A nurse-driven analgesia and sedation protocol reduces length of PICU stay and cumulative dose of benzodiazepines after corrective surgery for tetralogy of Fallot
Anja Hanser and Felix Neunhoeffer are co-first authorship.
Abstract
Purpose
Analgesia and sedation protocols are reported to reduce the requirement of sedative and analgesic agents, duration of mechanical ventilation, and length of pediatric intensive care unit (PICU) stay. However, these studies often were conducted based on inhomogeneous cohorts. The aim of this study was the evaluation of a nurse-driven analgesia and sedation protocol in a homogenous population of infants following corrective surgery for tetralogy of Fallot (TOF).
Design and Methods
This retrospective analysis was conducted in a cardiac PICU of a tertiary referral center. Two cohorts of patients who underwent corrective surgery for TOF below the age of 7 months, were retrospectively evaluated before and after implementation of a nurse-driven analgesia and sedation protocol. We compared peak and cumulative doses of midazolam, morphine, and clonidine, length of PICU stay and time on mechanical ventilation.
Results
A total of 33 patients were included in the preimplementation period and 32 during the postimplementation period. Implementation of the nurse-driven analgesia and sedation protocol had no effect on time on mechanical ventilation (72 hr [24–141] vs. 49 hr [24–98]), but significantly on length of PICU stay (7 days [5–14] vs. 5 days [4–7]). Cumulative doses of midazolam (7.37 mg/kg [4.70–17.65] vs. 5.0 mg/kg [2.70–9.12]) as well as peak doses of midazolam (0.22 mg·kg−1·hr−1 [0.20–0.33] vs. 0.15 mg·kg−1·hr−1 [0.13–0.20]) and morphine (50.0 µg·kg−1·hr−1 [39.7–79.9] vs. 42.5 µg·kg−1·hr−1 [29.7–51.8]) were significantly reduced. The postimplemantation group showed no increase in postoperative complications and adverse events.
Practice Implications
The implementation of a nurse-driven analgesia and sedation protocol is safe in infants following corrective surgery for TOF. It reduces significantly the length of PICU stay, cumulative and peak doses of midazolam and peak doses of morphine.
WHAT IS CURRENTLY KNOWN?
-
Analgesia and sedation protocols for pediatric intensive care patients are reported to be feasible and safe with no increase of adverse events
WHAT DOES THIS ARTICLE ADD?
-
This study evaluates the implementation of a goal-directed nurse-driven analgesia and sedation protocol in a homogenous cohort
-
Our nurse-driven sedation protocol can help to reduce doses of opioids and benzodiazepines as well as length of PICU stay
1 INTRODUCTION
Sedation strategies in pediatric intensive care units (PICUs) have undergone significant changes during the recent years. Validated assessment scales for sedation level and therapy protocols were established to promote individually adjusted therapy to pediatric patients (Curley et al., 2015; Deeter et al., 2011; Gaillard-Le Roux et al., 2017; Ista, de Hoog, Tibboel, & van Dijk, 2009; Ista, van Dijk, Tibboel, & de Hoog, 2005; Michel et al., 2017; Motta, Luglio, Delgado, & Carvalho, 2016; Neunhoeffer et al., 2015; Poh, Poh, Buang, & Lee, 2014; Simone et al., 2017; Staveski, Wu, Tesoro, Roth, & Cisco, 2017). They aim for a minimal sedation of wake, pain-free and calm children who tolerate therapy on PICU including mechanical ventilation and catheters. Oversedation with detrimental side effects especially on hemodynamics and ventilation should be minimized. In addition, higher doses of sedative drugs and longer exposure to sedatives may increase the occurrence of withdrawal symptoms and delirium (Best, Wypij, Asaro, Curley, & Randomized Evaluation of Sedation Titration For Respiratory Failure Study, 2017). Avoidance of oversedation should also help to minimize potential neurotoxic effects of benzodiazepines (e.g., midazolam). Nonetheless, sedation protocols must also prevent undersedation with potential risks for accidental extubation, agitation, anxiety, and patient discomfort.
