Pre-existing sleep problems as a predictor of post-acute sequelae of COVID-19
Additional members of the EPILOC Phase 1 Study Group: Dietrich August, Christoph Bauer, Benedict Blankenhorn, Ulrike Bopp-Haas, Stefanie Bunk, Peter Deibert, Armin Dietz, Birgit Friedmann-Bette, Roland Giesen, Veronika Götz, Sylvia Grote, Beate Grüner, Alexandra Junginger, Oliver Kappert, Johannes Kirsten, Hans-Georg Kräusslich, Anne Kühn, Nisar P. Malek, Barbara Müller, Andreas Niess, Stefanie Pfau, Isolde Piechotowski, Siegbert Rieg, Sibylle Röttele, Jana Schellenberg, Chantal Schröder, Rainer Schwertz, Monika Spannenkrebs, Gabriele Wagner, Birgit Walter-Frank, Kersten Wolfers.
Summary
Several months after COVID-19 many individuals still report persisting symptoms, the so-called ‘post-COVID-19 syndrome’. An immunological dysfunction is one of the main pathophysiological hypotheses. As sleep is central to the functioning of the immune system, we investigated whether self-reported pre-existing sleep disturbance might be an independent risk factor for the development of post-COVID-19 syndrome. A total of 11,710 participants of a cross-sectional survey (all tested positive for severe acute respiratory syndrome coronavirus-2) were classified into probable post-COVID-19 syndrome, an intermediate group, and unaffected participants at an average of 8.5 months after infection. The case definition was based on newly occurring symptoms of at least moderate severity and ≥20% reduction in health status and/or working capacity. Unadjusted and adjusted odds ratios were calculated to investigate the association between pre-existing sleep disturbances and subsequent development of post-COVID-19 syndrome while controlling for a variety of demographic, lifestyle, and health factors. Pre-existing sleep disturbances were found to be an independent predictor of subsequent probable post-COVID-19 syndrome (adjusted odds ratio 2.7, 95% confidence interval 2.27–3.24). Sleep disturbances as part of the post-COVID-19 syndrome were reported by more than half of the participants and appeared to be a new symptom and to occur independent of a mood disorder in most cases. Recognition of disturbed sleep as an important risk factor for post-COVID-19 syndrome should promote improved clinical management of sleep disorders in the context of COVID-19. Further, it may stimulate further research on the effect of improving sleep on the prognosis of COVID-19 long-term sequelae and other post-viral conditions.
1 INTRODUCTION
A considerable number of individuals affected by COVID-19 still report persistent symptoms several months after the acute infection (Fernández-de-Las-Peñas et al., 2021). If such symptoms persist for ≥4 weeks after infection, they are colloquially referred to as ‘long COVID’ (Nalbandian et al., 2021). For symptoms occurring 3 months from onset of COVID-19, persisting for ≥2 months and which cannot be explained by an alternative diagnosis, the World Health Organization (WHO) defined the term ‘post-COVID-19 condition’ (Soriano et al., 2022), also referred to as ‘post-COVID-19 syndrome’. The likely multifactorial pathogenesis of this disease is not yet well understood. Possible mechanisms include coagulopathy, the persistence of viral components as triggers for sustained immune activation, chronic low-grade inflammation, and autoimmune phenomena (Crook et al., 2021; Phetsouphanh et al., 2022; Spudich & Nath, 2022). Thus, immunological dysfunction is part of the main hypotheses of post-COVID-19 syndrome pathogenesis. Pandemic-related stress might also be a risk factor for immunological imbalance (Wang et al., 2022).
Sleep is central to the proper functioning of immunological processes (Irwin, 2019; Irwin & Opp, 2017; Kuna et al., 2022). On one hand, disturbed sleep contributes to immune dysfunction such as a higher risk of autoimmune diseases (Hsiao et al., 2015), reduced vaccine response (Athanasiou et al., 2023; Lange et al., 2011; Rayatdoost et al., 2022; Taylor et al., 2017) and increased risk of upper respiratory tract infections (Nieters et al., 2019; Robinson et al., 2021). Subjectively good sleep quality, on the other hand, protects young adults from respiratory infection risk in the case of sleep deprivation (Walsh et al., 2022). With respect to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infections, it has been shown that poor or reduced sleep as well as sleep–wake rhythm disturbance due to shift work are associated with an increased risk of contracting COVID-19 (Jones et al., 2022; Loef et al., 2022; Peng et al., 2022) and with an increased risk of a severe disease course (Huang et al., 2020; Jones et al., 2022).
