Circadian period and the timing of melatonin onset in men and women: predictors of sleep during the weekend and in the laboratory
Summary
Sleep complaints and irregular sleep patterns, such as curtailed sleep during workdays and longer and later sleep during weekends, are common. It is often implied that differences in circadian period and in entrained phase contribute to these patterns, but few data are available. We assessed parameters of the circadian rhythm of melatonin at baseline and in a forced desynchrony protocol in 35 participants (18 women) with no sleep disorders. Circadian period varied between 23 h 50 min and 24 h 31 min, and correlated positively (n = 31, rs = 0.43, P = 0.017) with the timing of the melatonin rhythm relative to habitual bedtime. The phase of the melatonin rhythm correlated with the Insomnia Severity Index (n = 35, rs = 0.47, P = 0.004). Self-reported time in bed during free days also correlated with the timing of the melatonin rhythm (n = 35, rs = 0.43, P = 0.01) as well as with the circadian period (n = 31, rs = 0.47, P = 0.007), such that individuals with a more delayed melatonin rhythm or a longer circadian period reported longer sleep during the weekend. The increase in time in bed during the free days correlated positively with circadian period (n = 31, rs = 0.54, P = 0.002). Polysomnographically assessed latency to persistent sleep (n = 34, rs = 0.48, P = 0.004) correlated with the timing of the melatonin rhythm when participants were sleeping at their habitual bedtimes in the laboratory. This correlation was significantly stronger in women than in men (Z = 2.38, P = 0.017). The findings show that individual differences in circadian period and phase of the melatonin rhythm associate with differences in sleep, and suggest that individuals with a long circadian period may be at risk of developing sleep problems.
Introduction
Irregular sleep patterns, delayed sleep phase syndrome, non-24-h sleep–wake syndrome and insomnia complaints are prevalent in modern societies (Sack et al., 2007). This may be in part because, with the advent of artificial light, sleep–wake timing is no longer dictated by the natural 24-h light–dark cycle. The exposure to artificial light associated with work schedules and evening behaviours, such as watching TV or working in front of computer screens, has been shown to affect circadian physiology and latency to sleep onset (Cajochen et al., 2011; Santhi et al., 2012), and appears to be a major driver of sleep timing. Our weekly work schedules often lead to a pattern of irregular sleep, with delayed and short sleep during workdays and sleep rebound during weekends/free days (Basner et al., 2007). To our knowledge, biological correlates of these sleep patterns and individual differences therein have not been identified. The involvement of circadian processes is often implied, although the available evidence primarily consist of the association between self-reported circadian preference and self-reported delayed sleep during free days (Roenneberg et al., 2003).
Circadian rhythms with their near-24-h period are generated by a network of transcriptional activators and repressors, and synchronised to the 24-h day by light (Takahashi et al., 2008). Laboratory studies have established that sleep timing and sleep quality are regulated by a fine-tuned interaction of sleep homeostasis and circadian rhythmicity (Dijk and Czeisler, 1995).
Based on our current understanding of the light-sensitivity and photic-entrainment of circadian clocks, and the homeostatic and circadian interaction in the regulation of sleep, it can be predicted that individuals with a long circadian period are at risk for delayed and irregular sleep patterns (Dijk and Lockley, 2002). This is because self-selected artificial light exposure in the evening will lead to delay of the onset of melatonin, sleepiness and latency to sleep onset (Santhi et al., 2012), and hence exacerbate the natural tendency to delay, especially, in individuals with a long circadian period. Whereas, during workdays this delay cannot be reflected in a delay of wake time, it will lead to later sleep times during weekends and thereby create irregular sleep patterns.
However, no direct evidence supporting this prediction is currently available. We assessed associations between the circadian period and entrained phase of the melatonin rhythm, and also compared men and women, because gender differences in entrained phase (Cain et al., 2010) and circadian period (Duffy et al., 2011) have been reported.
Materials and methods
The protocol received a favourable opinion from the Research Ethics Committee of the University of Surrey. All 35 healthy non-smoking participants (18 women) aged 20.5–32.4 years provided written informed consent and, after passing a screening night, entered a forced desynchrony protocol. Details of the recruitment, screening procedures and protocol have been reported elsewhere (Hasan et al., 2012). This sample was stratified on the basis of the rs57875989 PER3 VNTR polymorphism, and in vivo and in vitro circadian period were compared across genotypes, but no differences between genotypes were observed (Hasan et al., 2012) and genotype will not be considered in the current analyses.
