Interactions between insomnia, sleep duration and emotional processes: An ecological momentary assessment of longitudinal influences combining self-report and physiological measures
Chiara Baglioni and Anna F. Johann contributed equally.
Summary
Previous studies indicated that further investigation is needed to understand how insomnia disorder interacts with emotional processes. The present study is an ecological momentary assessment evaluating the link between emotional and sleep alterations in patients with insomnia. Physiological (heart rate and heart rate variability) and subjective (sleep and emotions) indices were observed for 5 days in patients with insomnia disorder (n = 97), good sleepers under self-imposed sleep restriction (n = 41), and good sleepers with usual amount of sleep (n = 45). We evaluated differences in emotion regulation strategies and in valence and variability of emotional experiences. Over 5 days, patients with insomnia showed increased sleep and emotional difficulties compared with both control groups. Independent from group allocation, days with more negative emotions were associated with higher sleep alterations. Longer wake episodes at night and higher diurnal heart rate were associated with increased variations in emotion experienced during the day. Only in patients with insomnia, use of adaptive emotion regulation strategies was associated with higher sleep efficiency. Our data showed that alterations in sleep and emotional processes are closely linked. A combination of strategies targeting both sleep and emotional processes appears promising in the prevention and treatment of insomnia disorder.
1 INTRODUCTION
Sleep and emotional processes are strongly interrelated, and are key factors for mental health and brain functioning (Harvey et al., 2011; Palmer & Alfano, 2017). The most commonly presented sleep disorder is insomnia, which is diagnosed when persistent subjective difficulty in initiating and/or maintaining sleep and/or early awakening is accompanied by daytime impairments is presented (DSM-5, American Psychiatric Association, 2013; ICSD-3, American Academy of Sleep Medicine, 2014). Increased psychophysiological arousal is a main feature of insomnia (Riemann et al., 2010), including elevated levels of emotional arousal (Baglioni, Spiegelhalder, et al., 2010; Espie, 2002; Harvey, 2002). Indeed, theoretical models of insomnia have pointed out that patients experience increased cognitive activity during the night, which is negatively toned, and associate feelings of discomfort and stress with the nights of poor sleep (Harvey, 2002). With sleep being an everyday experience, the increased negative emotions associated with sleep difficulties may likely spread to other aspects of daily life. In order to cope with intense negative emotions, patients with insomnia disorder may likely attempt to monitor and lower them. It is reasonable to assume that these attempts are often not effective and instead further increase the intensity of the negative emotions, maintaining a vicious cycle between disturbed sleep and emotional experience (Baglioni, Spiegelhalder, et al., 2010).
The ability to regulate emotions reflects variations in how well people adjust emotional responses to meet current situational demands (Gross & Thompson, 2007). An emotional response is shown when individuals direct their attention towards relevant internal or external stimuli causing changes in multiple systems: physiology, behaviour and self-perception (Mauss et al., 2005). Depending on the situation, individuals may be able to modulate this response. In Gross' conceptual framework, the Process Model of Emotion Regulation (Gross, 1998), individuals are stated to adopt a range of strategies to increase, maintain or decrease their affective experiences to adapt to the current context. Adaptive regulation strategies (such as acceptance, reappraisal and problem solving) guide the emotional response back to baseline values, while maladaptive strategies (such as rumination, avoidance and suppression) tend to maintain elevated emotional arousal levels for longer periods, which has often been observed in patients with mental disorders (Aldao et al., 2010; Sloan et al., 2017). Thus, increased use of maladaptive emotion regulation strategies is considered a transdiagnostic aspect in psychopathology (Aldao et al., 2010; Aldao et al., 2016; Sloan et al., 2017; Webb et al., 2012). The Process Model has certainly shed light on the individual differences that predispose to or precipitate psychopathology; nevertheless, emotional dysregulation is not an end-point but a complex process (Carpenter & Trull, 2013).
In recent years, a growing interest in the dynamics of affective processes has led to new models that aim at capturing moment-by-moment changes. In a model accounting for the dynamics of affect, the DynAffect model, Kuppens et al. (2010) point out that individuals differ in terms of the variability of their affective experiences across time. Individual differences concern valence and arousal of core affect (home base), the affective fluctuations in response to internal or external events (affective variability), and the regulatory processes that act to redirect affect back towards the home base (attractor strength). In the DynAffect model, the individual's affective experience behaves as a dynamic system. The home base represents the average value of the affective experience, in terms of valence and arousal, which is different from individual to individual. The system fluctuates with ups and downs from the home base in response to external and internal stimuli. The magnitude of these fluctuations is captured by the component of affect variability. After excursions away from average values, the home base acts as an attractor to the system, and the affective experience is pulled back towards an average value (Kuppens et al., 2010). Affect variability is considered as an individual characteristic that only partially depends on the specific reactions to life events. Evidence indicates that affect variability is associated with psychological wellbeing above average levels of positive and negative emotions (Houben et al., 2015). The DynAffect model provides a useful framework to capture the dynamics of affective experience over time. For instance, it has been applied to the assessment of the affective experience in borderline personality disorder, which is characterized by extreme ups and downs in the emotional experience and by slow emotional recovery (Ebner-Priemer et al., 2015). To capture the dynamic processes of affects, time-sensitive assessment and analysis are crucial. In particular, it is recommended to conduct a time-intensive assessment (in the laboratory or in the real world) to assess the affective dynamics within each individual and to investigate them in varying time frames (Trull et al., 2015).
Thus, while the Process Model is a widely used framework to assess individual ability to respond to emotions both as trait-like characteristics and in ecological contexts, the DynAffect model focuses on changes in emotions over time and can thus provide information on the different impact of average levels of negative and positive affect and their variability over time. Adopting these two models together may capture better different aspects of emotional functioning in terms of the trait tendency to adopt adaptive/maladaptive emotion regulation strategies and in home base affect dimensions, and in terms of affect variability dynamics. Figure 1 graphically describes the rationale of this work, i.e. an integrated approach considering two theoretical models of emotion functioning with respect to insomnia disorder.