Disturbed or inadequate sleep may not only contribute to post-COVID-19 syndrome via an increased risk of infection or an imperfect response towards vaccination but might also promote the development of post-acute sequelae via a disturbance of the body's immunological response to the infection. Accordingly, a study by Paul and Fancourt (2022) examined the influence of health behaviour during the month preceding SARS-CoV-2 infection on self-reported occurrence of persistent COVID-19 symptoms in 1581 participants of an online survey on psychosocial pandemic experiences and found an association between subjective poor sleep quality and persistent COVID-19 symptoms.
With regard to potential mechanisms of an interaction between sleep and persisting COVID-19 sequelae, both systemic immunological mechanisms, as well as local inflammatory processes may be considered. It is well known that disordered sleep may compromise immune system function (Ibarra-Coronado et al., 2015; Savard et al., 2003). Sleep disturbance and insomnia with reduced sleep duration are associated with systemic inflammation (Fernandez-Mendoza et al., 2017; Irwin et al., 2016). Also, deficient sleep may promote cellular stress and damage (Coulson et al., 2022) and blood–brain barrier leakage (Hurtado-Alvarado et al., 2016). This might enable antigens and immune mediators to enter the brain, which in turn might further aggravate neuroinflammation and subsequently disordered sleep in a vicious circle (Semyachkina-Glushkovskaya et al., 2021).
In addition to sleep disturbance as a potential risk factor for post-COVID-19 syndrome, disturbed sleep is one of the most prevalent symptoms in post-COVID-19 syndrome (Merikanto et al., 2022; Schilling et al., 2022). Its recognition is relevant not only because of the associated suffering of the patients, but also because of possible interaction with symptoms such as fatigue and cognitive disorders. Furthermore, in view of the role of sleep for immunological balance (Irwin, 2019; Kuna et al., 2022) and as a risk factor for neuropsychiatric morbidity (Harvey et al., 2011; Hertenstein et al., 2019), disordered sleep may also affect the further course of the COVID-19 disease as well as other comorbidities.
However, it is unclear to what extent sleep disturbances in post-COVID-19 conditions represent a newly occurring symptom, persistence of a pre-existing sleep disorder, or a symptom of comorbid depression. Also, despite increasing knowledge of disease mechanisms involved in the post-COVID-19 condition, our understanding of the role of pre-existing health factors for developing a post-COVID-19 condition is still limited.
On the basis of questionnaire data of the population-based Epidemiology of Long COVID (EPILOC) study (Peter et al., 2022), our main objective was to investigate whether pre-existing sleep disturbances might be an independent risk factor for the development of post-acute sequelae of COVID-19. We hypothesised that pre-existing sleep disturbance predicts the development of post-COVID-19 syndrome while controlling for other pre-existing health conditions, severity of acute illness, and demographic factors. In addition, we aimed at characterising current sleep disturbance in the post-COVID-19 condition. We investigated to what extent current sleep disturbances in post-COVID-19 subjects represent pre-existing or newly occurring sleep problems and to what extent newly occurring sleep problems occur in parallel with or independent of a newly occurring mood disorder.
2 METHODS
2.1 Study design and study population
The EPILOC is a non-interventional population-based study. The data were collected by the EPILOC group in four regions of Baden-Württemberg in southwestern Germany (Freiburg, Heidelberg, Tübingen, and Ulm). The study included subjects aged 18–65 years who tested positive in a SARS-CoV-2 polymerase chain reaction (PCR) between October 1, 2020 and April 1, 2021 and whose infection was notified (according to the German Infection Protection Act) to the local public health authorities. Surviving persons were contacted between late August and September 2021 via postal mail. All study materials (i.e., information about the study, informed consent form, and the questionnaire) were included in the letter. Subjects were asked to provide written informed consent and return the materials (pre-paid envelope was enclosed) to the study centre at the Freiburg University Medical Centre. First, informed consents were separated from the questionnaires, which were forwarded to the data management centre at Ulm University. The study was conducted according to the Declaration of Helsinki, and ethical approval was obtained from the respective ethical review boards of the study centres in Freiburg (21/1484) and Ulm (337/21). The study was pre-registered with the Deutsches Register Klinischer Studien (DRKS, German Clinical Trials Register) under the identification number of DRKS 00027012. A total of 50,457 adults with confirmed SARS-CoV-2 infection were invited to participate in the study, of whom 12,053 (24%) subjects responded, and 11,710 provided at least information on age and gender (for further information, see Peter et al., 2022).
