Association between proteomics and obstructive sleep apnea phenotypes in a community-based cohort of women
Summary
Proteomic-based technologies offer new opportunities to identify proteins that might reflect the cardiometabolic stress caused by different aspects of sleep-disordered breathing. We aimed to investigate whether severe obstructive sleep apnea and severe obstructive sleep apnea during rapid eye movement sleep are associated with changed levels of inflammatory and cardiac disease-related proteins in a population-based cohort of women. In the community-based “Sleep and Health in Women” (SHE) cohort study, 400 women underwent polysomnography, anthropometric measurements and blood sampling. Two proteomic assays (Olink Proseek® Inflammation panel and Olink Proseek® Cardiovascular II panel), each measuring 92 proteins, were analysed in a subsample of 253 women. p-Values were adjusted for multiple testing, with false discovery rate set at 10%. In unadjusted models, 57 proteins were associated with apnea−hypopnea index, 56 proteins with oxygen desaturation index and 64 proteins with rapid eye movement−apnea−hypopnea index. After adjustment for age, body mass index and plate, there were no significant associations between apnea−hypopnea index or oxygen desaturation index and any of the proteins. Severe obstructive sleep apnea during rapid eye movement sleep (rapid eye movement−apnea−hypopnea index ≥ 30) was associated with decreased levels of two anti-inflammatory proteins; Sirt2 (q-value .016) and LAP-TGF-β1 (q-value .016). There was also a negative association between rapid eye movement−apnea−hypopnea index of ≥ 30 and Axin1 (q-value .095), a protein thought to facilitate TGF-β-signalling. We conclude that severe obstructive sleep apnea during rapid eye movement sleep is associated with low levels of Sirt2, LAP-TGF-β1 and Axin1, anti-inflammatory proteins involved in metabolic regulation and in the atherosclerotic process. For obstructive sleep apnea based on a whole night, the associations with cardiac and inflammatory proteins are weaker, and explained to a large extent by age and body mass index.
1 INTRODUCTION
Obstructive sleep apnea (OSA) is associated with an increased risk of hypertension (Peppard, Young, Palta, & Skatrud, 2000), stroke (Campos-Rodriguez et al., 2014), heart failure (Ljunggren et al., 2016) and diabetes (Lindberg et al., 2012). Although the underlying pathophysiological mechanisms linking OSA to cardiovascular disease are not fully understood, they are thought to include the effects of repeated oxygen desaturation, the activation of the sympathetic nervous system and repeated episodes of negative intrathoracic pressure swings. In addition, sleep-stage-dependent mechanisms may be important; OSA during rapid eye movement (REM) sleep has been suggested to have more severe cardiometabolic consequences than OSA during non-REM sleep (Ljunggren et al., 2018; Mokhlesi et al., 2014).
The cardiometabolic stress caused by different measurements of sleep-disordered breathing might be reflected in changed levels of cardiac and inflammatory biomarkers. OSA has been associated with increased levels of inflammatory markers, including interleukin 6 (IL6) and tumour necrosis factor α (TNFα), both of which are primarily associated with measurements of hypoxia (Svensson, Venge, Janson, & Lindberg, 2012). Plasma Type B natriuretic peptide (BNP), a marker of heart failure, on the other hand, is associated with the apnea−hypopnea index (AHI), but not with the level of hypoxia associated with the AHI (Ljunggren, Lindahl, Theorell-Haglow, & Lindberg, 2012). Studying sleep-stage-dependent OSA, Chami et al. reported that, while non-REM−AHI was associated with fasting glucose levels, an independent association with insulin resistance was only seen for REM−AHI (Chami, Gottlieb, Redline, & Punjabi, 2015).
Proteomic-based technologies offer new opportunities to identify associations between different measurements of OSA and multiple cardiovascular and inflammatory proteins. This has the potential to add to our understanding of underlying pathophysiological mechanisms linking sleep-disordered breathing to cardiovascular disease, and to identify subpopulations of patients with OSA at risk of cardiovascular disease. There are a few clinic-based studies of proteomics in OSA that have contributed important information on how OSA affects protein expression in serum (Jurado-Gamez et al., 2012; Kim et al., 2009), red blood cells (Feliciano et al., 2017) and urine (Seetho et al., 2014). Most have only included men, while studies of women, larger community-based studies, and studies of the effect of OSA during REM sleep on cardiac and inflammatory proteins are lacking.