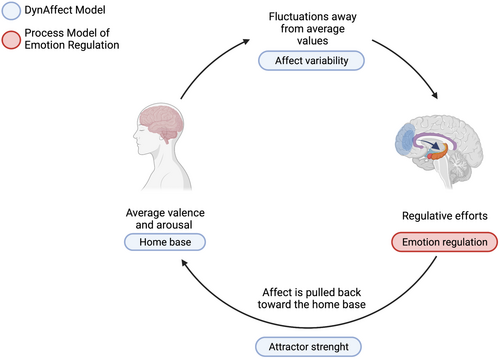
It is important to stress that insomnia disorder, while associated with polysomnographic alterations (Baglioni et al., 2013), does not necessarily reflect a reduction in sleep quantity, and that sleep deprivation is a different condition from insomnia. Sleep deprivation indeed reflects a reduction of sleep quantity and is generally temporary. Insomnia, instead, is a chronic disorder of sleep quality that could be associated with a sufficient amount of sleep. A complex interplay between both sleep duration and insomnia with emotion regulation has previously been demonstrated (Gruber & Cassoff, 2014). Nevertheless, sleep deprivation and insomnia could affect emotional processes or could be affected differently by emotional processes. However, data are still scarce, and repetitive measures in real life conditions combining subjective and physiological assessments of sleep and emotions are not yet available.
The present study adopts an ecological momentary assessment (EMA) design to evaluate the close relationship between emotional processes and insomnia drawing on the theoretical frameworks offered by the Process and the DynAffect models by repeated collection of real-time data on individuals' experience and behaviour in their natural environment (Shiffman et al., 2008). Specifically, physiological (heart rate [HR] and heart rate variability [HRV]) and subjective (sleep and emotion diaries) indices were observed for 5 days, in dynamical natural contexts, in patients with insomnia disorder (PID) compared with two groups of good sleepers: one control group with usual amount of sleep (GSC) and one with a restricted amount of sleep (no more than 5.5 hr per night; GSR). In order to evaluate mechanisms specifically linking insomnia disorder to emotional processes, only PID without comorbidities were recruited.
- PID, compared with GSC and GSR, over 5 days of observation in a natural context, present higher physiological arousal, more intense negative emotions, and less intense positive emotions;
- PID, compared with GSC and GSR, over 5 days of observation in a natural context, would adopt maladaptive emotion regulation strategies more frequently, and adaptive emotion regulation strategies less frequently.
- PID, over 5 days of observation in a natural context, compared with GSC and GSR, report more variations in their emotional experience during several assessments per day;
- independent of group assignment, over 5 days of observation in a natural context, emotional experience (intensity of negative versus positive emotions, and use of maladaptive versus adaptive emotion regulation strategies) is associated with variations in physiological arousal as measured through HR and HRV, and in sleep variables as measured through sleep diaries: sleep-onset latency (SOL), wake after sleep onset (WASO), and sleep efficiency index (SEI);
- PID, over 5 days of observation in a natural context, compared with GSC and GSR, present stronger associations between emotional experience (intensity of emotions and use regulation strategies) and variations in physiological arousal (HR and HRV), and in sleep variables as measured through sleep diaries (SOL, WASO and SEI).
2 METHODS
2.1 Recruitment and screening procedure and instruments
The full recruitment and screening procedure is graphically summarized in Figure 2.
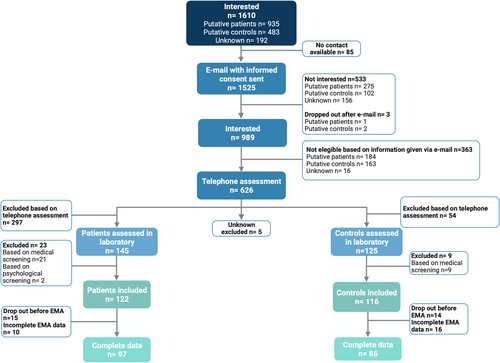
Potential participants were recruited for taking part to a study promoted by the German Society for Research (Deutsche Forschungsgemeinschaft, DFG), and conducted at the Sleep Laboratory of the Department of Psychiatry and Psychotherapy of the Faculty of Medicine of the University of Freiburg. In the advertisements it was specified that the study aimed to deepen the understanding of relationships between sleep and emotional processes. Potential participants were recruited through several procedures: (1) within patients of the Sleep Clinic of the Department of Psychiatry and Psychotherapy of the Faculty of Medicine of the University of Freiburg Medical Centre; (2) through newspapers and social media; (3) through local advertising actions. Those showing interest were asked to leave their email address to receive the study's informed consent to read and to sign. All potential participants provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki, and was approved by the Ethics Committee of the University Medical Center Freiburg. The study protocol was pre-registered in a public database (www.germanctr.de; DRKS00013071). Participants who returned the signed informed consent were contacted for a first clinical telephone interview. The phone interview was conducted by trained master's/PhD students in clinical psychology or medicine. It served to carefully screen potential participants for inclusion and exclusion criteria (see below), and lasted on average 30 min.
- An extensive psychological clinical interview (clinical interview for DSM-IV, Axis I, SKID-I; Lecrubier et al., 1999) and a semi-structured interview on sleep and insomnia. The latter asked for: (a) precise bedtimes and sleeping times during the week and at the weekend; (b) insomnia diagnostic criteria; (c) behaviours during nocturnal waking times; (d) ratings of day-time performance, well-being and sleeping times during the day; (e) chronobiological aspects with respect to sleep and daytime performance; (f) behaviour with respect to sleep hygiene rules, including information about physical exercise, coffee/tea/alcohol/chocolate consumption, pre-sleep habits, etc.; (g) typical symptoms of narcolepsy, restless legs syndrome, periodic leg movement disorder, sleep apnea syndrome and parasomnias; (h) medical and psychiatric disorders; (i) psychotropic medication known to affect sleep; (j) family history; and (k) in case of insomnia disorder, the onset and the course of the disorder as well as previous treatments.