During a screening visit, participants completed multiple questionnaires. Self-reported sleep habits during workdays and free days were assessed with the Munich Chronotype Questionnaire (MCTQ; Roenneberg et al., 2003). The MCTQ assesses prevailing sleep habits and addresses sleep–wake timing separately for workdays and free days. We extracted bedtime, wakeup time as well as sleep latency from the MCTQ. From these measures we derived reported time in bed (TIB) as the time period between reported bedtime and wakeup time. Sleep complaints were assessed with the Insomnia Severity Index (ISI). The ISI is a seven-item questionnaire assessing dissatisfaction with sleep during the last 2 weeks, with a maximum score of 28. A score of 0–7 indicates no clinically significant insomnia, and a score of 8–14 is considered sub-threshold insomnia (Bastien et al., 2001). Sleep disorders were excluded by a clinical polysomnographic assessment during the screening night.
In the week prior to the laboratory phase, participants were instructed to follow a regular sleep–wake schedule, and compliance was assessed by actigraphy. During the 9-day laboratory protocol, participants were resident in the Clinical Research Centre of the University of Surrey. After a baseline day, participants were scheduled to live on 28-h ‘days’ consisting of 9 h 20 min in bed in darkness and 18 h 40 min wakefulness in dim light (<5 lux) in the absence of external time cues (Fig. 1). This leads to desynchrony between the sleep–wake cycle and circadian rhythms, and allows the assessment of the near-24-h circadian period of the hypothalamic circadian pacemaker driving circadian rhythms such as plasma melatonin when the desynchrony is at least one beat cycle long as in the current protocol (Dijk and Czeisler, 1995; Klerman et al., 1996). During the sleep periods of the 1st, 4th and 7th sleep–wake cycle of the forced desynchrony protocol, blood samples were scheduled to be collected every 60 or 120 min for plasma melatonin analysis. The timing of the onset of nocturnal melatonin secretion while participants were in dim light (the dim light melatonin onset, defined as the time when the melatonin concentration was 25% of the measured peak value) was used as a circadian phase marker (see Hasan et al., 2012 for rationale and references). The timing of melatonin onset at baseline (the evening prior to sleep period 2) was assessed in 35 participants, and intrinsic circadian period was determined from the progression of the melatonin rhythm throughout the protocol in 31 participants, as described in Hasan et al. (2012). The data on circadian period, melatonin phase and diurnal preference, as reported in Hasan et al. (2012), were used as predictor variables in the current analyses.
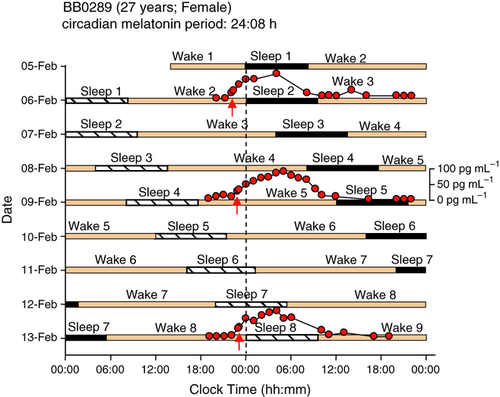
Throughout the laboratory segment, sleep was recorded polysomnographically on Siesta 802 devices (Compumedics, Abbotsford, Vic., Australia) using a 12-channel electroencephalogram montage. Sleep staging was performed according to the criteria of Rechtschaffen and Kales (1969), and standard sleep parameters were computed. In addition we computed latency to persistent sleep, a parameter often used in clinical trials in insomnia, and defined as the first occurrence of any sleep stage that is followed by 10 min of uninterrupted sleep.
Statistical analyses were based on Spearman correlations, multiple regression analyses and analyses of covariance as implemented in SAS version 9.1 (SAS Institute, Cary, NC, USA). Correlation coefficients were Fisher-Z transformed and statistically compared between the two gender groups using a t-test.
