The relationship between periodic limb movement during sleep and dyslipidaemia in patients with obstructive sleep apnea
Summary
Periodic limb movements during sleep and obstructive sleep apnea are both associated with increased sympathetic tone, and have been proposed as risk factors for heart diseases and, in particular, cardiovascular disease. As sympathetic system activation may lead to dyslipidaemia, periodic limb movements during sleep could be an additional risk factor for cardiovascular disease in patients with obstructive sleep apnea. The aim of the study was to determine whether the presence of periodic limb movements during sleep affects serum lipid levels in obstructive sleep apnea. Total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, non- high-density lipoprotein cholesterol and triglyceride levels were investigated in 4138 patients with obstructive sleep apnea in the European Sleep Apnea Database (ESADA) cohort, divided into those with periodic limb movements during sleep index ≥ 15 per hr (n = 628) and controls (n = 3510). ANCOVA adjusted for age, sex, body mass index, apnea–hypopnea index, alcohol intake, smoking status, diabetes, insomnia and study site was used to assess differences in lipids between periodic limb movements during sleep and controls. Patients with periodic limb movements during sleep (24% female, 54.4 ± 12.1 years, body mass index 31.9 ± 5.8 kg m−2, apnea–hypopnea index 36.7 ± 25.4 per hr) had higher triglyceride (1.81 ± 1.04 versus 1.69 ± 0.90 mmol L−1, p = 0.002) and lower high-density lipoprotein cholesterol (1.19 ± 0.34 versus 1.24 ± 0.37 mmol L−1, p = 0.002) levels, whilst there was no difference in either total cholesterol (4.98 ± 1.10 versus 4.94 ± 1.07 mmol L−1), low-density lipoprotein cholesterol (3.04 ± 0.96 versus 2.98 ± 0.98 mmol L−1) or non- high-density lipoprotein cholesterol (3.78 ± 1.10 versus 3.70 ± 1.05 mmol L−1) concentrations (all p > 0.05). The results remained unchanged after most sensitivity analyses. Patients with obstructive sleep apnea with periodic limb movements during sleep had more prevalent cardiovascular disease (11% versus 6%, p < 0.01). Periodic limb movements during sleep in obstructive sleep apnea is associated with dyslipidaemia independently of important confounders. Our results highlight periodic limb movements during sleep as an additional risk factor for cardiovascular disease in obstructive sleep apnea.
1 INTRODUCTION
Obstructive sleep apnea (OSA) is a frequent disorder that is characterised by repetitive collapse of the upper airways during sleep, resulting in chronic intermittent hypoxaemia and frequent arousals. OSA is a risk factor for cardiovascular disease (CVD; Salari et al., 2022). However, its treatment with continuous positive airway pressure (CPAP) does not reduce cardiovascular morbidity or mortality, at least not in recent randomised–controlled trial studies performed in asymptomatic OSA patients with CVD (McEvoy et al., 2016; Peker et al., 2016). Identifying treatable traits to prevent CVD in patients with OSA is still an unmet clinical need.
Periodic limb movements during sleep (PLMS) are repetitive, stereotypical limb movements that can lead to arousals and to sleep fragmentation. Their prevalence increases with age, affecting about 30% of people over the age of 50 years (Haba-Rubio et al., 2016). They are also more prevalent among patients with OSA than in the general population (Al-Alawi et al., 2006). PLMS are strongly associated with increased sympathetic activity. However, there is some debate whether PLMS-triggered arousals lead to sympathetic surges, or if the hyperexcitable sympathetic state precedes PLMS (Guggisberg et al., 2007; Ware et al., 1988). Nevertheless, increased sympathetic activity leads to rises in blood pressure (Hart, 2016), vascular inflammation (Hart, 2016), hypercoagulation (Bikov et al., 2021) and dyslipidaemia (Lambert et al., 2013), all being hallmarks of atherosclerotic processes. In line with this, PLMS are associated with all-cause mortality in patients with chronic heart failure (Yumino et al., 2011), incident CVD (Koo et al., 2011) and significant cardiac events (Winkelman et al., 2017) both in the general population and in patients with OSA (Kendzerska et al., 2014; J. W. Kim et al., 2020). PLMS are associated with a significant but small increase in daytime blood pressure values in patients with OSA (Lombardi et al., 2020), suggesting that additional mechanisms, such as dyslipidaemia, could be conceivable for PLMS-associated cardiovascular risk in OSA.
Only one study has investigated dyslipidaemia in patients with OSA with and without PLMS so far (Murase et al., 2014). There was no relationship between PLMS and dyslipidaemia, but the study was limited by its sample size (254 patients with OSA), which prevented multivariable and subgroup analyses. Nevertheless, the authors reported a direct link between PLMS and C-reactive protein (CRP) as well as fibrinogen levels (Murase et al., 2014).
The European Sleep Apnea Database (ESADA) is a large collaborative network that at the time of our analysis included > 30,000 patients (Hedner et al., 2011). As part of their assessment, many subjects had fasting blood samples, including lipid profile and polysomnography (PSG) as a diagnostic test. These features make the database suitable to investigate the relationship between PLMS and dyslipidaemia in patients with OSA.