- A standard physical examination conducted by MD medical students and supervised by a medical doctor (L.F.).
- Routine blood sampling conducted by MD medical students.
- Blood sampling for epigenetic analyses (which will be reported in a separate publication).
The following inclusion criteria were applied for all participants: age ≥18 years and fluent in German. Participants in the PID group were required to fulfil criteria for clinical insomnia disorder, while controls could be included if they did not show relevant sleep or health issues. The presence of insomnia disorder for patients and absence of sleep difficulties in controls were ascertained with clinical interviews.
- age under 18 years;
- the presence of any other psychiatric diagnosis or any relevant medical/neurological disorder as identified through standard clinical interview and physiological assessment;
- (1) for patients, the presence of any sleep disorder other than insomnia disorder as identified through clinical interview; (2) for controls, the presence of any sleep difficulty as identified through clinical interview;
- intake of any psychotropic medication in the last 2 weeks prior and during the study;
- report of a psychotherapy in the last 3 years;
- for controls, a family history of insomnia;
- participation to another research study in the last 30 days;
- blood donation in the last 2 months for men, and in the last 3 months for women;
- report of shift work and/or night work and/or travelling implying jet lag in the last 30 days.
- weekly team meetings were conducted to discuss each case.
In order to obtain a comprehensive description of the sample, a large number of self-report measures were used. It is indeed important to define and describe as much as possible the sample characteristics, as this can allow better interpretation and comparison with previous and future similar works. Participants were asked to fill in the German versions of the following questionnaires.
Questionnaires measuring insomnia symptoms and sleep quality: Insomnia Severity Index – ISI (Bastien et al., 2001), Pittsburgh Sleep Quality Index – PSQI (Buysse et al., 1989), brief version of the Dysfunctional Beliefs and Attitudes about Sleep Scale – DBAS-16 (Morin et al., 2007), Glasgow Sleep Effort Scale – GSES (Broomfield & Espie, 2005), Pre-Sleep Arousal Scale – PSAS (Nicassio et al., 1985), Epworth Sleepiness Scale – ESS (Johns, 1991), Ford Insomnia Response to Stress Test – FIRST (Drake et al., 2004).
Questionnaires measuring psychiatric symptomatology: Beck Depression Inventory – BDI-II (Beck et al., 1996), State–Trait Anxiety Inventory – STAI (Spielberger et al., 1983).
Questionnaires measuring emotion regulation: Emotion Regulation Questionnaire – ERQ (Gross & John, 2003); Difficulties in Emotion Regulation Scale – DERS (Gratz & Roemer, 2004).
All questionnaires were filled in during the screening, except for the emotion regulation measure ERQ and DERS, which participants filled in after the EMA procedure described below. This was done in order to reduce the time needed to fill in the questionnaires and thus reduce participants' burden.
2.2 Study procedure
Patients with insomnia disorders and good sleepers were matched for age and gender.
- good sleepers sleeping as usual (GSC);
- good sleepers partially sleep deprived (self-imposed sleep restriction to 5.5 hr; GSR).
Randomization was conducted by using closed envelopes marked with gender and age group (≤ 45 years, > 45 years) containing a pre-randomized subject ID allocated to one of the two groups, and the group. The envelopes were prepared before the start of the study through a computerized allocation procedure. Researchers and participants were blind to which IDs were assigned to which group, and only found out together by opening the envelope after completion of the screening phase.
Those participants who were randomly assigned to the group of GSR were instructed to go to bed whenever they wanted, but to set the alarm clock every night after 5.5 hr from bedtime and to try to get up as soon as possible. Adherence and compliance to the instructions was verified through sleep diaries.
- positive and negative affect taken from the Positive and Negative Affective Schedule (Watson et al., 1988);
- use of adaptive and maladaptive emotion regulation strategies.
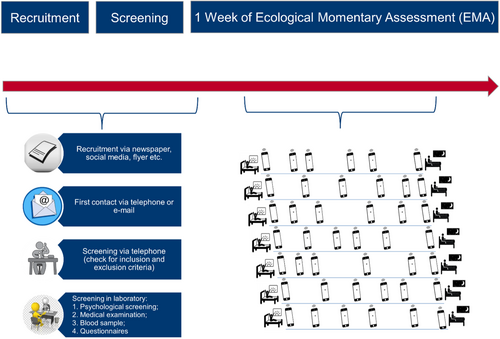
The emotional assessment was presented each morning, each evening and at four further random times during the day. For daytime assessments, the app alerted participants to answer the questions. Morning and evening questionnaires took about 5 min, while each daytime assessment took about 2 min. HR was recorded through the belt all day and night, with the exception of 3 hr in the morning in which participants were instructed to take the belt off to avoid skin problems. The observation was conducted over 7 days. On days 6 and 7, participants were invited to take part in two laboratory studies, thus, for this publication we considered only the first 5 days for each participant.
The procedure is graphically shown in Figure 3.
2.3 Data analyses
As shown in the study flowchart in Figure 2, a per protocol method was used by considering and analysing only complete data. HR and HRV were computed by the movisens algorithm (https://docs.movisens.com/Algorithms/ecg_hr_hrv/) by first detecting R peaks (Hamilton, 2002) and then computing beat-to-beat intervals (Clifford et al., 2002). Mean HR is computed over 60-s intervals, as well as HRV (SDNN, standard deviation of beat-to-beat intervals). Sections without signal (e.g. non-wear, bad electrodes or strong artefacts) are omitted from the timestamped output. Aggregated values over the day were computed over the existing values between the end of bedtime and start of the next bedtime.
