment options.
Sleep-restriction therapy (SRT)
The behavioural model of insomnia put forth by Spielman et al. (1987) identified time in bed extension as important in the maintenance of insomnia. Extending time in bed (including napping), often as a compensatory response to poor sleep, can lead to increased sleep latencies, sleep fragmentation, and increased variability in the timing of the sleep–wake pattern. SRT aims to restrict sleep opportunity and harness increased sleep pressure to consolidate sleep and regularise its occurrence (Spielman et al., 1987). Once sleep is consolidated (indexed by sleep efficiency thresholds of > 85% or 90%), the goal is to extend time in bed, often on a weekly basis, to arrive at a sleep opportunity that delivers nightly sleep need, improved sleep continuity, and optimised daytime functioning. There is, however, variation in the configuration of SRT in relation to defining the initial time in bed prescription, minimum time in bed (e.g. 4.5, 5 or 6 hr), position of the sleep window, and weekly titration parameters (Kyle et al., 2015). SRT is hypothesised to work through restricting, regularising and reconditioning sleep opportunity, which drives a cascade in cognitive, behavioural and physiological pathways to improve sleep and daytime functioning (Maurer et al., 2018; Spielman et al., 1987). Evidence from randomised–controlled trials (RCTs) shows that acute implementation of SRT increases sleepiness proximal to bedtime, decreases pre-sleep arousal, and improves markers of sleep continuity and sleep depth (Maurer, Schneider et al., 2022).
Stimulus control therapy (SCT)
The basic concept in stimulus control is that many patients with insomnia exhibit a learned association between the bed/bedroom and being awake (instead of sleeping). Bootzin (1972) conceived of SCT as an operant conditioning paradigm; we go to bed to get the reward of sleep. There is heavily reinforced instrumental learning in good sleepers because of the regular proximal relationship between bed and (rapid) sleep onset. However, this connection is broken in insomnia and counterproductive classical conditioning can occur. SCT comprises seven primary instructions with the aim of eliminating these maladaptive connections and reestablishing the connection where “bed” again equals “sleep” to the patient.
Cognitive therapeutics
Difficulties with cognitive arousal, mental events intruding upon sleep, heightened emotion and counterproductive sleep effort are very common in insomnia. Over the past few decades, numerous cognitive techniques have been developed and tested to address these problems. In most trials, and in routine clinical practice, these are commonly “bundled” within the “C” component of CBT-I. The interventions themselves are described in detail elsewhere (Baglioni et al., 2022; Espie, 2022a). In brief, they are cognitive control (“putting the day to rest” before going to bed), paradoxical intention (abandoning effort to sleep/attempting to remain awake), imagery training (active visualisation exercises that capture attention and manage rumination) and cognitive restructuring (challenging negative thoughts by means of Socratic dialogue or within the framework of behavioural experiments).
The evidence-base: CBT-I for insomnia without comorbidities
Meta-analyses concerning CBT-I are depicted in Table 6.
| Author (year) | Population | No. studies/patients | Intervention | Study endpoints | Effects on study endpoints |
|---|---|---|---|---|---|
| Morin et al. (1994) | Insomnia | 59/2102 | CBT-I and single components | SOL, WASO, NOA, TST | (a) Good effects of CBT-I on all parameters |
| (b) Good follow-up results | |||||
| Murtagh and Greenwood (1995) | Insomnia | 66/2007 | CBT-I and single components | SOL, NOA, TST, SQ | (a) Good effects of CBT-I on all parameters |
| (b) Good follow-up results | |||||
| Pallesen et al. (1998) | Insomnia, age > 50 years | 13/388 | CBT-I and single components | SOL, NOA, WASO, TST | (a) Good effects of CBT-I on all parameters |
| (b) Good follow-up results | |||||
| Montgomery and Dennis (2004) | Primary insomnia, age > 60 years | 7/322 | CBT-I, bright light and physical exercise | SOL, TST, SE, WASO | (a) Good effects of CBT-I on sleep maintenance |
| (b) Almost no effects of bright light and physical exercise | |||||
| Irwin et al. (2006) | Insomnia, age > 55 years versus younger patients | 23/NA | CBT-I and single components | SQ, SOL, TST, SE, WASO | Medium to strong effects in older patients |
| Okajima et al. (2011) | Primary insomnia | 14/927 | CBT-I | SOL, WASO, EMA, SE, PSG, ACT | (a) Good effects of CBT-I on all parameters |
| (b) Good follow-up results | |||||
| Miller et al. (2014) | Primary insomnia | 4/192 | SRT | SOL, WASO, TST, NOA, SE, SQ | Sleep restriction alone is effective |
| Koffel et al. (2015) | Insomnia | 8/659 | Group CBT-I | SOL, WASO, SE, SQ, TST, pain, depression | Group CBT-I is effective |
| Trauer et al. (2015) | Chronic insomnia | 20/1162 | CBT-I | SOL, WASO, TST, SE | Clinically relevant efficacy without undesired side-effects |
| Ballesio et al. (2018) | Insomnia | 47/4317 | CBT-I | Depression, fatigue | Small effects for depression, no significant effects for fatigue |
| Chung et al. (2018) | Insomnia | 15/1194 | Psychoeducation/sleep hygiene versus CBT-I | ISI, SE, TST, SOL, WASO, AKT, PSQI | Psychoeducation/sleep hygiene less effective than CBT-I for PSQI, ISI, SE, SOL, WASO (medium to large ES) |
| Mitchell et al. (2019) | Insomnia disorder | 15/1541 | CBT-I | PSG, ACT | Small effects on actigraphy, no effects on PSG parameters |
| Van der Zweerde, van Straten, et al. (2019) | Insomnia | 30/2835 | CBT-I | SE, SOL, ISI 3, 6 and 12 months after therapy | Small to medium ES for ISI and SE, small ES for SOL at follow-ups |
| Benz et al. (2020) | Insomnia disorder | 86/15,578 | CBT-I | Daytime impairments in the context of insomnia | Small to medium ES for depression, anxiety, fatigue, quality of life and daytime functioning |
| Thakral et al. (2020) | Insomnia | 16/1964 | CBT-I | Dysfunctional thoughts and beliefs about sleep | Large ES for dysfunctional thoughts and beliefs about sleep post-treatment and at follow-up |
| Ballesio et al. (2021) | Insomnia | 15/1058 | CBT-I | Repetitive negative thinking (e.g. ruminations) | Small ES for worry, no significant effect for rumination |
| Edinger et al. (2021) | Chronic insomnia | 89/not indicated | CBT-I and its single components | TST, SE, SOL, WASO, SQ, ISI, PSQI | Medium to large ES for sleep parameters, smaller ES for single components |
| Kwon et al. (2021) | Insomnia > 60 years | 28/2391 | CBT-I, BT, acupuncture | PSQI | All treatments effective against waitlist |
| Maurer, Schneider, et al. (2021) | Insomnia | 8/533 | Sleep restriction | TST, SE, SOL, WASO, ISI | Large effects on SE, WASO, ISI; medium effects on SOL; no effect on TST |
| Xu et al. (2021) | Insomnia | 31/2449 | F2F CBT-I | TST, SE, SOL, WASO, NOA, ISI, PSQI, depression, anxiety, fatigue, somatic and mental health | Significant effects for ISI, PSQI, TST, SE, SOL, WASO, NOA, depression and fatigue; no significant effects on anxiety and mental health |
| Yu et al. (2021) | Insomnia | 14/2263 | CBT-I | TST, SE, SOL, depression, anxiety | Significant effects for TST, SE, SOL, depression and anxiety |
| Alimoradi et al. (2022) | Insomnia | 24/1977 | CBT-I | Quality of life | Small to medium effects on quality of life |
| Huang, Li, et al. (2022) | Insomnia > 60 years | 14/792 | CBT-I | TST, SE, SOL, WASO | Significant effects for TST, SE, SOL, WASO |
| Jansson-Fröjmark et al. (2022) | Insomnia | 10/384 | Paradoxical intention | TST, SE, SOL, NOA | Large ES for SOL, NOA, medium ES for TST, no effect for SE |
| Kwon et al. (2022) | Insomnia | 10/496 | Brief behavioural therapy | TST, SE, SOL, WASO | Significant effects for SE, SOL, WASO 1–8 weeks after therapy |
- Abbreviations: ACT, actigraphy; BT, behavior therapy; CBT-I, cognitive-behavioural therapy for insomnia; EMA, early-morning awakening; ES, effect size; F2F, face-to-face; ISI, Insomnia Severity Index; NOA, number of awakenings; PSG, polysomnography; PSQI, Pittsburgh Sleep Quality Index; SE, sleep efficiency; SOL, sleep-onset latency; SQ, sleep quality; SRT, sleep-restriction therapy; TST, total sleep time; WASO, wake time after sleep onset.
Up to now, 25 meta-analyses have been published focusing on the effect of CBT-I on insomnia/insomnia disorder in those without comorbidities since 1994. It should be critically mentioned that many early studies used a waitlist group as a control group, which is not as powerful as a placebo group, typically used in pharmacological studies. Furthermore, these meta-analyses are not independent of each other, thus there may be overlap within the studies. These issues, therefore, may overemphasise the effects of CBT-I. That said, a number of early placebo-controlled studies with small sample sizes (Espie et al., 1989; Turner & Ascher, 1979) exist, and suggested positive effects of CBT-I beyond sham comparison groups.
Most recent meta-analyses on the efficacy of CBT-I in insomnia/insomnia disorder, without comorbidities, show large effects on the severity of insomnia symptoms (see summary by Edinger et al., 2021), whereby follow-up measurements up to a year later show small to medium effects (van der Zweerde, Bisdounis, et al., 2019).