Results
Circadian period varied among individuals between 23 h 50 min and 24 h 31 min, with an average of 24 h 10 min (SD = 10 min). This variation correlated significantly (n = 31, rs = 0.43, P = 0.017) with entrained phase of the melatonin rhythm such that, in individuals with a longer period, habitual bedtime (i.e. dark onset) occurred closer to the onset of melatonin secretion (Hasan et al., 2012). There was no statistically significant difference between the genders for either circadian period or the timing of the onset of melatonin secretion relative to habitual bedtime (Table 1).
| Variable | Men | Women | P-value | ||||
|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | ||
| Age | 17 | 24.3 | 3.0 | 18 | 26.7 | 3.5 | 0.04 |
| Circadian period (h) | 17 | 24.1 | 0.2 | 14 | 24.2 | 0.1 | ns |
| Melatonin onset relative to bedtime (h) | 17 | −2.2 | 1.2 | 18 | −1.6 | 1.2 | ns |
| TIB (min) | 15 | 560.0 | 0.0 | 17 | 560.0 | 0.0 | ns |
| TST (min) | 15 | 486.8 | 39.9 | 17 | 489.3 | 28.2 | ns |
| SE (%) | 15 | 86.9 | 7.1 | 17 | 87.4 | 5.0 | ns |
| SL (min) | 16 | 7.0 | 3.8 | 18 | 8.3 | 6.4 | ns |
| LPS (min) | 16 | 9.7 | 6.3 | 18 | 10.9 | 9.0 | ns |
| Stage 1 (%) | 15 | 10.8 | 2.6 | 17 | 9.5 | 3.2 | ns |
| Stage 2 (%) | 15 | 48.1 | 6.4 | 17 | 49.0 | 6.3 | ns |
| SWS (%) | 15 | 16.3 | 4.4 | 17 | 17.4 | 3.8 | ns |
| REM (%) | 15 | 24.8 | 3.7 | 17 | 24.1 | 4.2 | ns |
- TIB, time in bed; TST, total sleep time; SE, Sleep efficiency; SL, sleep latency; LPS, latency to persistent sleep; SWS, slow-wave sleep; REM, rapid eye movement sleep.
- Number of observations, mean values, standard deviations and one-way analysis effects are indicated. Sleep efficiency = TST/TIB; SL (min) = time period between lights out and the first sleep epoch; LPS (min) = time period between lights out and the first 10 minutes of continuous sleep; Stage 1 (%) = Time in stage 1 expressed as percentage of TST. Differences between the genders were assessed with analysis of covariance using age as a covariate.
The average ISI was 3.7 (SD = 3.5, n = 35), varied between individuals from 0 to 12, and correlated with circadian period (n = 31, rs = 0.36, P = 0.05). The ISI also correlated significantly (n = 35, rs = 0.47, P = 0.004) with a more delayed timing of the melatonin rhythm at baseline (Fig. 2a).
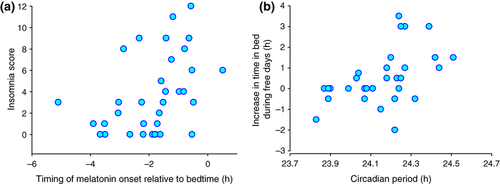
Average bed and wakeup times during free days were significantly later than during workdays [bedtime: 23 h 34 min (SD = 1 h 14 min) versus 00 h 28 min (SD = 1 h 17 min), n = 35, t = 6.365, P < 0.001; wakeup time: 07 h 45 min (SD = 1 h 13 min) versus 09 h 13 min (SD = 1 h 22 min), n = 35, t = 6.198, P < 0.001]. TIB during workdays averaged 8 h 10 min (SD = 1 h 1 min) whereas, during free days, it increased to 8 h 46 min (SD = 1 h 6 min, n = 35, t = −2.722, P < 0.01). An increase in TIB or no change was observed in the majority of participants, although in some participants a decrease was observed (Fig. 2b). On average, women showed a 61 min earlier bedtime (F1,32 = 6.44, P = 0.016) and 59 min earlier wakeup time (F1,32 = 5.74, P = 0.023) during free days as compared with males. However, no significant effect of gender was observed for sleep timing during workdays or any of the TIB measures.
Circadian period correlated with ‘weekend’ sleep timing parameters such that participants with a longer circadian period reported longer TIB during free days (n = 31, rs = 0.47, P = 0.007) and a greater increase in TIB from workdays to free days (n = 31, rs = 0.54, P = 0.002; Fig. 2b). Multivariate regression analyses showed that TIB during free days was independently and positively predicted by both circadian period (P = 0.009) and TIB during workdays (P = 0.02). The change in TIB from workdays to free days was also independently and positively predicted by circadian period (P = 0.009), and negatively by TIB during workdays (P = 0.007). Sleep–wake timing parameters during workdays were not associated with circadian period (P > 0.4 in all cases).
