Cryptic diversity and significant cophylogenetic signal detected by DNA barcoding the rust fungi (Pucciniaceae) of Cyperaceae–Juncaceae
Abstract
Plants play important roles as habitat and food for a tremendous diversity of specialist animals and fungi. The disappearance of any plant species can lead to extinction cascades of its associated biota. In consequence, documenting the diversity and specificity of plant-associated organisms is of high practical relevance in biodiversity conservation. Here, we present the first large-scale molecular investigation into the diversity, host specificity, and cophylogenetic congruence of an especially rich plant–fungal association, the rust fungi (Pucciniaceae) of Cyperaceae and Juncaceae. Using the largest rust fungi DNA barcoding dataset published to date (252 sequences, 82 taxa), we reject the presence of a global ITS2-28S barcode gap, but find a local gap in Cyperaceae–Juncaceae rusts, and suggest the existence of many cryptic species in North America, with some broadly circumscribed species possibly corresponding to >10 cryptic species. We test previous hypotheses of correlations between the phylogenies of rust fungi and their Cyperaceae–Juncaceae hosts using a combination of global-fit and event-based cophylogenetic methods. A significant cophylogenetic signal is detected between rusts and their hosts, but the small number of cospeciations argues for preferential host jumps as the driving process behind these correlations. In addition, temporal congruence between the origin of major Carex clades and their rusts suggests that host diversification may have promoted parasite diversification. Finally, we discuss the relevance of rust infection patterns to the systematics of Cyperaceae, highlight some taxonomic problems uncovered by the analyses, and call attention to the promise of DNA barcoding for bridging knowledge gaps in poorly studied plant-associated microorganisms.
1 Introduction
An important motivation for targeting vascular plants in conservation efforts is the crucial role they play as food and habitat for a tremendous diversity of specialist animals and fungi (Caro, 2010). Specialized associations are common in phytophagous arthropods and plant-associated fungi, and it is estimated that there could be three to six arthropods and two to five specialist fungi for every species of vascular plant (Ødegaard, 2000; Zhou & Hyde, 2001; Schmit & Mueller, 2007; Blackwell, 2011; Blackwell & Vega, 2018). The extinction of any plant species can thus trigger extinction cascades of its associated biota and of species that depend, in turn, on this biota (Koh, 2004; Brodie et al., 2014). Documenting diversity of plant-associated organisms and their degree of host specificity is thus of high practical relevance for conservation, especially considering that most plant-associated arthropods and microfungi remain undescribed (Hawksworth & Rossman, 1997; Larsen et al., 2017). In this study, we present the first large-scale molecular investigation into the diversity, host specificity, and cophylogenetic congruence of a particularly rich plant–fungal association, the rust fungi of Cyperaceae (sedges) and Juncaceae (rushes).
Cyperaceae (ca. 5500 species) and Juncaceae (ca. 440 species) are sister families of mostly wind-pollinated, grass-like plants that are particularly abundant in wetlands, temperate forest understories, and arctic–alpine habitats (Goetghebeur, 1998; Kirschner, 2002; Govaerts et al., 2007). As a consequence of their diversity and ecological prominence, Cyperaceae and Juncaceae host highly diverse fungal assemblages, being amongst the most heavily used families by two extremely diverse lineages of plant parasites, the smut (Ustilaginales) and rust (Pucciniales) fungi (Arthur, 1934; Savile, 1979; Cummins & Hiratsuka, 2003; Henderson, 2004; Vánky, 2012). The high diversity and specificity of fungal parasites of Cyperaceae and Juncaceae fostered early interest into their use as an aid for plant classification, with the underlying expectation that parasite phylogeny would mirror the phylogeny of their hosts, a principle known as “Fahrenholz′s rule” (Fahrenholz, 1913; Eichler, 1948; Klassen, 1992). Rust and smut fungi provided some of the earliest evidence supporting the close relationship between Cyperaceae and Juncaceae (Savile, 1990; Piepenbring et al., 1999; Riess et al., 2019), and they formed the basis for one of the first phylogenetic hypotheses for Carex L. (Savile & Calder, 1953), a genus comprising more than a third of all Cyperaceae species and ranking amongst the largest in the world (ca. 2000 species; Frodin, 2004; Global Carex Group, 2015). Molecular studies have since confirmed that phylogenies of Carex and their smut fungi are correlated (Hendrichs et al., 2005; Escudero, 2015). Likewise, the close relationship between Carex and Trichophorum Pers., first proposed by Kukkonen & Timonen (1979) based on the presence of Anthracoidea Bref. smuts on both genera, was recently confirmed by molecular phylogenetic studies (Léveillé-Bourret et al., 2014, 2018b).
In his seminal paper on the use of fungi as aids in plant classification, Savile (1979) proposed that young lineages of rust fungi infect young plant genera, whereas older rust lineages remain confined to their initial hosts, thereby providing a basis for the phylogenetic classification of plants. This work influenced many classical studies on higher-level plant taxonomy (Takhtajan, 1980; Dahlgren, 1983; Thorne, 2000), and it was cited as evidence for several infrafamilial classifications, including the most recent complete treatments of Cyperaceae tribes and genera (Bruhl, 1995; Goetghebeur, 1998). However, no study has yet re-examined Savile′s hypothesis of a correlation between the phylogenies of Cyperaceae genera and their rust parasites.
Rust fungi (>8400 species; Pucciniales, Basidiomycota) take their name from their usually bright-orange replicative spores (Aime et al., 2014). They are responsible for several economically devastating crop diseases such as crown rust of oats, stem rust of wheat, and white pine blister rust (Saari & Prescott, 1985; Geils et al., 2010; Liu & Hambleton, 2012, 2013), but they are also ecologically important as mediators of plant competition in natural environments (Rice & Westoby, 1982; Price et al., 1988; Barnes et al., 2005) and as food for many fungal and arthropod hyperparasites (Lutz et al., 2004a, 2004b; Nischwitz et al., 2005; Nelsen, 2010; Henk et al., 2011; Trakunyingcharoen et al., 2014).
Rusts are infamously known for possessing some of the most complex life cycles found in nature, often involving as many as five morphologically and functionally distinct spore stages (O basidiospores, I spermatia, II aeciospores, III urediniospores, IV teliospores) on two distantly related plant species (Jackson, 1931; Ono, 2002). These two plant species are called “telial” and “aecial” hosts, which refer to the main specialized spore type produced on each host. The monocotyledonous species of Cyperaceae and Juncaceae are telial hosts used for clonal population growth through the spread of urediniospores (stage III), and for the production of teliospores (IV), thick-walled spores used for overwintering. In the spring, teliospores (IV) germinate into basidia, producing basidiospores (O) that infect a new dicotyledonous aecial host. Spermatia (I) and receptive hyphae are produced on the aecial host, and their fusion eventually results in the production of aeciospores (II) that complete the cycle by infecting another telial host. From such complex host-alternating (heteroecious) life cycles, a great variety of reduced life cycles involving fewer spore types (microcyclic) or the infection of a single host (autoecious) have evolved (Jackson, 1931; Petersen, 1974; Ono, 2002).
Cyperaceae and Juncaceae are common hosts for rusts in one of the two main clades of the giant genus Puccinia Pers. (ca. 3000 species), including species of the nested polyphyletic genera Aecidium Pers. p.p. and Uromyces (Link) Unger (Aime, 2006; Maier et al., 2003, 2007; van der Merwe et al., 2007). These rusts alternate between Cyperaceae–Juncaceae and at least 15 families of dicotyledons, such as Asteraceae, Grossulariceae, or Urticaceae, but each rust species normally infects a single or a few closely related plant species for their telial and aecial life stages (Arthur, 1934; Savile, 1972). Host fidelity should be especially important in the aecial hosts, because the infection of different hosts would make outcrossing more difficult. This hypothesis would explain why cophylogenetic signal is higher and host jumps less frequent in aecial hosts than in telial hosts at higher taxonomic levels (Aime et al., 2018). However, no study has yet examined the pattern at lower taxonomic levels. We here hypothesize that a similar pattern will be found in the rusts of Cyperaceae–Juncaceae, with a higher cophylogenetic in aecial dicotyledon hosts than in telial Cyperaceae–Juncaceae hosts.