In this discovery study we aimed to investigate whether severe OSA and severe OSA during REM sleep are associated with changed levels of inflammatory and cardiac disease-related proteins in a population-based cohort of women.
2 METHODS
2.1 Study population
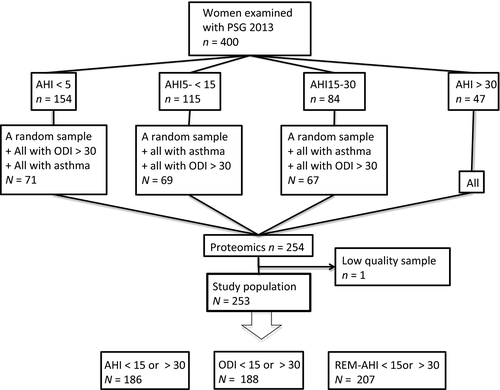
The current study was based on a subsample of women from the 10-year follow-up of the “Sleep and Health in Women” (SHE) study. In the SHE study, a population-based cohort study of randomly selected women from the City of Uppsala in Sweden, 400 women aged 20–70 years at baseline, with an oversampling of snorers, were examined with whole-night polysomnography (PSG), anthropometric measurements, questionnaires, blood sampling and blood pressure measurements for the first time in 2002–2004 (Theorell-Haglow, Berne, Janson, & Lindberg, 2008), and again in 2012–2014. Proteomic assays (Olink Proseek® Inflammation panel and Olink Proseek® Cardiovascular II panel) were available in a subsample of 254 women from the follow-up study. The subsample of 254 women included all the women with severe OSA (AHI ≥ 30 or oxygen desaturation index [ODI] ≥ 30), all the women with asthma, and a random sample of women with no (AHI < 5), mild (AHI 5– < 15) and moderate (AHI 15– < 30) OSA (Figure 1). One woman was excluded because of low-quality samples in both the Inflammation panel and the Cardiovascular II panel, leaving a total study population of 253 women. Two more women had low-quality samples in the Inflammation panel and were excluded from these analyses, and one woman had low-quality samples in the Cardiovascular II panel and was excluded from those analyses.
2.2 Ethical approval
The written informed consent of all the participants was obtained, and the Ethics Committee at Uppsala University, Uppsala, Sweden approved the study protocol (approval number 2009/379).
2.3 Assessment of obstructive sleep apnea
All the women underwent whole-night ambulatory PSG (EMBLA, Flaga), including continuous 16-channel recordings of two airflow leads (oronasal thermistor and nasal flow pressure sensor), two respiratory effort leads from piezoelectric belts (thoracic and abdominal), one pharyngeal sound lead (from a piezovibration sensor), one oximeter lead, two electroencephalography leads (C3-A2, C4-A1), two electrooculography leads, three electromyography leads (sub-mental, left and right anterior tibialis muscles), two electrocardiography leads and one body position lead. Women receiving treatment for sleep apnea (continuous positive airway pressure [CPAP] or a mandibular advancement device) were instructed to pause treatment for at least 3 nights prior to the recordings.
Sleep was scored manually in 30-s epochs. An obstructive apnea was defined as the complete cessation of nasal and oral airflow lasting 10 s or more with continuing abdominal and thoracic movements. A hypopnea was defined as a ≥ 50% reduction in both oronasal thermistor and nasal pressure for at least 10 s compared with baseline, in combination with a desaturation of ≥ 3% or an arousal (Iber et al., 2007). The AHI was defined as the mean number of apneas and hypopneas per hour of sleep. The ODI was calculated as the mean number of desaturations of ≥ 3% per hour of sleep. Severe OSA was defined as an AHI or ODI of ≥ 30, and no or mild OSA was defined as an AHI or ODI of < 15 (“Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force”, 1999). The REM−AHI was calculated as the number of apneas and hypopneas during REM sleep divided by the hours spent in REM sleep. Severe OSA during REM sleep was defined as an REM−AHI of ≥ 30, and no or mild OSA during REM sleep as an REM−AHI of < 15 (Ljunggren et al., 2018).