Therefore, the aim of this study was to compare blood lipid values in patients with OSA with and without PLMS. We also performed pre-defined sensitivity analyses to validate our findings.
2 METHODS
2.1 Study design and subjects
The ESADA is a multicentre, prospective study currently involving 29 sleep centres across Europe (Hedner et al., 2011). Patients referred with symptoms suggestive for OSA are invited to participate in the study. They had to speak, read and understand the local language, and possess the ability to respond to questions and follow instructions. Patients were included irrespective of comorbidity, concomitant medication and degree of sleepiness. However, they were not included if they had a limited life expectancy due to illness unrelated to sleep apnea (e.g. advanced renal disease, uncontrolled malignancies) or documented alcohol or drug abuse up to 1 year prior to inclusion in the study. The centres were encouraged to enrol as many patients as possible and no further specific selection criteria were applied to those to be enrolled.
For the current analysis, we screened patients who were not on any treatment for OSA. Out of the 34,259 patients aged between 18 and 91 years available at data extraction (06.04.2022), we also excluded: (1) subjects who had home sleep apnea test instead of PSG; (2) subjects who did not have a full lipid panel, including total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and triglycerides (TG); and (3) subjects with missing or incomplete PSG data. The final analysis included 4452 participants (4138 with OSA and 314 without OSA) from 22 sites (Figure 1). For this analysis we captured demographic data, comorbidities, medications, the Epworth Sleepiness Scale (ESS), lipid profile, and PSG sleep study data including PLM scoring. The study was approved by individual research ethics committees, and all patients gave their informed consent prior to the study.

2.2 Sleep studies
Sleep studies were performed using in-laboratory PSGs. For respiratory scoring, the American Academy of Sleep Medicine (AASM) 2007 criteria were used (Iber et al., 2007), whilst for limb movement scoring the criteria proposed by the AASM and the World Association of Sleep Medicine International Restless Legs Syndrome Study Group (IRLSSG) were applied (Ferri et al., 2016). For the analysis, we captured total sleep time, sleep efficiency, the percentage of N1, N2, N3 and rapid eye movement (REM) sleep, apnea–hypopnea index (AHI), oxygen desaturation index (ODI), the time spent with oxygen saturation below 90% (T90) and PLMS index (PLMSI). OSA was diagnosed if the AHI was ≥ 5 per hr, and PLMS was defined if the PLMSI was ≥ 15 per hr.
2.3 Statistical analyses
The JASP 0.14 (JASP Team, University of Amsterdam, Amsterdam, The Netherlands) software was used for statistical analysis. Demographic and clinical characteristics were compared using t-test, Mann–Whitney test and Chi-square test. ANCOVA adjusted for age, sex, body mass index (BMI), AHI, alcohol intake, smoking status, the presence of type II diabetes, insomnia and the study site was used to assess the difference in lipid levels between patients with and without PLMS.
The primary objective was to compare lipid levels in patients with OSA with (n = 628) versus without (n = 3510) PLMS. Further sensitivity analyses were performed in patients with OSA who had fasting blood sample analyses (n = 509 versus 3271), in patients with OSA with BMI < 30 kg m−2 (n = 268 versus 1348), in patients with OSA with BMI ≥ 30 kg m−2 (n = 360 versus 2162), in patients with OSA who did not take lipid-lowering medications (n = 506 versus 2707), and in patients with OSA who did not take dopaminergic/anti-epileptic medications (n = 599 versus 3449). We also compared participants in the ESADA cohort without OSA (n = 46 versus 268, with and without PLMS, respectively).
To further confirm the validity of the primary analysis, patients were weighted by the inverse of the probability (inverse probability treatment weighting [IPTW]) of having PLMS (inverse of the propensity score), and a weighted linear model was performed to assess the effect of PLMS on the different outcomes. Variables considered for the propensity score analysis were age, BMI, AHI and sex.
The results are expressed as mean ± standard deviation and beta coefficient with standard error for the IPTW analysis. A p-value < 0.05 was considered significant.
3 RESULTS
3.1 Comparison of clinical characteristics between patients with OSA with and without PLMS
Four-thousand, one-hundred and thirty-eight patients had OSA (AHI ≥ 5 per hr), 628 of them had PLMSI ≥ 15 per hr. The two groups were comparable in age, sex, BMI, and waist and hip circumference (Table 1). Patients with PLMS had slightly lower neck circumference, AHI, ODI and T90. The prevalence of current smokers was higher in the PLMS group. Despite that the prevalence of restless leg syndrome (RLS) and insomnia was higher, patients with PLMS tended to have a more consolidated sleep profile. In line with the better markers of sleep quality, patients with PLMS had lower ESS scores. In contrast, the prevalence of CVD (6% versus 11%, p < 0.01) and diabetes (20% versus 13%, p < 0.01) were higher in patients with PLMS.