The majority of studies showed improvement of sedation and analgesia following the implementation of sedation protocols (Curley et al., 2015; Deeter et al., 2011; Gaillard-Le Roux et al., 2017; Ista et al., 2009; Michel et al., 2017; Motta et al., 2016; Neunhoeffer et al., 2015; Poh et al., 2014; Simone et al., 2017; Staveski et al., 2017). Many of these protocols allow the assessment of sedation levels and the management of drug doses primarily by intensive care nurses, who usually are able to assess more fully the patient's needs due to their presence at the bedside compared to physicians. Several authors described positive effects such as the reduced requirement of sedative and analgesic agents, decreased duration of mechanical ventilation and length of PICU stay as well as reduced occurrence of withdrawal symptoms and delirium (Curley et al., 2015; Deeter et al., 2011; Gaillard-Le Roux et al., 2017; Ista et al., 2009; Michel et al., 2017; Motta et al., 2016; Neunhoeffer et al., 2015; Poh et al., 2014; Simone et al., 2017; Yaghmai, Di Gennaro, Irby, Deeter, & Zimmerman, 2016). Sedation protocols were reported to be feasible and safe in pediatric patients with no increase of adverse events like accidental extubations (Curley et al., 2015; Deeter et al., 2011; Gaillard-Le Roux et al., 2017; Ista et al., 2009; Michel et al., 2017; Motta et al., 2016; Neunhoeffer et al., 2015; Poh et al., 2014; Simone et al., 2017; Staveski et al., 2017). However, these studies often were conducted based on inhomogeneous cohorts reflecting the typical multidisciplinary nature of PICUs. Also, some of the studies showed that different effects on the target variables: for instance, almost all studies show that the implementation of sedation protocols is associated with the reduced requirement of sedative and analgesic agents. However, not all studies a shortened duration of mechanical ventilation (Curley et al., 2015; Deeter et al., 2011) and length of PICU stay (Deeter et al., 2011) could be found.
In contrast, the aim of the underlying study was to evaluate on the basis of a homogenous patient population to what extent the introduction of a nurse-driven and goal-directed analgesia and sedation protocol affects the duration of mechanical ventilation, length of PICU stay, peak and cumulative doses of analgesic and sedative agents and occurrence of adverse events like bleeding, unplanned extubations or arrhythmias. We decided to examine patients after corrective surgery for tetralogy of Fallot (TOF). In contrast to other cardiac defects such as children with complex univentricular hearts with rather individual and varying treatments, or children with simple heart defects with a usually short length of intensive stay, children with TOF require a rather standardized surgery, undergo similar peri- and postoperative treatment, and are all of the similar age from two to 6 months.
2 PATIENTS AND METHODS
2.1 Study location, population, and study design
The data of our retrospective study were obtained at the PICU (14 beds) of a tertiary referral center from the medical records. The purpose of the study was to evaluate the effects of the implementation of a nurse-driven sedation protocol on the duration of mechanical ventilation, length of PICU stay, peak and cumulative doses of analgesic and sedative agents and the occurrence of adverse events compared to the physician-driven preimplementation period. The sedation protocol was implemented with the idea that nurses can better assess and manage sedation levels and therapy than physicians, due to their presence at the bedside. This two-phase observational study consists of a preimplementation period (January 2005–October 2010) followed by a postimplementation period (January 2012–June 2017). A period of several months was interposed between the two observation periods to ensure that the new concept was implemented consistently. All patients, who underwent cardiac surgery for repair of TOF within the first 6 months of life, were eligible for enrollment in this study. Exclusion criteria included comorbidities like chromosomal abnormalities (e.g., 22q11 microdeletion syndrome, Trisomy 21) as well as significant extracardiac anomalies requiring specific treatment like esophageal atresia and anal atresia. The observation period ended on Day 5 after corrective surgery for TOF. Some patients were included also in other analgesia and sedation studies.
The study protocol was approved by the local ethics committee (578/2012R).