Using one of the largest rust fungi DNA barcoding datasets produced to date and recent developments in cophylogenetic analysis of host–parasite associations, we aim to answer the following questions: (i) how many species of rust fungi are there on Cyperaceae–Juncaceae hosts? (ii) Is the phylogeny of rust fungi correlated with the phylogeny of their Cyperaceae–Juncaceae hosts? (iii) If it is, what are the processes responsible for such cophylogenetic congruence? (iv) Are host jumps less frequent in the aecial dicotyledonous hosts (sexual stage) than in the telial Cyperaceae–Juncaceae hosts (asexual stage)?
2 Material and Methods
2.1 Taxonomic sampling
A total of 254 rust-infected herbarium specimens from the Canadian National Mycological Herbarium (DAOM) at Agriculture and Agri-Food Canada (AAFC) in Ottawa were selected for DNA extraction and sequencing. Included were representatives of 53 rust taxa (species, subspecies, or varieties) in the genera Aecidium (1), Puccinia (43), and Uromyces (9) and two unidentified specimens. In addition, 94 sequences previously generated for DAOM specimens using the same methods were also included in the analyses, representing an additional 29 Puccinia and four Uromyces taxa. The DAOM specimens successfully sequenced and included in the analyses are listed in Data S1 with their GenBank species names and accessions, collection metadata, and AAFC DNA numbers.
Of the total 348 specimens included in the study (254 newly sampled + 94 previously sequenced), 50 were collected before 1930, 260 from 1930 to 1990, 37 since 1990, and one had no date recorded. The majority of rusts sampled were collected on host plants in Cyperaceae (165) and Juncaceae (21) or alternate hosts in Asteraceae (96) and Grossularicaeae (21), but other represented families are Araliaceae, Celastraceae, Elaeagnaceae, Iridaceae, Lamiaceae, Lythraceae, Onagraceae, Orobanchaceae, Primulaceae, and Urticaeae. Specimens were primarily from Canada (184), the United States (71), and countries in the European Union (51), with the rest from Africa (5), Asia (8), New Zealand or Australia (12), South America (11), Bermuda (1), Mexico (4), and the Dominican Republic (1). Forty-four rust sequences representing 17 additional species were acquired from GenBank when they had a Cyperaceae or Juncaceae as host, were related to Cyperaceae–Juncaceae rusts as evidenced from BLAST searches and preliminary phylogenetic analyses, or to serve as outgroups (Austropuccinia Beenken and Dasyspora Berk. & M.A. Curtis, Puccinia spp. on Poaceae; Data S1).
2.2 DNA extraction and sequencing
The amount of infected leaf tissue sampled per specimen depended on the sizes of lesions and degree of infection. The infected leaf tissue was excised with scalpels or with 1-, 1.5-, 2-, or 3-mm biopsy punches. Most often, two 2-mm biopsy punches were used to excise infected regions of the plant tissue. Samples were similarly excised from uninfected areas, from the same leaf as the rust whenever possible, for host-only extractions. Methods for genomic DNA extraction were as described in Hambleton et al. (2019) using a Macherey-Nagel NucleoMag® 96 Trace kit (Macherey Nagel GmbH & Co. KG, Düren, Germany) and a KingFisher Flex magnetic particle processor (Thermo Fisher Scientific Oy, Vantaa, Finland). The protocol was modified as follows: before extraction, samples were homogenized using liquid nitrogen and sterile disposable micro-centrifuge tube pestles (PES-15-B-SI Axygen, Corning, NY, USA), before suspending the DNA in 70 µL of elution buffer.
Methods for polymerase chain reaction (PCR) amplification and sequencing of the nuclear rDNA ITS2 and partial 28S region (ITS2-28S) for the rusts, and a portion of the ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit (rbcL) gene for the hosts, were as described in Demers et al. (2017), with some modifications. For the rusts, PCR fragments were typically amplified and sequenced using primers Rust2inv (Aime, 2006) and ITS4Ru1 (Rioux et al., 2015), which target ITS2 plus ca. 300 bp of the 28S gene, or Rust2inv and ITS4 (White et al., 1990), which only target the ITS2 region, when the amplification of longer fragments failed. For select samples, the reverse primers LR5 or LR6 were used to target longer 28S fragments (Vilgalys & Hester, 1990). A few putatively misidentified host species were also barcoded for a fragment of the external transcribed spacer region of rDNA (ETS 1f; Starr et al., 2003) using primers ETS-1F and 18S-R or for the whole internal transcribed spacer region (ITS; Cheng et al., 2016) using primers ITS-p5 and ITS-p4. The same protocol for amplification was used for these regions as above, except for the following modifications: 40 cycles of 94 °C for 1 min, 48 °C for 30 s, and 72 °C for 2 min (ETS 1f) or 40 cycles of 94 °C for 30 s, 55 °C for 40 s, and 72 °C for 1 min (ITS). Voucher information and GenBank accession numbers of rust fungi and associated host sequences can be found in Data S1.
Many of the specimens sampled for DNA in this study were processed as part of a large-scale DNA barcoding project focused on generating reference sequence data for a broad diversity of obligate plant pathogenic fungi housed in the DAOM collection. There are challenges inherent in obtaining high-quality genomic data for herbarium specimens collected and initially dried under unknown conditions. DNA integrity is not necessarily related to age of the specimen (Liu & Hambleton, 2013), meaning that success or failure at DNA barcode amplification is mostly unpredictable. This routine initial PCR fragment for the rusts (comprising ITS2 and ~300 bp of the adjacent 28S amplified with primers Rust2inv/ITS4Ru1) was adopted because it can be sequenced bidirectionally with only two sequence reactions (one forward, one reverse). Its use facilitates a rapid processing of specimens for initial analyses and provides enough 28S signal to place the sequence near potential relatives when the ITS2 sequence has no close matches. In analyses, this region effectively groups specimens in operational taxonomic units (OTUs) that can then guide additional sequencing efforts (e.g., longer 28S) and the evaluation of other taxonomic characters.
We have observed that ITS2 is more amenable to direct sequencing than ITS1, because it possesses fewer indels and polybase regions that require cloning approaches to resolve. In targeting the ITS2 region, we recognize that important infraspecific variation may sometimes be difficult to interpret as compared with data derived from the more conserved 28S region (McTaggart & Aime, 2018). Longer 28S sequences were obtained for a select number of specimens, but often only ITS2 could be amplified and sequenced for many samples. No effort was made to sequence alternative genes due to the lack of universal primers that work well across the diversity of rust fungi, especially for DNA from herbarium specimens and single-copy genes (Hambleton et al., 2019), and the labor-intensive effort required to design and test specific primers for each new group being studied.
2.3 Rust and host identification
Rust identifications were based on the latest taxonomic treatments and floras available (Savile, 1965, 1970, 1972, 1979; Parmelee, 1967, 1969; Parmelee & Savile, 1981; Klenke & Scholler, 2015). Preliminary phylogenetic analyses showed that many of the species we sampled were split into two to many distinct lineages, sometimes distantly related. In consequence, we circumscribed OTUs for downstream analyses using a combination of genetic, host range, and geographic criteria. OTUs were circumscribed as monophyletic clades of ITS2-28S sequences that are consistently different from all other OTUs by at least one substitution or indel, and occur on different telial or aecial host species, or on a different continent from their closest relative(s). Using such a combination of phylogenetic, sequence similarity, ecological and geographic criteria can increase the chance that OTUs correspond to species, when compared with methods based on sequence similarity alone (Lücking et al., 2020). All analyses used OTUs in place of species. Samples were given a species name (New Determination/OTU in Data S1), appended with a number for species split into two or more OTUs (e.g., “Puccinia angustata 1,” “Puccinia angustata 2”). Host identifications were based on information present on herbarium labels, verified using the morphology of leaf, stem, and sometimes inflorescence fragments found in rust herbarium packets, as well as by BLAST or phylogenetic (parsimony) analysis of host rbcL, ETS 1f, or ITS barcodes.