2.2 Measurements
The questionnaire included sociodemographic characteristics, height, weight, and medically attended comorbidities already present before the acute SARS-CoV-2 infection. The participants were presented with a list of 30 symptoms: hair loss, headache, confusion, concentration difficulties, memory disturbance, anxiety, depressive mood, dizziness, altered smell, altered taste, cough, hoarseness, sore throat, wheezing, shortness of breath, chest pain, vomiting, nausea, abdominal pain, diarrhoea, chills, fever, limb pain, myalgia, arthralgia, paraesthesia, sleep disorder, chronic fatigue, rapid physical exhaustion, and skin rash. Other symptoms, if present, could be added. For each of these symptoms, we asked whether it was present before the SARS-CoV-2 infection, during the infection, and currently (i.e., 6–12 months after acute infection). For each current symptom, the participant was asked to estimate how strong the effect of this symptom is on daily life (‘How much do you feel impaired by this symptom at the moment?’), presenting a 4-point scale (‘not at all’, ‘mildly’, ‘moderately’, ‘severely’). In addition, the participants were asked whether they sought medical help during the acute COVID-19 infection or were treated in a hospital or an intensive care unit. Participants were asked to compare their current general health status and current working capacity with the situation before the acute SARS-CoV-2 infection on two 11-point Likert scales (10% steps from 0% to 100%).
The participants were asked whether they experienced sleep problems prior to the SARS-CoV-2 infection (‘yes’ or ‘no’). In addition, we also elicited whether sleep problems existed during acute infection and at time of the survey (6–12 months after infection).
2.3 Data processing and statistical methods
In order to group the participants as likely unaffected versus affected by post-COVID-19 syndrome, the following criteria were used: first, at least one of the 30 symptoms (except for fever, nausea, vomiting, abdominal pain, diarrhoea, chills) should have newly occurred after the acute infection and resulted in a moderate or severe impairment of daily life. In addition, the estimates of the subjects’ perceived current health status and/or the current working capacity should be ≤80% compared to the pre-infection levels (Peter et al. [2022]). The likely unaffected group was defined by 100% recovery of health and working capacity compared to the pre-infection levels and lack of any moderate or severe newly occurred symptoms. All other participants were grouped as ‘Intermediate’.
Statistical procedures were performed with the Statistical Analysis System (SAS) software package 9.4 (SAS Institute Inc., Cary, NC, USA). In order to determine the effect of pre-existing sleep disorders on probable post-COVID-19 syndrome and current health status, we computed ordinal regressions with multiple possible confounders including age, gender, level of education, country of birth, body mass index (BMI), smoking status, interval between infection and study participation, medical treatment during acute infection, inpatient treatment during acute infection, intensive care unit treatment, and pre-existing health conditions (injuries due to accidents, musculoskeletal disorders, cardiovascular disorders, respiratory diseases, mental disorders, neurological or sensory disorders, illnesses related to the digestive system, diseases of the urogenital tract, dermatological diseases, cancer, metabolic disorders, blood-related disorders [e.g., anaemia]), and hereditary diseases (all variables were entered simultaneously). For computing unadjusted and adjusted odds ratios (ORs, effect of pre-existing sleep disturbances on post-COVID-19 symptomatology), logistic regressions were performed. Missing data were dealt with by listwise deletion of participants’ data. The intercorrelations of the variables included in the ordinal regression ranged from r = −0.285 to r = 0.473. Thus, there was no indication of multicollinearity. In order to compare different impacts between the variables, effect sizes for the standardised estimates of the ordinal regressions were computed according to the formula given by (Cohen, 1988) using the algorithms implemented by Lenhard and Lenhard (2017). To illustrate the association between pre-existing sleep disturbance and probable post-COVID-19 syndrome, we calculated the ORs for the total sample and for men and women separately. ORs were adjusted for all demographic and health status-related variables listed above.
Study method and results are reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement for cross-sectional studies (von Elm et al., 2007).