The evidence-base: CBT-I component-related efficacy
In relation to the monotherapy of insomnia, from the active elements of CBT-I, data up to now suggest efficacy of SRT (Edinger et al., 2021; Kyle et al., 2023; Maurer, Schneider, et al., 2021), SCT (Edinger et al., 2021), relaxation therapy (Edinger et al., 2021) and paradoxical intention as one specific cognitive therapeutic (Jansson-Fröjmark et al., 2022). Psychoeducation/sleep hygiene alone does not appear to be very effective (Chung et al., 2018; Edinger et al., 2021), probably because many patients are already aware of these recommendations (Lacks & Rotert, 1986). Thus, sleep hygiene advice given alone is not recommended in the treatment of chronic insomnia disorder and, in RCTs among patients with insomnia, sleep hygiene is often used as the “placebo” condition (Bjorvatn et al., 2011).
A network meta-analysis (Steinmetz et al., 2022, 2023) that aimed to delineate the efficacy of the different components of CBT-I showed that sleep restriction and stimulus control seem to be the most effective components of CBT-I. Nevertheless, interventions based on single strategies should be offered in full consideration of motivational and safety issues. Especially the behavioural strategies because sleep restriction and stimulus control may be very challenging for the patients; thus, the intervention may require careful consideration of the patient's motivation and readiness to apply the strategy with systematic regularity. Furthermore, these therapeutics may temporarily increase daytime sleepiness and fatigue in the early interventional phases, thus safety issues should be fully discussed with the patient.
The evidence-base: CBT-I effects beyond sleep complaints
Importantly, beyond beneficial effects on sleep-related outcomes, CBT-I also has positive effects on associated subclinical depressive symptoms, anxiety, daytime sleepiness and fatigue (Benz et al., 2020), dysfunctional cognitions related to sleep (Thakral et al., 2020), worry (Ballesio et al., 2021), and quality of life (Alimoradi et al., 2022).
The evidence-base: CBT-I for insomnia with comorbidities
The CBT-I is also effective when insomnia is comorbid with other medical disorders (Table 7).
| Author (year) | Population | No. studies/patients | Intervention | Study endpoints | Effects on study endpoints |
|---|---|---|---|---|---|
| Belleville et al. (2011) | Insomnia with/without comorbid anxiety | 50/2690 | CBT-I | Anxiety scales | Moderate effects on anxiety |
| Geiger-Brown et al. (2015) | Comorbid insomnia (somatic/mental) | 23/1379 | CBT-I | SOL, WASO, TST, SE, ISI, PSQI | Good efficacy; long-term effects at 18 months |
| Wu et al. (2015) | Comorbid insomnia (somatic/mental) | 37/2189 | CBT-I | SOL, WASO, SQ, TST, remission, comorbid symptoms | Good efficacy; smaller effects on comorbid symptoms; better effects for mental outcomes |
| Ho et al. (2016) | Insomnia + PTSD | 11/593 | CBT-I | SOL, WASO, SE, TST, PTSD symptoms | Good sleep effects, good effects on PTSD symptoms |
| Johnson et al. (2016) | Insomnia + cancer | 8/752 | CBT-I | SE, WASO, ISI, cancer symptoms | Good sleep effects, good effects on cancer symptoms |
| Tang et al. (2015) | Insomnia + pain | 11/1066 | CBT-I | SQ, fatigue, pain | Good sleep effects, good effects on comorbid symptoms |
| Van Straten et al. (2018) | Insomnia, with and without comorbidities | 87/6303 | CBT-I | SE, SOL, ISI | Significant medium to large ES for SE, SOL, ISI |
| Feng et al. (2020) | Insomnia disorder + depression | 17/1756 | CBT-I | ISI, PSQI, depressive symptoms | Significant ES for ISI/PSQI, weaker effects for depressive symptoms |
| Zhou et al., 2020 | Insomnia disorder + psychiatric/somatic comorbidity | 13/853 | CBT-I | SOL, WASO, SQ, ISI | Medium ES for ISI, SQ; weaker effects for SOL, WASO |
| Curtis et al. (2021) | Insomnia + tinnitus | 4/470 | CBT-I | ISI | Medium ES for ISI |
| Ma et al. (2021) | Insomnia disorder + breast cancer | 14/1363 | CBT-I | ISI | Medium ES for ISI |
| Selvanathan et al. (2021) | Insomnia + chronic pain | 12/762 | CBT-I | Global sleep parameters, pain, depressive symptoms | Strong ES for global sleep parameters, smaller ES for pain and depressive symptoms |
| Gao, Liu, et al. (2022) | Insomnia + cancer | 16/1523 | CBT-I | SE, SOL, WASO, ISI | Small to medium ES for ISI, SOL, WASO |
| Hertenstein et al. (2022) | Insomnia + comorbid mental disorders | 22/1083 | CBT-I | ISI, comorbid symptom severity | Significant medium to large ES for ISI, significant ES for symptoms of comorbid disorder |
| Squires et al. (2022) | Insomnia + cancer | 22/1461 | CBT-I | ISI, TST, SE, SOL, WASO, SQ, anxiety, depressive symptoms, fatigue, quality of life | Medium ES for ISI, small to large ES for TST, SE, SOL, WASO, SQ, anxiety, depressive symptoms, fatigue, quality of life |
- Abbreviations: CBT-I, cognitive-behavioural therapy for insomnia; ES, effect size; ISI, Insomnia Severity Index; PSQI, Pittsburgh Sleep Quality Index; PTSD, posttraumatic stress disorder; SE, sleep efficiency; SOL, sleep-onset latency; SQ, sleep quality; TST, total sleep time; WASO, wake time after sleep onset.
There have been 15 published meta-analyses concerning CBT-I for insomnia when comorbid with other conditions since 2011.
In the area of mental disorders, medium to large effects have been reported on insomnia severity when insomnia is comorbid with depression, posttraumatic stress disorder or alcohol dependency (Hertenstein et al., 2022). There is not sufficient evidence concerning insomnia comorbid with bipolar disorders (Bisdounis et al., 2022) or psychotic disorders (Hertenstein et al., 2022). CBT-I is also effective when insomnia is comorbid with tinnitus (Curtis et al., 2021), chronic pain (Selvanathan et al., 2021), cancer (Gao, Liu, et al., 2022; Squires et al., 2022), sleep apnea (COMISA; Sweetman et al., 2023), and in patients with neurological disorders such as traumatic brain injury, stroke or Parkinson's disease (Ford et al., 2023; Lebrun et al., 2020). Interestingly, especially in the area of mental disorders, the evidence indicates that CBT-I not only has sleep-related effects, but also has a positive effect on the symptoms of the comorbid disorder/condition (Hertenstein et al., 2022).
The evidence-base: CBT-I in self-help formats or delivered digitally
Over the past 10 years there has been a growing literature on the application of CBT-I in self-help formats, particularly using either self-help books/texts or, now, dCBT-I. To date, 11 meta-analyses are available on this topic (Table 8). A meta-analysis by Gao, Ge, et al. (2022), explored a range of delivery formats, and reported that CBT-I in single therapy, in group therapy and in digital form, with or without support by therapists, is effective. Single therapy, group therapy and digital therapy with personal support were most effective (Gao, Ge, et al., 2022). A further recent meta-analysis by Hasan et al. (2022) also suggests that personal support is to be preferred when CBT-I is administered digitally.
| Author (year) | Population | No. studies/patients | Intervention | Study endpoints | Effects on study endpoints |
|---|---|---|---|---|---|
| Van Straten and Cuijpers (2009) | Insomnia | 10/1000 | Self-help CBT-I | SOL, WASO, SE, SQ, TST | Small to moderate effects |
| Cheng and Dizon (2012) | Insomnia | 6/433 | dCBT-I | SOL, WASO, SE, SQ, TST | Small to moderate effects |
| Ho et al. (2015) | Insomnia | 20/2411 | Self-help + dCBT-I | SOL, WASO, SE, SQ, TST | Self-help CBT-I is effective and acceptable as a starter for treatment |
| Ye et al. (2015) | Insomnia with comorbid conditions | 9/776 | dCBT-I | Anxiety, depression | Moderate effect sizes for comorbid symptoms |
| Zachariae et al. (2016) | Insomnia | 11/1460 | dCBT-I | ISI, SOL, WASO, NOA, TST, SQ | Comparable to F2F CBT-I |
| Seyffert et al. (2016) | Insomnia | 15/2392 | dCBT-I | ISI, SOL, TST, WASO, NOA, SQ, PSQI | Good efficacy for sleep parameters, good follow-up results |
| Soh et al. (2020) | Insomnia | 33/9364 | dCBT-I | ISI, other sleep parameters | Small ES for ISI |
| Ho et al. (2020) | Insomnia + depression | 30/5945 | Self-help CBT-I | Insomnia symptoms, depressive symptoms | Medium ES for insomnia symptoms, smaller ES for depressive symptoms |
| Gao, Ge, et al. (2022) | Insomnia | 61/11,571 | CBT-I in several formats including dCBT-I | TST, SE, SOL, WASO, ISI | Significant effects of individual and group CBT-I and dCBT-I with or without assistance; largest for individual and group CBT-I and dCBT-I with assistance |
| Hasan et al. (2022) | Insomnia | 54/11,815 | dCBT-I | TST, SE, SOL, WASO | Significant effects of assisted dCBT-I for TST, SE, SOL, WASO |
| Forma et al. (2022) | Chronic insomnia | 20/5659 | dCBT-I | SOL, WASO, ISI | Significant effects for ISI, WASO |
- Abbreviations: CBT-I, cognitive-behavioural therapy for insomnia; dCBT-I, digital-cognitive behavioural therapy for insomnia; ES, effect size; F2F, face-to-face; ISI, Insomnia Severity Index; NOA, number of awakenings; PSQI, Pittsburgh Sleep Quality Index; SE, sleep efficiency; SOL, sleep-onset latency; SQ, sleep quality; TST, total sleep time; WASO, wake time after sleep onset.