| Without PLMS (n = 3510) | With PLMS (n = 628) | p-Value | |
|---|---|---|---|
| Age (years) | 54.7 ± 12.7 | 54.4 ± 12.1 | 0.368 |
| Sex (females %) | 26 | 24 | 0.357 |
| BMI (kg m−2) | 32.4 ± 6.2 | 31.9 ± 5.8 | 0.059 |
| Waist circumference (cm) | 110.7 ± 14.6 | 109.2 ± 13.5 | 0.085 |
| Hip circumference (cm) | 111.7 ± 12.6 | 110.4 ± 10.3 | 0.127 |
| Neck circumference (cm) | 42.1 ± 4.1 | 41.5 ± 4.2 | < 0.001 |
| Systolic blood pressure (mmHg) | 131.7 ± 15.2 | 133.4 ± 18.6 | 0.190 |
| Diastolic blood pressure (mmHg) | 80.6 ± 10.7 | 78.4 ± 11.1 | < 0.001 |
| Heart rate (1/min) | 71.2 ± 11.3 | 76.9 ± 14.5 | < 0.001 |
| FEV1 (litres)a | 3.14 ± 0.84 | 3.16 ± 0.94 | 0.590 |
| FVC (litres)a | 3.97 ± 1.02 | 4.03 ± 1.15 | 0.305 |
| FEV1/FVC (%)a | 79 ± 7 | 79 ± 7 | 0.210 |
| Hypertension (%) | 45 | 47 | 0.354 |
| CVD (%) | 6 | 11 | < 0.001 |
| Cerebrovascular disease (%) | 2.1 | 1.9 | 0.829 |
| Type II diabetes (%) | 13 | 20 | < 0.001 |
| COPD (%) | 6.9 | 4.7 | 0.038 |
| Asthma (%) | 5.9 | 7.7 | 0.097 |
| RLS (%) | 0.3 | 3.9 | < 0.001 |
| Insomnia (%) | 0.6 | 5.8 | < 0.001 |
| Alcohol intake (units) | 2.0 ± 4.2 | 2.8 ± 6.4 | 0.003 |
| Smokers (%) | 24 | 28 | 0.037 |
| Total sleep time (minutes) | 362 ± 100 | 366 ± 70 | 0.443 |
| Sleep efficiency (%) | 78.8 ± 16.4 | 81.4 ± 12.6 | 0.039 |
| N1 (%) | 13.9 ± 13.5 | 11.2 ± 11.6 | < 0.001 |
| N2 (%) | 60.9 ± 16.7 | 58.7 ± 14.4 | < 0.001 |
| N3 (%) | 13.2 ± 11.3 | 15.4 ± 10.6 | < 0.001 |
| REM (%) | 12.0 ± 7.2 | 14.8 ± 7.8 | < 0.001 |
| AHI (per hr) | 39.7 ± 26.0 | 36.7 ± 25.4 | 0.003 |
Mild OSA (AHI 5–14.9 per hr, %) Moderate OSA (AHI 15–29.9/h, %) Severe OSA (AHI ≥ 30.0 per hr, %) |
17.6 26.9 55.5 |
22.6 29.8 47.6 |
< 0.001 |
| ODI (per hr) | 36.5 ± 27.0 | 28.6 ± 24.6 | < 0.001 |
| T90 (min) | 63 ± 86 | 36 ± 62 | < 0.001 |
| ESS score | 9.9 ± 5.3 | 9.2 ± 5.4 | < 0.001 |
- Note: Data are expressed as mean ± standard deviation or percentages. Significant differences are highlighted in bold.
- Abbreviations: AHI, apnea–hypopnea index; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; ESS, Epworth Sleepiness Scale; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; PLMS, periodic limb movements during sleep; REM, rapid eye movement; RLS, restless legs syndrome; T90, time spent with oxygen saturation below 90%.
- a Data are available for 1316 and 306 patients without and with PLMS.
3.2 Comparison of lipid profile between patients with OSA with and without PLMS
Patients with PLMS had lower HDL-C and higher TG levels (both p < 0.001; Table 2). There was no difference in TC or LDL-C concentrations between the two groups. PLMSI correlated significantly with TG levels (Spearman's rho 0.05, p < 0.01), but not with any of the other lipid parameters.