3 RESULTS
Overall, 11,710 participants with previous PCR-confirmed SARS-CoV-2 infection (6881 women, 4829 men) completed the questionnaire. For characteristics of the study population see Table 1. The most frequent pre-existing health conditions were musculoskeletal disorders (Table 1).
| Characteristic | Number of valid answers | Value |
|---|---|---|
| Age, years, mean (SD) | 11,710 | 44.1 (13.7) |
| Gender, n (%) | 11,710 | |
| Male | 4829 (41.2) | |
| Female | 6881 (58.8) | |
| University entrance qualification, n (%) | 11,678 | 6065 (51.9) |
| Germany as place of birth, n (%) | 11,668 | 10,355 (88.8) |
| Smoking status, n (%) | 11,678 | |
| Current smoker | 1192 (10.2) | |
| Former smoker | 2882 (24.7) | |
| Never smoked | 7604 (65.1) | |
| Body mass index, kg/m2, mean (SD) | 11,619 | 26.08 (5.3) |
| Pre-existing health conditions, n (%) | ||
| Injuries due to accidents | 11,414 | 1282 (11.2) |
| Musculoskeletal disorders (including rheumatism) | 11,376 | 3283 (28.5) |
| Cardiovascular disorders (including hypertension) | 11,410 | 1925 (16.9) |
| Respiratory diseases | 11,436 | 1354 (11.8) |
| Mental disorders | 11,440 | 1431 (12.5) |
| Neurological or sensory disorders | 11,438 | 1813 (15.9) |
| Illnesses related to the digestive system | 11,555 | 967 (8.4) |
| Diseases of the urogenital tract | 11,532 | 811 (7.0) |
| Dermatological diseases | 11,516 | 1226 (10.7) |
| Cancer | 11,313 | 376 (3.3) |
| Metabolic disorders | 11,494 | 1954 (17.0) |
| Blood-related disorders (e.g., anaemia) | 11,531 | 250 (2.2) |
| Hereditary diseases | 11,439 | 301 (2.6) |
| Time since positive PCR test, months, mean (SD) | 11,521 | 8.5 (1.6) |
| Treatment of acute SARS-CoV-2 infection, n (%) | ||
| No medical care | 11,599 | 9000 (77.6) |
| Any medical treatment | 11,599 | 2599 (22.4) |
| Inpatient care (without intensive care) | 11,688 | 410 (3.5) |
| Intensive care | 11,689 | 97 (0.8) |
- Abbreviations: PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; SD, standard deviation.
Out of the total number of 11,255 valid answers, 1698 participants (15.09%) stated that they had sleep problems prior to the COVID-19 infection (18.87% in women, 9.71% in men). Of the participants with a pre-existing mental disorder, 43.43% also reported sleep problems; thus, these two items did not overlap fully. In 9.49% of all participants, pre-existing sleep problems were reported in the absence of any mental comorbidity.
The overall sample size for the classification into the three groups of probable post-COVID-19 syndrome, unaffected and intermediate subjects was 11,077. Based on the criteria given in the method section, 29.56% of the participants were categorised as part of the probable post-COVID-19 syndrome group, while 40.16% were considered unaffected and the remaining subjects (30.29%) were considered as the intermediate group.
3.1 Pre-existing and newly occurring sleep problems in probable post-COVID-19 syndrome, unaffected and intermediate subjects
As shown in Figure 1, current sleep problems were reported in 54.55% of the subjects with probable post-COVID-19 syndrome and were much more common than in the two other groups (26.4% in the intermediate group, and 8.4% in the no post-COVID-19 syndrome group, respectively). More subjects in the probable post-COVID-19 syndrome group reported newly-occurring sleep problems (31.79%) than pre-existing plus current sleep problems (22.76%).
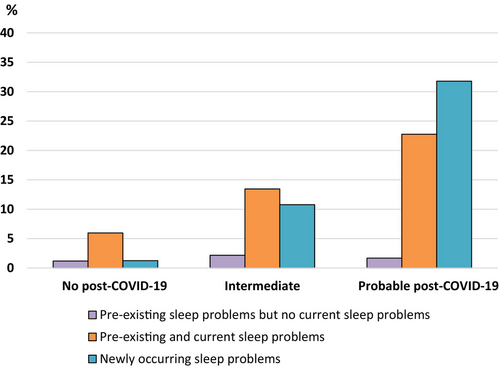
Of those participants who reported newly occurring sleep problems after the SARS-CoV-2 infection, 33.66% also reported newly occurring depressive symptoms; that is, one third of the newly occurring sleep problems might be associated with a mood disorder.