2.4 Assessment of covariates
The women answered questionnaires including questions on lifestyle factors, medical history and smoking history. Excessive daytime sleepiness was assessed with the Epworth Sleepiness Scale (ESS; Johns, 1991). The participants' height and weight were measured, and the body mass index (BMI) was calculated. Blood pressure was measured in the right arm in a supine position after 15 min of rest.
2.5 Proteomic analysis
Fasting blood samples were drawn between 07:00 hours and 09:00 hours in the morning after the PSG recordings. EDTA plasma samples for the protein analyses were stored at −70°C until analysis. The blood samples for the protein biomarkers were analysed at Olink Analysis Service (Olink Proteomics) using the Olink Proseek® Multiplex Inflammation kit and the Olink Proseek® Cardiovascular II95x96 kit, each measuring 92 proteins (https://www.olink.com/products/document-download-center/). The kits use proximity extension assay (PEA) technology (Assarsson et al., 2014; Lundberg, Eriksson, Tran, Assarsson, & Fredriksson, 2011). Data are presented as normalized protein expression values on a log2 scale, where a high value corresponds to a high protein concentration but not an absolute quantification. The samples were analysed on three different plates. The intra-assay and inter-assay coefficients of variation were 4% and 7% for the Olink Proseek Multiplex Inflammation panel, and 4% and 8% for the Olink Proseek Cardiovascular II panel. Proteins with more than 90% of the values below the lower limit of detection (LOD) were excluded from the analyses (artemin, interferon gamma, IL-1alpha, IL-2, IL-2 receptor subunit beta, IL-13, IL-20, IL-22 receptor subunit alpha-1, IL-24, IL-33, leukaemia inhibitory factor, neuturin, thymic stromal lymphopoietin, tumour necrosis factor), leaving 78 proteins in the Inflammation panel and 92 proteins in the Cardiovascular II panel.
2.6 Statistical analysis
The statistical analyses were performed using R version 3.4.4.
Proteins with 20%–90% of the values below the LOD (eight proteins in the Inflammation panel and three proteins in the Cardiovascular II panel) were discretized as detectable (above the LOD) or undetectable (below the LOD). For all proteins with less than 20% values below the LOD, the values below the LOD were imputed by replacing the missing value by the value of the LOD.
Associations between measurements of sleep-disordered breathing (AHI, ODI and REM−AHI) and protein values were assessed using linear regression for continuous protein values and logistic regression for discretized protein values. AHI, ODI and REM−AHI were discretized, comparing women with severe sleep-disordered breathing (AHI, ODI or REM-AHI ≥ 30) with women with no or mild sleep-disordered breathing (AHI, ODI or REM-AHI < 15). The regression models were adjusted for age, BMI and plate, and the adjusted association between protein and measurements of sleep-disordered breathing was assessed using a likelihood ratio test. Because the women had been selected for proteomic analyses based not only on OSA status but also on the presence or absence of asthma, asthma was also considered as a possible confounding factor. However, asthma showed little impact on the results and was omitted from the final model, but the results from models also adjusting for asthma are presented in Tables S1−S3.
The p-values were adjusted for multiple testing using the Benjamini and Hochberg method for controlling the false discovery rate (FDR), setting the FDR at 10%.
3 RESULTS
Of the 253 women, 18.6% had severe sleep-disordered breathing, defined as an AHI of ≥ 30, 21.7% when defined as an ODI of ≥ 30, and 49.4% when defined as an REM−AHI of ≥ 30. The characteristics of the participants and PSG data are presented in Tables 1 and 2. A total of 48 women were receiving treatment for sleep apnea; of them, 22 women were on CPAP treatment and 24 women had a mandibular advancement device.