| Without PLMS (n = 3510) | With PLMS (n = 628) | Adjusted p-value | |
|---|---|---|---|
| All patients in the analysis cohort | |||
| TC (mmol L−1) | 4.94 ± 1.07 | 4.98 ± 1.10 | 0.767 |
| LDL-C (mmol L−1) | 2.98 ± 0.98 | 3.04 ± 0.96 | 0.578 |
| HDL-C (mmol L−1) | 1.24 ± 0.37 | 1.19 ± 0.34 | 0.005 |
| Non-HDL-C (mmol L−1) | 3.70 ± 1.05 | 3.78 ± 1.10 | 0.223 |
| TG (mmol L−1) | 1.69 ± 0.90 | 1.81 ± 1.04 | 0.005 |
| Without PLMS (n = 3271) | With PLMS (n = 509) | Adjusted p-value | |
|---|---|---|---|
| Sensitivity analysis—fasting condition | |||
| TC (mmol L−1) | 4.93 ± 1.07 | 4.96 ± 1.10 | 0.906 |
| LDL-C (mmol L−1) | 2.97 ± 0.99 | 3.00 ± 0.97 | 0.918 |
| HDL-C (mmol L−1) | 1.24 ± 0.37 | 1.19 ± 0.32 | 0.003 |
| Non-HDL-C (mmol L−1) | 3.69 ± 1.05 | 3.77 ± 1.11 | 0.393 |
| TG (mmol L−1) | 1.66 ± 0.87 | 1.77 ± 1.00 | 0.021 |
| Without PLMS (n = 1348) | With PLMS (n = 268) | Adjusted p-value | |
|---|---|---|---|
| Sensitivity analysis—non-obese OSA patients (BMI < 30 kg m−2) | |||
| TC (mmol L−1) | 5.05 ± 1.06 | 5.07 ± 1.09 | 0.643 |
| LDL-C (mmol L−1) | 3.09 ± 0.99 | 3.13 ± 0.99 | 0.797 |
| HDL-C (mmol L−1) | 1.33 ± 0.41 | 1.26 ± 0.36 | 0.113 |
| Non-HDL-C (mmol L−1) | 3.73 ± 1.07 | 3.81 ± 1.10 | 0.928 |
| TG (mmol L−1) | 1.52 ± 0.86 | 1.59 ± 0.87 | 1.000 |
| Without PLMS (n = 2162) | With PLMS (n = 360) | Adjusted p-value | |
|---|---|---|---|
| Sensitivity analysis—obese OSA patients (BMI ≥ 30 kg m−2) | |||
| TC (mmol L−1) | 4.86 ± 1.07 | 4.91 ± 1.09 | 0.454 |
| LDL-C (mmol L−1) | 2.90 ± 0.98 | 2.97 ± 0.94 | 0.343 |
| HDL-C (mmol L−1) | 1.19 ± 0.33 | 1.14 ± 0.31 | 0.021 |
| Non-HDL-C (mmol L−1) | 3.68 ± 1.05 | 3.77 ± 1.10 | 0.143 |
| TG (mmol L−1) | 1.79 ± 0.91 | 1.97 ± 1.12 | < 0.001 |
| Without PLMS (n = 2707) | With PLMS (n = 506) | Adjusted p-value | |
|---|---|---|---|
| Sensitivity analysis –OSA patients without lipid-lowering medication | |||
| TC (mmol L−1) | 5.03 ± 1.03 | 5.12 ± 1.08 | 0.351 |
| LDL-C (mmol L−1) | 3.07 ± 0.96 | 3.18 ± 0.94 | 0.289 |
| HDL-C (mmol L−1) | 1.25 ± 0.38 | 1.20 ± 0.34 | 0.049 |
| Non-HDL-C (mmol L−1) | 3.78 ± 1.03 | 3.92 ± 1.08 | 0.231 |
| TG (mmol L−1) | 1.68 ± 0.92 | 1.79 ± 1.03 | 0.043 |
| Without PLMS (n = 3449) | With PLMS (n = 599) | Adjusted p-value | |
|---|---|---|---|
| Sensitivity analysis –OSA patients without dopaminergic/anti-epileptic medication | |||
| TC (mmol L−1) | 4.94 ± 1.07 | 4.98 ± 1.09 | 0.826 |
| LDL-C (mmol L−1) | 2.98 ± 0.98 | 3.04 ± 0.95 | 0.664 |
| HDL-C (mmol L−1) | 1.24 ± 0.37 | 1.19 ± 0.34 | 0.004 |
| Non-HDL-C (mmol L−1) | 3.69 ± 1.05 | 3.79 ± 1.10 | 0.246 |
| TG (mmol L−1) | 1.68 ± 0.89 | 1.81 ± 1.04 | 0.003 |
| Without PLMS (n = 268) | With PLMS (n = 46) | Adjusted p-value | |
|---|---|---|---|
| Sensitivity analysis—patients without OSA | |||
| TC (mmol L−1) | 4.92 ± 1.10 | 5.10 ± 1.15 | 0.331 |
| LDL-C (mmol L−1) | 2.99 ± 0.91 | 2.97 ± 0.90 | 0.543 |
| HDL-C (mmol L−1) | 1.32 ± 0.40 | 1.35 ± 0.39 | 0.865 |
| Non-HDL-C (mmol L−1) | 3.60 ± 1.10 | 3.75 ± 1.17 | 0.365 |
| TG (mmol L−1) | 1.48 ± 1.36 | 1.71 ± 1.12 | 0.272 |
- Note: Data are expressed as mean ± standard deviation. Significant differences are highlighted in bold.
- Abbreviations: BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; OSA, obstructive sleep apnea; PLMS, periodic limb movements during sleep; TC, total cholesterol; TG, triglycerides.
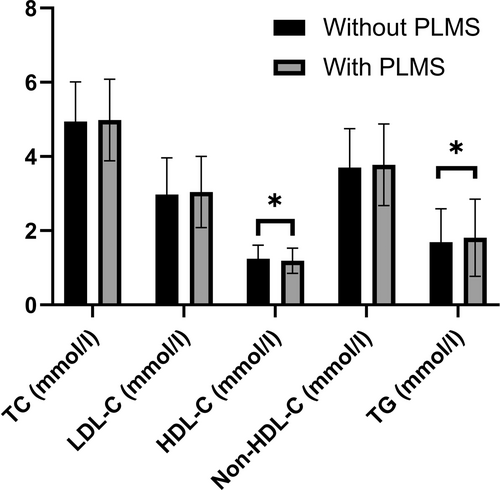
Sensitivity analysis performed in patients in whom blood samples were taken in the fasting condition confirmed the significant group differences for HDL-C and TG (Table 2; Figure 2). Further sensitivity analyses were performed in obese and non-obese patients, as well as in patients where treatment with ongoing lipid-lowering or dopaminergic/anti-epileptic medications were excluded. The difference in HDL-C and TG levels between the two groups remained significant in these sub-analyses except in non-obese patients; in this subgroup there was no difference in any of the lipid concentrations (Table 2; Figure 3). Furthermore, there was no difference between the two groups in any lipid parameter in control subjects without OSA.