2.4 Evaluating the barcode gap and diversity of rust fungi
An ideal molecular marker for species identification and discovery would show higher interspecific than intraspecific sequence divergence, that is, a discrete DNA barcode gap (Stielow et al., 2015). We assessed the utility of the ITS2-28S region for species identification of Cyperaceae–Juncaceae rust fungi by testing for the presence of global and local barcode gaps. Pairwise distances between every sample were measured using the F84 substitution model in the ape v5.3 package and an alignment-free k-mer approach (with k = 7) using the kmer v1.1.2 package in R v3.6.3 (Edgar, 2004; Wilkinson, 2018; Paradis & Schliep, 2019; R Core Team, 2020). Distances were calculated on the central portion of the alignment, which was covered by 95% of the samples, essentially consisting of the whole ITS2 and 25 bp of the downstream 28S. There was a strong linear relationship (R² = 0.86) between F84 and k-mer distances, so we report only results obtained with k-mers, which are not affected by alignment errors.
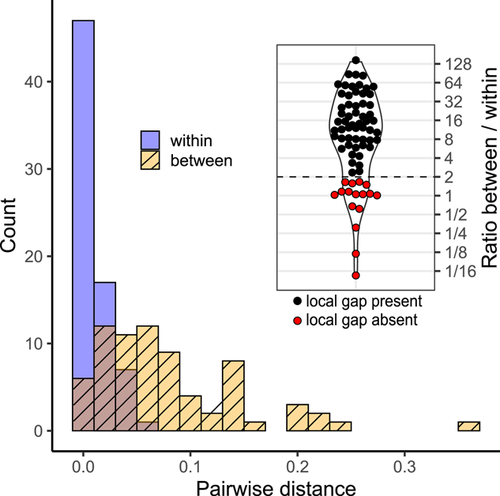
To detect a global barcode gap, the distribution of pairwise distances within OTUs was plotted alongside the distribution of distances between OTUs, with the expectation that a global gap would show as a break or minimal overlap between the two distributions. Even in the absence of a global gap, each OTU can still form a cluster that is distinct from all other clusters, or in other words a “local barcode gap.” To detect a local barcode gap, we focused on OTUs represented by at least two samples and compared the minimum between-OTU distance with the maximum within-OTU distance for each of these. A local barcode gap is supported for an OTU if its minimum between-OTU distance is at least twice as large as the maximum within-OTU distance. In other words, the length of the branches separating individuals of two different OTUs should be at least twice as long as the length of the branches separating individuals of the same OTU for a local barcode gap to be recognized. This is a conservative criterion, compared with previous studies (e.g., Robinson et al., 2009; Steinke et al., 2009), which considered a local barcode gap to exist when the between-OTU distance was greater than the within-OTU distance. More sampling within OTUs is expected to increase the maximum distance between individuals of the same OTU. To determine whether uneven sampling of OTUs could drive differences in the width of the local barcode gap, we ran an ordinary least-squares analysis with number of samples per OTU as predictor and maximum within-OTU distance as response.
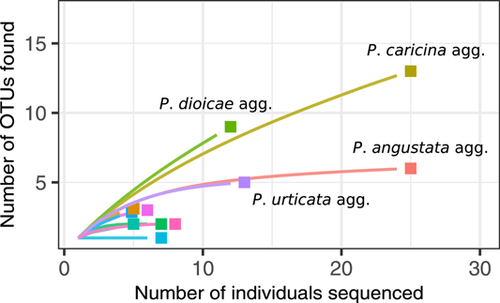
To estimate the magnitude of undescribed diversity existing in Cyperaceae–Juncaceae rust fungi, richness accumulation curves were calculated for 11 morphologically defined North American species (or species aggregates) where more than five samples were available. We focused only on samples from the United States and Canada, because other countries were poorly represented in the dataset. A rarefaction curve was fit by treating OTUs as “species” and samples as “individuals” in the R package vegan v2.5-6 (Oksanen et al., 2019), and the presence or absence of a plateau was checked. The Chao1 and ACE estimators of OTU richness (O′Hara, 2005; Chiu et al., 2014) were also calculated as approximations of the total number of OTUs (putative undescribed cryptic species) existing in each of the studied rust species aggregates in North America north of Mexico.
2.5 Host phylogenetic data
We assembled phylogenetic datasets of monocot and dicot hosts using sequence data available on GenBank (Data S2). For monocots, four plastid regions (rbcL, matK, ndhF, trnL-F) and two nuclear ribosomal regions (ITS, ETS 1f) were selected due to their good coverage across Cyperaceae and Juncaceae genera. Monocot hosts were selected on the basis of the hosts on which rust OTUs were found and supplemented with host information from the literature when there was no ambiguity about the rust species identity. In a few cases, sequence data were unavailable or coverage was poor for a monocot host species; in such cases, we selected a representative species that is putatively closely related on the basis of morphology. For these reasons, Rhynchospora capitellata was used as an examplar host for rusts on Rhynchospora gigantea, R. rariflora, and R. glomerata; Cyperus papyrus was used as a substitute for Cyperus latifolius (both C4 Cyperus); Carex pellita for C. lasiocarpa (both sect. Paludosae); and Carex lurida for C. frankii (both sect. Vesicariae). For dicot hosts, sequences of two plastid barcodes (rbcL and matK) were compiled for one examplar species of each host genus from GenBank. Voucher information and GenBank accession numbers of host sequences used for these analyses can be found in Data S2.
2.6 Phylogenetic analyses
Rust relationships were estimated by Bayesian analysis using MrBayes v3.2.7a (Ronquist et al., 2012) on the Cipres Science Gateway (Miller et al., 2010). Two GTR + G + I partitions were used: (i) 5.8S + 28S rDNA, (ii) ITS2. We used a two-exponential branch length prior with a mean of 0.01 for internal and 0.1 for external branches, following recommendations for minimizing overestimation of posterior probabilities (Yang & Rannala, 2005). To speed up convergence, searches were started on a tree derived from a previous short approximate maximum likelihood analysis in FastTree v2.1.11 (Price et al., 2010), with 50 random perturbations. Two independent MC3 chains were run for 50 million generations sampling trees every 5000 generations, with 11 heated chains using a temperature parameter of 0.02.
Branch support for rust fungi relationships was further assessed by maximum parsimony and distance-based bootstrapping (Felsenstein, 1985). Maximum parsimony bootstrap (PBS) searches were done in PAUP* 4.0a166 for Linux (Swofford, 2003) using 1000 replicates, with each replicate consisting of three random addition sequences (RAS), retaining three trees per RAS, and using the strict consensus bootstrap (GRPFREQ = NO). In addition, 1000 distance-based bootstrap (DBS) searches were done in PAUP* using a GTR + Gamma model with empirical base frequencies and neighbor-joining (NJ) for tree estimation.