| All | AHI < 15 | AHI ≥ 30 | ODI < 15 | ODI ≥ 30 | REM− AHI < 15 | REM− AHI ≥ 30 | |
|---|---|---|---|---|---|---|---|
| No. of subjects | 253 | 139 | 47 | 133 | 55 | 83 | 124 |
| Age, years | 60.1 (10.7) | 56.3 (10.9) | 66.0 (7.8) | 56.6 (11.2) | 65.6 (8.2) | 53.1 (11.1) | 64.6 (7.9) |
| BMI, kg m−2 | 27.2 (4.8) | 26.1 (4.1) | 29.5 (5.4) | 25.7 (4.0) | 30.4 (6.1) | 25.4 (3.7) | 28.4 (5.3) |
| Smoking status, n (%) | |||||||
| Never | 118 (46.8) | 67 (48.2) | 18 (39.1) | 66 (49.6) | 21 (38.9) | 47 (56.6) | 57 (46.3) |
| Former | 111 (44.1) | 59 (42.5) | 23 (50.0) | 54 (40.6) | 28 (51.9) | 26 (31.3) | 57 (46.3) |
| Current | 23 (9.1) | 13 (9.4) | 5 (10.9) | 13 (9.8) | 5 (9.3) | 10 (12.1) | 9 (7.3) |
| Diabetes mellitus, n (%) | 17 (6.8) | 4 (2.9) | 4 (8.7) | 4 (3.0) | 9 (16.7) | 5 (6.1) | 10 (8.1) |
| Systolic blood pressure, mmHg | 132.3 (17.9) | 129.3 (17.9) | 138.3 (14.7) | 128.9 (18.0) | 138.8 (18.0) | 125.7 (15.3) | 136.4 (17.9) |
| Diastolic blood pressure, mmHg | 77.8 (9.1) | 77.4 (9.2) | 78.7 (8.5) | 77.0 (9.0) | 77.8 (8.9) | 76.3 (9.0) | 79.0 (9.2) |
| ESS, points | 7.9 (4.3) | 8.0 (4.4) | 8.6 (3.5) | 7.8 (4.4) | 8.9 (3.7) | 8.3 (4.3) | 7.7 (4.3) |
| Antihypertensive medication, n (%) | 85 (33.6) | 34 (24.5) | 26 (55.3) | 30 (22.6) | 29 (52.7) | 16 (19.3) | 57 (46.0) |
| Lipid-lowering drugs, n (%) | 36 (14.2) | 15 (10.8) | 8 (17.0) | 14 (10.5) | 13 (23.6) | 7 (8.4) | 23 (18.6) |
| Antiplatelet or anticoagulant drugs, n (%) | 23 (9.1) | 10 (7.2) | 8 (17.0) | 8 (6.0) | 11 (20.0) | 4 (4.8) | 13 (10.5) |
- Data are presented as the means (SD) for normally distributed data, as the median (IQR) for not normally distributed data or as n (%).
- AHI, apnea−hypopnea index; BMI, body mass index; ESS, Epworth Sleepiness Scale; ODI, oxygen desaturation index; REM, rapid eye movement.
| All | AHI < 15 | AHI ≥ 30 | ODI < 15 | ODI ≥ 30 | REM− AHI < 15 | REM− AHI ≥ 30 | |
|---|---|---|---|---|---|---|---|
| No. of subjects | 253 | 139 | 47 | 133 | 55 | 83 | 124 |
| TST, min | 401.2 (60.9) | 405.7 (56.7) | 390.3 (59.1) | 404.5 (58.2) | 398.4 (71.9) | 407.2 (60.6) | 391.8 (59.5) |
| AHI, events per hr | 12.6 (4.1–24.8) | 4.8 (1.3–9.0) | 46.0 (36.2–68.5) | 4.4 (1.3–8.2) | 42.8 (31.2–65.5) | 2.0 (0.9–4.4) | 24.0 (16.0–38.7) |
| ODI, events per hr | 14.0 (5.2–26.9) | 5.7 (2.2–9.8) | 49.3 (36.5–68.1) | 5.5 (2.1–9.4) | 44.1 (33.8–63.9) | 2.9 (0.9–5.8) | 23.5 (16.1–37.7) |
| REM−AHI, events per hr | 29.5 (7.5–45.2) | 9.2 (2.4–24.8) | 55.3 (47.0–62.2) | 9.1 (2.3–25.2) | 53.2 (42.8–62.1) | 3.6 (1.1–7.5) | 45.3 (37.9–55.5) |
| Mean saturation, % | 94.4 (1.7) | 95.0 (1.5) | 93.3 (1.7) | 95.1 (1.4) | 93.1 (1.8) | 95.4 (1.5) | 93.8 (1.5) |
| Lowest saturation, % | 83.5 (6.9) | 86.4 (6.1) | 78.3 (6.6) | 86.5 (6.1) | 78.1 (6.0) | 87.4 (6.8) | 80.7 (6.0) |
| % of TST with saturation < 90% | 0.7 (0.1–3.3) | 0.1 (0.0–0.7) | 5.4 (2.7–11.5) | 0.1 (0.0–0.6) | 6.1 (2.8–12.2) | 0.0 (0.0–0.3) | 2.5 (0.8–5.8) |
| % of TST with REM sleep | 17.9 (6.2) | 19.0 (5.7) | 14.9 (7.3) | 19.1 (5.8) | 14.9 (7.2) | 19.3 (5.9) | 16.8 (6.1) |
| % of TST in supine position | 43.0 (21.4–64.9) | 35.0 (17.3–63.6) | 53.6 (36.5–71.4) | 34.2 (17.0–60.6) | 53.3 (36.5–72.0) | 34.8 (17.3–63.6) | 49.8 (26.7–67.3) |
- Data are presented as the means (SD) for normally distributed data, as the median (IQR) for not normally distributed data or as n (%).