3.2 Predictors of probable post-COVID-19 syndrome
According to ordinal regression, the strongest factor was medical treatment during acute infection (a proxy for illness severity) (Table 2). The second strongest factor in this analysis was pre-existing sleep disturbance. The effect sizes associated with all other variables were smaller: higher BMI, female sex, smoking, lower level of education, musculoskeletal disorders, cardiovascular disorders, respiratory diseases, mental disorders, neurological or sensory disorders, illnesses related to the digestive system, and blood-related disorders (e.g., anaemia) (Table 2). As the present case definition of probable post-COVID-19 syndrome included newly occurred sleep problems as a symptom, a second ordinal regression using ‘change in health status’ as a variable that is independent of our definition of post-COVID-19 condition, was computed. As depicted in Table 2, the effect sizes of associated factors showed a pattern almost identical to that of the regression analysis using the case definition variable.
| Variable | Case definition (1, 0.5, 0) (N = 9464) | Current health status (in relation to pre-infection status) (N = 9634) | ||||||
|---|---|---|---|---|---|---|---|---|
| SE | χ2 | p | Effect size | SE | χ2 | p | Effect size | |
| Demographic variables and lifestyle factors | ||||||||
| Age | −0.0061 | 0.2 | 0.6268 | 0.009 | −0.0040 | 0.1 | 0.7439 | 0.006 |
| Gender (female vs. male) | 0.1092 | 87.1 | <0.0001 | 0.193 | −0.0787 | 47.3 | <0.0001 | 0.141 |
| Level of education | −0.0788 | 45.9 | <0.0001 | 0.140 | 0.1019 | 82.6 | <0.0001 | 0.186 |
| Country of birth (Germany vs. other) | −0.0013 | 0.0 | 0.9043 | 0.002 | 0.0068 | 0.4 | 0.5223 | 0.013 |
| Body mass index | 0.0712 | 34.1 | <0.0001 | 0.120 | −0.0703 | 36.8 | <0.0001 | 0.124 |
| Smoking (current/former vs. never) | 0.0703 | 39.5 | <0.0001 | 0.130 | −0.0521 | 23.2 | <0.0001 | 0.098 |
| Acute infection-related factors | ||||||||
| Time of infection (0 = October 2020 to 6 = April 2021) | 0.0091 | 0.7 | 0.4058 | 0.017 | −0.0158 | 2.2 | 0.1386 | 0.030 |
| Medical treatment during acute infection | 0.2767 | 522.5 | <0.0001 | 0.484 | −0.2657 | 552.3 | <0.0001 | 0.493 |
| Inpatient treatment during acute infection | 0.0201 | 2.2 | 0.1347 | 0.031 | −0.0345 | 8.3 | 0.0045 | 0.059 |
| Intensive care unit treatment | 0.0073 | 0.3 | 0.5762 | 0.011 | −0.0215 | 3.6 | 0.0568 | 0.039 |
| Pre-existing health problems | ||||||||
| Pre-existing sleep problems | 0.1310 | 123.9 | <0.0001 | 0.230 | −0.1027 | 87.4 | <0.0001 | 0.191 |
| Pre-existing conditions | ||||||||
| Injuries due to accidents | 0.0051 | 0.2 | 0.6485 | 0.009 | −0.0039 | 0.1 | 0.7220 | 0.007 |
| Musculoskeletal disorders | 0.0782 | 44.1 | <0.0001 | 0.137 | −0.0646 | 32.4 | <0.0001 | 0.116 |
| Cardiovascular disorders | 0.0489 | 16.9 | <0.0001 | 0.085 | −0.0419 | 13.8 | 0.0002 | 0.076 |
| Respiratory diseases | 0.0550 | 23.9 | <0.0001 | 0.101 | −0.0542 | 26.4 | <0.0001 | 0.105 |
| Mental disorders | 0.0640 | 29.8 | <0.0001 | 0.112 | −0.0466 | 17.9 | <0.0001 | 0.086 |
| Neurological or sensory disorders | 0.0359 | 10.0 | 0.0016 | 0.065 | −0.0364 | 11.4 | 0.0007 | 0.069 |
| Illnesses related to the digestive system | 0.0340 | 9.1 | 0.0025 | 0.062 | −0.0366 | 12.1 | 0.0005 | 0.071 |
| Diseases of the urogenital tract | 0.0076 | 0.5 | 0.4957 | 0.014 | −0.0061 | 0.3 | 0.5652 | 0.012 |
| Dermatological diseases | 0.0032 | 0.1 | 0.7780 | 0.006 | −0.0024 | 0.0 | 0.8245 | 0.005 |
| Cancer | 0.0077 | 0.5 | 0.4829 | 0.014 | −0.0082 | 0.6 | 0.4368 | 0.016 |
| Metabolic disorders | 0.0119 | 1.0 | 0.3092 | 0.021 | −0.0139 | 1.5 | 0.2153 | 0.025 |
| Blood-related disorders (e.g., anaemia) | 0.0252 | 5.2 | 0.0224 | 0.047 | −0.0361 | 12.3 | 0.0004 | 0.072 |
| Hereditary diseases | 0.0124 | 1.2 | 0.2646 | 0.023 | −0.0088 | 0.7 | 0.3966 | 0.017 |
- Note: All variables were entered simultaneously. Grey tones highlight the two most important factors.