As global-fit cophylogenetic methods work best with ultrametric trees, rust relationships and divergence times were also jointly estimated in MrBayes v3.2.7a. Settings were the same as above, except for the use of a birth–death (clock) branch length prior with an exponential speciation prior of 10, flat beta (1,1) extinction prior, and sampling probability of 5% assuming random sampling. For dating, we used an uncorrelated gamma (IGR) clock (Lepage et al., 2007) with a lognormal clock rate of 0.0025 substitutions/site/My with a standard deviation (SD) of 0.5 and an exponential hyperparameter of 10. Following previously estimated divergence times of rust fungi (Aime et al., 2018), we placed a truncated normal secondary prior on the divergence between the Austropuccinia–Dasyspora outgroup and the Puccinia–Uromyces–Aecidium ingroup with an average of 71 My, an SD of 8, and a minimum of 40 My. Two independent MC3 chains were run for 120 million generations sampling trees every 10 000 generations, with 11 heated chains using a temperature parameter of 0.02.
Monocot and dicot host relationships and divergence times were also jointly estimated by Bayesian analysis using MrBayes v3.6.7a, to generate ultrametric trees for cophylogenetic analysis. Fossil and secondary calibrations were placed on select nodes for dating (Smith et al., 2010; Barreda et al., 2015; Magallón et al., 2015; Jiménez-Mejías et al., 2016; Mandel et al., 2019). Detailed methods can be found in the supplementary material (Doc. S1).
For all Bayesian phylogenetic analyses, parameter convergence was assessed in Tracer v1.7.1 (Rambaut et al., 2014), with 10% of runs discarded as burnin, and a maximum clade credibility chronogram (MCCT) estimated using functions of the paleotree R package (Bapst, 2012). Clade support was subjectively characterized as poor (<0.90 pp), moderate (0.90–0.94 pp), and strong (0.95–1 pp).
2.7 Quantifying cophylogenetic signal
A cophylogenetic signal between rust fungi and their monocot and dicot hosts was quantified on four datasets. Two “complete” datasets with all rust–monocot and rust–dicot associations, including rust OTUs that are autoecious (with only one host type) or where one of the alternate hosts was unknown, thus maximizing the amount of available data. Two “reduced” datasets were also generated (rust–monocot and rust–dicot) by pruning from phylogenies rust OTUs that are autoecious or with unknown alternate hosts, thus keeping only OTUs that have known hosts for both monocots and dicots. The “reduced” datasets are more appropriate when comparing cophylogenetic signal between host types.
For each dataset, we ran two global-fit methods (PACo and Random TaPas) and one event-based (Jane4) method. Global-fit approaches work on distance matrices (genetic or cophenetic), and are therefore robust to phylogenetic uncertainty. They are most appropriate for the type of datasets presented here, where a single barcode locus is used to estimate the parasite phylogeny, affording limited signal to resolve backbone relationships. Event-based methods are less accurate when phylogenetic uncertainty is high, but they have the advantage of directly estimating the number of cospeciation and host shift events.
PACo is based on Procrustes superimposition of principal coordinates derived from cophenetic matrices of host and parasite trees (Balbuena et al., 2013). Perfect cospeciation results in host and parasite trees that are exactly identical in topology and branch lengths, whereas other scenarios increase the distance between host and parasite cophenetic matrices. The significance of cophylogenetic signal is tested by random permutations of the host–parasite associations and comparing the observed Procrustes distance to the distribution of distances obtained by permutations. We ran PACo analyses on all datasets using 10 000 permutations to test for significance. Although PACo is fast and relatively powerful at quantifying the cophylogenetic signal, the statistics obtained are not comparable between host and parasite systems.
Random TaPas is a recently developed method that provides an absolute measure of cophylogenetic signal (G*) that can be compared across different host–parasite systems. The G* statistic ranges from G* = 0 for perfect cospeciation to G* = 2/3 for random associations, and up to G* = 1 for highly uneven signal where most clades are incongruent or random, but a few clades are highly congruent (Balbuena et al., 2020). A significant cophylogenetic signal is detected when the confidence interval of G* does not overlap with 2/3. Random TaPas was run by calculating PACo fits on N = 10 000 random partial tanglegrams of size n = 10% of the total number of associations, which are optimal settings for quantification of cophylogenetic signal (Balbuena et al., 2020). We obtained a point estimate of G* on the MCCT and calculated 95% credibility intervals by re-running Ramdom TaPas on 500 randomly selected host and parasite trees from the MrBayes posterior samples.
Finally, we used Jane4, an event-based approach that estimates the number of cospeciations and host switches (Conow et al., 2010). Jane4 was run with a population size of 200 for 20 generations using the following default costs (in steps): cospeciation = 0, duplication = 1, host switch = 2, loss = 1, and failure to diverge = 1. The significance of the cophylogenetic signal was estimated by re-running the algorithm on 100 randomly permuted host–parasite associations with the same settings and comparing the resulting cost distribution with the observed cost.
3 Results
3.1 Barcoding results
ITS2 barcodes were obtained for 157 of the 254 specimens sampled for this study (62% sequencing success), including one specimen (DAOM 181219) yielding ITS2 barcodes for two different rust species from separate DNA extractions of aecial and uredinial pustules. These 158 sequences were combined with previously sequenced and unpublished ITS2 barcodes for 94 additional DAOM specimens. Sequencing of the rust failed for 84 newly sampled specimens with collection dates between 1883 and 2013, and sequences were too short or difficult to interpret for another 13. In addition to the ITS2 spacer, the 28S was also successfully sequenced for a short length (97–346 bp) in 211 sequences (83%) and for a longer length (421–1174 bp) in 37 sequences (15%). Good amplicons were obtained for specimens that ranged in age from <1 to 136 years (oldest specimen collected in 1880), with the majority of sequences from specimens collected before 1962. Basic sequence statistics can be found in Table 1. Rust sequences were obtained to represent all the diversity of Cyperaceae hosts, including 12 genera representing 11 tribes; however, sampling was biased toward North America and Europe. Additional sequences were obtained on related Juncaceae and Iridaceae rusts, and on alternate dicot (aecial) hosts, including samples from Adoxaceae, Celastraceae, Elaeagnaceae, Grossulariaceae, Lamiaceae, Orobanchaceae, Primulaceae, Urticaceae, and 37 genera and 9 tribes of Asteraceae.
|
Data set |
Aligned length |
Terminals missing |
Gaps, missing, and ambiguous characters |
Variable characters |
Parsimony-informative characters |
Consistency index (CI) |
Retention index (RI) |
|---|---|---|---|---|---|---|---|
| 5.8S | 159 | 10 (3.7%) | 48% | 22 (14%) | 15 (9.4%) | 0.585 | 0.877 |
| ITS2 | 360 | 0 | 57% | 257 (71%) | 199 (55%) | 0.234 | 0.761 |
| 28S | 1244 | 9 (3.0%) | 71% | 321 (26%) | 176 (14%) | 0.529 | 0.671 |
| Full alignment | 1763 | 0 | 72% | 600 (34%) | 390 (22%) | 0.304 | 0.737 |
We obtained rbcL host plant barcodes for 130 of the 158 (82%) specimens successfully sequenced for the rust in this study and included previously generated rbcL barcodes for 70 of the other 94 specimens. We also selectively generated ETS 1f (21) or ITS (1) barcodes for 22 of those specimens and ETS 1f (2) or ITS (3) for five specimens for which rbcL sequencing failed. The rbcL host plant barcodes revealed two host genus misidentifications; the 23 ETS 1f barcodes revealed three host genus misidentifications and three host species misidentifications, and the four ITS barcodes revealed a single host genus misidentification. Overall, host genus misidentification rate was around 2.9% (6 misidentifications out of 205 specimens with host barcodes). Host misidentification is difficult to assess at the species level, due to the poor resolution of rbcL barcodes and limited number of more informative ETS 1f and ITS barcodes.