- AHI, apnea−hypopnea index; ODI, oxygen desaturation index; REM, rapid eye movement; TST, total sleep time.
In unadjusted models, 57 proteins were significantly associated with AHI, 56 proteins with ODI and 64 proteins with REM−AHI (data not shown). Associations between severe sleep-disordered breathing and proteins, after adjustment for age, BMI and plate, are given in Figures 2 and 3. The results for the top 10 proteins for the AHI, ODI and REM−AHI are given in Tables 3−5. After adjustment for age, BMI and plate, there was no significant association between AHI or ODI and any of the proteins.
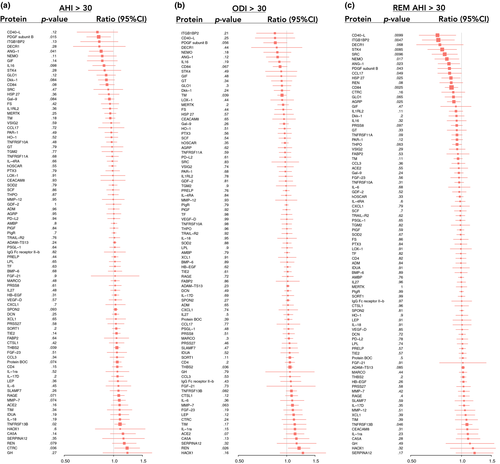
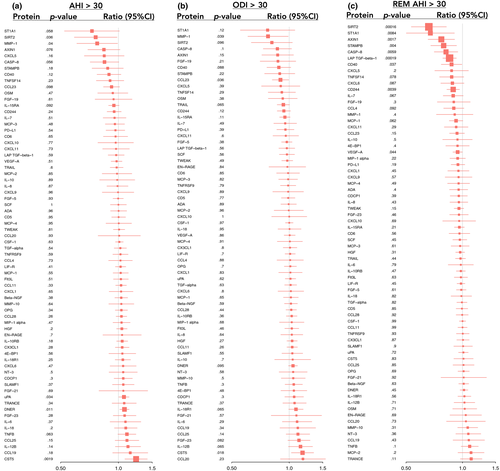
| Regression coefficient | Protein concentration ratio (AHI ≥ 30/AHI < 15) | p-value | q-value | |
|---|---|---|---|---|
| CST5 | .31 | 1.24 | .0019 | .32 |
| DNER | .11 | 1.08 | .011 | .62 |
| PDGF subunit B | −.30 | 0.81 | .015 | .62 |
| TNFSF13B | .13 | 1.09 | .020 | .62 |
| uPA | .10 | 1.07 | .034 | .62 |
| CTRC | .27 | 1.21 | .036 | .62 |
| SIRT2 | −.39 | 0.76 | .036 | .62 |
| THBS2 | .07 | 1.05 | .039 | .62 |
| MMP-1 | −.37 | 0.78 | .042 | .62 |
| ANG-1 | −.25 | 0.84 | .043 | .62 |
- Results from linear regression models adjusted for age, BMI and plate. The ratio = 2^coef. The q-value is the FDR adjusted p-value.