Interestingly, the most contemporary network meta-analysis on all forms of CBT-I administrations (i.e. onsite versus digital and other settings; Simon, Steinmetz, et al., 2023) demonstrated that individual F2F CBT-I, group F2F CBT-I, telehealth (videoconference, phone call) and guided bibliotherapy conveyed the strongest effects on insomnia, whereas guided and unguided dCBT-I yielded medium effect sizes, slightly favouring guided over unguided dCBT-I. Smart phone applied CBT-I did not attain significant effects compared with control.
In Europe, several dCBT-I treatment programs are now available in web and/or mobile application format. Table 9 presents information on these programs, their evidence base, and levels of adoption.
| Language (country of origin) | Name of product | Web address | Computer/app based | Reimbursement by national healthcare | Reference (s) |
|---|---|---|---|---|---|
| Dutch The Netherlands | i-Cycle | https://slaapregister.nl | Yes/Yes (partly) + assistancea | No | Leerssen et al. (2022) |
| Dutch The Netherlands | Somnio | https://somnio.nl | Yes/Yes + assistancea | Yes | Dekker et al. (2020) |
| Dutch The Netherlands | i-Sleep | + assistancea | Yes | Baka et al. (2022); Van der Zweerde, van Straten, et al. (2019); Van der Zweerde et al. (2020); Van Straten et al. (2014) | |
| English UK | SLEEPIO | https://www.sleepio.com/ | Yes/Yes | Yes (Scotland) | More than 30 publications; Selection: Espie et al. (2012, 2019); Espie, Kyle, Miller, et al. (2014); Felder et al. (2020); Freeman et al. (2017) |
| French France | TheraSomnia | https://www.therasomnia.com/ | Yes/Yes + assistancea | No | Lopez et al. (2019) |
| German Austria | NUKKUAA | www.nukkuaa.com | No/Yes | No | Schabus et al. (2022); Eigl et al. (2022); Hinterberger et al. (2022, 2023) |
| German Germany | SOMNIO | https://somn.io/ | Yes/Yes | Yes | Lorenz et al. (2019) |
| German Germany | HelloBetter Schlafen/HelloBetter sleep | https://hellobetter.de/online-kurse/schlafen/ | + assistancea | Yes | Behrendt et al. (2020); Ebert et al. (2015); Thiart et al. (2015) |
| German Switzerland | Meinstresscoach/SweetDreams | https://www.meinstresscoach.ch/kurse | No/Yes + assistancea | No | Hürlimann et al. (2023) |
| Swedish Sweden | Internetpsykiatri Sömnproblem—insomni | https://www.internetpsykiatri.se/behandling/somnproblem-insomni/ | Yes/No + assistance | Yes | Kaldo et al. (2015); Blom et al. (2016) |
| Swedish Sweden | Livanda Sömnproblem (insomnia) | https://www.livanda.se/kbt-internetterapi-somnproblem.aspx | Yes/No + assistancea | No | Ström et al. (2004) |
- a Assistance: mail/phone interaction with a health professional possible.
To obtain this information, all members of the EIN (n = 250) and all authors of this guideline (n = 44) were asked to complete a questionnaire about digital tools available in their home country, which resulted in the above-mentioned tables. The evidence base reported reflects publications available from peer-review journals. For a systematic overview encompassing the wider number of apps claiming to improve sleep that are available through App stores, see Simon, Reimann, et al. (2023).
Table 9 summarises dCBT-I treatments where effectiveness for insomnia is supported through at least one pre-registered published randomised–controlled trial (RCT). As described in the table, some programs incorporate guidance from health professionals whilst others are fully automated. Guidance means participants are offered support (either by mail or phone) to discuss critical issues and progress. Almost half of the evidence-based dCBT-I programs are now reimbursed by the local/national health authorities, and can be recommended or prescribed at the cost of local health insurance. Sleepio from the UK (also available in the USA) is the most extensively researched dCBT-I application, having generated 13 published RCTs including a placebo-controlled trial.
Table S1 (supplemental material) summarises programs which, up to now, lack evidence through an RCT. Some of these programs have also been tested in uncontrolled single-arm trials.
To summarise, at present there is a rapidly developing market of digital health applications targeting the treatment of insomnia, with some of the applications already having a considerable database and being reimbursed by local health authorities. Concerning RCT supported applications (Table 9), five European languages (including Dutch, English, French, German and Swedish) are already covered. This development definitely reflects the overall trend for digitalisation in healthcare, but may also be seen as a consequence of our previous guideline (Riemann, Baglioni, et al., 2017; Riemann, Baum, et al., 2017), in which we recommended CBT-I as first-line treatment for insomnia. Digital CBT-I surely will make access to CBT-I easier than before (particularly when fully automated and 24/7 available), meeting the needs of many patients with insomnia who have no access to CBT-I therapists. In any case, at present, it must be stressed that this type of therapy has to undergo rigorous quality control as well (e.g. does it really contain all important ingredients of CBT-I?) and is it evidence-based, meaning has it been subjected to at least one high-quality RCT? Gold-standard evidence criteria for the regulation of dCBT-I (digital therapeutics) have been published (Espie, Torous, & Brennan, 2022; Table 10). Importantly, developers should be aware that an evidence-informed intervention (broadly based on CBT-I but not specifically subjected to trials) is not an adequate substitute for being evidence based (Espie, Firth, & Torous, 2022).
| dCBT criteria | Demonstrated by … |
|---|---|
| W: The therapeutic has to WORK | Treatment efficacy needs to be supported by adequately powered RCT(s) to demonstrate level 1 evidence of change that is attributable to the digital therapeutic. The trial and its primary and secondary outcomes should be pre-registered, and those data should be published following peer-review |
| H: The therapeutic has to HELP | Statistically significant between-group effects of the digital therapeutic over a control condition are important. However, RCT(s) need to demonstrate clinically meaningful differences or clinical remission endpoints relating to the target therapeutic condition as assessed by clinically accepted and standardised pre-registered measures |
| A: The therapeutic has to be ACCESSIBLE | A digital therapeutic has to use high-quality technology to deliver a reliable and acceptable patient experience within the target population. There needs to be evidence that the digital therapeutic is capable of scaling safely and effectively to population level distribution in the real world |
| T: The therapeutic has to be TRUSTED | The digital therapeutic needs to adhere to clinical governance and risk management standards for software as a medical device, privacy and data security certifications, and reflect national and international clinical guidelines for information storage and data transfer |
- Abbreviations: dCBT, digital cognitive-behavioural therapy; RCT, randomised–controlled trial.
We also stress that local health authorities in the various European countries should take over this regulatory function—in Germany and the UK, for example, the same authority that regulates medications has embraced this task. Future research will have to demonstrate whether digital CBT-I is really equivalent to in-person/F2F therapy and to which degree increased rates of dropouts/attrition may occur, especially in cases of comorbid insomnia. Caution is urged in cases of comorbid insomnia, especially when severe acute mental disorders are present. It is important that the pathways to the various CBT-I formats are based on professional expertise. Also, the question of adverse events and how these are dealt with needs to be considered thoroughly (in all CBT-I programs). Finally, sleep trackers, with often inaccurate sleep feedback, need to be more thoroughly tested and should in general be discouraged for patients with insomnia until published validation of their accuracy against PSG is available.
Side-effects and contraindications of CBT-I
Typical of the psychotherapy literature more generally, adverse effects of CBT-I have received limited attention in clinical trials (Condon et al., 2021). This situation, however, is changing as trial methodology and reporting improve. Clinical experience shows that SRT is coupled with increased tiredness and sleepiness, and there is evidence from both controlled and uncontrolled trials of objectively reduced vigilance during the first weeks of implementation (Kyle et al., 2014; Maurer et al., 2022; Maurer, Schneider, et al., 2021). Indeed, the initial restriction of time in bed and its associated sleepiness may be central to therapeutic effects of behavioural treatment (Maurer et al., 2020). In a large trial (n = 1711) of dCBT-I, Espie and colleagues (2019) showed that CBT-I was associated with higher reports of intervention-attributed adverse effects (extreme sleepiness, fatigue or exhaustion, attention and concentration problems, low motivation and energy, headaches, memory difficulties and irritability) relative to sleep hygiene. Further work by Sunnhed et al. (2020) localised adverse effects, especially fatigue and sleepiness, to behavioural therapy (SRT, SCT) relative to cognitive therapy. As such, care should be taken when administering SRT and/or stimulus control protocols, including initial assessment for suitability and ongoing reviews of sleepiness (and impact on driving) during treatment, with appropriate adaptations put in place where necessary. In the largest test of SRT to date (n = 642), Kyle et al. (2023) found no evidence that nurse-delivered SRT was associated with serious adverse events, or increased incidence of important pre-defined adverse events (falls, work-related or motor vehicle accidents, near-miss driving incidents, or falling asleep while driving) relative to sleep hygiene control. Contraindications for treatments like stimulus control or sleep restriction that may entail partial sleep deprivation include any kind of epilepsy and conditions that might be aggravated by sleep loss. It should be noted of course, that as with medications, patients and clinicians should generally be familiar with risk–benefit considerations and associated decision-making.