3.2 Barcode gap analysis
Barcode gap analyses were done on the final rust alignment including a total of 296 sequences (252 sequences published here + 44 sequences from Genbank). The distribution of within-OTU pairwise k-mer distances overlapped and was completely included within the distribution of distances between OTUs, rejecting the presence of a global barcode gap (Fig. 1). However, 56 out of the 72 OTUs (78%) represented by more than one sample showed a local barcode gap, because the distance to the nearest OTU was more than twice as large as the maximum within-OTU distance (Figs. 1, S1), supporting the status of most OTUs as distinct entities (differentiated populations or species). The maximum within-OTU distance, which is used to identify the width of the local barcode gap, was only weakly and non-significantly associated with the number of individuals sampled per OTU (least squares p = 0.067, R 2 = 0.047). A t-test did not detect a significant difference (p = 0.37) in sampling depth between OTUs that showed a local barcode gap (averaging 3.3 samples per OTU) and those that did not (averaging 2.9 samples per OTU). Depth of sampling within OTUs is, therefore, unlikely to have influenced the detection of the local barcode gap.
3.3 Rarefaction and richness analysis
Eleven rust species or aggregates were represented by enough North American samples to be used in rarefaction and richness analyses. Of those, Puccinia mcclatchieana Dietel & Holw. was the only taxon where all samples formed a clade with little molecular variation. All others showed sample paraphyly, polyphyly, or sufficient molecular variation for multiple OTUs (possible cryptic species) to be recognized. The chao1 and ACE statistics estimated between two and four OTUs for most rust aggregates, including the Puccinia asteris Duby agg., P. eriophori Thüm. agg., P. lagenophorae Cooke agg., Uromyces junci Tul. agg., and U. silphii (Syd. & P. Syd.) Arthur agg.
A few aggregates stood out by their high number of observed and estimated OTUs. The rarefaction curve for the P. angustata Peck agg. was nearly flat at 25 samples; thus, chao1 and ACE estimated the same number of OTUs as observed: 6 OTUs in total (Fig. 2). The situation was similar for the P. urticata DC. agg. with 14 North American samples included and 5 OTUs observed and estimated. The rarefaction curve was still growing fast for the P. obtecta Peck agg. with only 5 sampled specimens and ca. 3 OTUs observed, so that chao1 and ACE estimated, respectively, 4 and 6.7 OTUs. The rarefaction curve for the P. caricina DC. agg. was also still growing fast at 28 included North American samples, so that 20 and 29.8 total OTUs were estimated, much more than the 13 observed OTUs. The rarefaction curve for the highly paraphyletic Puccinia dioicae Magnus agg. also showed no sign of leveling-off at 12 samples, and chao1 and ACE were consequently high, respectively, estimating a total of 19.5 and 26.8 OTUs for this aggregate in North America.
3.4 Phylogenetic results
Austropuccinia psidii and Dasyspora amazonica were sister to a strongly supported Puccinia + Uromyces + Aecidium clade (1.00 pp; 99% PBS; 93% DBS). Within the latter, rusts of Poaceae [Puccinia coronata Corda, P. graminis Pers., P. heterospora Berk. & M.A. Curtis, P. kuehnii (W. Krüger) E.J. Butler], P. malvacearum Bertero ex Mont., and P. myrsiphylli (Thüm.) G. Winter formed a grade of lineages (outgroups, not shown in Figs. 3, 4) leading to a nested clade comprising all Cyperaceae–Juncaceae rusts and related microcyclic species (Figs. 3, 4). The rusts on Cyperaceae–Juncaceae (ingroup) were monophyletic in all analyses, though support was low (<0.50 pp; <50% PBS; 74% DBS; Figs. 3, 4). However, a single Carex rust, Puccinia microsora Körn., was placed with low support as part of the early-diverged grade of Poaceae rusts that was included in the outgroup. Within the clade of Cyperaceae–Juncaceae rusts, backbone relationships also received poor support, but 143 OTUs were determined, including 43 identified at the species level and 100 corresponding best to 50 species in 27 species aggregates (Data S1). Several moderately to strongly supported clades emerged from a highly paraphyletic group of microcyclic Asteraceae rusts and samples identified as a part of the Puccinia dioicae agg. This aggregate accommodates nearly all rusts alternating between Carex and Asteraceae. The 12 sampled specimens of the P. dioicae agg. formed 9 distinct lineages placed throughout the phylogeny. Rusts on Juncaceae (Juncus L. and Luzula DC.) formed seven lineages scattered throughout the phylogeny and often associated with specimens of the P. dioicae agg. The crown age of Cyperaceae–Juncaceae rusts was estimated to be Late Eocene (ca. 35 Mya), and most major rust clades appeared between 34 and 12 Mya, coincident with the crown age of Carex (34–37 Mya) and the divergence of its major clades (23–18 Mya; Martín-Bravo et al., 2019; Figs. 3, 4).
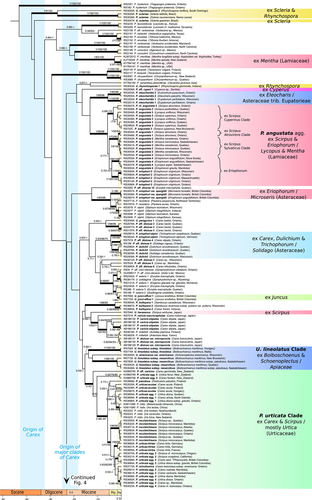
Rusts of Scirpeae formed several distantly related clades throughout the phylogeny. A strongly supported P. angustata agg. (1.00 pp, 99% DBS, 98% PBS; Fig. 3) comprised rusts with telia on Scirpus Tourn. ex L. or Eriophorum L. (Scirpeae) and aecia on Mentha L. or Lycopus L. (Lamiaceae tribe Mentheae). Within the P. angustata agg., five subclades received high support (>0.95 pp), with one of these subclades comprising rusts of Eriophorum originally identified as “P. eriophori.” Other samples of P. eriophori formed two distantly related clades. One comprising accessions of Puccinia eriophori var. apargidii Savile, including the holotype, formed a strongly supported clade (1.00 pp, <50% DBS, 96% PBS; Fig. 3) that was distantly related to other accessions of “P. eriophori 1” (1.00 pp, 83% DBS, 97% PBS; Fig. 4). Puccinia mcclatchieana, a species on Scirpus without known aecial host, formed a strongly supported subclade (1.00 pp, 95% DBS, 95% PBS; Fig. 3) within the P. urticata Clade, which mostly comprises rusts of Carex. Puccinia mcclatchieana showed no important sequence variation; however, two samples originally identified as “P. mcclatchieana” on Scirpus from Oregon and California (DAOM 70596 and DAOM 134101) were found to be distantly related to other P. mcclatchieana accessions. They clustered instead with samples of P. urticata and were accordingly re-identified as “P. urticata agg. 2.”
A moderately supported P. eriophori-alpini Allesch.–P. dulichii P. Syd. & Syd. clade consisted of species that alternate between Solidago L. (Asteraceae tribe Astereae) and members of the Scirpo-Caricoid Clade: Trichophorum, Carex, and Dulichium Pers. (0.93 pp, 98% DBS, 70% PBS; Fig. 3). Samples from Solidago confirmed this aecial host for P. dulichii and one P. dioicae agg. species, but no samples were available to confirm the aecial host of P. eriophori-alpini, which is known from a single record on Solidago in Europe (Jørstad, 1942).
A moderately supported “Uromyces lineolatus Clade” (0.94 pp, <50% DBS and PBS; Fig. 3) comprised all rusts of Fuireneae s.lat. with unicellular teliospores and aecia on Apiaceae: Uromyces lineolatus (Desm.) Schröt. subsp. lineolatus, U. lineolatus subsp. nearcticus Savile, and U. americanus Speg. Within this clade, U. lineolatus subsp. lineolatus, an Old World rust on Bolboschoenus (Asch.) Palla, was separated by a long branch from a poorly supported New World clade consisting of U. lineolatus subsp. nearcticus, and U. americanus, a rust that uses Schoenoplectus (Rchb.) Palla as a host.