- AHI, apnea−hypopnea index.
| Regression coefficient | Protein concentration ratio (ODI ≥ 30/ODI < 15) | p-value | q-value | |
|---|---|---|---|---|
| CST5 | .23 | 1.17 | .018 | .72 |
| REN | .26 | 1.19 | .026 | .72 |
| THBS2 | .07 | 1.05 | .036 | .72 |
| CCL23 | −.15 | 0.90 | .036 | .72 |
| MMP−1 | −.37 | 0.77 | .039 | .72 |
| TM | −.10 | 0.93 | .039 | .72 |
| PAPPA* | −1.26 | 0.28 | .051 | .72 |
| PDGF subunit B | −.24 | 0.85 | .056 | .72 |
| IL−17A* | .80 | 2.24 | .058 | .72 |
| MMP−7 | .12 | 1.09 | .063 | .72 |
- Results from linear (or logistic for those marked with *) regression models adjusted for age, BMI and plate. The ratio = 2^coef, the ratios for logistic regressions are odds ratios. The q-value is the FDR adjusted p-value.
- ODI, oxygen desaturation index.
| Regression coefficient | Protein concentration ratio (REM− AHI ≥ 30/REM− AHI < 15) | p-value | q-value | |
|---|---|---|---|---|
| SIRT2 | −.60 | 0.66 | .00016 | .016 |
| LAP TGF-β1 | −.23 | 0.85 | .00019 | .016 |
| AXIN1 | −.37 | 0.77 | .0017 | .095 |
| CD84 | −.20 | 0.87 | .0025 | .11 |
| CD244 | −.17 | 0.89 | .0039 | .11 |
| STAMPB | −.33 | 0.79 | .0040 | .11 |
| ITGB1BP2 | −.46 | 0.73 | .0047 | .11 |
| CASP−8 | −.29 | 0.82 | .0059 | .12 |
| ST1A1 | −.57 | 0.67 | .0084 | .14 |
| STK4 | −.38 | 0.77 | .0085 | .14 |
- Results from linear regression models adjusted for age, BMI and plate. The ratio = 2^coef. The q-value is the FDR adjusted p-value.
- AHI, apnea−hypopnea index; REM, rapid eye movement.
Severe OSA during REM sleep was associated with decreased levels of SIR2-like protein 2 (Sirt2), latency-associated peptide transforming growth factor beta-1 (LAP-TGF-β1) and Axin1, after adjustment for age, BMI and plate (Table 5). For Sirt2 and LAP-TGF-β1, severe OSA during REM sleep and BMI had opposite effects, where increasing BMI was associated with increased levels of Sirt2 and LAP-TGF-β1, while an REM−AHI of ≥ 30 was associated with decreased levels. Sirt2, LAP-TGF-β1 and Axin1 were all positively correlated with one another.
Rapid eye movement sleep corresponded on average to 17.9% of total sleep time in the whole study population. Nineteen women had less than 30 min of REM sleep. Severe OSA during REM sleep was still associated with decreased levels of Sirt2 (coef −.66, q-value .0066), LAP-TGF-β1 (coef −.26, q-value .0066) and Axin1 (coef −.42, q-value .046), after adjustment for age, BMI and plate when these women were excluded.
The associations between severe OSA during REM sleep and Sirt2, LAP-TGF-β1 and Axin1 were further analysed, excluding women with diabetes (n = 15) and adding supine AHI as a possible confounder to the models also adjusted for age BMI and plate. Severe OSA during REM sleep was still associated with decreased protein levels with similar protein concentration ratios; for Sirt2 ratio 0.64 (p = .00086), LAP-TGF-β1 ratio 0.83 (p = .00010) and Axin1 ratio 0.76 (p = .00403).
Additional analyses of the effect of daytime sleepiness on Sirt2, LAP-TGF-β1 and Axin1 showed no significant correlations between the ESS and protein values.
4 DISCUSSION
In this population-based cohort study of women, severe OSA during REM sleep was associated with changed levels of the inflammatory and cardiac proteins Sirt2, LAP-TGF-β1 and Axin1. For OSA based on a whole night, the associations with these and other examined circulating protein markers were weaker and not statistically significant after adjustment for age, BMI and plate. These are novel data that add to our understanding of the associations between sleep-disordered breathing and cardiovascular dysregulation, and are in accordance with several recent studies reporting an association between OSA during REM sleep and adverse cardiometabolic outcomes (Acosta-Castro et al., 2018; Appleton et al., 2016; Aurora, Crainiceanu, Gottlieb, Kim, & Punjabi, 2018; Chami et al., 2015; Mokhlesi et al., 2014).