- Abbreviation: SE, standardised estimates.
In order to illustrate the effect of pre-existing sleep disorders on the risk of developing probable post-COVID-19 condition, we calculated unadjusted and adjusted ORs. For the total sample, the adjusted OR was >2 (95% confidence interval 2.27–3.24). The ORs for women and men were similar. For the risk to be classified into the intermediate group, the ORs were somewhat smaller (Table 3).
| Variable | Intermediate group vs. unaffected group | Probable post-COVID-19 syndrome group vs. unaffected group | |||
|---|---|---|---|---|---|
| OR (95% CI) | Sample size | OR (95% CI) | Sample size | ||
| Total sample | Unadjusted | 2.40 (2.07–2.79) | 7642 | 4.20 (3.65–4.84) | 7522 |
| Adjusted* | 1.86 (1.57–2.22) | 6819 | 2.70 (2.27–3.24) | 6539 | |
| Women | Unadjusted | 2.25 (1.88–2.70) | 4269 | 3.77 (3.18–4.47) | 4352 |
| Adjusted* | 1.95 (1.58–2.41) | 3783 | 2.85 (2.30–2.54) | 3728 | |
| Men | Unadjusted | 2.41 (1.85–3.17) | 3373 | 4.38 (3.39–6.55) | 3170 |
| Adjusted* | 1.80 (1.32–2.45) | 3036 | 2.50 (1.81–3.46) | 2811 | |
- Note: *Adjusted for: age, gender, level of education, country of birth, body mass index, smoking, interval between infection and study participation, medical treatment during acute infection, inpatient treatment during acute infection, intensive care unit treatment, and pre-existing health conditions (injuries due to accidents, musculoskeletal disorders, cardiovascular disorders, respiratory diseases, mental disorders, neurological or sensory disorders, illnesses related to the digestive system, diseases of the urogenital tract, dermatological diseases, cancer, metabolic disorders, blood-related disorders [e.g., anaemia], and hereditary diseases) (see also Table 3). Abbreviations: CI, confidence interval; OR, odds ratio.
4 DISCUSSION
This study investigated the relationship of self-reported pre-existing sleep problems prior to SARS-CoV-2 infection with the risk of developing post-COVID-19 syndrome in a cross-sectional study of 11,710 adults in southern Germany. Controlling for other health problems and demographic variables, we found pre-existing sleep problems to be an independent predictor of probable post-COVID-19 syndrome. In addition, we found that sleep was impaired in more than half of the participants with probable post-COVID-19 syndrome and that within this group, in about two thirds of the cases, the sleep disturbances were newly occurring. Of these newly occurring sleep disorders, only one third were associated with a newly occurring mood disorder, whereas two thirds occurred independently of a mood disorder.
In accordance with data from other epidemiological studies (Bahmer et al., 2022), we also found several other pre-existing health problems to be associated with an enhanced risk of long-term sequelae of COVID-19. This was the case for musculoskeletal disorders, cardiovascular disorders, respiratory diseases, mental disorders, as well as the demographic factors BMI, female sex, and smoking, even though their effect sizes were all quite small (<0.2). Severity of acute illness (with medical treatment during acute infection as a proxy) had the strongest effect on the likelihood of probable post-COVID-19 syndrome, consistent with findings from other epidemiological studies (Bahmer et al., 2022; Merikanto et al., 2022). To validate our result, we calculated a second regression using ‘change in health status’ as an alternative outcome variable independent of individual symptoms. This produced an almost identical pattern of factors associated with post-COVID-19 syndrome risk with only minor differences regarding effect sizes, thus strengthening our result.