Sister to the U. lineolatus Clade was a strongly supported “Puccinia urticata Clade” (1.00 pp, <50% DBS, 51% PBS; Fig. 3), comprising all rusts of Carex with 3+ pores on the urediniospores and with aecia usually on Urtica L. (Urticaceae). Samples identified as “P. urticata” formed five distinct lineages separated by long branches. One sample of a segregate species recognized in Europe, P. urticae-acutae Kleb., was not positioned with the other samples of this species. Nested within the P. urticata clade were a few species with different host alternations: P. iridis Wallr. alternating between Iris L. (Iridaceae) and Valeriana L. (Valerianaceae); P. paludosa Plowr. between Carex and Pedicularis L. (Orobanchaceae); and P. minutissima Arthur between Carex and Decodon J.F.Gmel. (Lythraceae).
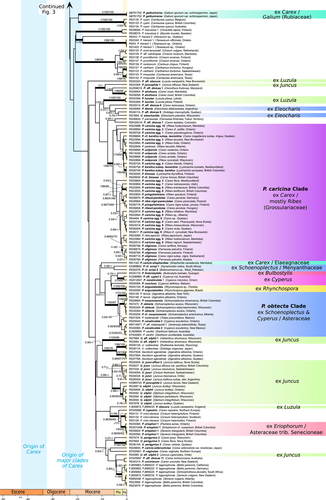
A strongly supported “Puccinia caricina Clade” included all samples of P. caricina and segregate species (1.00 pp, 66% DBS, 70% PBS; Fig. 4). These heteroecious rusts alternate between Carex and Ribes L., Lysimachia L., or Parnassia L. The autoecious derivative species P. parkerae Dietel & Holw. and P. ribis-japonici Henn. of Ribes were nested in this clade. Species with distinctive characters such as single-pored urediniospores (P. uniporula Orton) or aecia on genera other than Ribes (P. karelica Tranzschel, P. limosae Magnus, P. uliginosa Juel) were nested within a highly paraphyletic “P. caricina agg.” that formed subclades without obvious structure in host ranges, but usually restricted either to samples from North America or from Europe.
A strongly supported “Puccinia obtecta Clade” (1.00 pp, 83% DBS, 97% PBS; Fig. 4) consisted of all heteroecious rusts of Schoenoplectus and Cyperus L. with telia in locules or surrounded by fused paraphyses, and aecia on Asteraceae, as well as a few autoecious rusts on related genera of Asteraceae. Within this clade, a strongly supported subclade contained intermixed samples of P. obtecta and P. osoyoosensis Savile (1.00 pp, 66% DBS, 98% PBS; Fig. 4), two species infecting Schoenoplectus and alternating either on Bidens L. or Xanthium L. (Asteraceae tribe Heliantheae) that differ by several morphological characters. Sister to this subclade was a strongly supported subclade including P. canaliculata (Schwein.) Lagerh. 2 (0.95 pp, 83% DBS, 68% PBS; Fig. 4), a rust of Cyperus also thought to alternate on Xanthium, and autoecious derivatives on Xanthium and various other Heliantheae, including accessions identified as P. xanthii Schwein. and P. melampodii Dietel & Holw. that were not monophyletic.
3.5 Cophylogenetic signal
A significant cophylogenetic signal was detected by global-fit (PACo, Random TaPas) and event-based (Jane4) methods for complete and reduced datasets, and for dicot and monocot hosts (Table 2). Yet, the event-based approach, Jane4, estimated comparatively few cospeciations and a large number of host switches and duplications, suggesting that the significant cophylogenetic signal is not driven by strict cospeciation.
| Statistics | Complete datasets | Reduced datasets | ||
|---|---|---|---|---|
| Monocot hosts | Dicot hosts | Monocot hosts | Dicot hosts | |
| PACo | ||||
| p Value | <0.0001* | <0.0001* | <0.0001* | <0.0001* |
| Random TaPas | ||||
| G* coefficient | 0.706 [0.704–0.732]* | 0.747 [0.733–0.760]* | 0.735 [0.708–0.744]* | 0.741 [0.709–0.763]* |
| Jane4 | ||||
| p Value | <0.01* | <0.01* | <0.01* | <0.01* |
| cost | 284 | 211 | 209 | 105 |
| events | 240 | 176 | 191 | 90 |
| cospeciations | 14 (5.8%) | 14 (8.0%) | 10 (5.2%) | 6 (6.7%) |
| duplications | 18 (7.5%) | 42 (24%) | 19 (10%) | 30 (33%) |
| host switches | 58 (24%) | 52 (30%) | 28 (15%) | 21 (23%) |
| losses/sorting | 127 (53%) | 49 (28%) | 113 (59%) | 21 (23%) |
| failures to diverge | 23 (9.6%) | 16 (9.2%) | 21 (11%) | 12 (13%) |
- An asterisk (*) indicates a statistically significant value at α = 0.05.
The Random TaPas G* coefficient was >2/3 for all datasets, indicating that the cophylogenetic signal was split between highly congruent clades (blue colors in Fig. 5) and highly discordant clades (red colors in Fig. 5). In monocots, a high cophylogenetic signal was visible in the deeper nodes of the Carex phylogeny (Cariceae) and in the Scirpus–Eriophorum clade (Scirpeae; Fig. 5A). A highly discordant signal comes from genera of Fuireneae s.lat., Eleocharis R.Br. (Eleocharideae), and Cyperus (Cypereae), as well as Juncaceae. In dicots, the clade comprising Grossulariaceae, Lythraceae, Celastraceae, Elaeagnaceae, and Urticaceae (Rosids) showed a particularly discordant cophylogenetic signal, as they were hosts to several unrelated rust lineages (Fig. 5B). No other dicot lineage showed a particularly strong cophylogenetic signal.
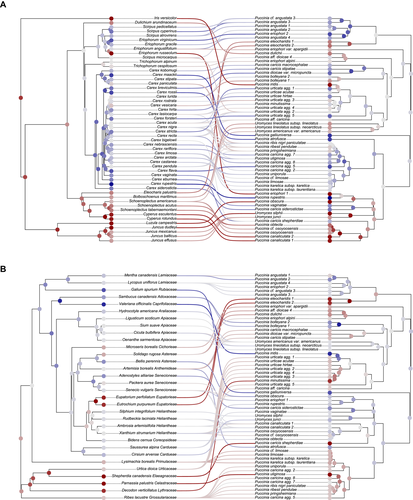
In the reduced datasets, Jane4 estimated slightly more cospeciations and fewer host switches in dicots as compared with monocots, suggesting higher cophylogenetic signal on dicot hosts. However, the confidence interval of the G* coefficients of monocots and dicots overlapped in the reduced datasets (Table 2), and there is thus no significant difference in cophylogenetic signal between monocot and dicot hosts.
4 Discussion
4.1 Cophylogenetic signal without cospeciation in Cyperaceae–Juncaceae rusts
Using the largest barcoding dataset of rust fungi to date (Feau et al., 2011; Beenken et al., 2017), we demonstrate the utility of ITS2-28S barcodes for species identification and discovery in North American Cyperaceae–Juncaceae rust fungi. Perhaps surprisingly, our analyses suggest large numbers of host jumps and a relatively minor role for cospeciation, in spite of the significant cophylogenetic signal detected by both global-fit and event-based methods. A strict cospeciation scenario can confidently be rejected, but what then explains the correlation we observed between host and parasite phylogenies?