3.3 IPTW sensitivity analysis
To further validate our results, we performed an IPTW analysis. After weighting, the results were similar to the original analysis (Table 3).
| Beta | SD | Adjusted p-value | |
|---|---|---|---|
| TC (mmol L−1) | 0.0403 | 0.047 | 0.3870 |
| LDL-C (mmol L−1) | 0.0640 | 0.042 | 0.1316 |
| HDL-C (mmol L−1) | −0.049 | 0.016 | 0.0021 |
| Non-HDL-C (mmol L−1) | 0.0892 | 0.045 | 0.0522 |
| TG (mmol L−1) | 0.1311 | 0.040 | 0.0010 |
- Abbreviations: HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides.
4 DISCUSSION
Our study performed in a European multi-centric clinical cohort of patients with OSA demonstrated that PLMS are associated with dyslipidaemia. This finding was confirmed in several sensitivity analyses addressing aspects of co-medication, obesity and analysis methodology. Our data propose PLMS as a potential mechanism for the increased risk of cardiometabolic disease, in particular CVD and diabetes, in patients with OSA.
Periodic limb movements may elevate cardiovascular risk through surges in sympathetic system activity (Guggisberg et al., 2007; Ware et al., 1988). Indeed, physiological studies showed increases in muscle sympathetic nerve activity (MSNA) during PLMS. In line with this, MSNA surges were associated with increased blood pressure (Pennestri et al., 2007) and heart rate (Winkelman, 1999), the latter of which is reflected in the current results. In fact, in the previous study of the same cohort, increased blood pressure values were detected in patients with PLMS when higher PLMSI (25 events per hr) was used as a cut-off (Lombardi et al., 2020). In the current analysis, we did not detect significantly higher blood pressure values or higher prevalence of hypertension in patients with PLMS. This finding may be masked by the ongoing treatment with antihypertensive drugs or the higher OSA severity in the non-PLMS group. Further data suggest that the relationship between PLMS and long-term cardiovascular risk depends on PLM disease severity (Koo et al., 2011). Our findings are in agreement with a study in patients with OSA that also used a PLMSI of 15 per hr to define the PLMS group (Murase et al., 2014), whereas another study using a lower PLMSI cut-off of 5 per hr showed that PLMS were not associated with hypertension (Al-Alawi et al., 2006). Another line of evidence suggests that sympathetic nervous activity (Hart, 2016; Pöyhönen-Alho et al., 2008), and most particularly PLMS (Trotti et al., 2012), could also lead to increased vascular inflammation. Indeed, a previous study showed increased CRP values in the PLMS group (Murase et al., 2014).
The effect of PLMS on lipid parameters has only been examined in a limited number of studies. In patients with renal transplantation, PLMS (defined as PLMSI ≥ 25 per hr) were associated with significantly lower HDL-C and a tendency for higher TG values (Lindner et al., 2012). In contrast, Murase et al. could not show an association between PLMS and lipid values in patients with OSA (Murase et al., 2014). These contrasting results could be explained by a number of factors. Most importantly, Murase et al. included a significantly lower number of subjects (n = 208 patients without and only 46 with PLMS) and excluded all patients with CVD. They also used a higher cut-off for OSA with an AHI of 15 per hr compared with 5 per hr in our study. Conditions associated with PLMS have been studied in the context of dyslipidaemia. For instance, microarousals, which are often associated with PLMS, could lead to dyslipidaemia (Ekstedt et al., 2004). Furthermore, PLMS are found in 80% of the patients with RLS (Montplaisir et al., 1997), and several studies provide evidence for a strong association between dyslipidaemia and RLS. Firstly, hypercholesterolaemia is a risk factor for incidental RLS (De Vito et al., 2014). Secondly, patients with RLS had lower HDL-C and a tendency for higher TG concentrations in a large cohort study (Schlesinger et al., 2009). Thirdly, there is a genetic link between RLS and HDL-C metabolism (Didriksen et al., 2020). Unfortunately, none of the studies cited above investigated PLMS as a link between RLS and dyslipidaemia.
We found significantly higher TG and lower HDL-C levels in patients with PLMS after adjustment for most relevant factors. These findings were confirmed when patients without fasting lipid values, and those on lipid-lowering, anti-Parkinson and anti-epileptic medications were excluded. Most importantly, we confirmed these differences with IPTW, a robust method for propensity score matching in large observational studies (Chesnaye et al., 2022). Interestingly, in patients with BMI < 30 kg m−2 no significant difference in lipid levels was found. This could potentially indicate that a certain amount of adipose tissue is needed for the sympathetic surges to liberate free fatty acids, which ultimately form TGs. Of note, this subgroup analysis could have been underpowered, as the direction for differences was similar to the whole cohort. The patients in the study by Murase et al. also had a lower BMI compared with our population, which could also explain the discrepancies (Murase et al., 2014). We compared lipid levels in non-OSA control subjects with and without PLMS. However, due to the low number of subjects, this subgroup analysis may have been underpowered to detect small differences.