A study using data from the UK Biobank investigating health behaviours, including sleep duration and quality in the month before infection and the development of self-reported long COVID, also found poor sleep quality to increase the odds of long COVID (Paul & Fancourt, 2022). However, there are a variety of methodological differences between their study and ours: Unlike the UK Biobank study, our sample comes from a geographically defined, population-based study and included only subjects with PCR-confirmed SARS-CoV-2 infection instead of self-reported infection status. Further, in the study by Paul and Fancourt (2022), long COVID was operationalised using a single binary variable in response to a self-report question of whether the participant considered himself/herself to have or have had long COVID. In contrast, our study operationalised long-term sequelae of COVID-19 using a combination of criteria consisting of new onset symptoms of ≥3 months duration and moderate severity from a comprehensive list of symptoms and requested a significant impact on health status or working capacity, thus more closely approximating the WHO definition of the post-COVID-19 condition. The fact that, despite these methodological differences, both studies consistently identify disturbed sleep as an important predictor variable for the development of persistent symptoms after COVID-19 (with ORs between 2 and 3), supports the hypothesis that sleep disorders might be a risk factor for a postulated pathophysiological concept of post-COVID-19 syndrome, namely an imbalanced immune system (Irwin, 2019; Irwin & Opp, 2017; Kuna et al., 2022). Similarly, disturbed sleep also was a risk factor for milder forms of post-COVID-19 symptoms (intermediate group).
In our cohort, pre-existing sleep problems were more prevalent in women than in men, as reported in the literature for the general population (Zhang & Wing, 2006). In view of the impact of pre-existing sleep problems on post-COVID-19 syndrome risk, this may contribute to the higher risk of a post-COVID-19 condition in women compared to men. The finding of Wang et al. (2022) that depression, anxiety, and perceived stress prior to infection are associated with the risk of a post-COVID-19 condition might be partly explained by sleep disturbances as all of these conditions are typically accompanied by sleep disturbances and the authors did not control their analyses for pre-existing sleep disturbances.
With regard to potential pathomechanisms, such as the promotion of local cerebral and/or systemic inflammatory processes by disordered sleep, further studies should investigate immune mediators and markers of neuroinflammation in patients with post-COVID-19 syndrome with and without pre-existing sleep disorders. The link between pre-existing sleep disturbance and the risk of post-COVID-19 syndrome adds to previous evidence for somatic disease mechanisms.
A second finding of the present study was that more than half of the participants with probable post-COVID-19 syndrome currently had sleep problems, most often having developed after the acute infection and not being pre-existent. This indicates that a significant proportion of current sleep problems may be attributable to COVID-19. In addition, we found that two thirds of the newly occurring sleep problems after COVID-19 occurred independently of depressed mood. Thus, sleep disturbances after COVID-19 are more likely to be considered as COVID-19- and/or pandemic-related sleep disturbances independent of depression.
As sleep disorders impair immunological balance (Irwin, 2019; Irwin & Opp, 2017; Kuna et al., 2022), they could act as a disease-sustaining factor. Thus, further studies should investigate the course of post-COVID-19 syndrome in groups with and without sleep disturbances and whether treatment of insomnia improves the prognosis of post-COVID-19 syndrome.
The high prevalence of sleep disturbances in the post-COVID-19 condition found in our study is in accordance with literature data reporting sleep disturbance in post-COVID-19 cohorts during the first year after disease onset on average between 30% and 50% (Bahmer et al., 2022; Schilling et al., 2022). A recent international survey study on sleep symptoms in long COVID consisting of 13,628 participants reported insomnia symptoms in 49.6% of respondents reporting long-lasting symptoms after COVID-19 (Merikanto et al., 2022). Interestingly, prominent sleep problems had already been observed in subjects with ongoing symptoms after SARS infection during the 2003 epidemic (Moldofsky & Patcai, 2011). Although disease severity in acute infection also has an influence on the development of post-COVID-19 syndrome, which in turn is strongly associated with sleep disorders, hospitalisation and intensive care unit treatment in our cohort was rare (<5%) and had no additional effect on post-COVID-19 syndrome risk and thus probably not on the increased incidence of sleep disorders.
4.1 Strengths and limitations
Particular strengths of our study are its large number of almost 12,000 participants and its population-based approach. Even if a possible selection bias due to a response rate of 24% is relevant for estimating the prevalence of post-COVID-19 syndrome in the general population, it is unlikely to affect our analyses because we only looked at relationships between variables, and our sample included a sufficient number of non-post-COVID-19 syndrome affected individuals, thus the variances of these variables were not reduced.