2.2 Surgical management and anesthesia
Anesthesia for pediatric cardiac surgery was initiated with midazolam, an opioid and a muscle relaxant and continued with sevoflurane in both groups. Surgical access was gained via a median sternotomy, and a cardiopulmonary bypass was established with aorto-bicaval cannulation. The surgical technique of repair was adjusted according to the patient's specific anatomy of the right ventricular outflow tract. Closure of the VSD was achieved by pericardial patch insertion. In patients with an adequate size of the pulmonary valve, ring repair was performed using a transatrial approach including resection of obstructing muscle bundles in the right ventricular outflow tract and additional commissurotomy of the pulmonary valve if required. Patients with hypoplastic pulmonary annulus underwent transannular patch enlargement.
2.3 Postoperative intensive care in the preimplementation period
In the preimplementation period, postoperative care did not include a standardized procedure, when and how to change the dosages of analgesics and sedatives in case of undersedation or oversedation. Nurses informed the physician about their observations and discussed the requirements of the patients' sedation, but they were not authorized to change the sedation regime without the physicians' order. The medication included continuous i.v. infusion of opioids (morphine: 5–100 µg·kg−1·hr−1; starting dose: 30 µg·kg−1/hr−1) and continuous i.v. infusion of benzodiazepines (midazolam: 0.05–0.42 mg·kg−1·hr−1; starting dose: 0.05 mg·kg−1·hr−1). Clonidine was introduced several days following surgery to support tapering of opioids and sedation (i.v. infusion of clonidine: 0.2–2 µg·kg−1·hr−1, starting dose: 0.04 µg·kg−1/hr−1). Oral melatonin (3–5 mg/day) was started to regulate the day-night cycles after 3 days stay on PICU. Chloral hydrate (up to 25 mg/kg four times daily) was administered additionally after the second postoperative day to promote sleep.
2.4 Postoperative intensive care in the postimplementation period
To standardize the management of analgesia and sedation, to make it independent of the experience or personal opinion of PICU staff, and to put it in the hands of nurses who have more bedside presence, a nurse-driven analgesia and sedation protocol were introduced in our department. The standard medication consisting of continuous i.v. infusion of morphine and midazolam and their starting doses did not change in the postimplementation period. Also, the adjuvant therapy with oral melatonin and chloral hydrate was the same in the preimplementation and postimplementation period. In the postimplementation period, continuous i.v. infusion of clonidine (0.2–2 µg·kg−1·hr−1, starting dose: 0.04 µg·kg−1·hr−1) was added in all patients after 24 hr on PICU.
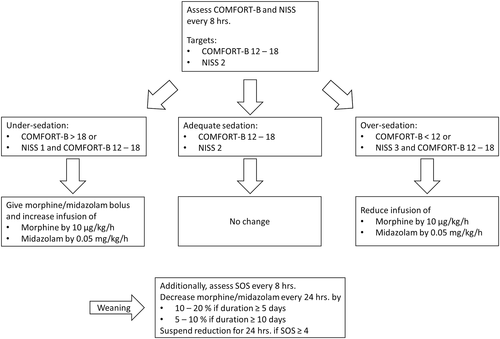
In the postimplementation period levels of sedation and analgesia were measured routinely at least every 8 hr with COMFORT Behavior (COMFORT-B) scale and the nurse interpretation of sedation scale (NISS; Neunhoeffer et al., 2015). The COMFORT-B score is an adjusted version of the COMFORT score, based on observational items such as alertness, calmness, respiratory response in ventilated patients or crying in nonventilated patients, muscle tone, physical movement, and facial tension. It has been introduced and validated for pain assessment as well as for sedation management in the setting of PICUs. COMFORT-B scoring of 12–18 was assumed as an optimal range of analgesia and sedation (Ista et al., 2005; Ista et al., 2009; Michel et al., 2017). A COMFORT-B score higher than 18 reflects undersedation, whereas a score of <12 indicates oversedation of the patient.
In addition, the NISS was used to evaluate the level of analgesia and sedation based on the nurses' personal opinion of the sedation depth. The scale has three levels: NISS 1 indicates insufficient sedation, NISS 2 indicates adequate sedation while NISS 3 indicates oversedation (Michel et al., 2017). This scale was used in addition to the COMFORT-B scale to ensure that the assessment and management of sedation are not based on a single scale alone and to include expert judgement and experience of intensive care nurses. In addition, the scale is considered a fallback level in case of inconsistencies in the COMFORT-B scale. There are no objectifiable variables based on which the nurses must make the assessment. The nurses decided about the NISS score based on their experience and patient observation.