Both higher TG and lower HDL-C levels are independently associated with cardiovascular risk (Miller et al., 2011; Wilson et al., 1998). However, the exact cardiovascular risk is difficult to estimate as various cohort studies were conducted in different populations and follow-up durations. Nevertheless, a meta-analysis concluded that each 1 mmol L−1 increase in TG levels is associated with relative risks for significant cardiovascular events of 1.14/1.05–1.28/ for men (8.4 years average follow-up) and 1.37/1.13–1.66/ for women (11.4 years average follow-up; Hokanson & Austin, 1996). On the other hand, the association between HDL-C and cardiovascular risk is U-shaped (Madsen et al., 2017). In the investigated HDL-C concentration range, an 0.026 mmol L−1 increment in HDL-C reduced the cardiovascular risk by 2% in men and 3% in women (Gordon et al., 1989). These data suggest that the differences in HDL-C and TG between the PLMS and control groups could attribute to a 10-year cardiovascular risk increase of 1%–4% in men and 4%–10% in women. However, these estimations need to be confirmed in large cohort studies in patients with PLMS and OSA.
Although it was not our primary study aim, we were able to compare clinical characteristics and PSG data of patients with and without PLMS. It has been reported that PLMS is more frequent in OSA than in the general population, and is related to male sex, age, depression and caffeine intake (Budhiraja et al., 2020). Similarly to our findings, patients with PLMS had slightly lower BMI (Murase et al., 2014), but a higher prevalence of type II diabetes (Al-Alawi et al., 2006). The latter association is known in the literature and is potentially related to sympathetic nervous activation (Rizzi et al., 2011). We reported a higher prevalence of smoking in PLMS, which replicates a previous report from a large general population study (Lavigne et al., 1997). Of note, smoking could also have been the reason for increased prevalence of CVD in patients with PLMS in our population. Despite smoking was more common, the prevalence of chronic obstructive pulmonary disease (COPD) was lower in the PLMS group. Unfortunately, only one-third of patients had lung function tests, hence no further analyses were performed to explore this relationship. In contrast to previous reports (Budhiraja et al., 2020; H. J. Kim & Lee, 2020; Murase et al., 2014), patients with PLMS had more consolidated deep- and REM-sleep. Although the exact reason for this discrepancy is not known, the most likely explanation could be the more severe OSA among patients without PLMS. Whilst the difference in average AHI was trivial, patients with PLMS had significantly lower prevalence of severe OSA. In line with a better sleep quality, patients with PLMS had a lower degree of daytime sleepiness as assessed with the ESS. These findings are in line with the previous reports that PLMS does not necessarily worsen sleepiness in OSA (Chervin, 2001; H. J. Kim & Lee, 2020). The prevalence of insomnia was higher in patients with PLMS. Insomnia is associated with dyslipidaemia (Song et al., 2020; Zhan et al., 2014). Therefore, our analyses were adjusted for this symptom.
The strengths of the study include its large sample size, the diversity of the participating sites and patients, the comprehensive assessment of sleep disorders, laboratory values and comorbidities. These merits allowed us to perform robust subgroup analyses, and it increases the generalisability of our findings. The limitation of the analysis is its cross-sectional nature; therefore, a causal relationship between PLMS and dyslipidaemia can only be hypothesised. Prospective, interventional studies are required to analyse this in detail. It was previously shown that the reduction of PLMS with rotigotine was significantly associated with a decrease in blood pressure (Bauer et al., 2016); however, lipid values were not measured. Of note, rotigotine attenuated cholesterol uptake in endothelial cells thus having an anti-atherosclerotic effect (Kang et al., 2021). We hypothesised that increased MSNA is the reason for dyslipidaemia in PLMS. However, whilst higher heart rate in the PLMS group in the current study is suggestive for increased MSNA, this can be directly tested with microneurography, which was not performed. Nevertheless, former and the current studies highlight the possibility that PLMS could be a treatable cardiovascular trait in OSA. In this context, a recent meta-analysis has concluded that CPAP may elevate the PLMSI in patients with OSA (Lin et al., 2022). Considering this together with our result, this may explain why there is only a modest change in lipid values following CPAP treatment, as rising PLMS may counterbalance the beneficial effect of CPAP (Gunduz et al., 2020). To confirm this hypothesis, future analyses addressing CPAP effects on blood lipids after controlling for the potential change in PLMS are warranted.
In summary, we have reported that PLMS is associated with high TG and low HDL-C levels in patients with OSA. This highlights the significance of PLMS as a potential cardiovascular trait. However, this hypothesis needs to be tested by interventional studies. We believe that our findings could serve as a potential basis to design these trials.