Up to now, no mention has ever been made that CBT-I is associated with any risks of abuse/dependency, “dose increase”, tolerance or rebound insomnia—all problems associated with some of the hypnotic medications. The low probability of serious or enduring adverse effects with CBT-I is considered a strong advantage over medications.
Combining CBT-I with pharmacotherapy
Concerning the question of whether a combination of CBT-I and pharmaceutical treatment is clinically superior to either therapy in isolation, there are two high-quality original studies, from some years ago, using temazepam or zolpidem as medications (Morin et al., 1999, 2009). Both studies showed that during the acute treatment phase a synergistic effect of CBT-I and pharmacotherapy can be observed; however, after acute therapy mono-treatment with CBT-I is superior to the combination of CBT-I and pharmacotherapy. More recent work demonstrated that sequential treatment, starting with behavioural therapy and subsequently zolpidem, resulted in the largest percentage response and remission in the longer term (1 year), indicating that secondary administration of medication could be an effective strategy in insomnia management (Morin et al., 2020). This high-quality controlled study also found that approximately half of the patients remitted with these interventions, underscoring the urgency for the development of more effective treatments, as opposed to CBT-I or pharmacotherapy, for the other half. This importance is further underlined by one of the few meta-analyses that reports actual post-CBT-I sleep efficiency scores, as opposed to effect sizes only (Seyffert et al., 2016): sleep efficiency does not normalise in more than half of those treated.
Insomnia care in Europe: the role of CBT-I
In conclusion for this section, the published evidence suggests that CBT-I should be used as first-line treatment for patients with chronic insomnia. This recommendation is independent of the question whether the insomnia is the sole presenting problem, or comorbid to another medical disorder. Single components (especially sleep restriction and stimulus control) may be effective on their own, and there is evidence that brief treatments (consisting of one–two sessions) may also have some effect on their own (Kwon et al., 2022). A major advantage of CBT-I over pharmacological treatment is its treatment sustainability—in contrast to pharmacotherapy where symptoms frequently recur or even rebound after treatment discontinuation, where no such effect has been reported for CBT-I. Furthermore, as suggested by the evidence, the effects of CBT-I (Tables 6–8) appear to be stable over time (follow-ups up to 10 years have been published; Jernelöv et al., 2022) and may only gradually weaken. Booster sessions may be needed to refresh the initial treatment gains.
However, a huge problem presently in healthcare across Europe is the fact that up to now in-person/F2F CBT-I is not available for all patients suffering from insomnia. It needs to be seen whether this gap in clinical care can be closed by digitally based interventions. The accelerating and substantial evidence from trials of dCBT-I applications are very promising and may greatly improve guideline-based insomnia care in Europe.
Another important issue at this point (which also applies to pharmacotherapy) is the question as to what extent CBT-I (in all its forms of applications) is associated with attrition/adherence, and at what point are patients considered mere responders (showing some meaningful clinical benefit) or remitters (being fully resolved, i.e. not fulfilling threshold values, e.g. on the ISI). These issues are especially pertinent for fully automated digital treatments, i.e. applied without any human guidance, where reported dropout rates have been high (> 50%). It is recommended that future RCTs and, even more so, comparative meta-analyses correct their effect sizes for the differential attrition they have (i.e. intention to treat). Few studies have investigated the predictors of dropout. As such, it is imperative to determine who are most likely to drop out of F2F or dCBT-I, and which alternative treatments they might best respond to.
Future research will also have to address the issue of head-to-head comparisons between all types of treatments (both psychological and/or pharmacological). Furthermore, beyond well-controlled randomised clinical trials we are in need of data from real-world everyday clinical practice regarding treatment acceptance, adherence, attrition, side-effects and outcome.
4.1.2 Other cognitive-behavioural/psychotherapeutic interventions
As previously mentioned, the range of treatments for insomnia has expanded over the past few decades, and these have often been combined into the multicomponent approach. Recent expansions in this literature have included the so-called “third-wave” therapies such as mindfulness, and acceptance and commitment therapy (ACT; n.b. “first-wave”: behavioural; “second-wave”: cognitive).
Two contemporary meta-analyses concerning mindfulness-based treatments for insomnia show unequivocally positive effects (Chen, Chang, et al., 2020; Wang, Wang, et al., 2020). The number of studies in this area, however, is still relatively small, and the authors of both meta-analyses stress that long-term effects need to be investigated more thoroughly. ACT has been studied only in small randomised clinical studies. While ACT was shown to be superior to an active control condition (Zakiei et al., 2021), it was inferior to CBT-I (El-Rafihi-Ferreira et al., 2022). Moreover, replacing the cognitive therapy aspect of CBT-I with ACT did not enhance efficacy (El Rafihi-Ferreira et al., 2021). Finally, one study of a web-based treatment platform using ACT, SRT and SCT found it was superior to waitlist control conditions (Chapoutot et al., 2021). In summary, there is evidence that ACT may be effective in insomnia; however, larger randomised clinical studies are desperately needed.
A meta-analysis by Lam et al. (2015) on hypnotherapy for insomnia suggested some positive effects. The methodological quality of the original studies, however, was judged as poor, which casts doubt on the benefits of this approach at this time.
Intensive sleep retraining is a newer form of behavioural treatment, requiring PSG monitoring. Scott et al. (2023) summarise promising evidence for this new type of training; however, at present data are still too scarce to provide a recommendation.
Summarising, further research is needed for these other cognitive-behavioural forms of psychotherapy before they can be recommended for the treatment of insomnia.
4.1.3 Exercise, light therapy, music and non-invasive brain stimulation
Concerning the efficacy of exercise in the treatment of insomnia, there is some evidence; however, further high-quality studies are necessary (published meta-analyses: Amiri et al., 2021; Baglioni, Bostanova, et al., 2020; Banno et al., 2018; Choong et al., 2022; D'Aurea et al., 2022; Li, Li, et al., 2021; Mercier et al., 2017; Rubio-Arias et al., 2017 T). One RCT showed that the addition of exercise to CBT-I helped maintain its initial beneficial effects and this was largely due to CBT-I (Dekker et al., 2020). Tai-Chi is a specific form of exercise suggested to be effective by most recent meta-analyses; however, it was highlighted that more high-quality studies are still needed (Han et al., 2022; Li, Chen, et al., 2020).
A meta-analysis on light therapy for insomnia showed small positive effects on sleep (Van Maanen et al., 2016) and this is an area worthy of further investigation, especially because a circadian sleep–wake rhythm disorder is frequently comorbid with insomnia. Chambe et al. (2023) came to similar conclusions: light therapy may have some promise, but further RCTs are necessary. One RCT showed that the addition of light to CBT-I helped maintain its initial beneficial effects due to CBT-I (Dekker et al., 2020).
The most recent meta-analyses on the effects of listening to music on sleep in patients with insomnia also suggest a possible positive effect (Chen et al., 2021; Feng et al., 2018; Jespersen et al., 2015). However, there are few original studies on these topics and the quality of the available literature was criticised in these meta-analyses.
Several non-invasive brain stimulation techniques have been tested as potential treatment approaches for insomnia, in particular repetitive transcranial magnetic stimulation (rTMS), transcranial electric stimulation (tES) comprising transcranial direct current stimulation and transcranial alternating current stimulation, as well as vagus nerve stimulation and brain cooling. While several RCTs report positive results, methodological issues such as the lack of blinding or insufficient sham controls limit their interpretability. The therapeutic benefits of current non-invasive brain stimulation protocols for insomnia treatment are likely overestimated and, at present, no recommendation can be given for the use of brain stimulation approaches as a treatment strategy for insomnia (Krone et al., 2023).
In light of the available evidence, or lack thereof in some cases, it is considered premature to recommend any of these treatments as standalone interventions for insomnia. We do, however, suggest that elements of exercise and light therapy be integrated with CBT-I and may deliver additional benefits.
4.2 Pharmacological treatments
A summary of the available and often used medications (on-label and off-label) for insomnia in Europe is provided in Table 11.
| BZ | Diazepam, flunitrazepam, flurazepam, lormetazepam, nitrazepam, oxazepam, temazepam, triazolam |
| BZRA | Zaleplone, zolpidem, zopiclone, eszopiclone |
| Sedating antidepressants | Agomelatine, amitriptyline, doxepin, mianserin, mirtazapine, trazodone, trimipramine |
| Antipsychotics | Chlorprothixene, levomepromazine, melperone, olanzapine, pipamperone, prothipendyl, quetiapine |
| Antihistamines | Diphenhydramine, doxylamine, hydroxyzine, promethazine |
| Phytotherapeutics | Hops, kava-kava, melissa, passiflora, valerian, lavender |
| Melatonin receptor agonists | Fast-release melatonin, ramelteon, PR melatonin |
| Orexin receptor antagonists | Daridorexant |
- Abbreviations: BZ, benzodiazepines; BZRA, benzodiazepine receptor agonists; PR, prolonged-release.