- questionnaires measuring insomnia symptoms, sleep quality, psychiatric symptomatology and emotion regulation;
- sleep diary variables: SEI (the ratio of total sleep time [TST] and total time in bed [TIB]); SOL (time from light out until sleep onset); WASO (total time awake after sleep onset and before last awakening).
- intensity of positive (PA) and negative (NA) affective states;
- physiological arousal variables: HR and HRV.
- Exploratory testing was used for secondary hypotheses employing a single multivariate mixed-effects analysis of variance (rm-MANOVA) with group, age, sleep in the preceding night (SOL, WASO and SEI) as well as physiological variables (HR, HRV) as independent variables. Within- and between-subject effects of sleep and physiological variables and their group interactions were assessed separately. Considering hypothesis (c), variations of experience of emotions were operationalized as standard deviation of the difference between positive and negative emotions in the PANAS questionnaire across the day, which included six assessments per individual, and included as dependent variable in the rm-MANOVA. For hypotheses (d) and (e), the rm-MANOVA contained emotional valence (positive and negative emotions) and ER strategies (adaptive and maladaptive) as independent variables. In each case, we aimed to describe the relationship between the target variable and group, its dependence upon prior sleep and its relationship with physiological parameters during the day, both as within- and between-subject effect.
The rm-MANOVA test statistic was Wilk's Λ. Values of p < 0.05 were considered as statistically significant. All statistical analysis was done using the software R (version 4.0.4, The R Foundation for Statistical Computing 2021, https://www.r-project.org/).
3 RESULTS
3.1 Study groups description
The study flowchart is reported in Figure 2. Summarizing, 1610 persons showed interest per phone or per mail. Eighty-five persons showing interest per phone did not leave their email address. That is, 1525 persons received an email with detailed study information and the consent form to read and to sign. Of those, 533 reported no interest in taking part to the study and three returned the consent form, but after that decided to interrupt their participation. Nine-hundred and eighty-nine potential participants returned the consent form and showed their interest to the participation to the research study. Of those, 363 persons were excluded as they reported per email information indicating the presence of one or more exclusion criteria.
Six-hundred and twenty-six potential participants were interviewed per telephone call. Of those, 54 potential GSC and 297 potential patients were excluded after phone interview. Thus, 125 GSC and 145 PID took part to the comprehensive lab screening. Of those, 32 were excluded because of medical screening (21 PID and nine GSC) or psychological screening (two PID). Thus, 122 PID and 116 controls were assigned with participants IDs. Of those, 29 (15 PID and 14 controls) dropped out before completing the 5 days of observation. Finally, for 26 participants (10 PID and 16 controls), data were incomplete.
A total of 97 PID and 86 controls (41 GSR and 45 GSC) were recruited. PID were slightly older (38.32 ± 17.15 years) than both GSR (35.39 ± 15.62 years) and GSC (35.89 ± 13.77 years). The t-tests revealed no statistically significant difference in mean age and sex ratio between groups. The sample characteristics are summarized in Table 1.
| PID (N = 97) | GSR (N = 41) | GSC (N = 45) | |
|---|---|---|---|
| Age (years) | 38.32 ± 17.10 | 35.89 ± 13.77 | 37.39 ± 15.62 |
| Sex | 25M/72F | 17M/24F | 15M/30F |
| Insomnia Severity Index (ISI)a,b | 11.97 ± 2.66 | 1.22 ± 1.47 | 1.33 ± 1.54 |
| Pittsburgh Sleep Quality Index (PSQI)a,b | 8.91 ± 2.41 | 2.29 ± 1.55 | 2.27 ± 1.39 |
| Epworth Sleepiness Scale (ESS)a,b | 8.27 ± 4.16 | 5.78 ± 2.95 | 6.09 ± 3.37 |
| Dysfunctional Beliefs and Attitudes about Sleep (DBAS)a,b | 71.09 ± 19.75 | 38.17 ± 20.47 | 39.07 ± 20.80 |
| Glasgow Sleep Effort Scale (GSES)a,b | 6.72 ± 2.72 | 1.34 ± 1.37 | 1.44 ± 1.08 |
| Ford Insomnia Response to Stress Test (FIRST)a,b | 14.67 ± 5.37 | 7.05 ± 4.04 | 7.13 ± 3.62 |
| Pre-Sleep Arousal Scale (PSAS) – somatic arousala,b | 11.63 ± 3.23 | 9.00 ± 1.40 | 9.20 ± 1.52 |
| Pre-Sleep Arousal Scale (PSAS) – cognitive arousala,b | 19.53 ± 4.87 | 11.17 ± 2.99 | 11.89 ± 2.36 |
| Beck Depression Inventory (BDI)a,b | 6.46 ± 4.65 | 1.80 ± 2.85 | 1.80 ± 2.11 |
| State–Trait Anxiety Inventory (STAI-trait version)a,b | 37.08 ± 7.57 | 27.17 ± 6.07 | 27.78 ± 5.60 |
| Emotion Regulation Questionnaire (ERQ) – cognitive reappraisal | 27.14 ± 6.72 | 28.80 ± 5.08 | 29.04 ± 7.22 |
| Emotion Regulation Questionnaire (ERQ) – behavioural suppression | 12.91 ± 4.31 | 11.78 ± 5.21 | 13.04 ± 4.94 |
| Difficulties in Emotion Regulation Scale (DERS) – non-acceptance of emotional responses (NONACCEPT) | 11.91 ± 4.18 | 11.00 ± 4.59 | 10.80 ± 3.96 |
| Difficulties in Emotion Regulation Scale (DERS) – difficulty engaging in goal-directed behaviour (GOALS)a | 13.08 ± 4.31 | 11.49 ± 4.34 | 10.47 ± 3.56 |
| Difficulties in Emotion Regulation Scale (DERS) – impulse control difficulties (IMPULSE)a,b | 10.39 ± 3.88 | 8.90 ± 2.93 | 8.33 ± 2.23 |
| Difficulties in Emotion Regulation Scale (DERS) – lack of emotional awareness (AWARENESS)b | 16.19 ± 4.28 | 14.00 ± 3.94 | 14.89 ± 3.82 |
| Difficulties in Emotion Regulation Scale (DERS) – limited access to emotion regulation strategies (STRATEGIES)a,b,c | 16.13 ± 5.41 | 13.44 ± 4.67 | 11.64 ± 2.90 |
| Difficulties in Emotion Regulation Scale (DERS) – lack of emotional clarity (CLARITY)a | 9.66 ± 2.75 | 8.61 ± 2.90 | 8.22 ± 2.23 |
| Difficulties in Emotion Regulation Scale (DERS) – totala,b | 77.34 ± 17.39 | 67.44 ± 16.56 | 64.36 ± 11.78 |
- Abbreviations: GSC, good sleepers with usual amount of sleep; GSR, good sleepers with restricted amount of sleep; PID, patients with insomnia disorder.