AUTHOR CONTRIBUTIONS
Andras Bikov: Conceptualization; data curation; formal analysis; methodology; writing – original draft. Sébastien Bailly: Data curation; formal analysis; writing – review and editing. Dries Testelmans: Data curation; project administration; writing – review and editing. Francesco Fanfulla: Data curation; project administration; writing – review and editing. Athanasia Pataka: Data curation; project administration; writing – review and editing. Izolde Bouloukaki: Data curation; project administration; writing – review and editing. Holger Hein: Data curation; project administration; writing – review and editing. Zoran Dogas: Data curation; project administration; writing – review and editing. Ozen K. Basoglu: Data curation; project administration; writing – review and editing. Richard Staats: Data curation; project administration; writing – review and editing. Gianfranco Parati: Data curation; project administration; writing – review and editing. Carolina Lombardi: Data curation; project administration; writing – review and editing. Ludger Grote: Data curation; project administration; writing – review and editing. STEFAN MIHAICUTA: Conceptualization; data curation; project administration; writing – original draft.
ACKNOWLEDGEMENTS
Andras Bikov is supported by the NIHR Manchester BRC. The ESADA network was founded during the COST action B26 supported by the European Union. The ESADA network is one of the Clinical Research Collaborations (CRC) funded by the European Respiratory Society (ERS). Unrestricted seeding grants from the ResMed Foundation and the Philips Respironics Foundation for establishment of the database in 2007 and 2011 are gratefully acknowledged. The ESADA has received an unrestricted grant from Bayer AG for collaborative data analysis. LG and JH are supported by the Swedish Heart and Lung Foundation (20180567 and 20180585) and the agreement concerning research and education of doctors (ALFGBG 725601 and ALFGBG 721521). Support was also provided by the European Sleep Research Society (ESRS) and the ERS in terms of logistics for communication, meetings and data presentations for the ESADA collaborators. SB is supported by the French National Research Agency in the framework of the “Investissements d'avenir” program (ANR-15-IDEX-02) and the “e-health and integrated care and trajectories medicine and MIAI artificial intelligence” (ANR-19-P3IA-0003). The authors thank Mr Andrew Chai for his grammatical corrections.
CONFLICT OF INTEREST STATEMENT
LG reports no COI for the content of the current manuscript. Outside the scope of the manuscript, LG reports non-financial and other support from Itamar Medical, Resmed, Philips, Astra Zeneca, Breas, Lundbeck, and Desitin outside the submitted work. Outside the scope of the manuscript, GP reports honoraria for lectures by Omron, Merck and Somnomedics. In addition, LG reports ownership in a patent on sleep apnea therapy licensed. None of the other authors report any conflict of interest.
APPENDIX
- Alexandroupolis, Greece
- Steiropoulos P, Sleep Unit, Department of Pneumonology, Democritus University of Thrace, Alexandroupolis, Greece
- Antwerp, Belgium
- Verbraecken J, Multidisciplinary Sleep Disorders Centre, Antwerp University Hospital and University of Antwerp, Antwerp, Belgium
- Petiet E, Multidisciplinary Sleep Disorders Centre, Antwerp University Hospital and University of Antwerp, Antwerp, Belgium
- Athens, Greece
- Georgia Trakada, Pulmonary Medicine, National and Kapodistrian University of Athens, Athens, Greece
- Berlin, Germany
- Fietze I, Schlafmedizinisches Zentrum, Charité – Universitätsmedizin Berlin, Germany
- Penzel T, Schlafmedizinisches Zentrum, Charité – Universitätsmedizin Berlin, Germany
- Brno, Czech Republic
- Ondrej Ludka, Department of Cardiology, University Hospital Brno and International Clinical Research Center, St. Ann's University Hospital, Brno, Czech Republic
- Crete, Greece
- Bouloukaki I. Sleep Disorders Unit, Department of Respiratory Medicine, Medical School, University of Crete, Greece
- Schiza S, Sleep Disorders Unit, Department of Respiratory Medicine, Medical School, University of Crete, Greece
- Dublin, Ireland
- McNicholas WT, Department of Respiratory Medicine, St. Vincent's University Hospital, Dublin, Ireland
- Ryan S, Pulmonary and SleepDisorders Unit, St. Vincent'sUniversityHospital, Dublin, Ireland
- Edinburgh, United Kingdom
- Riha RL, Department of Sleep Medicine, Royal Infirmary Edinburgh, Scotland
- Förde, Norway
- Kvamme JA, Sleep Laboratory, ENT Department, Førde Central Hospital, Førde, Norway
- Gothenburg, Sweden
- Grote L, Sleep Disorders Center, Pulmonary Department, Sahlgrenska University Hospital, and Center of Sleep and Wake Disorders, Sahlgrenska Academy, Gothenburg University, Göteborg, Sweden