4.2.1 Benzodiazepines (BZ) and benzodiazepine receptor agonists (BZRA)
The BZ and BZRA medications that are available in Europe are listed in Table 11. Table 12 summarises the meta-analyses on the efficacy of BZs and BZRAs.
| Author (year) | Population | No. studies/patients | Intervention | Study endpoints | Effects on study endpoints |
|---|---|---|---|---|---|
| Nowell et al. (1997) | Primary insomnia | 22/1894 | BZ + zolpidem versus placebo, short-term treatment | SOL, NOA, TST, SQ | Significant improvement of sleep |
| Soldatos et al. (1999) | Insomnia | 75/1276 | Rapidly eliminated BZ + BZRA hypnotics versus placebo | SOL, TST, WASO (all PSG) | (a) Strong evidence for significant initial efficacy |
| (b) Increased risk for development of tolerance and rebound phenomena; difference between substances | |||||
| Holbrook et al. (2000) | Primary insomnia | 45/2672 | BZ + zopiclone versus placebo, short-term treatment | SOL, TST, USE | (a) Significant improvement of sleep (b) Increased risk for USE |
| Dündar et al. (2004) | Insomnia | 24/3909 | BZ versus BZRA, short-term treatment | SOL, TST, NOA, WASO, SQ, USE | (a) No difference between substances |
| Glass et al. (2005) | Insomnia, age > 60 years | 24/2417 | BZ + BZRA versus placebo, short-term treatment | SQ, SOL, TST, NOA, USE | (a) Significant improvement of sleep |
| (b) Increased risk for USE | |||||
| Buscemi et al. (2007) | Chronic insomnia | 105/5582 | BZ + BZRA + sedating antidepressants | SOL + secondary outcomes, USE | BZ and BZRA are effective; more USE with active drugs versus placebo |
| Huedo-Medina et al. (2012) | Insomnia | 13/4378 | BZRA (zolpidem, zaleplone, eszopiclone) | SOL + secondary outcomes | Small but significant effects on subjective and objective SOL |
| Winkler et al. (2014) | Insomnia | 31/3820 | BZ, BZRA, sedating antidepressants, melatonin | PSG and subjective sleep parameters | BZ and BZRA have significant effects on subjective and objective outcomes; smaller effects for antidepressants |
| Rösner et al. (2018) | Insomnia disorder | 14/4732 | Eszopiclone versus placebo | TST, SE, SOL, WASO | Significant effects for TST, SE, SOL, WASO, persisting up to 6 months |
| Liang et al. (2019) | Primary insomnia | 6/2809 | Eszopiclone versus placebo | TST, SOL, WASO, NOA | Significant effects for TST, SOL, WASO, NOA, persisting up to 6 months |
| Xiang et al. (2021) | Insomnia disorder | 6/1068 | Zolpidem versus placebo (treatment at least 1 month) | TST, SOL, WASO, SQ | Significant effects for TST, SOL, SQ, but not for WASO |
- Abbreviations: BZ, benzodiazepines; BZRA, benzodiazepine receptor agonists; NOA, number of awakenings; PSG, polysomnography; SE, sleep efficiency; SOL, sleep-onset latency; SQ, sleep quality; TST, total sleep time; USE, undesired side-effects; WASO, wake time after sleep onset.
Most of the meta-analyses demonstrate that BZ and BZRA drugs have positive effects on sleep when taken up to a maximum of 4 weeks. Beyond that timeframe, the meta-analyses by Liang et al. (2019) and Rösner et al. (2018) suggest positive effects of eszopiclone for a period of application of up to 6 months in certain cases, but this is to be decided on an individual basis.
Side-effects associated with BZs and BZRAs used include the development of tolerance and dependence, nocturnal confusion and falls (Treves et al., 2018), negative effects on cognitive functioning including memory impairment (Barker et al., 2004; Stranks & Crowe, 2014), hangover effects with associated impairments in driving capability, especially from substances with long half-lives (Rapoport et al., 2009), as well as rebound insomnia after withdrawal. Concerning the development of dependence from hypnotics, some authors postulate that in Germany up to a million people may be affected (Soyka, 2021).
Considering the risks of long-term use of these substances especially when considering the comparative efficacy of other treatment options, the scientific evidence, according to our evaluation, does not support a general positive recommendation for BZ and BZRA in the longer-term treatment of insomnia beyond 4 weeks. Although eszopiclone has been investigated in studies for 3 and 6 months and with long-term data for up to 12 months (see overview by Rösner et al., 2018), it should be used with caution when used beyond 4 weeks given its mechanism of action, as with other BZRAs. In general, longer-term treatment (mostly off-label use) with BZ or BZRA, either daily or preferably intermittently, may be initiated in some cases but the advantages and disadvantages need to be discussed on a case-by-case basis.
4.2.2 Low-dose sedating antidepressants
The sedating antidepressants, which are frequently used in Europe to treat not only comorbid insomnia but also insomnia without comorbidities, are listed in Table 11. None of these substances has a general indication on the market for insomnia, in the absence of comorbid depression. Off-label use, therefore, is usually standard. Typically, sedative antidepressants are prescribed in insomnia at lower doses than are used in depression treatment. The meta-analytic literature on sedating antidepressants for insomnia is summarised in Table 13.
| Author (year) | Population | No. studies/patients | Intervention | Study endpoints | Effects on study endpoints |
|---|---|---|---|---|---|
| Buscemi et al. (2007) | Chronic insomnia | 105/873 | BZ + BZRA + sedating antidepressants | SOL | Sedating antidepressants are less effective than BZ/BZRA |
| Winkler et al. (2014) | Insomnia | 31/3820 | BZ + BZRA + sedating antidepressants + melatonin | Subjective and objective sleep parameters | Sedating antidepressants are less effective than BZ/BZRA |
| McCleery et al. (2014) | Insomnia comorbid with M. Alzheimer | 5/313 | Trazodone + melatonin + ramelteon | SOL, TST, WASO, SE | Trazodone improves TST and SE |
| Yeung et al. (2015) | Insomnia | 9/1983 | Low-dose doxepin | Subjective and objective sleep parameters | Small to moderate effects for sleep maintenance and TST, but no effects for SOL |
| Liu et al. (2017) | Insomnia disorder | 9/968 | Tricyclic antidepressants | TST, SE, SOL, WASO (all PSG) | Medium effects for TST, WASO; smaller effects for SE, SOL; more USE than placebo |
| Everitt et al. (2018) | Insomnia | 23/2806 | Antidepressants | TST, SE, SOL (PSG), SQ | Tricyclics have significant small effects on SQ and on TST and SE, but not on SOL. Small effects of trazodone on various parameters; Side-effects have not been systematically studied. |
| Yi et al. (2018) | Insomnia disorder | 7/429 | Trazodone | PSG: SE, TST, SOL, WASO, NOA, SQ | Significant effects for NOA and SQ; none for SE, TST, SOL, WASO |
| Zheng et al. (2022) | Insomnia disorder | 11/466 | Trazodone | PSG: TST, SOL, WASO, NOA, N1, N2, SWS, REM | Significant increase of TST, SWS; significant reduction of SOL, WASO, NOA, N1; no effects on N2, REM |
- Abbreviations: BZ, benzodiazepines; BZRA, benzodiazepine receptor agonists; NOA, number of awakenings; PSG, polysomnography; REM, rapid eye movement; SE, sleep efficiency; SOL, sleep-onset latency; SQ, sleep quality; SWS, slow-wave sleep; TST, total sleep time; USE, undesired side-effects; WASO, wake time after sleep onset.
The meta-analysis by Everitt et al. (2018) suggests that there is only scant evidence for indicating sedating antidepressants in the treatment of insomnia. Significant but small effects were noted for doxepin and trazodone in the short term, up to 4 weeks. Undesired side-effects must always be considered carefully. Longer-term treatment of insomnia disorder (without comorbidities; off-label use) with low-dose sedating antidepressants may be initiated in some cases but the advantages and disadvantages need to be discussed on a case-by-case basis.
4.2.3 Antipsychotics
The antipsychotic medications that are used quite frequently for the treatment of insomnia, especially where there are comorbid mental disorders, are listed in Table 11. The antipsychotics that explicitly mention insomnia, as an indication in the drug information, are melperone and pipamperone. There are, however, no randomised controlled clinical studies on these substances concerning insomnia disorder, either with or without comorbidities. Therefore, at present the scientific evidence does not recommend the use of antipsychotics (including quetiapine) in the treatment of insomnia without comorbidities, in either the short or long term.
4.2.4 Dual orexin receptor antagonists (DORAs)
The introduction of DORAs has probably been the most significant recent development in the pharmacological treatment of insomnia. Among this family of drugs, the only one approved by the European Medicines Agency (EMA, 2022, 2023) so far has been daridorexant, which is indicated for treating adults with insomnia for a duration of least 3 months and has a considerable impact on how they function during the day (https://www.ema.europa.eu/en/medicines/human/EPAR/quviviq). This approval is mainly based on two controlled phase-III studies that compared daridorexant with placebo in 1854 patients with insomnia over a period of 3 months, and constitutes, so far, the largest clinical program among the available drugs in Europe. Over that period, a dose of 50 mg daridorexant showed significant effects on PSG-defined sleep parameters (sleep latency and wake after sleep onset) as well as increases in subjective total sleep time and reductions in daytime sleepiness/fatigue, with small to medium effect sizes (Mignot et al., 2022). While daridorexant improved objective (PSG-defined) parameters, no subjectively reported improvements in sleep-onset latency or sleep maintenance disturbances were observed. Overall, adverse effects were mild, and their incidence was comparable between daridorexant and placebo (with nasopharingitis, headache and somnolence being most common). Furthermore, an open extension study for up to 1 year in 804 patients demonstrated a maintained efficacy over this period, with no next-morning sleepiness and no withdrawal-related symptoms or rebound being observed after treatment discontinuation. Most importantly, no new safety problems, to the ones already known, arose during this long-term treatment period (Kunz et al., 2023).