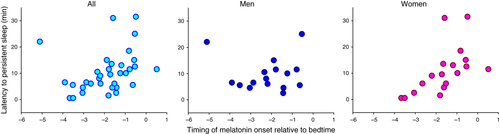
A more delayed melatonin rhythm was associated with a longer sleep latency (n = 34, rs = 0.39, P = 0.024) as well as a longer latency to persistent sleep (n = 34, rs = 0.48, P = 0.004), as assessed polysomnographically when participants were sleeping at their habitual bedtime. These correlations were significantly stronger in women than in men for both sleep latency (Z = −2.02, P = 0.043) and latency to persistent sleep (Z = 2.38, P = 0.017; Fig. 3). Other baseline sleep parameters were not associated with either circadian period or phase at baseline, and no additional gender differences were observed (Table 1).
Discussion
The data show that the circadian period of the melatonin rhythm and the timing of the melatonin rhythm relative to habitual bedtime correlated with TIB during free days, the increase in TIB from workdays to free days and the subjective sleep complaints as measured by the ISI, as well as polysomnographically assessed sleep latency when participants were sleeping at their habitual sleep time. The direction of these correlations is such that individuals with a long circadian period and delayed melatonin rhythm appear most at risk of delayed sleep during weekends, and experience less sleep satisfaction in general. The observation that sleep timing during workdays did not correlate with circadian period or the entrained phase of the melatonin rhythm confirms that, in contrast to free days, sleep–wake timing during workdays is primarily determined by social demands such as work schedules and social activities.
The most parsimonious interpretation of these observations is that while living in an environment with access to artificial light, a long circadian period is associated with a delay of the melatonin rhythm relative to bedtime (Wright et al., 2005). During workdays, when sleep timing is primarily determined by social factors, sleep occurs at a sub-optimal phase relative to the sleep propensity and melatonin rhythm, and this leads to sleep complaints. It is only during the weekend that sleep occurs in phase with the sleep propensity rhythm, which in individuals with a long circadian period occurs later than in those with a shorter circadian period. The accumulated sleep debt carried over from workdays is greater in individuals with longer intrinsic circadian period, who thus show a greater increase in sleep duration during free days. This interpretation is supported by the multiple regression analyses, which showed that the increase in TIB during free days is correlated positively with circadian period and negatively with TIB during workdays. This implies that the increase in TIB during free days reflects both the timing of the circadian sleep propensity rhythm and a homeostatic response to short sleep during workdays. Based on subjective reports, it appeared that some participants had shorter sleep on weekends than on weekdays. This may be related to late social activities on weekends, or reflect inaccuracies in the subjective data. More objective long-term data on sleep timing during workdays and free days are required to fully understand the dynamics of sleep duration in the real world.
Although in this small sample (n = 31) with limited statistical power we did not replicate the previously reported gender differences in the entrained phase (Cain et al., 2010; n = 57) and circadian period (Duffy et al., 2011; n = 157), we did, however, confirm gender differences in sleep timing. The observed gender differences in the strength of the correlation between circadian parameters and sleep physiology add to the growing body of evidence of gender differences in circadian rhythmicity (Mong et al., 2011).
The current sample was stratified with respect to the rs57875989 PER3 VNTR polymorphism and it can be argued that this may have influenced some of the results also because the PER34/4 genotype has been associated with insomnia severity in alcohol dependence (Brower et al., 2012). However, there were no significant genotype-dependent differences in circadian period, entrained phase or habitual sleep–wake timing. Nevertheless, this study population, as well as other study populations in this research area, was highly selected, and how these results will generalise to a more representative study population remains to be investigated.
Overall, our data show that intrinsic characteristics of the circadian timing system assessed in the absence of the confounding influence of work, sleep and light exposure schedules associate with sleep characteristics during the weekend and while sleeping at habitual bedtime in the laboratory. The data imply that individuals with a long circadian period may be more at risk of developing irregular sleep–wake patterns, sleep complaints and a delayed sleep phase syndrome.
Acknowledgements
This study was funded by BBSRC grant (BB/F022883/1). We thank the staff of the Clinical Research Centre for their help in data collection, and Patrick McCabe for statistical advice.
Conflict of interest
This is not an industry-sponsored study. Prof. Dijk has received research funding from, and served as a consultant to, Philips Lighting, and several pharmaceutical companies with an interest in sleep and circadian rhythms. He is also Editor-in-Chief of Journal of Sleep Research, but he has had no involvement in the processing of this manuscript. Drs Lazar, Santhi, Hasan, Lo, Johnston, Archer and von Schantz declare no conflicts of interest.