- a A significant statistical difference was observed comparing PID versus GSC.
- b A significant statistical difference was observed comparing PID versus GSR.
- c A significant statistical difference was observed comparing GSR versus GSC.
Prior to sleep manipulation, GSCs or GSRs did not show significant differences in any psychological or sleep variable. No significant group differences were found between PID and both GSC and GSR in mean age and sex ratio.
At screening, PID reported on average higher levels of insomnia symptoms on the ISI (11.97 ± 2.66) compared with both GSC (1.33 ± 1.54; t = 30, p < 0.001) and GSR (1.22 ± 1.47; t = 30.26, p < 0.001). PID also showed higher daytime sleepiness, sleep effort, stress-related insomnia, pre-sleep somatic and cognitive arousal, dysfunctional beliefs and attitudes about sleep, and lower sleep quality compared with both control groups (details in Table 1).
With reference to psychological distress, group differences were found regarding levels of depression as measured by the BDI (PID: 6.46 ± 4.65; GSC: 1.80 ± 2.11; GSR: 1.80 ± 2.85; p < 0.001) and trait anxiety (PID: 37.08 ± 7.57; GSC: 27.78 ± 5.60; GSR: 27.17 ± 6.07; p < 0.001). No significant group difference was found regarding emotion regulation in the use of either “suppression” or “cognitive reappraisal” strategies.
As reported in Table 1, DERS total scores and subscales showed significant group differences: PID had a higher total score (77.34 ± 17.39), indicative of higher emotion regulation difficulties, compared with both GSR (67.44 ± 16.56; p = 0.002) and GSC (64.36 ± 11.78; p < 0.001). Subscales scores indicate that PID, compared with GSC, had higher difficulties in engaging in goal-directed behaviour (GOALS), impulse control (IMPULSE), emotional awareness (AWARENESS), emotional clarity (CLARITY) and limited access to emotion regulation strategies (STRATEGIES; all statistically significant at p < 0.001). Furthermore, a significant difference emerged between PID and GSR in the subscales IMPULSE, AWARENESS and STRATEGIES, indicating that PID had higher difficulties (p < 0.05). Confronting the two control groups, only STRATEGIES showed a significant difference, with GSR having higher scores than GSC (13.44 ± 4.67 versus 11.64 ± 2.90; p < 0.05).
3.2 Descriptive results: Sleep diary variables (5-day means)
Table 2 summarizes the variables from monitoring sleep in the three groups. Group differences were found for the following sleep variables: over 5 consecutive nights of recording PID showed longer SOL (34.69 ± 26.94) and WASO (42.70 ± 29.87) and lower SEI (76.35 ± 11.15) compared with both control groups (p < 0.001). As expected, no differences were found between GSC and GSR with respect to SOL and SEI, while GSC displayed higher WASO compared with GSR (t = −2.06, p = 0.043). GSR showed significantly shorter TST and TIB than GSC (p < 0.001), which also confirmed that GSR were adherent to the sleep restriction protocol (TST = 5.32 ± 0.55).
| PID (N = 97) | GSR (N = 41) | GSC (N = 45) | |
|---|---|---|---|
| SOLa,b | 34.69 ± 26.94 | 11.42 ± 7.34 | 11.84 ± 7.58 |
| WASOa,b,c | 42.70 ± 29.87 | 8.91 ± 9.84 | 16.28 ± 21.63 |
| SEIa,b | 76.35 ± 11.15 | 88.90 ± 11.15 | 87.98 ± 8.53 |
| TSTa,b,c | 6.47 ± 1.16 | 5.32 ± 0.55 | 7.26 ± 0.86 |
| TIBb,c | 8.51 ± 1.07 | 6.04 ± 0.58 | 8.33 ± 1.22 |
- Abbreviations: GSC, good sleepers with usual amount of sleep; GSR, good sleepers with restricted amount of sleep; PID, patients with insomnia disorder; SEI, sleep efficiency index; SOL, sleep-onset latency; TIB, time in bed; TST, total sleep time; WASO, wake after sleep onset.
- a A significant statistical difference was observed comparing PID versus GSC.
- b A significant statistical difference was observed comparing PID versus GSR.
- c A significant statistical difference was observed comparing GSR versus GSC.
3.3 Primary hypotheses (a) and (b): Does the emotional experience of PID differ from good sleepers and sleep-deprived controls?
The PANAS scores, ER strategies and physiological variables averaged over 5 days are reported in Table 3. PID reported higher intensity of negative emotions and increased use of maladaptive ER strategies compared with both control groups over 5 days of observation.
| PID (N = 97) | GSR (N = 41) | GSC (N = 45) | |
|---|---|---|---|
| Negative emotions (PANAS)a,b | 1.22 ± 0.27 | 1.07 ± 0.10 | 1.09 ± 0.11 |
| Positive emotions (PANAS)a,b | 2.44 ± 0.47 | 2.76 ± 0.64 | 3.03 ± 0.62 |
| Adaptive ERa,c | 2.87 ± 0.48 | 2.89 ± 0.51 | 3.14 ± 0.51 |
| Maladaptive ERa,b | 1.98 ± 0.56 | 1.56 ± 0.47 | 1.59 ± 0.45 |
| HRV diurnal | 33.66 ± 15.64 | 35.54 ± 21.20 | 33.49 ± 14.79 |
| HR diurnal | 82.78 ± 9.56 | 82.48 ± 12.44 | 84.75 ± 10.42 |
- Abbreviations: GSC, good sleepers with usual amount of sleep; GSR, good sleepers with restricted amount of sleep; HR, heart rate; HRV, heart rate variability; PID, patients with insomnia disorder.