The nurses tried to attain an optimal range of analgesia and sedation according to the implemented standardized, goal-directed nurse-driven analgesia and sedation protocol using the COMFORT-B scale and NISS (Appendix; Michel et al., 2017; Neunhoeffer et al., 2015). In case of a discrepancy between both scales regarding the adequacy of analgesia and sedation levels, NISS took precedence. Protocol deviation by the nursing staff or attending intensivists to protect patients' safety was allowed.
In case of insufficient analgesia or sedation (COMFORT-B >18 and NISS 1) continuous intravenous infusion of morphine and midazolam was increased (morphine by 10 µg·kg−1/hr−1, midazolam by 0.05 mg·kg−1·hr−1). To ensure the immediate establishment of adequate anesthesia, a bolus of morphine (dose is equivalent to 1-hr bolus) was given in addition to the increased infusion rate. In the case of oversedation (COMFORT-B <12 and NISS 3) continuous intravenous infusion was decreased (morphine by 10 µg·kg−1·hr−1, midazolam by 0.05 mg·kg−1/hr−1).
Opioid and sedative use for more than 3 days, upcoming extubation, and decreasing requirement of analgesia based on COMFORT-B and NISS scores triggered starting of the medication tapering plan. The medication tapering plan included reduction of opioids and benzodiazepines by 50% of the dose every 24 hr in case of therapy shorter than 5 days, and by 10–20% every 24 hr in case of therapy longer than 5 days. The medication tapering plan was paused for 24 hr if withdrawal symptoms occurred.
2.5 Statistical analysis
Clinical data of the patients including age, gender, weight, diagnosis, date of surgery, length of stay on PICU, duration of mechanical ventilation, cumulative dose of morphine, midazolam, and clonidine were collected from the patient data management system (IntelliSpace Critical Care and Anesthesia, Koninklijke Philips N.V., The Netherlands and CareVue, Medsphere Systems Corporation, Carlsbad, CA). Scoring data of the COMFORT-B scale and NISS were also extracted from the electronic patient data management system (PDMS).
Statistical analysis and the creation of charts were performed using SPSS (Version 24; IBM, Armonk, NY) and SigmaPlot (Version 12.5 for Windows; Systat Software, Inc., San Jose, CA). Data were tested for normal distribution with a Shapiro–Wilks test. Because most of the data were not normally distributed, they are presented as median (first/third interquartile). For not normally distributed values the Wilcoxon rank-sum test, and for normally distributed values Student's t test was applied. A probability of p < .05 was defined as statistically significant.
3 RESULTS
Between January 2005 and October 2010, 33 patients were included in the preimplementation group. Following the implementation of our nurse-driven analgesia and sedation protocol, 32 patients were included in the postimplementation group in the period from January 2012 to July 2017. All 65 patients underwent surgical repair of TOF and met study criteria.
As shown in Table 1, there were no statistically significant differences between the preimplementation and the postimplementation group regarding age (3.7 months [2.1–4.8] vs. 4.3 months [3.6–5.4], p = .056) and weight (5.7 kg [4.2–6.6] vs. 6.2 kg [5.2–6.8], p = .168). Both groups were homogenous and comparable to each other regarding underlying diagnosis, surgery, and having no other comorbidities. After implementation of the analgesia and sedation protocol, the median time on a ventilator was shorter (72 [24–141] hours vs. 49 hr [24–98], p = .407), but not statistically significant. The length of PICU stays reduced significantly (7 days [5–14] vs. 5 days [4–7], p = .017*; Figure 1).
| All patients (n = 65) | Preimplementation | Postimplementation | p |
|---|---|---|---|
| Patients | 33 | 32 | |
| Age (months) | 3.7 (2.1–4.8) | 4.3 (3.6–5.4) | .111 |
| Weight (kg) | 5.7 (4.2–6.6) | 6.2 (5.2–6.8) | .168 |
| Time on mechanical ventilation (hr) | 72 (24–141) | 49 (24–98) | .407 |
| PICU length of stay (day) | 7 (5–14) | 5 (4–7) | .017* |
- Note: Patients’ characteristics and outcome measures of the preimplementation group and the postimplementation group. Data are given as median, interquartile range (IQR1-3), a p < .05 was considered statistically significant *.