Even though there are a multitude of studies investigating one or a few cardiac and inflammatory biomarkers in OSA, few other proteomic studies of adult OSA exist (Feliciano et al., 2017; Jurado-Gamez et al., 2012; Kim et al., 2009; Seetho et al., 2014). In clinic-based studies with male study populations, changes in the levels of proteins involved in lipid metabolism, vascular pathways, the complement system, oxidative stress and the acute phase response in association with OSA have been reported (Feliciano et al., 2017; Jurado-Gamez et al., 2012; Kim et al., 2009). In a larger study investigating urinary proteomics in obese subjects, including both women and men, 24 proteins showed changed levels in OSA, but the differences did not reach significance after adjustment for multiple testing (Seetho et al., 2014). To the best of our knowledge, this is the first proteomic study of sleep-disordered breathing not only looking at OSA based on a whole night but also investigating the effect of OSA during REM sleep on protein expression.
An association between Sirt2 and OSA during REM sleep has not previously been reported. There are, however, some studies of the closely related Sirt1 protein and OSA (Chen et al., 2015; Lin, Wang, Liaw, Chiu, & Lin, 2018). In patients with OSA, Sirt1 levels were lower than in healthy controls and, after CPAP treatment (Chen et al., 2015) or oral appliance treatment (Lin et al., 2018), the levels increased. There are no previous studies of LAP-TGF-β1 and OSA during REM sleep, and for OSA based on a whole night the results of previous studies are conflicting (Alberti et al., 2003; Hernandez-Jimenez et al., 2017; Steffanina et al., 2015). To the best of our knowledge, Axin1 has not previously been studied in association with OSA.
The proteomic assays included a few proteins that have been reported to be associated with OSA in previous studies, but did not correlate with measurements of OSA in the present study. This might be explained by differences in the study population. In clinic-based studies, there are more patients with recurrent deep desaturations, while very few women in our population-based study had severe desaturations. This likely explains why proteins mainly associated with hypoxia, like IL-6, IL-8 and IL-10, did not show any significant alterations in our study. Other markers that have been suggested to be associated with OSA, like leptin (Pan & Kastin, 2014), showed an association in the unadjusted analysis but were explained by BMI.
Sirt2 is a cytoplasmic NAD-dependent deacetylase and a part of the sirtuin family of proteins (Gomes, Fleming Outeiro, & Cavadas, 2015). The homologue, Sir2, in yeast, worms and flies is associated with longevity, and Sirt2 has been shown to exhibit anti-inflammatory effects by the inhibition of NF-κB activity (Gomes et al., 2015; Tissenbaum & Guarente, 2001). Sirt2 is also thought to play an important role in metabolic regulation, but its exact effects are not fully understood (Gomes et al., 2015). Sirt2 inhibits adipogenesis and facilitates hepatic glucose uptake, and an inverse relationship between Sirt2 expression and insulin resistance has been reported (Lemos et al., 2017). It was recently reported that OSA during REM sleep is associated with insulin resistance (Chami et al., 2015), diabetes (Acosta-Castro et al., 2018) and poorer glycaemic control in diabetes (Grimaldi, Beccuti, Touma, Van Cauter, & Mokhlesi, 2014). The association between severe OSA during REM sleep and decreased levels of Sirt2 in the present study points to a possible pathway by which OSA during REM sleep could contribute to this metabolic dysregulation.
TGF-β1, produced as the latent form LAP-TGF-β1, is an anti-inflammatory cytokine involved in the regulation of numerous cellular functions (Redondo, Navarro-Dorado, Ramajo, Medina, & Tejerina, 2012). In atherosclerosis, TGF-β1 is considered to be an anti-atherogenic factor in the early stage of the disease. TGF-β1 inhibits excessive vascular smooth-muscle cell accumulation in the neointima and stimulates tissue repair (Redondo et al., 2012). Decreased plasma levels of TGF-β1 have been correlated with clinical atherosclerosis (Toma & McCaffrey, 2012). The association between severe OSA during REM sleep and decreased levels of LAP-TGF-β1 might reflect a pathway by which sleep-disordered breathing could contribute to the development of atherosclerosis. This is consistent with the results from our previous study where severe OSA during REM sleep was associated with increased intima thickness (Ljunggren et al., 2018), an early sign of atherosclerosis, and with data from the Sleep Heart Health study, where severe OSA during REM sleep was associated with a higher incidence of cardiovascular insults in persons with cardiovascular disease (Aurora et al., 2018).