These interesting data remain to be validated through practical experience in everyday practice. Considering that at the time of writing daridorexant has not yet been launched in most European countries, caution should prevail. In general, orexin receptor antagonists can be used for periods of up to 3 months. In individual cases, treatment can be extended for longer-term periods (up to 1 year) following discussion with the patient regarding the advantages and disadvantages reported in the 1-year study.
4.2.5 Antihistaminergic drugs
Available antihistamines in Europe are depicted in Table 11. The table summarises over-the-counter (OTC) medication (diphenhydramine, doxylamin), and substances like hydroxyzine and promethazine that have to be prescribed by a physician. The status of these drugs (OTC versus prescription medicine) may vary from one European country to the other.
The evidence concerning antihistaminics in the treatment of insomnia is insufficient. One systematic review stated that there were no high-quality randomised–controlled clinical studies demonstrating efficacy compared with placebo—concluding that the evidence for these drugs is small and there might also be a rapid development of tolerance (Vande Griend & Anderson, 2012). Therefore, the scientific evidence at this point does not support a recommendation for antihistaminergic drugs in the treatment of insomnia either short or long term. Recently published evidence (Oyekan et al., 2021), indicating an association of antihistamines with increased mortality, suggests further caution.
4.2.6 Melatonin
Melatonin is freely available in most European countries and can also be prescribed by physicians in most European countries for patients with insomnia. One substance (prolonged-release melatonin = PR melatonin) has been approved for the treatment of insomnia in patients older than 55 years (EMA, 2007/2010/2023—Circadin, https://www.ema.europa.eu/en/medicines/human/EPAR/Circadin). Ramelteon, a synthetic melatonin receptor agonist, is not available in Europe presently. The meta-analytic literature on melatonin in insomnia is shown in Table 14.
| Author (year) | Population | No. studies/patients | Intervention | Study endpoints | Effects on study endpoints |
|---|---|---|---|---|---|
| Brzezinski et al. (2005) | Different populations including insomnia | 17/284 | Melatonin 0.3–40 mg versus placebo | SOL, TST, SE | SOL ↓; TST ↑; SE ↑ |
| Buscemi et al. (2005) | Primary sleep disorders | 14/425 | Melatonin 1–5 mg versus placebo | SOL, WASO, TST, SE, SQ, USE | SOL ↓; best effect in sleep phase delay |
| Buscemi et al. (2006) | Secondary sleep disorders | 15/524 | Melatonin 1–10 mg versus placebo | SOL, USE | No effect on SOL, no USE |
| Braam et al. (2009) | Sleep problems with intellectual dysfunction | 9/183 | Melatonin 0.5–9 mg versus placebo | SOL, TST, NOA | SOL ↓; TST ↑; NOA ↑ |
| Ferracioli-Oda et al. (2013) | Primary sleep disorders | 19/1683 | Melatonin 1—ca. 10 mg versus placebo | SOL, TST, SQ | Moderate effects on sleep continuity |
| Liu and Wang (2012) | Chronic insomnia | 8/4055 | Ramelteon 4–32 mg versus placebo | SOL, USE | Positive effects on subjective/objective SOL/no USE |
| McCleery et al. (2014) | Insomnia with Alzheimer's disease | 5/313 | Trazodon, melatonin, ramelteon | SOL, TST, WASO, SE | No evidence supporting melatonin/ramelteon |
| Kuriyama et al. (2014) | Insomnia | 13/5812 | Ramelteon | SOL, TST, SQ | SOL ↓; SQ ↑; clinically small effects |
| Winkler et al., 2014 | Insomnia | 31/3820 | BZ, BZRA, sedating antidepressants, melatonin | PSG and subjective sleep parameters | Melatonin less effective than BZ/BZRA |
| Zhang et al. (2016) | Sleep disorders with neurodegenerative disorders | 9/370 | Melatonin | PSQI | Positive effects on PSQI and RBD |
| Auld et al. (2017) | Primary insomnia | 5/1145 | Melatonin | SOL | Significant effects for SOL |
| Marupuru et al. (2022) | Chronic insomnia > 60 years | 17/2462 | Melatonin, ramelteon | TST, SE, SOL, SQ | Significant effects for TST (PSG), SOL, SQ (subjective and PSG); no effect for SE |
- Abbreviations: BZ, benzodiazepines; BZRA, benzodiazepine receptor agonists; NOA, number of awakenings; PSG, polysomnography; PSQI, Pittsburgh Sleep Quality Index; RBD, rapid eye movement sleep behaviour disorder; SE, sleep efficiency; SOL, sleep-onset latency; SQ, sleep quality; TST, total sleep time; USE, undesired side-effects; WASO, wake time after sleep onset.
The meta-analysis by Marupuru et al. (2022) summarises studies on melatonin (including PR melatonin) and ramelteon. The authors report small to medium effects on sleep-related parameters in elderly patients and patients with insomnia, in short-term studies. Several very recent all-encompassing (network) meta-analyses included studies on fast-release, PR melatonin and ramelteon (De Crescenzo et al., 2022; Hasan et al., 2023; Maruani et al., 2023; Yue et al., 2023); however, the results are conflicting. Considering this evidence, fast-release melatonin and ramelteon are not recommended for insomnia treatment (exception: if there are circadian factors involved) due to lack of efficacy. Given the evidence from studies with PR melatonin, and the fact that many health authorities in Europe have sanctioned its use beyond 4 weeks, PR melatonin, short- and longer-term administration can be considered (≥ 55 years.) after weighing the advantages and disadvantages.
A major caveat with melatonin is that it is manufactured by very different sources and can be purchased through a variety of avenues like supermarkets, internet, chemists and pharmacies. This probably results in marked variations in pharmacological quality. We thus urge caution and suggest users should obtain melatonin only through licensed pharmacies or through a prescription by their physician.
4.2.7 Herbal remedies/phytotherapeutics
Table 11 lists herbal/phytotherapeutic substances available in Europe, and Table 15 presents all published meta-analyses on this topic, up until now.
| Author (Year) | Population | No. studies/patients | Intervention | Study endpoints | Effects on study endpoints |
|---|---|---|---|---|---|
| Bent et al. (2006) | Insomnia | 16/1093 | Valerian versus placebo, short-term treatment | SQ, SOL | (a) Slight improvement in sleep quality |
| (b) No improvement on other sleep parameters | |||||
| c) Poor quality of studies | |||||
| Fernández-San-Martín et al. (2010) | Insomnia | 18/1317 | Valerian versus placebo | SQ | No effects on quantitative parameters, slight effects for SQ |
| Leach and Page (2015) | Insomnia | 14/1602 | Valerian, chamomile, kava, wuling | SOL, SE, TST, SQ | No significant effects |
| Ni et al. (2015) | Insomnia | 76/7240 | CHM versus placebo versus BZ | PSQI, CGI | CHM better than placebo, but poor quality studies |
| Khadivzadeh et al. (2018) | Insomnia (peri- and post-menopausal) | 12/1257 | Phytotherapeutics | Subjective parameters | Evidence considered as poor |
| Hieu et al. (2019) | Insomnia | 1/34 | Chamomilla | ISI | No evidence |
| Zhang, Liu, et al. (2019) | Primary insomnia | 15/1500 | CHM | PSQI, AIS, TST, SE, SOL | Significant effects for PSQI, AIS, TST, SE, SOL |
| Birling et al. (2020) | Insomnia disorder | 19/1780 | Zaoren Anshen (CHM) | PSQI, USE | Significant effects for Zaoren Anshen versus placebo; poor methodological quality studies |
| Chen, Yi, et al. (2020) | Insomnia | 13/1175 | Zaoren Anshen (CHM) | PSQI, USE | No evidence |
| Fan et al. (2020) | Insomnia disorder | 13/1181 | Long Dan Xie Gan Tang (CHM) versus BZ/BZRA/barbiturate | Remission rate | Significant better remission with Long Dan Xie Gan Tang compared with BZ/BZRA/barbiturates |
| Li, Xu, et al. (2020) | Insomnia disorder | 14/1549 | Yangxin Anshen (CHM) versus placebo/BZ/BZRA | PSQI, PSG parameter | Significant effects of Yangxin Anshen versus placebo for PSQI, PSG-parameter; no difference to BZ/BZRA |
| Shinjyo et al. (2020) | Diverse populations | 60/6894 | Valerian | Diverse outcomes | No clear effects |
| Wang et al.(2020) | Insomnia disorder | 22/2029 | Chaihu Longgu Muli (CHM) | PSQI, clinical effectiveness, USE | Significant effects of Chaihu Longgu on PSQI und clinical effectiveness; small USE |
| Hu et al. (2021) | Insomnia disorder + anxiety | 9/681 | Xiao Yao San (CHM) | PSQI | Significant effects of Xiao Yao San for PSQI |
| Ji et al. (2021) | Insomnia | 72/7392 | CHM | Various sleep parameters | Significant effects, very heterogenous quality of studies |
| Lin et al. (2021) | Insomnia disorder | 14/910 | Banxia (CHM) | PSQI | Significant effects for Banxia |
| Yoon et al. (2021) | Insomnia + cancer | 14/1020 | Phytotherapeutics | PSQI | Significant effects, low-quality studies |
| Huang, Huang, et al. (2022) | Insomnia | 4/224 | Viola odorata | SE, SOL, SQ, ISI, PSQI | Significant effects for SQ, ISI, PSQI; no effects for SE, SOL |
| Kim and Lim (2022) | Insomnia post-stroke | 24/1942 | Far eastern phytotherapeutics | PSQI | Significant effects on PSQI, low-quality studies |
| Lian et al. (2022) | Insomnia | 8/611 | Saffron | ISI, PSQI | Significant effects on ISI, PSQI |
| Luan et al. (2022) | Insomnia disorder | 16/1260 | Traditional chinese medicine | TST, SE, SOL, NOA, ISI, PSQI | Significant effects for many parameters, low-quality studies |
| Munirah et al. (2022) | Insomnia disorder | 8/431 | Crocus Sativus | TST, SQ, ISI | Significant effects for TST, SQ, ISI; low-quality studies |
| Zhou et al. (2022) | Insomnia disorder | 19/1850 | Wuling | PSQI | Significant effects on PSQI |
- Abbreviations: BZ, benzodiazepines; BZRA, benzodiazepine receptor agonists; CGI, clinical global impression; CHM, Chinese herbal medicine; ISI, Insomnia Severity Index; NOA, number of awakenings; PSG, polysomnography; PSQI, Pittsburgh Sleep Quality Index; SE, sleep efficiency; SOL, sleep-onset latency; SQ, sleep quality; TST, total sleep time; USE, undesired side-effects.