An emerging consensus is that strict cospeciation is exceedingly rare in nature, and that other processes are responsible for the cophylogenetic signal observed in most host–parasite associations (Nylin et al., 2018). The most recent review of the empirical literature on the subject found only seven convincing examples of systems dominated by cospeciation out of 103 cophylogenetic studies (de Vienne et al., 2013). All conclusive cases were between invertebrates and internal mutualistic prokaryotes that are transmitted internally from parent to offspring, limiting the possibility of dispersal to new hosts (Bright & Bulgheresi, 2010). In external, horizontally transmitted parasites such as smut and rust fungi (Refrégier et al., 2008), host jumps were the dominant mode of evolution. This could be due to the fact that chance encounters with new hosts are more likely in external parasites. Indeed, rust spores are mostly wind-dispersed, and are therefore frequently deposited on non-host species (Savile, 1976), providing constant opportunities to colonize new species. Jumps may be further favored when related plant species live in close sympatry, as is often the case in Cyperaceae (Elliott et al., 2016). Jumps may also be common due to the fact that they can rescue parasites from extinction in the coevolutionary arms race with their hosts (Thines, 2019).
One way to explain the significant cophylogenetic signal we detected in Cyperaceae–Juncaceae rusts, even in the absence of cospeciation, would be to assume that host jumps occur most often between closely related hosts (Charleston & Robertson, 2002). Such a model of “preferential host jumps” is supported by inoculation experiments that showed fungal pathogens better able to infect novel host plants when they are closely related to their normal hosts (Gilbert & Webb, 2007; de Vienne et al., 2009). The same pattern was demonstrated in RNA viruses, with probability of host jumps following a sigmoidal relationship with phylogenetic distance between hosts, indicating that jumps to closely related hosts are easy, whereas moderately and distantly related hosts become quickly unreachable (Cuthill & Charleston, 2013). Preferential host jumps have now been documented in a large variety of plant and animal systems, and they appear to be the rule in most cases (de Vienne et al., 2013). Preferential host jumps were also suggested to be the main driver behind cophylogenetic congruence of Anthracoidea smuts and their Carex hosts (Hendrichs et al., 2005; Escudero, 2015), a classic example of host–parasite correlation in Cyperaceae (Savile, 1952; Savile & Calder, 1953; Kukkonen & Timonen, 1979). The rust fungi of Cyperaceae and Juncaceae appear to be yet another example of cophylogenetic signal driven by preferential host jumps.
4.2 Cophylogenetic signal does not differ significantly between aecial and telial hosts
A previous study found that correlations between rusts and host phylogenies are stronger on aecial than on telial hosts (Aime et al., 2018), a pattern we call here “differential host conservatism.” Differential host conservatism could be explained by the fact that sexual reproduction occurs on dicot aecial hosts, therefore making infection of novel aecial hosts more disadvantageous than infection of novel telial hosts. Indeed, a novel aecial host reduces the rust′s outcrossing opportunities, whereas a novel telial hosts does not affect any component of the rust reproductive cycle.
We find indications of differential host conservatism in the complete datasets, when cophylogenetic signal is compared in dicot (mostly aecial) and monocot (telial) hosts. However, analyses on reduced datasets where both aecial and telial hosts are known for all terminals find no significant difference in cophylogenetic signal. Thus, the differential host conservatism detected on the complete datasets is likely caused by the different size of the datasets, with the complete monocot dataset containing a larger number of terminals and hence higher chance of catching instances of incongruent signal. In consequence, the lack of a significant difference in cophylogenetic signal between telial and aecial hosts in Cyperaceae–Juncaceae rusts contrasts with the much stronger differential host conservatism reported by Aime et al. (2018) at higher taxonomic levels in rust fungi.
Our results would fit a pattern where differential host conservatism is stronger in more ancient rust lineages, such as between rust families (Aime et al., 2018), but weaker in recent lineages such as within rust genera (present results). Such a decay of differential host conservatism rejects the explanation that aecial hosts are conserved due to reduced mating opportunities on novel aecial hosts, because this phenomenon should act relatively fast in creating differences in host jump probabilities. Hence, if this was true, it should be equally detectable at all taxonomic levels. Consequently, the decay we observed points to a role for slowly acting evolutionary forces that would only create differences at longer evolutionary timescales. Such forces could include differential speed of the coevolutionary arms race in aecial and telial hosts or the effect of one of the many biological characteristics that differ between spore stages, such as ploidy (Ono, 2002) or virulence (Rice & Westoby, 1982). More studies are needed to determine the extent of differential host conservatism and to identify the causal factors behind the pattern.
4.3 Utility of rust fungi relationships in Cyperaceae–Juncaceae classification
The fact that cophylogenetic signal is driven by preferential host jumps in the Cyperaceae–Juncaceae rusts does not exclude the possibility that rust relationships might corroborate proposed host relationships. In some of his most influential studies, Savile (1972, 1979) hypothesized a close affinity between several groups of Cyperaceae genera that shared the same rust lineages. For instance, he proposed that Carex, Trichophorum, Scirpus, Eriophorum, and Dulichium were closely related on the basis of the occurrence of rusts classified within the “dioicae-hieracii lineage” infecting all these genera (Savile, 1970). Such a close relationship among these temperate sedge genera had never been proposed before, but molecular phylogenetic studies have since placed them all in a strongly supported “Scirpo-Caricoid Clade” (Léveillé-Bourret et al., 2014, 2015, 2018a; Léveillé-Bourret & Starr, 2019; Semmouri et al., 2019). Nevertheless, molecular analysis of the rusts invalidates Savile′s hypothesis, because members of his “dioicae-hieracii lineage,” such as the Puccinia dioicae agg., P. dulichii, P. eriophori, and U. perigynius Halst., are scattered throughout the rust phylogeny, as in previous analyses (van der Merwe et al., 2007, 2008). It, therefore, seems probable that characters used to delimit Savile′s “dioicae-hieracii lineage,” such as bizonate aeciospores, flattened urediniospores with two super-equatorial pores, and telia without paraphyses, are plesiomorphic or convergent in Cyperaceae–Juncaceae rusts (Puccinia–Uromyces clade I sensu Maier et al., 2007).
The close relationship of Bulbostylis Kunth and Eleocharis, confirmed by all phylogenetic analyses of Cyperaceae (e.g., Muasya et al., 2009; Hinchliff & Roalson, 2013; Semmouri et al., 2019), was also thought to be supported by rust fungi (Savile, 1979). However, the rusts of Bulbostylis and Eleocharis are here shown to form three separate and seemingly distant clades. Likewise, the fact that all rusts infecting Fuireneae s.lat. (Bolboschoenus, Schoenoplectus, Schoenoplectiella Lye, Fuirena Rottb.) and Eleocharis possess urediniospores with 3+ equatorial pores and loculate telia has been suggested to reflect correlated evolution of rusts and their hosts (Savile, 1979; Goetghebeur, 1998). However, urediniospores with 3+ pores and loculate telia are here shown to have evolved in four lineages from two-pored, non-loculate ancestors: (i) in the Uromyces lineolatus Clade; (ii) in Puccinia liberta F. Kern; (iii) in P. canaliculata 1; (iv) and in the P. obtecta Clade. Repeated evolution of loculate telia might be better understood as another example of parallel adaptation in rusts subject to similar ecological pressures (Savile, 1976, 1978), as most Fuireneae s.lat. and Eleocharideae produce emergent, leafless culms in open marshes. Their culms offer a substrate subject to intense sunlight and heat that may have favored rust species able to protect their resting spores by enclosing them within locules.
A stronger cophylogenetic signal is observed at lower taxonomic levels. Notably, subclades within the Puccinia angustata agg. are each restricted to different subclades of Scirpus and Eriophorum species, suggesting greater host specificity than previously recognized (Scirpeae; Gilmour et al., 2013; Léveillé-Bourret et al., 2014, 2015; Starr et al., 2019). Rusts on genera of Fuireneae s.lat. (Bolboschoenus, Schoenoplectus), a Cyperaceae tribe long suspected to be unnatural (Shiels et al., 2014; Glon et al., 2017; Semmouri et al., 2019), formed two distantly related clades that diverged in the Mid-Miocene (ca. 13 Mya), despite sharing highly distinctive loculate telia. These two rust clades differ in important morphological and ecological features (Savile, 1972; Klenke & Scholler, 2015), and their divergence supports the recognition of separate tribes for the genera Bolboschoenus and Schoenoplectus (Bolboschoeneae and Schoenoplecteae; see Starr et al., 2021 in this issue). A more detailed discussion of cophylogenetic patterns and their taxonomic implications can be found in the supplementary material (Doc. S2).