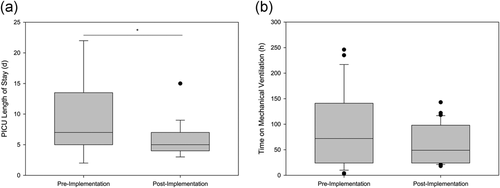
Table 2 demonstrates that patients of both groups received similar cumulative doses of morphine (1,961 µg/kg [1,113–3,162] vs. 1,920 µg/kg [904–2,687], p = .54). As in the postimplementation group, clonidine was started already after 24 hr, patients in the postimplementation period received significantly more clonidine (0.53 mg/kg [0.24–0.92] vs. 6.05 mg/kg [0.81–22.53], p = .001*). However, the cumulative dose of benzodiazepines was significantly reduced in the postimplementation period (7.37 mg/kg [4.70–17.65] vs. 5.0 mg/kg [2.70–9.12], p = .019*; Figure 2).
| Drug | Preimplementation | Postimplementation | p |
|---|---|---|---|
| Cumulative dose of morphine (µg/kg) | 1,961 (1,113–3,162) | 1,920 (904–2,687) | .54 |
| Cumulative dose of midazolam (mg/kg) | 7.37 (4.70–17.65) | 5.0 (2.70–9.12) | .019* |
| Cumulative dose of clonidine (mg/kg) | 0.53 (0.24–0.92) | 6.05 (0.81–22.53) | .001* |
- Note: Data are given as median, interquartile range (IQR1-3), p < .05 was considered statistically significant *.

Table 3 shows the statistically significant reduction of peak dosages of morphine (50.0 µg·kg−1·hr−1 [39.7–79.9] vs. 42.5 µg·kg−1·hr−1 [29.7–51.8], p = .004*) and midazolam (0.22 mg·kg−1·hr−1 [0.20–0.33] vs. 0.15 mg·kg−1·hr−1 [0.13–0.20], p = .001*).The use of clonidine increased significantly (0.014 µg·kg−1·hr−1 [0.009–0.021] vs. 0.674 µg·kg−1·hr−1 [0.02–1.095], p = .001*; Figure 3). Propofol and neuromuscular-blocking drugs were not used in the examined patients.
| Drug | Preimplementation | Postimplementation | p |
|---|---|---|---|
| Peak dose of morphine (µg·kg−1·hr−1) | 50.0 (39.7–79.9) | 42.5 (29.7–51.8) | .004* |
| Peak dose of midazolam (mg·kg−1·hr−1) | 0.22 (0.20–0.33) | 0.15 (0.13–0.20) | .001* |
| Peak dose of clonidine (mg·kg−1·hr−1) | 0.014 (0.009–0.021) | 0.674 (0.02–1.095) | .001* |
- Note: Data are given as median, interquartile range (IQR1-3), a p < 0.05 was considered statistically significant *.

Adequate sedation according to the evaluation based on the COMFORT-B (13.8 [11.5–15.0]) and NISS (2.0 [1.7–2.0]) scores was obtained in the postimplementation group. COMFORT-B scoring showed adequate analgesia and sedation in 71% of all observations (COMFORT-B 12-18), oversedation occurred in 25% (COMFORT-B <12), and undersedation (COMFORT-B >18) in 4%. According to NISS, patients were sedated adequately in 84% (NISS = 2) of all observations, oversedated in 6% (NISS = 3), and under-sedated in 10% (NISS = 1). Both COMFORT-B and NISS scores were not obtained in the preimplementation group because they were introduced with the nurse-driven protocol.
Complications including postoperative arrhythmias (12 patients of preimplementation group vs. eight patients of postimplementation group), extubation failure (four vs. two patients), and acute renal failure requiring temporary hemodialysis (two vs. zero patients) occurred in both groups without significant difference.