Meta-analyses suggest small superiority of valerian compared with placebo (Leach & Page, 2015; Shinjyo et al., 2020). That said, this is in the context of rating the original studies to be of low quality. There is also evidence, also from low-quality original studies (Ji et al., 2021), that Saffron (Lian et al., 2022; Munirah et al., 2022), Viola odorata (Huang, Huang, et al., 2022) and diverse plant cocktails, from traditional Chinese medicine, may be effective to a low degree (Birling et al., 2020; Chen et al., 2022; Chen, Yi, et al., 2020; Fan et al., 2020; Li, Xu, et al., 2020; Lin et al., 2021; Wang et al., 2021; Wang, Ju, et al., 2020; Yang et al., 2019; Zhang, Liu, et al., 2019; Zhou et al., 2022). It is problematic for the authors of this guideline to evaluate these original studies, within the context of traditional Chinese medicine, as most of the original studies were not published in English language. Caution is therefore suggested given the conclusion by most authors of the meta-analyses that they consider the methodological quality of included studies as rather weak. As such, herbal/phytotherapeutic interventions cannot be recommended for either short- or long-term use to treat insomnia.
4.2.8 Pharmacological long-term treatment
Studies on the pharmacotherapy of insomnia have mainly investigated treatment in the short term. Most studies on the BZs/BZRAs and low-dose sedating antidepressants had treatment durations shorter than 4 weeks, and the phase-III studies on daridorexant were for 3 months (with an extension up to 12 months). From a clinical perspective, given insomnia disorder is typically persistent, what would be more relevant would be to know the efficacy and safety of pharmacotherapy over a longer-term treatment period. It must be borne in mind, however, that all hypnotics are symptomatic treatments and insomnia symptoms typically return after discontinuation of these substances. Concerning longer-term treatment, therefore, up to this point, the scientific evidence remains insufficient to give a general positive recommendation for any medication beyond 4 or 12 weeks (depending on the type of drug being used). We do, however, acknowledge that in some cases, after carefully weighing the advantages and disadvantages, longer treatment periods might be initiated. Whether daridorexant or PR melatonin might become an exception to the rule remains to be seen, as more long-term data (in addition to practical experience) are needed. Thus, administration of these drugs for at least 3 months is an option, given that precautions should be taken to avoid the potential for tolerance and dependence.
4.3 Comparative meta-analyses
The Institute of Quality and Economy in Health Care System (IQWiG) in Germany has prepared two evidence reports for the comparative treatment for insomnia in Germany. Both reports encompass meta-analytic summaries of the relevant literature.
The first report investigated internet- or mobile-based CBT-I (IQWiG-report—Nr. 1223; IQWIQ, 2021b), and compared efficacy with conventional CBT-I in adults with insomnia. Five studies were included in the report. With respect to the ISI, significant superiority for conventional CBT-I over dCBT-I was observed. This effect was measurable at the end of therapy and at follow-ups. The quality of evidence was considered, moderate.
The second report with the title “Cognitive Behavioural Therapy for Insomnia” (IQWiG-report—Nr. 1224; IQWIG, 2021a) investigated the efficacy of CBT-I compared with BZ and BZRA in adults with insomnia. Even 50 years after the introduction of both treatments, only five studies could be identified, making this comparison difficult. In some outcome parameters CBT-I was superior and in others pharmacological treatment was superior. The quality of evidence was rated as low for all outcomes, hindering valid conclusions. That said, a meta-analysis by Zhang et al. (2022) comparing CBT-I with any kind of pharmacological treatment for insomnia concluded that CBT-I may be superior to pharmacological treatment.
Recently a meta-analysis was published in the Lancet, comparing all pharmacological treatments for insomnia with each other (De Crescenzo et al., 2022). In this study, eszopiclone had the highest efficacy for insomnia-related parameters; however, this was explicitly tempered against the side-effects associated with this medication. Other drugs like BZ, doxepin and daridorexant were missing data on the efficacy in short- and long-term use according to the authors (De Crescenzo et al. (2022). Further, melatonin and other OTC medications were not considered effective according to this analysis.
4.4 Daily use of medication versus as needed
With regard to sleep medications, it is mostly assumed that they are taken daily over short (up to 4 weeks) or longer-term (beyond 4 weeks) periods. This is probably engrained by the way clinical studies are conducted, mostly administering medication/placebo daily over a fixed time. Few studies have investigated intermittent use versus continuous use (Perlis et al., 2008), concluding that intermittent dosing does not lead to increased rebound phenomena. However, it could not be determined whether intermittent dosing is equal or superior to continuous dosing.
Given that chronic insomnia (beyond 3 months) is characterised by fluctuations in sleep quality, the question of intermittent versus continuous medication intake is of high interest. In everyday clinical practice there is evidence that sleep medication is often prescribed/used on an “if needed” basis as opposed to a daily basis. The question now is how to define “as needed”—does this entail usage when somebody directly experiences sleep-onset problems or nocturnal awakening, or should the drug be taken prophylactically, i.e. the night before, when next-day challenges are expected that require a proper sleep beforehand. Depending on its half-life, drugs should not be taken too late in the night to avoid “hangover” problems, precluding middle of the night use.
Given these highly important questions, much more clinical routine data are needed to determine how to proceed. As of now, our recommendations refer to daily use (as informed by RCTs), but we do acknowledge that in daily clinical practice things might be handled very differently—so some patients may be long-term users over years or months, but may use one or two pills a week. Learning theory has made a strong point against intermittent use of potentially abusive substances, stating that intermittent positive reinforcement guarantees the strongest ties between a given substance and drug-taking behaviour (see overview by Perlis et al., 2008).
This issue becomes even more pertinent when we consider acute insomnia, an issue largely neglected by the insomnia field (with a few exceptions: see Ellis et al., 2012; Ellis, 2019). Our guideline refers to chronic insomnia (duration beyond 3 months) and we do not make any recommendations for acute/short-term insomnia. Nevertheless, in the medical arena, acute insomnia is a clinical issue facing many doctors routinely every day, for example, in acute situations like emergency visits or emergency admissions to hospitals or patients having to undergo impending life-saving procedures/surgery. We would assume that under these circumstances, swiftly acting medication may be very useful, according to the clinical picture and all ramifications of a given “case”. We do assume that working on specific guidelines concerning these extreme scenarios will deserve special consideration and should be addressed in the near future.
4.5 Tapering off medications
One major open question is whether CBT-I should be initiated when patients are on concomitant medication and request to be tapered off because they experience unpleasant side-effects, or feel they need support to discontinue the medication. For BZ/BZRA, specific schemata have been suggested with good success (Gould et al., 2014). The critical issue is whether all sleep medications should be tapered off prior to initiation of CBT-I or if an overlap can be accepted. There is evidence that CBT-I works better after discontinuation of sleep medication (Morin et al., 1994); however, in clinical practice, this may just not be feasible. It is suggested that those in the insomnia field work more closely together with experts in addiction medicine to realise feasible solutions in this important area. Another important consideration is how to taper off any sleep medication if a patient is motivated to do so and explicitly requires to be helped. There may be cases of comorbid insomnia where one would want to argue against drug discontinuation, if there is expectancy of an extreme recurrence of insomnia symptoms during withdrawal that would negatively interfere with a medical comorbidity—for example, in cases of acute psychosis or mania.
5 FINAL REMARKS ABOUT THE MANAGEMENT OF INSOMNIA AND FUTURE RESEARCH
This guideline tries to bring together state of the science evidence, generated over the last 6 years, to improve the management of chronic insomnia. The diagnostic recommendations have been summarised in Table 4, and the therapeutic recommendations are given in Table 16 and Figure 1.
| Treatment considerations |
|
|
| CBT-I |
|
|
|
| Pharmacological interventions |
|
|
|
|
|
|
|
|
|
|
|
| Other therapies |
|
- Abbreviations: BZ, benzodiazepines; BZRA, benzodiazepine receptor agonists; CBT-I, cognitive-behavioural therapy for insomnia; OTC, over-the-counter; PR, prolonged-release.