- a A significant statistical difference was observed comparing PID versus GSC.
- b A significant statistical difference was observed comparing PID versus GSR.
- c A significant statistical difference was observed comparing GSR versus GSC.
3.3.1 Intensity of positive (PA) and negative (NA) affective states (5-day means)
The PID showed fewer positive emotions compared with both control groups (PID: 2.44 ± 0.47; GSC: 3.03 ± 0.62; GSR: 2.76 ± 0.64; p < 0.001). Significant differences were found confirming that PID experience more negative emotions compared with GSC (PID: 1.22 ± 0.27; GSC: 1.09 ± 0.11; p < 0.001) and GSR (1.07 ± 0.10; p = 0.005).
3.3.2 Intensity of use of maladaptive versus adaptive ER (5-day means)
The use of maladaptive ER strategies was more frequent in PID compared with both control groups (PID: 1.98 ± 0.56; GSC: 1.59 ± 0.45; GSR: 1.56 ± 0.47; p < 0.001). Regarding the use of adaptive ER, no significant difference was found between PID and GRS (t = −0.17, p = 863). Both PID (t = −2.91, p = 0.005) and GSR (t = 2.23, p = 0.28) reported less use of adaptive ER strategies compared with GSC controls.
3.3.3 Physiological arousal variables: HR and HRV (5-day means)
No group differences were found in either diurnal HR or diurnal HRV.
3.4 Secondary hypotheses
Hypothesis (c):.Which influences determine the within-day variability of emotions?
The rm-MANOVA results are reported in Tables 4 and 5. Within-subjects results showed that the nights with longer WASO (F1;493 = 7.02; p = 0.008; Table 4) and higher diurnal HR (F1;493 = 6.33; p = 0.012; Table 4) were associated with increased variations in emotional experience during the day, independent of group assignment. A significant GROUP × SOL interaction was observed for both control groups; particularly in the group with sleep restriction, nights with longer SOL were linked with days of higher emotional variations (F8;342 = 3.27; p < 0.05; Table 5).
Hypothesis (d):.How does sleep affect emotional experience?
| Multivariate test (Wilk's lambda) | SOL | SEI | WASO | HR | HRV | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | F | p | C | F | p | C | F | p | C | F | p | C | F | p | |
| Within-subject | |||||||||||||||
| 0.95 | 0.000 | 0.99 | 0.706 | 0.95 | 0.000 | 0.96 | 0.001 | 0.99 | 0.577 | ||||||
| Negative emotions | 0.001 | 10.26 | 0.001 | 0.001 | 0.05 | 0.824 | 0.001 | 9.82 | 0.002 | −0.002 | 0.35 | 0.556 | −0.002 | 1.93 | 0.165 |
| Positive emotions | −0.001 | 2.69 | 0.102 | 0.002 | 0.65 | 0.420 | 0.000 | 0.28 | 0.595 | 0.008 | 10.14 | 0.002 | 0.003 | 0.66 | 0.418 |
| Adaptive ER | −0.001 | 1.34 | 0.248 | 0.001 | 0.14 | 0.711 | 0.000 | 2.27 | 0.133 | 0.003 | 4.25 | 0.040 | 0.002 | 1.65 | 0.199 |
| Maladaptive ER | −0.001 | 0.87 | 0.351 | 0.000 | 0.29 | 0.590 | 0.000 | 0.02 | 0.885 | −0.006 | 5.01 | 0.026 | −0.002 | 0.74 | 0.389 |
| Affect variability | 0.001 | 2.25 | 0.134 | −0.001 | 1.58 | 0.210 | 0.001 | 7.02 | 0.008 | 0.004 | 6.33 | 0.012 | 0.000 | 0.30 | 0.585 |
| Between-subject | |||||||||||||||
| 0.94 | 0.058 | 0.95 | 0.180 | 0.98 | 0.541 | 0.93 | 0.048 | 0.95 | 0.117 | ||||||
| Negative emotions | 0.001 | 1.36 | 0.246 | −0.003 | 0.07 | 0.792 | −0.001 | 0.82 | 0.365 | −0.002 | 0.11 | 0.735 | 0.001 | 4.71 | 0.031 |
| Positive emotions | 0.000 | 0.32 | 0.572 | −0.006 | 0.51 | 0.477 | −0.001 | 0.60 | 0.438 | −0.003 | 1.23 | 0.270 | 0.003 | 0.70 | 0.405 |
| Adaptive ER | 0.004 | 5.68 | 0.018 | 0.002 | 5.83 | 0.017 | −0.002 | 0.82 | 0.366 | 0.003 | 1.82 | 0.179 | −0.002 | 1.59 | 0.209 |
| Maladaptive ER | 0.000 | 0.20 | 0.656 | 0.008 | 2.36 | 0.127 | −0.001 | 0.50 | 0.480 | 0.000 | 1.14 | 0.287 | 0.003 | 0.98 | 0.323 |
| Affect variability | 0.000 | 2.58 | 0.110 | −0.006 | 0.06 | 0.806 | −0.001 | 0.58 | 0.446 | 0.002 | 1.57 | 0.213 | 0.000 | 0.01 | 0.908 |
- Note: Bold values denote statistical significance at p < 0.05.