4 DISCUSSION
The underlying study is based on two cohorts of a homogenous patient population regarding diagnosis, operation, age, and weight. It confirms that the implementation of a nurse-driven sedation protocol is safe in infants following corrective surgery for TOF. Regular assessment of sedation levels by the nursing-staff revealed desired sedation goals for most of the observation period. There was no increase in adverse events including bleeding, arrhythmia, the requirement of hemodialysis, and extubation failure. In addition, peak doses of midazolam and morphine, as well as the cumulative dose of midazolam, were significantly lower following the introduction of our sedation protocol. The length of PICU stay was significantly shorter in the postimplementation group, while the difference in duration of mechanical ventilation, although shorter, did not reach statistical significance.
Regarding the interpretation of the results of our study it has to be taken into consideration, however, that with the implementation of the sedation protocol in our postimplementation group, we also changed our policy regarding the administration of clonidine. In the preimplementation group administration of clonidine was started several days following the surgical procedure to support tapering of analgesic and sedative agents. After the implementation of the protocol, the children usually received clonidine as a continuous infusion starting 24 hr after the operation. Therefore, the changes found in our study may be at least partially related to this change in our pharmacological policy. Alpha-2-agonists like clonidine and dexmedetomidine have been described to provide opioid-sparing effects in neonates and can be used as alternative or adjunctive sedative agents in combination with benzodiazepines (Czaja & Zimmerman, 2009; Hayden et al., 2016). Especially, the reduced requirement of midazolam during the first 5 days after surgery in the postimplementation cohort could be attributed to the earlier introduction of clonidine. However, the mean peak dose of clonidine that was applied in the postimplementation group was moderate (0.67 µg·kg−1·hr−1) as compared to dose ranges from 0.5 to 2.0 µg/kg−1·hr−1 which have been reported in the literature (Kleiber et al., 2016; Kleiber, van Rosmalen, Tibboel, & de Wildt, 2018). Due to this fact, we do not believe that the changes noted in our postimplementation group can be explained exclusively to our altered medication of clonidine.
Although this was not part of the study purpose, we did not observe significant negative effects on hemodynamics with the increased use of clonidine, which is supported by previous findings that the use of clonidine is safe in critically ill children (Kleiber et al., 2018). Alpha-2-agonists like clonidine and dexmedetomidine appear to be the least neurotoxic sedative agents and even neuroprotective effects have been discussed (Andropoulos & Greene, 2017; de Graaff, Houmes, & Tibboel, 2018; Zhang & Kimelberg, 2005). Therefore, the results of our study are encouraging with respect to the potential of alpha 2-agonists to achieve a reduction of the more harmful sedative agents of the benzodiazepine group.
So far there have been inconsistent data regarding the impact of nurse-driven analgesia and sedation protocols on the dosage of sedative agents, length of PICU stay and duration of mechanical ventilation. The results of our study provide further evidence that analgesia and sedation managed by the nursing staff based on a sedation protocol is safe in the setting of a pediatric ICU. We strongly believe that assessment of sedations levels at regular intervals is advantageous to provide a tailored therapy resulting in better satisfaction of the individual requirements of the patients (Staveski et al., 2017). The successful introduction of a sedation protocol requires stringent evaluation of sedation levels of the patients as well as regular updates of protocols on the basis of current knowledge (Yaghmai et al., 2016).
5 CONCLUSION
The evaluation of an analgesia and sedation protocol in a homogenous patient population shows that the implementation of a nurse-driven sedation protocol is can help to reduce doses of opioids and benzodiazepines, as well as the length of PICU, stay. Pediatric intensive care nurses and physicians should be encouraged to establish and use sedation protocols.
6 HOW MIGHT THIS INFORMATION AFFECT NURSING PRACTICE?
Since nurses usually spend more time at the patient's bedside than physicians, it is mostly the nurses who carry out the evaluation of sedation depth and pain level. The study should encourage physicians and nurses that the introduction of sedation protocols is easy to implement and that the management of analgesia and sedation by nurses seems useful.
ACKNOWLEDGEMENTS
This study was supported by the Stiftung KinderHerz. Dr. Hanser received funding from the Stiftung zur Förderung der Erforschung von Zivilisationserkrankungen (Baden-Baden, Germany)
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.