Axin1 functions as a negative regulator in the Wnt signalling pathway (Salahshor & Woodgett, 2005). It is regarded as a tumour suppressor, and mutations have been linked to several types of cancer, but the significance of low circulating levels of Axin1 is not known. Axin1 also facilitates TGF-β signalling (Furuhashi et al., 2001).
The mechanism by which OSA during REM sleep might affect the levels of Sirt2, LAP-TGF-β1 and Axin1 is unclear. The effect of intermittent hypoxia, the activation of the sympathetic nervous system and sleep fragmentation are all possible mechanisms. OSA during REM sleep is associated with longer apneas and more pronounced desaturations than OSA during non-REM sleep (Findley, Wilhoit, & Suratt, 1985), and the effect of high sympathetic activity during REM sleep, further augmented by OSA, might also contribute. Sirtuins are involved in circadian rhythm regulation. It was recently reported that, in Drosophila, Sirt2 is suppressed in a rhythmic manner in pacemaker neurons, and that flies with amorphic Sirt2 experience an increase in sleep duration (Chen & Rosbash, 2017). In mice, intermittent short sleep caused a reduction in sirtiun 1 and 3 in wake-activated neurons (Zhu et al., 2016). It can be hypothesized that sleep fragmentation caused by OSA could cause a decrease in the expression of Sirt2, and that the fragmentation of REM sleep has a greater impact than non-REM sleep fragmentation. We did not, however, find any association between Sirt2 levels and daytime sleepiness as measured by the ESS.
The strengths of this study include the large sample of women, the population-based design, reliable PSG data with information on AHI in different sleep stages, and the broad spectrum of proteins analysed with precise PEA technology. One limitation is that our findings come from a single cohort, and the results need to be confirmed in further studies of independent cohorts. Also, even though we adjusted for age and BMI, there could be a risk of residual confounding. Despite these limitations, our data provide novel insights into possible pathways linking sleep-disordered breathing with cardiometabolic disease, and highlight the significance of OSA during REM sleep.
Severe OSA during REM sleep has been associated with hypertension (Appleton et al., 2016; Mokhlesi et al., 2014), insulin resistance (Chami et al., 2015), diabetes (Acosta-Castro et al., 2018), signs of atherosclerosis (Ljunggren et al., 2018) and cardiovascular insults (Aurora et al., 2018). Our data suggest that, even though REM sleep only accounts for about 20% of total sleep time, severe OSA during REM sleep might have an effect on the plasma levels of important regulatory proteins linked to cardiovascular and metabolic disease. This implies that severe sleep apnea during REM sleep needs to be taken into account when deciding which patients to treat for sleep-disordered breathing and how to treat them. To cover REM sleep, CPAP treatment has to include the early morning hours (Grimaldi et al., 2014). Although far in the future, the identification of intermediate mechanisms linking OSA to cardiovascular disease could also result in new treatment options to prevent the development of cardiovascular disease in OSA. In a rat model of OSA, the antioxidant, reservatol, upregulated Sirt1 levels and prevented the development of insulin resistance (Wang et al., 2015). It is possible that, in the future, targeting the intermediate mechanisms linking OSA to cardiovascular disease could be an alternative in non-sleepy OSA with poor compliance with CPAP therapy.
In conclusion, severe OSA during REM sleep was associated with decreased plasma levels of Sirt2, LAP-TGF-β1 and Axin1, proteins with anti-inflammatory effects involved in numerous cellular functions, in metabolic regulation and in the atherosclerotic process. For OSA based on a whole night, the associations with cardiac and inflammatory proteins were weaker, and explained to a large extent by age and BMI.
ACKNOWLEDGEMENTS
This study was supported financially by the Swedish Heart Lung Foundation, with grant number 20130299, the Uppsala County Association against Heart and Lung Diseases, and the Bror Hjerpstedt Foundation.
Conflicts of interest
The authors have no conflicts of interest to declare.
Author contributions
All authors contributed to study concept and design. EF and ML carried out the data analyses. ML and EL wrote the first draft of the manuscript. All authors have revised the manuscript critically for important intellectual content and approved the final version.