- Abbreviations: HR, heart rate; SEI, sleep efficiency index; SOL, sleep-onset latency; WASO, wake after sleep onset.
| C | ||||
|---|---|---|---|---|
| GSC-PID | GSR-PID | F | p | |
| GROUP × SOL | ||||
| 0.93 | 0.280 | |||
| Negative emotions | 0.002 | 0.001 | 0.11 | 0.894 |
| Positive emotions | 0.011 | 0.005 | 0.22 | 0.800 |
| Adaptive ER | 0.022 | 0.011 | 1.23 | 0.294 |
| Maladaptive ER | 0.013 | 0.013 | 1.49 | 0.228 |
| Affect variability | 0.006 | 0.012 | 3.27 | 0.041 |
| GROUP × SEI | ||||
| 0.89 | 0.047 | |||
| Negative emotions | 0.003 | 0.004 | 0.18 | 0.837 |
| Positive emotions | 0.051 | 0.021 | 5.15 | 0.007 |
| Adaptive ER | 0.041 | −0.005 | 3.27 | 0.041 |
| Maladaptive ER | −0.010 | −0.013 | 0.81 | 0.446 |
| Affect variability | 0.011 | 0.010 | 2.31 | 0.103 |
| GROUP × WASO | ||||
| 0.96 | 0.725 | |||
| Negative emotions | 0.001 | 0.001 | 0.05 | 0.954 |
| Positive emotions | 0.005 | 0.018 | 1.31 | 0.274 |
| Adaptive ER | 0.009 | 0.007 | 1.09 | 0.338 |
| Maladaptive ER | 0.002 | 0.005 | 0.09 | 0.913 |
| Affect variability | 0.001 | 0.005 | 0.89 | 0.414 |
| GROUP × HR | ||||
| 0.96 | 0.702 | |||
| Negative emotions | 0.002 | −0.003 | 0.23 | 0.792 |
| Positive emotions | 0.009 | −0.011 | 1.49 | 0.228 |
| Adaptive ER | 0.009 | −0.016 | 1.83 | 0.164 |
| Maladaptive ER | −0.001 | −0.005 | 0.32 | 0.729 |
| Affect variability | −0.001 | −0.001 | 0.08 | 0.921 |
| GROUP × HRV | ||||
| 0.96 | 0.800 | |||
| Negative emotions | −0.002 | −0.003 | 0.37 | 0.689 |
| Positive emotions | −0.010 | −0.004 | 0.94 | 0.395 |
| Adaptive ER | 0.003 | 0.000 | 0.10 | 0.901 |
| Maladaptive ER | 0.001 | 0.004 | 0.12 | 0.887 |
| Affect variability | 0.000 | −0.003 | 0.30 | 0.744 |
- Note: Bold values denote statistical significance at p < 0.05.
- Abbreviations: GSC, good sleepers with usual amount of sleep; GSR, good sleepers with restricted amount of sleep; HR, heart rate; HRV, heart rate variability; PID, patients with insomnia disorder; SEI, sleep efficiency index; SOL, sleep-onset latency; WASO, wake after sleep onset.
The MANOVA performed on affect and ER strategies showed significant effects for some sleep and physiological variables. Over 5 days of recording, independent of group allocation, nights of longer SOL (F1;493 = 10.26; p = 0.001) and WASO (F1;493 = 9.82; p = 0.002) were associated with more negative emotions the following day. The experience of positive emotions was associated with increased HR during the day (F1;493 = 10.14; p = 0.002). No significant MANOVA effects of emotions and ER strategies were detected for HRV and SEI.
Hypothesis (e):.Is the association between sleep and emotional experience stronger in insomnia disorder?
Table 5 shows the results of MANOVA for GROUP interactions. Significant GROUP × SEI interactions were found, respectively, for positive emotions (F8;342 = 5.15, p = 0.007) and adaptive ER strategies (F8;342 = 3.27, p = 0.041), indicating that in PID only, compared with GSC and GSR, nights with higher SEI were followed by days of more use of adaptive ER strategies and of experience of more intense positive emotions (shown in Figure S1).
4 DISCUSSION
The EMA data on 5 days of physiological measures (continuous recording of HR and HRV) and subjective perception (sleep and emotion diaries) are reported. Three groups were compared: patients with insomnia; good sleeper controls in a self-imposed sleep restriction protocol; and good sleeper controls sleeping as usual. To our knowledge, this is the largest study that was conducted so far including longitudinal associations between sleep and emotional processes and using both physiological and subjective data.
As expected, patients with insomnia reported higher scores than control groups in all insomnia/sleep-related questionnaires. Similarly, patients with insomnia showed increased SOL, time awake during the night and consequent poor sleep efficiency compared with both control groups during 5 days of observation. It is not surprising that we detected a significant difference in time awake during the night between the two control groups, indicating that the sleep deprivation group reported less time awake during the night compared with the control group sleeping as usual. Based on behavioural principles of sleep, sleep restriction effects result in compressing sleep, thus reducing night awakenings, and increasing sleep depth (Espie, 2022).
Considering primary confirmatory hypotheses, patients with insomnia reported difficulties in several areas of emotion regulation abilities compared with both control groups. Specifically, patients reported to have more difficulties in regulating behaviour (impulsive behaviours) and engaging in goal-directed cognitions when distressed, increased lack of emotion awareness and clarity than both control groups. Both patients and controls in the sleep deprivation condition reported a greater lack of access to strategies for feeling better when distressed compared with controls sleeping as usual. As emotion regulation questionnaires were filled in after 5 days of observation, it is likely that for the control group in the sleep deprivation condition an association between short sleep duration and difficulties in selecting adaptive emotion regulation strategies could be observed. The effect of the study procedure is less likely in the patient group and in the control group sleeping as usual as for these participants no manipulation of their habitual sleep was conducted, apart from systematic self-monitoring through diaries.