4.4 Challenges and prospects of ITS2-28S for the discovery and identification of rust species
The ITS2-28S provides good resolution for the identification of rust fungi and enough variation to serve as a first step in the discovery of new species, as demonstrated here and in previous studies (e.g., McTaggart & Aime, 2018). The absence of a global barcode gap is in line with previous studies on this locus (Stielow et al., 2015), but it is compensated by the presence of a local gap in most species or OTUs (Robinson et al., 2009; Steinke et al., 2009). Combined with host and distribution information, we expect ITS2-28S to provide good resolution for rust identification, even in the most species-rich lineages. The large number of indels in ITS2 and the short length of the 28S sequence usually obtained with our primers provided only a limited phylogenetic signal, but its relative ease of amplification and specificity enable the rapid and extensive study of hundreds of taxa, even when old specimens (>100 years) must be used. This is an important feature for barcoding a group of fungi that is very diverse, but also severely undercollected in recent years (Liu & Hambleton, 2010). In the future, increasingly sensitive genome sequencing techniques and an acceleration in the availability of a diversity of rust genomes will lead to the recognition of optimal regions for molecular phylogenetic analyses and facilitate the development of new robust PCR primers for the rusts.
Our barcoding approach highlights the need for more in-depth studies of the taxonomy of Cyperaceae–Juncaceae rusts, by demonstrating that the broad species limits still in use in North America are in many cases unnatural (high levels of sample paraphyly or polyphyly). We estimate that between 5 and 24 potential cryptic species (OTUs) could exist within each of the four most common and abundant North American species aggregates on Carex, Scirpus and Eriophorum, the Puccinia angustata s.lat., P. caricina agg., P. dioicae agg., and P. urticata agg. This leads us to believe that the total number of rust species on Cyperaceae–Juncaceae recognized in North America will more than double when detailed taxonomic studies combining molecular and ecological data are carried out. In fact, if every OTU delimited here corresponds to one rust species and the richness estimates are reliable, a minimum of 90 rust species of Cyperaceae and 10 of Juncaceae would be expected in North America north of Mexico. This would be roughly one rust species for every ten Cyperaceae–Juncaceae species (Brooks & Clemants, 2000; Ball et al., 2002), which is well below the ratio of one rust species for every two to three Cyperaceae species reported from Germany (Klenke & Scholler, 2015). Such numbers are unsurprising, given that similarly large numbers of cryptic species are being discovered in many other groups of fungal plant parasites (Roy et al., 1998; Pažoutová et al., 2015; Riess et al., 2019; Shoukouhi et al., 2019; Kemler et al., 2020; Liu et al., 2020). Moreover, taxonomists have long recognized the likelihood of cryptic speciation in several of the rust complexes we studied, but were unable to discern robust diagnostic features due to the limited number of characters that can be studied with traditional morphological approaches (Savile, 1972, 1973, 1984; Parmelee, 1989).
In Europe, narrowly circumscribed species have been in use for a long time in the notoriously difficult heteroecious rusts of Carex, giving greater weight to host range and ecology as compared with the broader “morphological species” recognized in North America (Banz & Zwetko, 2018). This was made possible by abundant cross-inoculation studies and careful observations in the field, data sources that are more limited in North America. Our molecular results suggest that the narrower species limits used in Europe may be more appropriate; however, future studies will need to confirm that the OTUs delimited here correspond to species rather than intraspecific variants. DNA barcoding promises to play an important role in rust species delimitation by replacing labor-intensive cross-inoculations and field observations with easily obtained molecular data when linking life stages on telial (monocot) and aecial (dicot) hosts. Combined with other modern techniques such as multi-locus approaches to species delimitation (Rintoul et al., 2012) and the re-examination of morphological features using electron microscopy and morphometric analysis (Zwetko & Blanz, 2012; Liu & Hambleton, 2013), the tools are now available to document the diversity of undescribed microfungi that occur on Cyperaceae and Juncaceae in North America. A detailed discussion of the taxonomic implications of the phylogenetic results for a few important rust species aggregates and their correlated microcyclic or autoecious relatives can be found in the supplementary material (Doc. S2).
4.5 Diversity begets diversity in the rusts of Carex
The genus Carex (>2000 species) is larger than 92% of all flowering plant families. With a distribution that spans all the continents except Antarctica, its species can be found in habitats as varied as tropical rain forests, deserts, prairies, and arctic tundra (Global Carex Group, 2015; Starr et al., 2015; Martín-Bravo et al., 2019). This broad diversity is probably the driving factor behind the importance of Carex as telial host to such a phylogenetically and taxonomically diverse collection of rusts. Our results provide evidence that Carex diversity has promoted the diversification of their rust parasites, because (i) the timing of origin of Carex and its major clades appears to coincide roughly with the origin and diversification of the Cyperaceae–Juncaceae rusts (Puccinia–Uromyces clade I sensu Maier et al., 2007), and (ii) several major rust lineages such as the P. urticata Clade and the P. caricina Clade are mostly restricted to a single small aecial host genus (respectively, Urtica and Ribes), and yet they appear to comprise numerous cryptic rust species or races that have probably evolved by specialization on one or a few Carex hosts. This is not entirely unexpected, as studies on other parasitic associations have shown that the diversification of plant hosts drives the diversification of their insect associates (Janz et al., 2006; Cruaud et al., 2012), and that taxonomically diverse host communities increase parasite diversity (e.g., in amphibians, Johnson et al., 2016; birds, Hechinger & Lafferty, 2005; and insect protective symbionts, Hafer & Vorburger, 2019). Simulations also suggest that rapidly diversifying host clades promote parasite diversification in the presence of preferential host shifts (Engelstädter & Fortuna, 2019).
The high rate of ecological niche evolution observed in rapidly radiating lineages of Carex (Pender, 2015; Spalink et al., 2016; Pender et al., 2021 in this issue) may also have played a role in the diversification of their rust fungi, by facilitating the evolution of new aecial host associations or by promoting ecological specialization. As more phylogenetic data accumulate on rust fungi and as taxonomic studies clarify species limits, diversity, and host ranges, it should soon be possible to explicitly test for correlations between rust and host diversification rates. We suspect that such studies will find that the amazing diversity of rust fungi on Carex is more than mere coincidence.
Acknowledgements
Development of molecular data for DAOM specimens was supported by funding from the Genomics Research and Development Initiative (GRDI-QIS, Project ID 2679) of the Government of Canada (https://grdi.canada.ca/en). For assistance in locating herbarium specimens and permitting DNA work, the authors thank Jennifer Wilkinson and Scott Redhead (Canadian National Mycological Herbarium, Ottawa, Canada). They also thank Sylvia Wilson and the Molecular Technologies Laboratory (MTL) at the Ottawa Research & Development Centre, specifically Julie T. Chapados, Kasia Dadej, Wayne McCormick, and Lisa Koziol, for technical assistance. Finally, they thank Julie Carey for preparing all the GenBank submissions. A part of this research was conducted while the first author was a Ph.D. student at the University of Ottawa (UofO) with support from an Alexander Graham Bell NSERC Research Scholarship, an FRQNT Doctoral Scholarship, and a UofO Excellence Scholarship. This work was also supported by National Science and Engineering Research Council of Canada (NSERC) Discovery Grants to JRS (RGPINs 342278-2013 and 2018-04115). Two anonymous reviewers provided helpful comments on an early version of this manuscript.