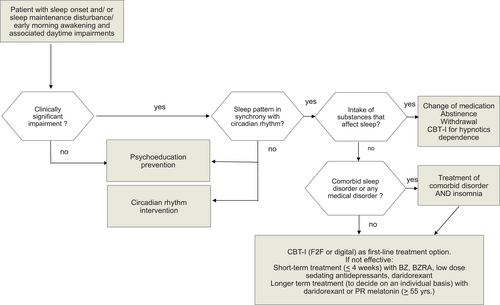
Guidelines are attempts to reconcile the arenas of evidence-based medicine and clinical reality, between which there is usually a huge gap. With this guideline, we have tried, as we should, to collect and analyse the available evidence at its highest level, i.e. published meta-analyses. On the other hand, and as documented throughout this guideline, we are aware that many clinicians consider this aspect of their discipline as artificial or not rooted in clinical reality. However, RCTs on their own and/or summarised in meta-analyses still constitute the most powerful instruments to tease out what works and what does not. Individual clinical knowledge and intuition is important; however, so-called eminence-based medicine was disregarded for a reason!
Although there are new data available on the diagnosis and treatment of insomnia, there are still several areas in need of more intensive research.
The chapter on comparative meta-analyses (Section 3) clearly shows that there are only a few studies that directly compare different treatment options for insomnia. Although guidelines now universally favour CBT-I, it would be useful to have studies directly comparing CBT-I with pharmacotherapy. Moreover, pharmacological treatments have rarely been compared in high-quality randomised clinical studies. It is very difficult, therefore, to establish whether new medications are inferior, equal or superior to established treatments.
The chapter on longer-term pharmacological treatment (Section 2.8) highlights a distinct need for longer-term treatment studies of insomnia using pharmacological agents. This lack of evidence has resulted in continued cautioning when considering pharmacotherapy in the long-term treatment of insomnia. We see a certain potential for PR melatonin and orexin-receptor-antagonists under these circumstances, especially in those patients for whom CBT-I was not effective.
An important question when judging treatment options for insomnia is also the question of placebo effects. Placebos have been shown to have small effects on subjective insomnia symptoms and even medium effects on global measures of sleep quality (Yeung et al., 2018). As such, the use of adequate placebos is a very important methodological precondition to judge the efficacy of treatment options in insomnia. Presently, there is a paucity of appropriate placebo-controlled studies with CBT-I, with the majority of studies using waiting list or treatment as usual control conditions. That said, Van Straten et al. (2018) compared waitlist CBT-I studies with placebo-controlled CBT-I studies, and concluded that the effects were comparable. It also has been demonstrated that Sleepio (one of the available dCBT-I platforms) is superior to placebo (Espie et al., 2012). Future clinical insomnia research on non-pharmacological treatments should therefore pay greater attention to the fact that it is feasible to construct valid placebo conditions. Additionally, the effects of CBT-I on objectively measured sleep parameters, from PSG, remain under-researched (Mitchell et al., 2019).
Another important issue concerns the question of remission versus response/non-response in both CBT-I and pharmacological trials. Morin et al. (2020) showed that in a comparative trial of psychological therapies versus zolpidem, clinically meaningful response occurred in 45% of psychological therapies (remission in slightly less than 40%) and in 49% of zolpidem-treated subjects (remission in slightly less than 30%). Most studies in the field (concerning both CBT-I and pharmacotherapy) usually report pre- to post-comparisons of outcome variables and effect strengths. Future research will have to take a closer look at response versus remission rates and present strategies to achieve higher remission rates, as in studies with sequential designs such as the one carried out by Morin et al. (2020).
The increasing trials pipeline on single-component CBT-I interventions is to be encouraged (Kyle et al., 2023), both with respect to the assessment of mechanisms and their unique efficacy. Recognition that CBT-I is an amalgam of numerous cognitive-behavioural options, each of which may have a discrete evidence base, offers considerable promise, and further research on potentially differing dimensions or sequencing of behavioural and cognitive therapeutics and their comparative effectiveness appears to be warranted.
A strong emphasis should be put on researching combination therapies, especially between medications and psychotherapeutic approaches. We need to know whether patients should be tapered off medication prior to CBT-I or not—will CBT-I then work better? Might a combination of medication and CBT-I work better in some patients or not?
What about patients who are dependent on hypnotic medication—how can we help them if they are motivated to abstain? Tapering strategies need to be developed and integrated with CBT-I strategies.
Further treatment options, which currently lack a solid empirical basis, need to be taken account of in future investigations, such as the cannabinoids (Bhagavan et al., 2020), and tES or TMS (Krone et al., 2023).
The treatment of insomnia and comorbid medical disorders is still under-researched. Attention should be drawn for example to “comorbid insomnia and sleep apnea” (COMISA). Some initial evidence seems to support CBT-I alongside treatment of sleep apnea, but further trials would be welcome.
A further research step will have to include the practical implementation of any kind of insomnia management in everyday clinical practice. Our knowledge is primarily based on good-quality evidence from RCTs; however, it is well known that high-quality RCTs may be very far from what happens in clinical routine. Thus, we need data from everyday clinical practice about diagnostic procedures that are accepted and executed, treatment acceptance, adherence, attrition, side-effects and therapeutic efficacy. For example, pragmatic adaptations of CBT-I for patients with insomnia in acute psychiatric care are currently developed and very promising (Schneider et al., 2020).
6 GUIDELINE RECOMMENDATIONS AND CLINICAL ALGORITHM
Recommendations for the diagnostic management of chronic insomnia and its comorbidities in adults of all ages have been summarised in Table 4. Recommendations for insomnia treatment are shown in Table 16.
A clinical algorithm summarising the diagnostic and therapeutic process is shown in Figure 1.
AUTHOR CONTRIBUTIONS
Dieter Riemann: Conceptualization; methodology; data curation; supervision; project administration; writing – original draft; writing – review and editing; validation; formal analysis; visualization; resources. Colin A. Espie: Conceptualization; methodology; writing – original draft; writing – review and editing; project administration. Ellemarije Altena: Conceptualization; writing – original draft; writing – review and editing. Erna Sif Arnardottir: Conceptualization; writing – original draft; writing – review and editing; project administration. Chiara Baglioni: Conceptualization; methodology; writing – original draft; writing – review and editing. Claudio L. A. Bassetti: Conceptualization; writing – original draft; writing – review and editing. Celyne Bastien: Conceptualization; writing – original draft; writing – review and editing. Natalija Berzina: Conceptualization; writing – original draft; writing – review and editing. Bjørn Bjorvatn: Conceptualization; methodology; writing – original draft; writing – review and editing. Dimitris Dikeos: Conceptualization; writing – original draft; writing – review and editing. Leja Dolenc Groselj: Conceptualization; writing – original draft. Jason G. Ellis: Conceptualization; methodology; writing – original draft; writing – review and editing. Diego Garcia-Borreguero: Conceptualization; methodology; writing – original draft; writing – review and editing. Pierre Geoffroy A: Conceptualization; writing – original draft; writing – review and editing. Michaela Gjerstad: Conceptualization; writing – original draft; writing – review and editing. Marta Gonçalves: Conceptualization; writing – original draft; writing – review and editing. Elisabeth Hertenstein: Conceptualization; methodology; writing – original draft; writing – review and editing. Kerstin Hoedlmoser: Conceptualization; writing – original draft; writing – review and editing. Tuuliki Hion: Conceptualization; writing – original draft; writing – review and editing. Brigitte Holzinger: Conceptualization; writing – original draft; writing – review and editing. Karolina Janku: Conceptualization; writing – original draft; writing – review and editing. Markus Jansson-Fröjmark: Conceptualization; writing – original draft; writing – review and editing. Heli Järnefelt: Conceptualization; writing – original draft; writing – review and editing. Susanna Jernelöv: Conceptualization; methodology; writing – original draft; writing – review and editing. Poul Jørgen Jennum: Conceptualization; writing – original draft; writing – review and editing. Samson Khachatryan: Conceptualization; writing – original draft; writing – review and editing. Lukas Krone: Conceptualization; methodology; writing – original draft; writing – review and editing. Simon D. Kyle: Conceptualization; methodology; writing – original draft; writing – review and editing. Jaap Lancee: Conceptualization; writing – original draft; writing – review and editing. Damien Leger: Conceptualization; writing – original draft; writing – review and editing. Adrian Lupusor: Conceptualization; writing – original draft; writing – review and editing. Daniel Ruivo Marques: Conceptualization; writing – original draft; writing – review and editing. Christoph Nissen: Conceptualization; methodology; supervision; writing – original draft; writing – review and editing. Laura Palagini: Conceptualization; supervision; writing – original draft; writing – review and editing. Tiina Paunio: Conceptualization; writing – original draft; writing – review and editing. Lampros Perogamvros: Conceptualization; writing – original draft; writing – review and editing. Dirk Pevernagie: Conceptualization; methodology; writing – original draft; writing – review and editing. Manuel Schabus: Conceptualization; writing – original draft; writing – review and editing. Tamar Shochat: Conceptualization; writing – original draft; writing – review and editing. Andras Szentkiralyi: Conceptualization; writing – original draft; writing – review and editing. Eus Van Someren: Conceptualization; methodology; supervision; writing – original draft; writing – review and editing. Annemieke van Straten: Conceptualization; writing – original draft; writing – review and editing. Adam Wichniak: Conceptualization; writing – original draft; writing – review and editing. Kai Spiegelhalder: Conceptualization; methodology; data curation; supervision; formal analysis; writing – original draft; writing – review and editing; project administration.
ACKNOWLEDGEMENTS
This guideline was supported and endorsed by the European Sleep Research Society (ESRS) and by the European Insomnia Network. We would like to thank especially the board of the ESRS in its present composition for having the confidence to trust us in delegating us to write this guideline. The ESRS generously provided € 1000 for financing English proof reading prior to JSR submission. Beyond, no financial support was available. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST STATEMENT
All conflict of interests forms are available as supplemental material.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