Consistent with previous literature (Baglioni, Lombardo, et al., 2010; Palmer & Alfano, 2017), patients with insomnia reported more intense negative emotions, less intense positive emotions, and a more frequent use of maladaptive emotion regulation strategies compared with both control groups. Interestingly, compared with controls sleeping as usual, both patients and controls in the sleep deprivation condition reported a less frequent use of adaptive emotion regulation strategies, which is also consistent with the results from questionnaire data.
Considering exploratory secondary analyses, increased variation in emotional experience is associated with increased vulnerability for mental health (Trull et al., 2015; Wichers et al., 2015). Independent from group allocation, nights with increased nocturnal time awake were linked with self-report of higher variation in emotional experience the following day, expressed as the variability in the difference between intensity of positive and negative emotions. Furthermore, nights with increased SOL and wake during night were linked to days with higher self-perception of negative emotions. Only for both control groups, we observed that nights with longer SOL were associated with greater emotional variability the following day. This effect was more pronounced in the sleep deprivation condition. As insomnia is linked with habituation processes, it is likely that patients report less variation in their emotional experience in association to sleep quality compared with good sleepers. This result may suggest that variations in emotional experience may be an index of vulnerability especially in acute insomnia. Instead, in chronic insomnia, alterations in core affect valence and intensity may get more stable. Further research is needed as this may have implications for clinical protocols, which might focus on emotion fluctuations during the day as an early marker of chronic insomnia.
Our data showed that when sleep is disrupted, emotional responses are altered. Longer awakening times during night are associated with increased variations in emotional experience and report of more intense negative emotions. Longer SOLs are also linked with more intense negative emotions. Sleep deprivation is associated with lower ability in selecting adaptive strategies to monitor emotional responses when distressed. Finally, chronic insomnia is associated with alterations in several areas of emotional functioning: core valence and intensity of affect and abilities in regulating emotional responses. On the other hand, patients show a positive association between daily use of adaptive emotion regulation strategies and report of intense positive emotions with better sleep efficiency. This may suggest a protective effect of positive emotions and adaptive emotion regulation strategies on body functions and sleep processes.
4.1 Limitations
To our knowledge, this is the largest study evaluating different emotional aspects in PID, compared with healthy controls sleeping as usual and healthy controls with sleep restriction. Extensive screening including physiological and psychological assessment was conducted. Nonetheless, our results should be seen in light of some limitations. Firstly, although our EMA data were prospective in nature, their analysis could only inform on daily associations. Longitudinal data with appropriate length of follow-ups could further enhance our understanding of long-term impacts of emotional process on sleep (and vice versa). Secondly, EMA-assessed emotional processes have higher ecological validity and reduced biased recall compared with self-report questionnaire, nonetheless our data were still necessarily based on participants' perceptions. Thirdly, while we performed accurate screening procedures including for comorbidities, our study design did not allow for laboratory-controlled conditions, which would increase detection of other possible confounding variables. Furthermore, the inclusion of patients with insomnia with comorbid mental and/or somatic conditions would allow more generalizability of the results, as insomnia disorder is a condition that often co-occurs with another illness. Future studies should compare with respect to emotional process different samples of patients with insomnia disorder, in order to provide a more extensive and comprehensive understanding of the topic. Lastly, the analyses here reported are based on subjective sleep variables. Insomnia disorder is indeed defined by subjective complaints regarding sleep, although some mild form of physiological alterations of sleep may be present. While physiological assessment of sleep, such as polysomnography, is less biased than subjective ones, in our EMA design it would have carried considerable costs and it would have been highly invasive for participants. Future studies could provide a more physiological detailed assessment of sleep in association with emotional process in a smaller number of participants and in a shorter time period.
4.2 Clinical implications
New emergent therapies in the treatment of insomnia direct their attention to emotional aspects associated to the disorder and have been linked with first promising results (Baglioni, 2022; Hertenstein et al., 2022; Ong et al., 2018). We suggest that aspects of emotional experience should be assessed in a clinical context when treating insomnia. Improving skills in emotion regulation may improve treatment outcomes.
AUTHOR CONTRIBUTIONS
Chiara Baglioni: Conceptualization; data curation; funding acquisition; investigation; methodology; project administration; writing – original draft; writing – review and editing. Anna Friederike Johann: Conceptualization; data curation; investigation; methodology; project administration; writing – original draft; writing – review and editing. Fee Benz: Conceptualization; data curation; investigation; methodology; writing – original draft; writing – review and editing. Lisa Steinmetz: Data curation; investigation; writing – review and editing. Debora Meneo: Writing – original draft; writing – review and editing. Lukas Frase: Data curation; investigation; writing – original draft; writing – review and editing. Marion Kuhn: Data curation; investigation; writing – original draft; writing – review and editing. Michelle Ohler: Data curation; investigation; writing – original draft; writing – review and editing. Sonja Haurt: Data curation; investigation; writing – original draft; writing – review and editing. Nathalie Speiser: Data curation; investigation; writing – original draft; writing – review and editing. Brunna Tuschen-Caffier: Methodology; supervision; writing – review and editing. Dieter Riemann: Methodology; project administration; supervision; writing – review and editing. Bernd Feige: Conceptualization; data curation; formal analysis; methodology; supervision; writing – original draft; writing – review and editing.
ACKNOWLEDGEMENTS
The authors wish to thank all participants who took part to the study. Furthermore, the authors wish to thank Bernd Tritschler for his constant help and support. Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
The study was founded by the German Society for Research (Deutsche Forschungsgemeinschaft, DFG; Individual Research Grant: DFG BA 5261/2–1). DFG had no role in the collection, analysis and interpretation of data, nor it was involved in the writing of the report or in the decision to submit the article for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare no financial involvement or affiliation with any organization whose financial interests may be affected by material in the manuscript, or which might potentially bias it.
Open Research
DATA AVAILABILITY STATEMENT
Further data that support the findings of this study, in addition to those available in the manuscript and in supplemental material, are available from the corresponding author upon reasonable request.