A further strength is the differentiation between pre-existing symptoms, symptoms during the acute illness and persisting symptoms, and the comprehensive collection of pre-existing health conditions. In this context, the cross-sectional design of our study with retrospective data on pre-existing sleep problems is a limitation of the study. However, a possible recall bias should be negligible because the prevalence of pre-existing sleep disorders in our study is in concordance with population-based surveys of sleep problems (Harsanyi et al., 2022). Moreover, our study produced similar results as the longitudinal UK Biobank study (Paul & Fancourt, 2022) with regard to the impact of sleep quality prior to infection on the development of post-acute COVID-19 sequelae.
Another limitation is that, due to the epidemiological nature of our study, the case definition of probable post-COVID-19 syndrome is not based on a formal medical diagnosis. However, our case definition uses a combination of criteria consisting of new onset symptoms of at least moderate severity and requests a significant impact on health status or working capacity as an indicator of functional relevance, thus approximating the WHO definition of the post-COVID-19 condition. Further, we additionally used a second outcome variable for validation of the results.
It is not unlikely that in addition to putative direct consequences of the viral infection, effects of the pandemic might also contribute to the symptoms reported by the participants. However, this applies equally to all three groups, and the case definition of probable post-COVID-19 syndrome was based on a change in health status and newly occurring symptoms in temporal context with the SARS-CoV-2 infection. Furthermore, stress and discomfort resulting from pandemic measures might have contributed to pre-existing sleep disturbance. However, what underlies the sleep disturbance is not relevant to the question, whether pre-existing sleep impairment increases the relative risk of developing post-COVID-19 syndrome.
4.2 Future directions
The impact of disordered sleep on the development and course of persistent sequelae after COVID-19 and other post-viral syndromes should be investigated in further longitudinal studies. Also, the underlying neurophysiological and immunological pathomechanisms should be explored in more detail. Importantly, insomnia and other sleep problems are conditions for which we have effective treatments such as cognitive behavioural therapy or sleep-promoting medication. Thus, intervention studies should investigate the effect of improving sleep on the development and further course of the post-COVID-19 condition and other post-viral syndromes.
5 CONCLUSIONS
In conclusion, disturbed sleep, which is a highly prevalent condition in the general population, appears to predict an enhanced risk of developing post-acute sequelae of COVID-19. Thus, recognition of disturbed sleep as an important and treatable risk factor for post-COVID-19 syndrome may promote a thorough clinical management of sleep disorders in this context and stimulate further research on the effect of improving sleep on the prognosis of COVID-19 long-term sequelae and other post-viral syndromes.
AUTHOR CONTRIBUTIONS
Winfried Kern, Alexandra Nieters, Raphael S. Peter, Dietrich Rothenbacher, Claudia Schilling and Michael Schredl were involved in the study conceptualisation and the development of the research question. Winfried Kern, Alexandra Nieters, and Dietrich Rothenbacher supervised the study. Stefan O. Brockmann and Gerhard Kindle contributed to the design of the study. Raphael S. Peter, Alexandra Nieters, and Dietrich Rothenbacher were involved in data acquisition. Michael Schredl performed the statistical analysis. Stefan O. Brockmann, Siri Göpel, Uta Merle, and Jürgen M. Steinacker contributed to the acquisition and interpretation of the data. Claudia Schilling and Michael Schredl wrote the first draft of the manuscript. All authors were involved in drafting or critically revising the manuscript, and all authors approved the final version. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
ACKNOWLEDGEMENTS
We thank the Baden-Württemberg Federal State Ministry of Science and Art (grant number MR/S028188/1) and the German pension fund (‘Deutsche Rentenversicherung’) Baden-Württemberg for their financial support of the work. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST STATEMENT
The authors declare financial support from the Baden-Württemberg Federal State Ministry of Science and Art and the German pension fund (‘Deutsche Rentenversicherung’) Baden-Württemberg for the submitted work. The funders had no role in considering the study design or in the collection, analysis and interpretation of the data, the writing of the report, or the decision to submit the article for publication. I declare that the authors have no financial or non-financial competing interests or other interests that might be perceived to influence the interpretation of the article.
Open Research
DATA AVAILABILITY STATEMENT
Data from EPILOC phase 1 are available for research purposes upon request from the EPILOC Phase 1 Study Group at [email protected].