- Hedner J, Sleep Disorders Center, Pulmonary Department, Sahlgrenska University Hospital, and Center of Sleep and Wake Disorders, Sahlgrenska Academy, Gothenburg University, Göteborg, Sweden
- Zou D, Sleep Disorders Center, Pulmonary Department, Sahlgrenska University Hospital, and Center of Sleep and Wake Disorders, Sahlgrenska Academy, Gothenburg University, Göteborg, Sweden
- Gent, Belgium
- Katrien Hertegonne, Department of Respiratory Medicine, Ghent University Hospital, and Department of Internal Medicine and Paediatrics, Faculty of Medicine and Health Sciences, Ghent University, Gent, Belgium
- Dirk Pevernagie, Department of Respiratory Medicine, Ghent University Hospital, and Department of Internal Medicine and Paediatrics, Faculty of Medicine and Health Sciences, Ghent University, Gent, Belgium
- Grenoble, France
- Bailly S, Université Grenoble Alpes, INSERM HP2 (U1042) and Grenoble University Hospital, Grenoble, France
- Pépin JL, Université Grenoble Alpes, INSERM HP2 (U1042) and Grenoble University Hospital, Grenoble, France
- Tamisier R, Université Grenoble Alpes, INSERM HP2 (U1042) and Grenoble University Hospital, Grenoble, France
- Hamburg, Germany
- Hein H, Sleep Disorders Center, St. Adolf Stift, Reinbeck, Germany
- Izmir, Turkey
- Basoglu OK, Department of Chest Diseases, Ege University, Izmir, Turkey
- Tasbakan MS, Department of Chest Diseases, Ege University, Izmir, Turkey
- Klecany, Czech Republic
- Buskova J, Department of Sleep Medicine, National Institute of Mental Health, Klecany, Czech Republic
- Kosice, Slovakia
- Joppa P, Department of Respiratory Medicine and Tuberculosis, Faculty of Medicine, P.J.Safarik University and L. Pasteur University Hospital, Kosice, Slovakia
- Lisbon, Portugal
- Staats R, Thorax Department, CHULN; Instituto de Saúde Ambiental – ISAMB; Faculty of Medicine, University of Lisbon, Lisbon, Portugal
- Leuven, Belgium
- Dries Testelmans, Sleep Disorders Centre, University Hospital Gasthuisberg, Leuven, Belgium
- Mainz, Germany
- Haralampos Gouveris, ENT department at Mainz University Hospital, Mainz, Germany
- Ludwig K, ENT department at Mainz University Hospital, Mainz, Germany
- Milano, Italy
- Lombardi C, Istituto Auxologico Italiano, IRCCS, Department of Cardiovascular, Neural and Metabolic Sciences, St. Luke Hospital, Milan & Department of Medicine and Surgery; University of Milano-Bicocca, Milan, Italy
- Parati G, Istituto Auxologico Italiano, IRCCS, Department of Cardiovascular, Neural and Metabolic Sciences, St. Luke Hospital, Milan & Department of Medicine and Surgery; University of Milano-Bicocca, Milan, Italy
- Palermo, Italy
- Bonsignore MR, PROMISE Dept., University of Palermo, Palermo, Italy
- Pavia, Italy
- Francesco Fanfulla, Unità Operativa di Medicina del Sonno, Istituto Scientifico di Pavia IRCCS, Pavia, Italy
- Porto, Portugal
- Drummond M, Pulmonology Department Hospital São João, Medicine Faculty of Porto University, Porto, Portugal
- van Zeller M, Pulmonology Department Hospital São João, Medicine Faculty of Porto University, Porto, Portugal
- Solingen, Germany
- Randerath W, Sleep Disorders Centre, Pulmonary Clinic, Solingen, Germany
- Marcel Treml, Respiratory Research Institute, Pulmonary Clinic, Solingen, Germany
- Split, Croatia
- Dogas Z, Sleep Medicine Center, Department of Neuroscience, University of Split School of Medicine, Split, Croatia
- Pecotic R, Sleep Medicine Center, Department of Neuroscience, University of Split School of Medicine, Split, Croatia
- Thessaloniki, Greece
- Pataka A, Respiratory Failure Unit, G. Papanikolaou Hospital, Aristotle University of Thessaloniki, Greece
- Timisoara, Rumania
- Mihaicuta S, Pulmonary Department, Victor Babes University of Medicine and Pharmacy, Victor Babes Hospital, Timisoara, Rumania
- Turku, Finland
- Anttalainen U, Division of Medicine, Department of Pulmonary Diseases, Turku University Hospital and Sleep Research Centre, Department of Pulmonary Diseases and Clinical Allergology, University of Turku, Finland
- Saaresranta T, Division of Medicine, Department of Pulmonary Diseases, Turku University Hospital and Sleep Research Centre, Department of Pulmonary Diseases and Clinical Allergology, University of Turku, Finland
- Warsaw, Poland
- Sliwinski P, 2nd Department of Respiratory Medicine, Institute of Tuberculosis and Lung Diseases, Warsaw, Poland
Steiropoulos P, Verbraecken J, Petiet E, Georgia Trakada, Fietze I, Penzel T, Ondrej Ludka, Bouloukaki I, Schiza S, McNicholas WT, Ryan S, Riha RL, Kvamme JA, Grote L, Hedner J, Zou D, Katrien Hertegonne, Dirk Pevernagie, Bailly S, Pépin JL, Tamisier R, Basoglu OK, Tasbakan MS, Buskova J, Joppa P, Staats R, Dries Testelmans, Haralampos Gouveris, Ludwig K, Lombardi C, Parati G, Bonsignore MR, Francesco Fanfulla, Drummond M, van Zeller M, Randerath W, Marcel Treml, Dogas Z, Pecotic R, Pataka A, Mihaicuta S, Anttalainen U, Saaresranta T, Sliwinski P.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors.




